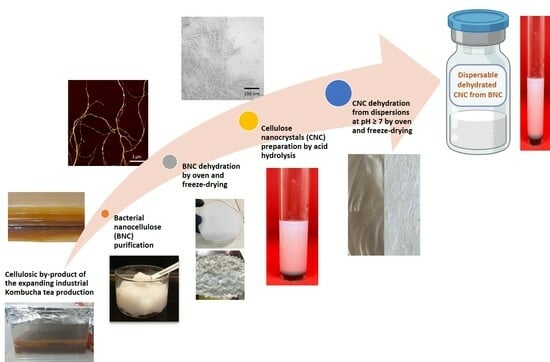
Valorization of a Residue of the Kombucha Beverage Industry Through the Production of Dehydrated Water Dispersible Cellulose Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
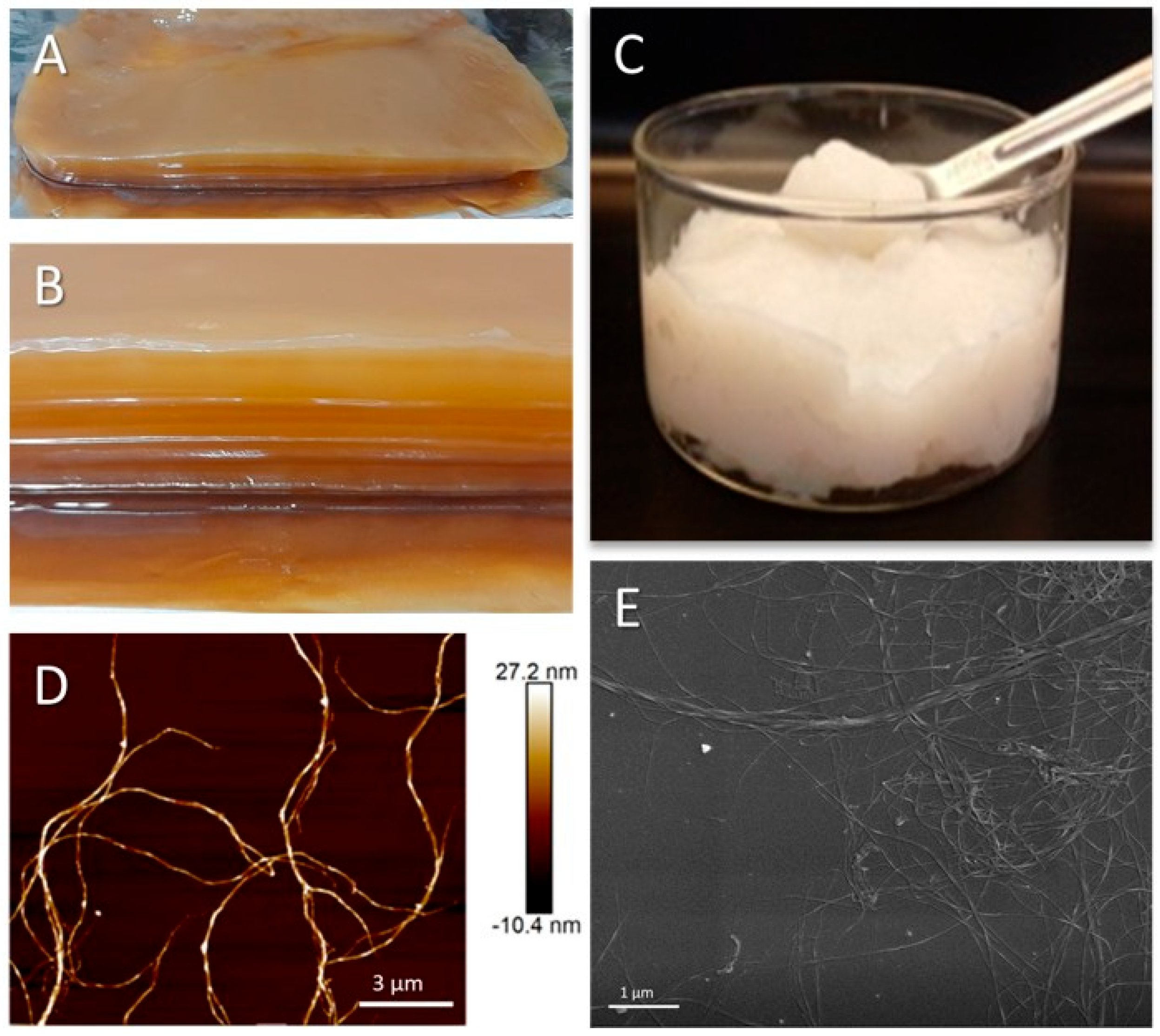
2.2. Production of Bacterial Nanocellulose
2.3. Production of Cellulose Nanocrystals
2.4. Characterization of Nanocelluloses
2.4.1. Field Emission Scanning Electron Microscopy (FE-SEM)
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. Atomic Force Microscopy (AFM)
2.4.4. Zeta Potential
2.4.5. X-Ray Diffraction (XRD)
2.4.6. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Dehydration of CNC Suspensions
2.6. Water Dispersion of Dehydrated CNC Samples
2.7. Statistical Analysis
3. Results and Discussion
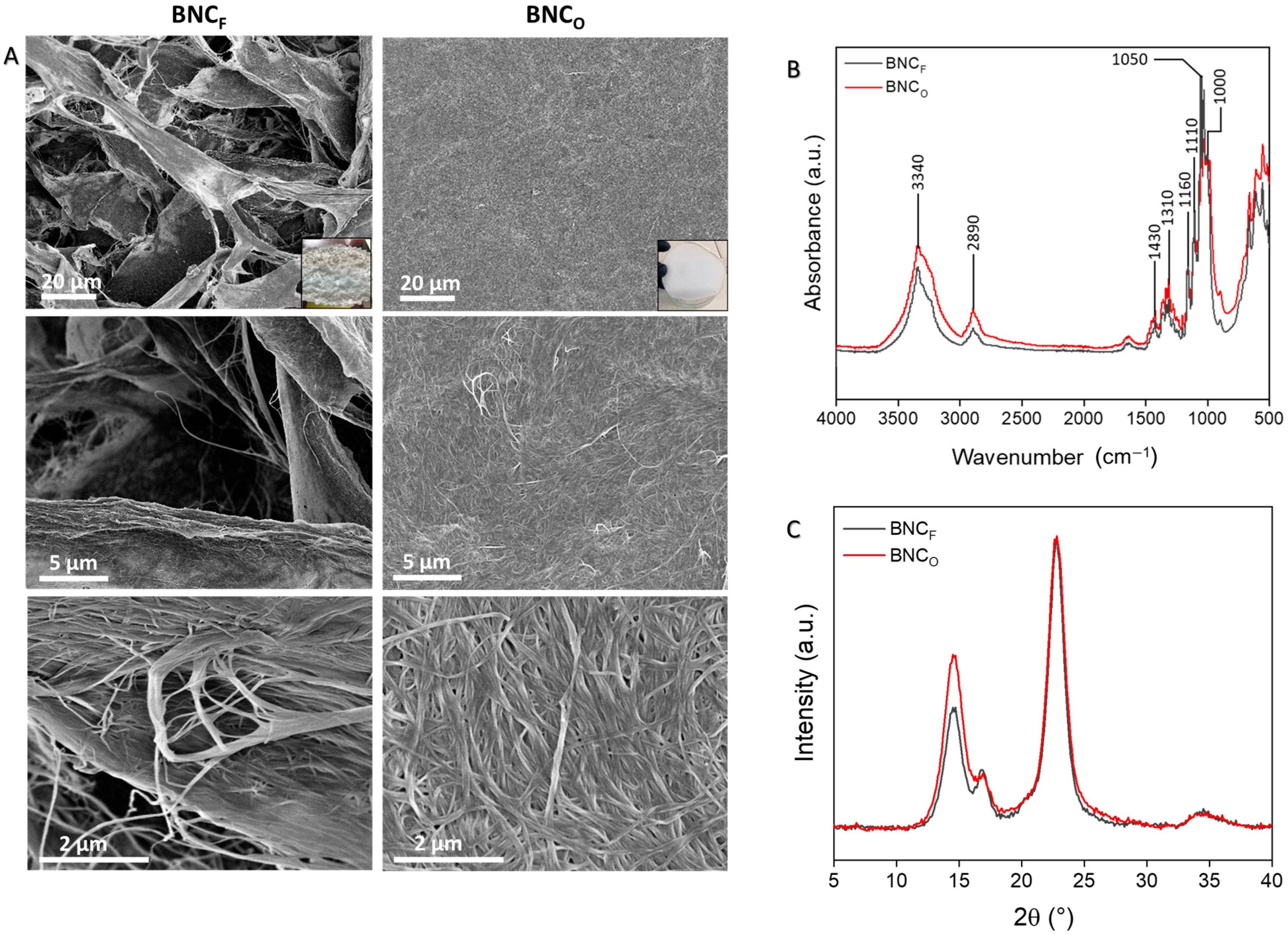
3.1. Production and Characterization of BNC
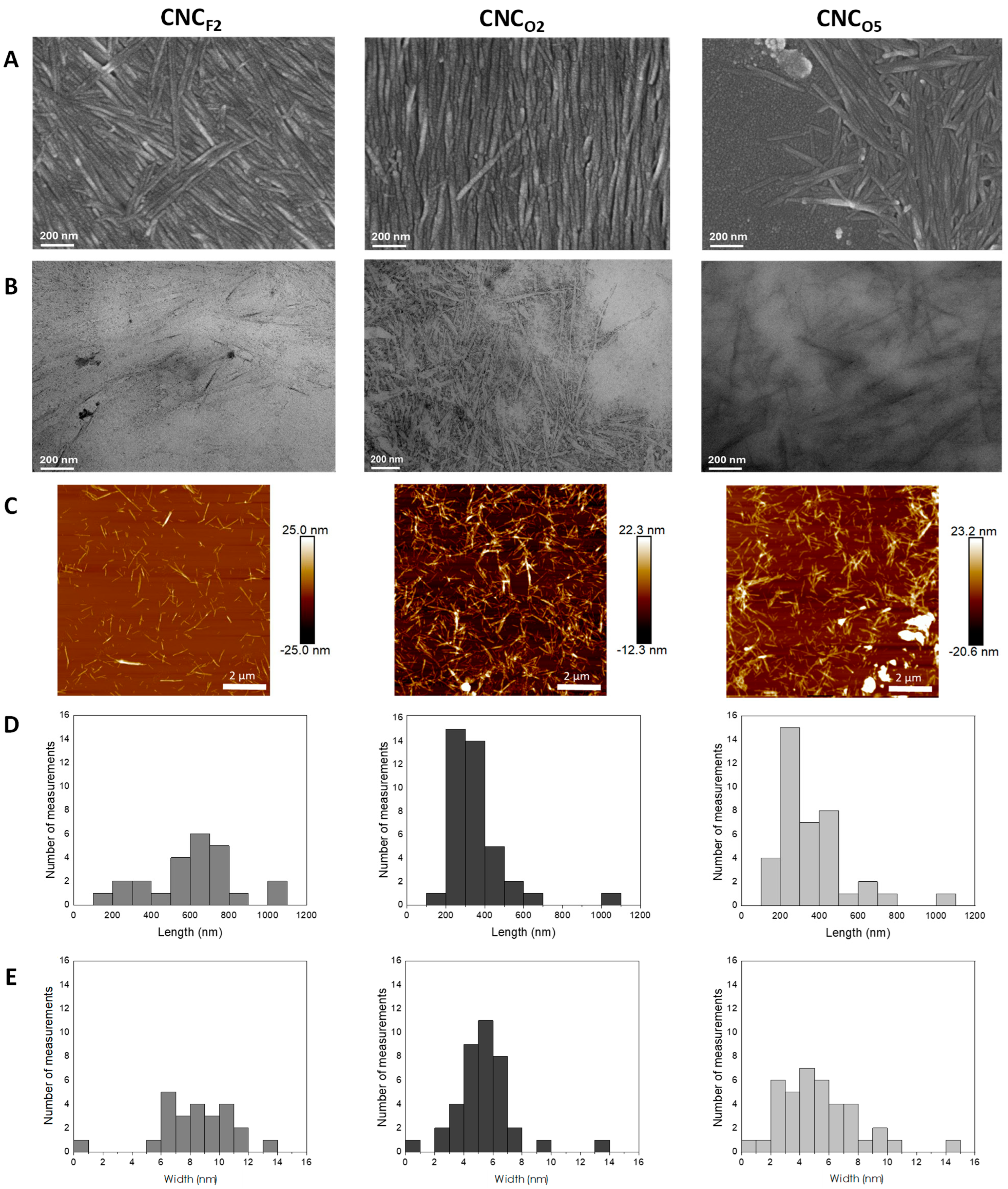
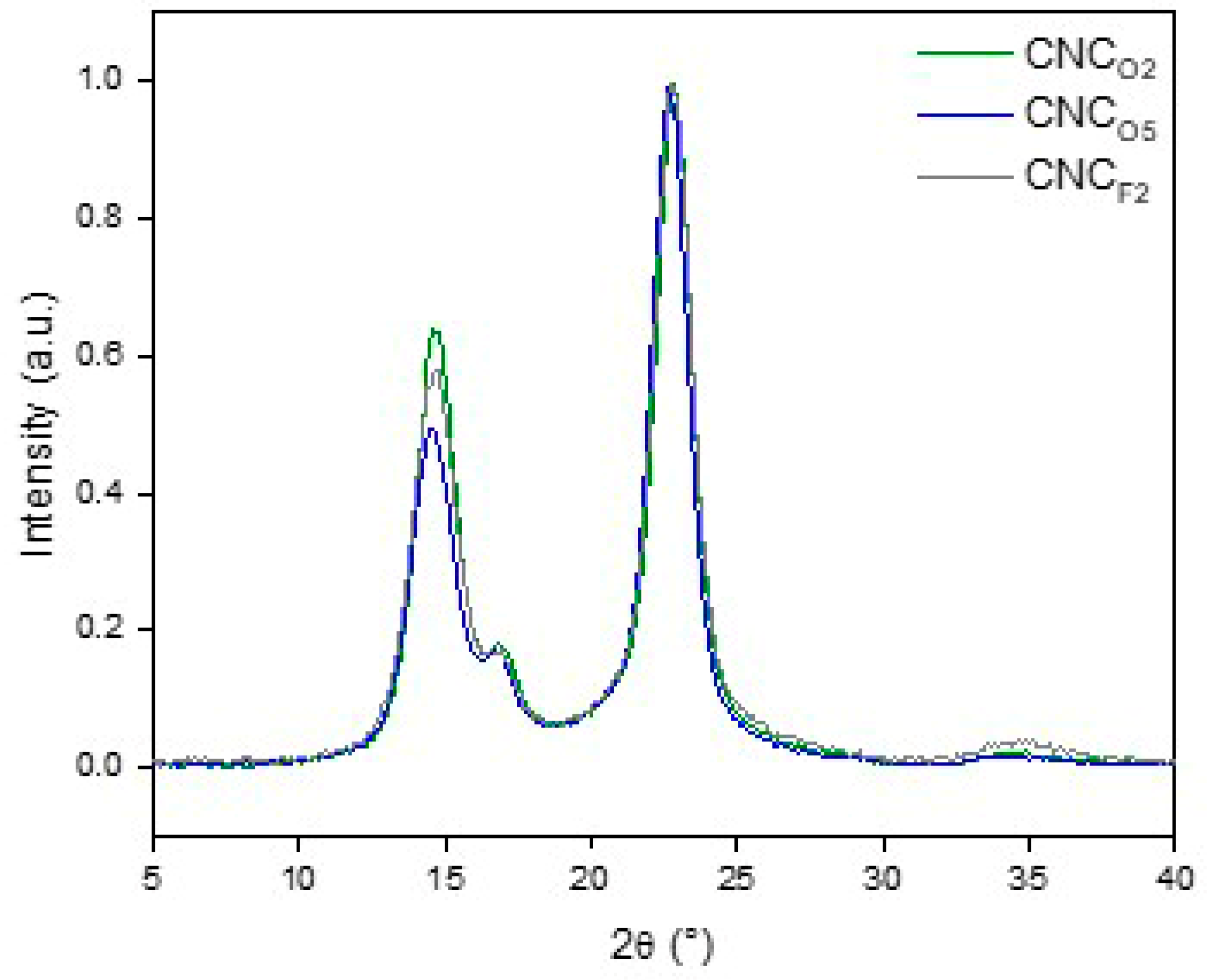
3.2. Production and Characterization of CNCs
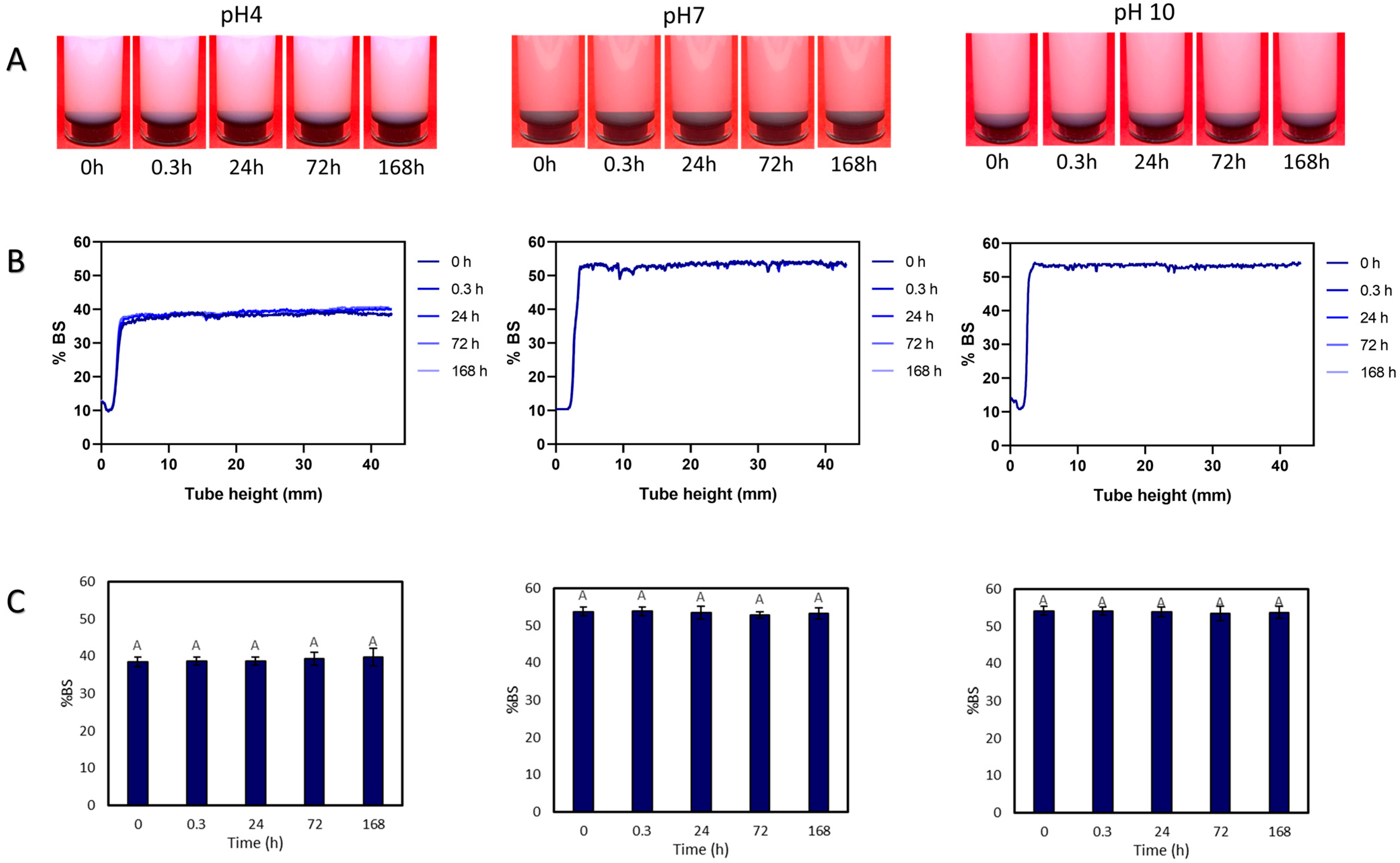
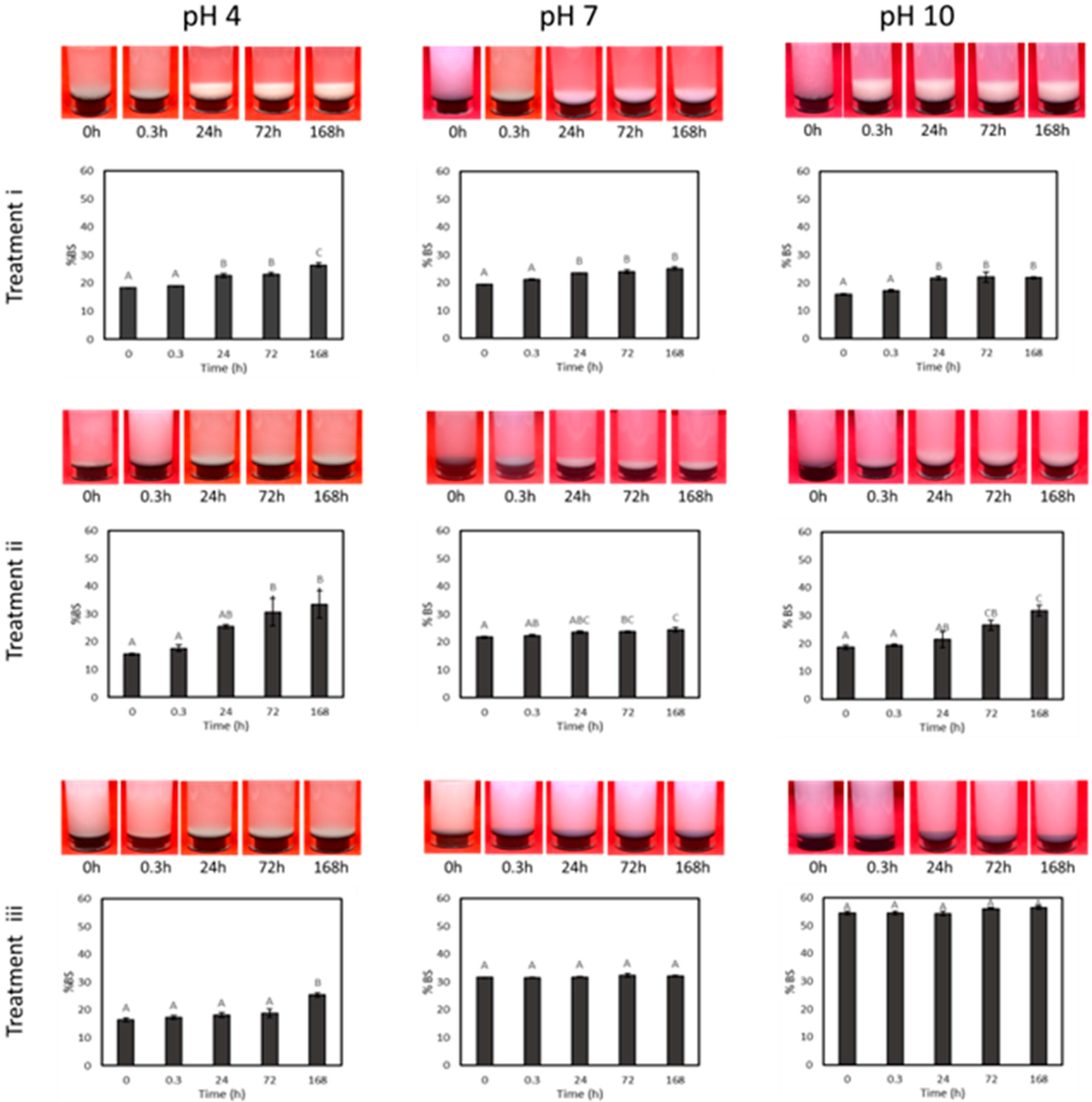
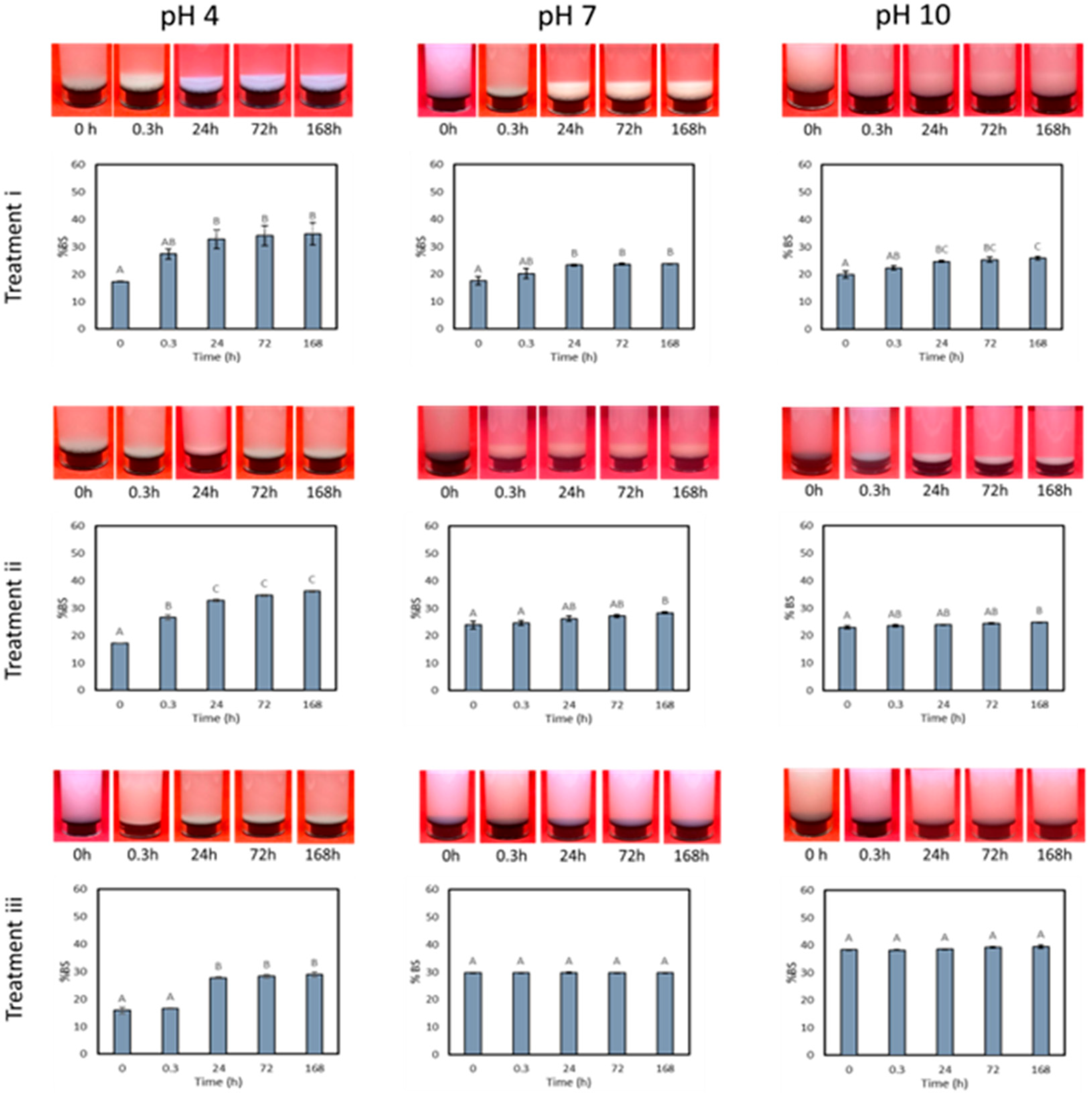
3.3. Drying and Dispersion of CNCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- ISO-TS 20477; 2017 (E): Nanotechnologies: Standard Terms and Their Definition for Cellulose Nanomaterial. International Organization for Standardization: Geneva, Switzerland, 2017.
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; Walter de Gruyter GmbH & Co KG.: Berlin, Germany; Boston, MA, USA, 2017. [Google Scholar]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Boix, A.C. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kassie, B.B.; Daget, T.M.; Tassew, D.F. Synthesis, functionalization, and commercial application of cellulose-based nanomaterials: A review. Int. J. Biol. Macromol. 2024, 278, 134990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.P.; Peng, F.; Bian, J.; Yuan, T.Q.; Xu, F.; Sun, R.C. Microwave-assisted solvent-free acetylation of cellulose with acetic anhydride in the presence of iodine as a catalyst. Molecules 2009, 14, 3551–3566. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Cellulose isolation and core–shell nanostructures of cellulose nanocrystals from chardonnay grape skins. Carbohydr. Polym. 2012, 87, 2546–2553. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Kishore, K.A. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, Y.; Liza, A.A.; Yang, G.; Sipponen, M.H.; Guo, J.; Li, H. Nanocellulose: A review on preparation routes and applications in functional materials. Cellulose 2023, 30, 4115–4147. [Google Scholar] [CrossRef]
- Rashid, A.B.; Hoque, M.E.; Kabir, N.; Rifat, F.F.; Ishrak, H.; Alqahtani, A.; Chowdhury, M.E. Synthesis, properties, applications, and future prospective of cellulose nanocrystals. Polymers 2023, 15, 4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, H.; Xu, X.; Li, L.; Kang, H.; Li, D. Recent advancements in the synthesis, functionalization, and utilization of cellulose nanocrystals. Resour. Chem. Mater. 2024, 4, 100073. [Google Scholar] [CrossRef]
- Arserim-Uçar, D.K.; Korel, F.; Liu, L.; Yam, K.L. Characterization of bacterial cellulose nanocrystals: Effect of acid treatments and neutralization. Food Chem. 2021, 336, 127597. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, N.B.; Almeida-Lima, J.; Rocha, H.A.O.; Medeiros, S.R.B.; Alves Jr, C.; Gama, F.M. BC nanofibres: In vitro study of genotoxicity and cell proliferation. Toxicol. Lett. 2009, 189, 235–241. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial efficacy of nisin-loaded bacterial cellulose nanocrystals against selected meat spoilage lactic acid bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial cellulose-based materials as dressings for wound healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; de Souza, M.D.S.M.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Bovi, J.; Butto, M.; Arroyo, S.; Bernal, C.; Foresti, M.L. Bacterial cellulose nanofibrils from a by-product of the growing kombucha business: Obtention and use in the development of poly (Lactic Acid) Composites. Lat. Am. Appl. Res.-Int. J. 2024, 54, 55–62. [Google Scholar] [CrossRef]
- Behera, B.; Laavanya, D.; Balasubramanian, P. Techno-economic feasibility assessment of bacterial cellulose biofilm production during the Kombucha fermentation process. Bioresour. Technol. 2022, 346, 126659. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Tapias, Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by Kombucha symbiotic community cultured on different herbal infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef]
- Posada, P.; Velásquez-Cock, J.; Gómez-Hoyos, C.; Serpa Guerra, A.M.; Lyulin, S.V.; Kenny, J.M.; Gañán, P.; Castro, C.; Zuluaga, R. Drying and redispersion of plant cellulose nanofibers for industrial applications: A review. Cellulose 2020, 27, 10649–10670. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Martín, L.; Salgado, P.R.; Mauri, A.N. Synthesis and conservation of cellulose nanocrystals. Carbohydr. Polym. 2020, 238, 116187. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Nanocelluloses from phormium (Phormium tenax) fibers. Cellulose 2020, 27, 4975–4990. [Google Scholar] [CrossRef]
- Porfiri, M.C.; Wagner, J.R. Extraction and characterization of soy hull polysaccharide-protein fractions. Analysis of aggregation and surface rheology. Food Hydrocoll. 2018, 79, 40–47. [Google Scholar] [CrossRef]
- Segal, L.G.J.M.A.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of nativecellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bovi, J.; Delgado, J.F.; de la Osa, O.; Peltzer, M.A.; Bernal, C.R.; Foresti, M.L. Biobased composites of poly (Lactic Acid) Melt compounded with bacterial and vegetal nanocelluloses incorporated through different strategies. Polymers 2024, 16, 898. [Google Scholar] [CrossRef]
- Clasen, C.; Sultanova, B.; Wilhelms, T.; Heisig, P.; Kulicke, W.M. Effects of different drying processes on the material properties of bacterial cellulose membranes. Macromol. Symp. 2006, 244, 48–58. [Google Scholar] [CrossRef]
- Illa, M.P.; Sharma, C.S.; Khandelwal, M. Tuning the physiochemical properties of bacterial cellulose: Effect of drying conditions. J. Mater. Sci. 2019, 54, 12024–12035. [Google Scholar] [CrossRef]
- Pa’e, N.; Hamid, N.I.A.; Khairuddin, N.; Zahan, K.A.; Seng, K.F.; Siddique, B.M.; Muhamad, I.I. Effect of different drying methods on the morphology, crystallinity, swelling ability and tensile properties of nata de coco. Sains Malays. 2014, 43, 767–773. [Google Scholar]
- Rossi, E.; Salvay, A.G.; Errea, M.I.; Foresti, M.L. Dried water-redispersible bacterial nanocellulose with sorbitol as capping agent. Food Hydrocoll. 2023, 143, 108916. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133, 43109–43118. [Google Scholar] [CrossRef]
- Arteaga-Ballesteros, B.E.; Guevara-Morales, A.; Martín-Martínez, E.S.; Figueroa-López, U.; Vieyra, H. Composite of polylactic acid and microcellulose from kombucha membranes. e-Polymers 2020, 21, 015–026. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Bacterial cellulose nanocrystals (BCNC) preparation and characterization from three bacterial cellulose sources and development of functionalized BCNCs as nucleic acid delivery systems. ACS Appl. Nano Mater. 2017, 1, 209–221. [Google Scholar] [CrossRef]
- Pääkkönen, T.; Spiliopoulos, P.; Nonappa, N.; Kontturi, K.S.; Penttilä, P.; Viljanen, M.; Svedström, K.; Kontturi, E. Sustainable high yield route to cellulose nanocrystals from bacterial cellulose. ACS Sustain. Chem. Eng. 2019, 7, 14384–14388. [Google Scholar] [CrossRef]
- Pirich, C.L.; de Freitas, R.A.; Woehl, M.A.; Picheth, G.F.; Petri, D.F.; Sierakowski, M.R. Bacterial cellulose nanocrystals: Impact of the sulfate content on the interaction with xyloglucan. Cellulose 2015, 22, 1773–1787. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Yang, F.; Dong, H.; Bian, Y.; Jia, H.; Zhang, J. Using Cellulose Nanocrystal as Adjuvant to Improve the Dispersion Ability of Multilayer Graphene in Aqueous Suspension. Front. Bioeng. Biotechnol. 2021, 9, 638744. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Coulter QuickSCAN 2024. Available online: http://www.cyto.purdue.edu/cdroms/cyto2/6/coulter/ss000098.htm (accessed on 9 May 2024).
- Beuguel, Q.; Tavares, J.R.; Carreau, P.J.; Heuzey, M.C. Ultrasonication of spray-and freeze-dried cellulose nanocrystals in water. J. Colloid Interface Sci. 2018, 516, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.; Holt, G. Sonication amplitude and processing time influence the cellulose nanocrystals morphology and dispersion. Nanocomposites 2020, 6, 41–46. [Google Scholar] [CrossRef]
- Miao, S.; Roos, Y.H. Comparison of nonenzymatic browning kinetics in spray-dried and freeze-dried carbohydrate-based food model systems. J. Food Sci. 2004, 69, 322–331. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos. Trans. R. Soc. A 2018, 376, 20170041. [Google Scholar] [CrossRef]
| Sample | BNC Drying Treatment | BNC Suspension Content (Dry Weight, wt.%) | ||
|---|---|---|---|---|
| Freeze-Drying (F) | Oven-Drying (O) | 2 | 5 | |
| CNCF2 | x | - | x | - |
| CNCF5 | x | - | - | x |
| CNCO2 | - | x | x | - |
| CNCO5 | - | x | - | x |
| Sample | Yield (%) | Length (nm) | Width (nm) | Z-Potential (mV) | CrI (%) (Segal’s Method) | CrI (%) (Two Phase Method) |
|---|---|---|---|---|---|---|
| CNCF2 | 83 ± 0.3 | 630 ± 201 | 9 ± 2 | −23 ± 2.4 | 93 ± 1 | 78 ± 1 |
| CNCO2 | 85 ± 2.5 | 331 ± 103 | 6 ± 2 | −38 ± 3.3 | 92 ± 1 | 80 ± 1 |
| CNCO5 | 67 ± 3.9 | 350 ± 127 | 5 ± 3 | −17 ± 1.4 | 93 ± 1 | 77 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, L.G.; Di Giorgio, L.; Foresti, M.L.; Mauri, A.N. Valorization of a Residue of the Kombucha Beverage Industry Through the Production of Dehydrated Water Dispersible Cellulose Nanocrystals. Polysaccharides 2025, 6, 44. https://doi.org/10.3390/polysaccharides6020044
Alonso LG, Di Giorgio L, Foresti ML, Mauri AN. Valorization of a Residue of the Kombucha Beverage Industry Through the Production of Dehydrated Water Dispersible Cellulose Nanocrystals. Polysaccharides. 2025; 6(2):44. https://doi.org/10.3390/polysaccharides6020044
Chicago/Turabian StyleAlonso, Laura Giselle, Luciana Di Giorgio, María Laura Foresti, and Adriana Noemi Mauri. 2025. "Valorization of a Residue of the Kombucha Beverage Industry Through the Production of Dehydrated Water Dispersible Cellulose Nanocrystals" Polysaccharides 6, no. 2: 44. https://doi.org/10.3390/polysaccharides6020044
APA StyleAlonso, L. G., Di Giorgio, L., Foresti, M. L., & Mauri, A. N. (2025). Valorization of a Residue of the Kombucha Beverage Industry Through the Production of Dehydrated Water Dispersible Cellulose Nanocrystals. Polysaccharides, 6(2), 44. https://doi.org/10.3390/polysaccharides6020044