Functionalized Bacterial Cellulose: A Potential Sustainable Adsorbent for Methylene Blue Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production and Functionalization of Bacterial Cellulose (BC)
2.3. Characterization of Bacterial Cellulose
2.4. Adsorption Experiments
2.5. Statistical Analysis
3. Results
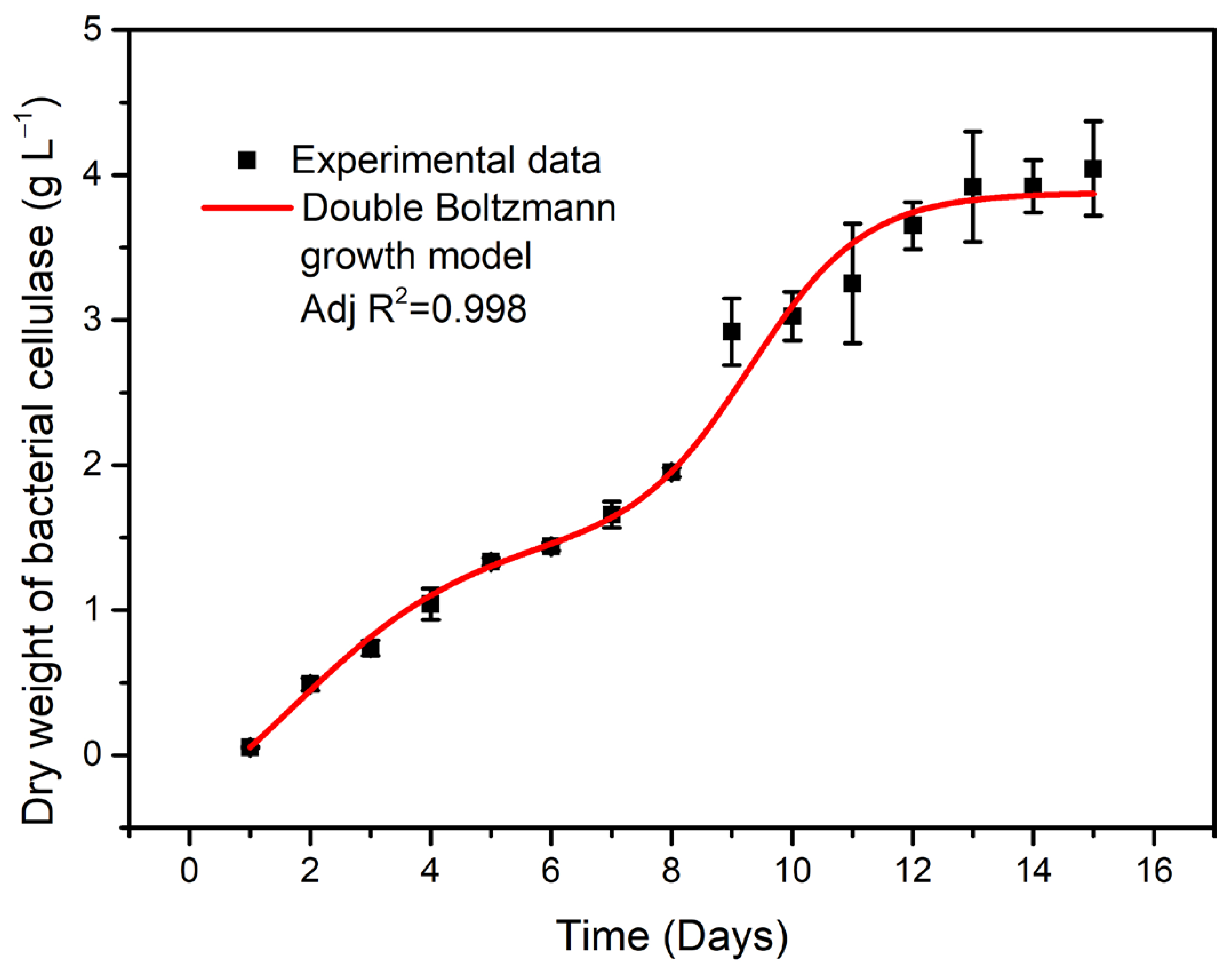
3.1. Production and Functionalization of Bacterial Cellulose (BC)
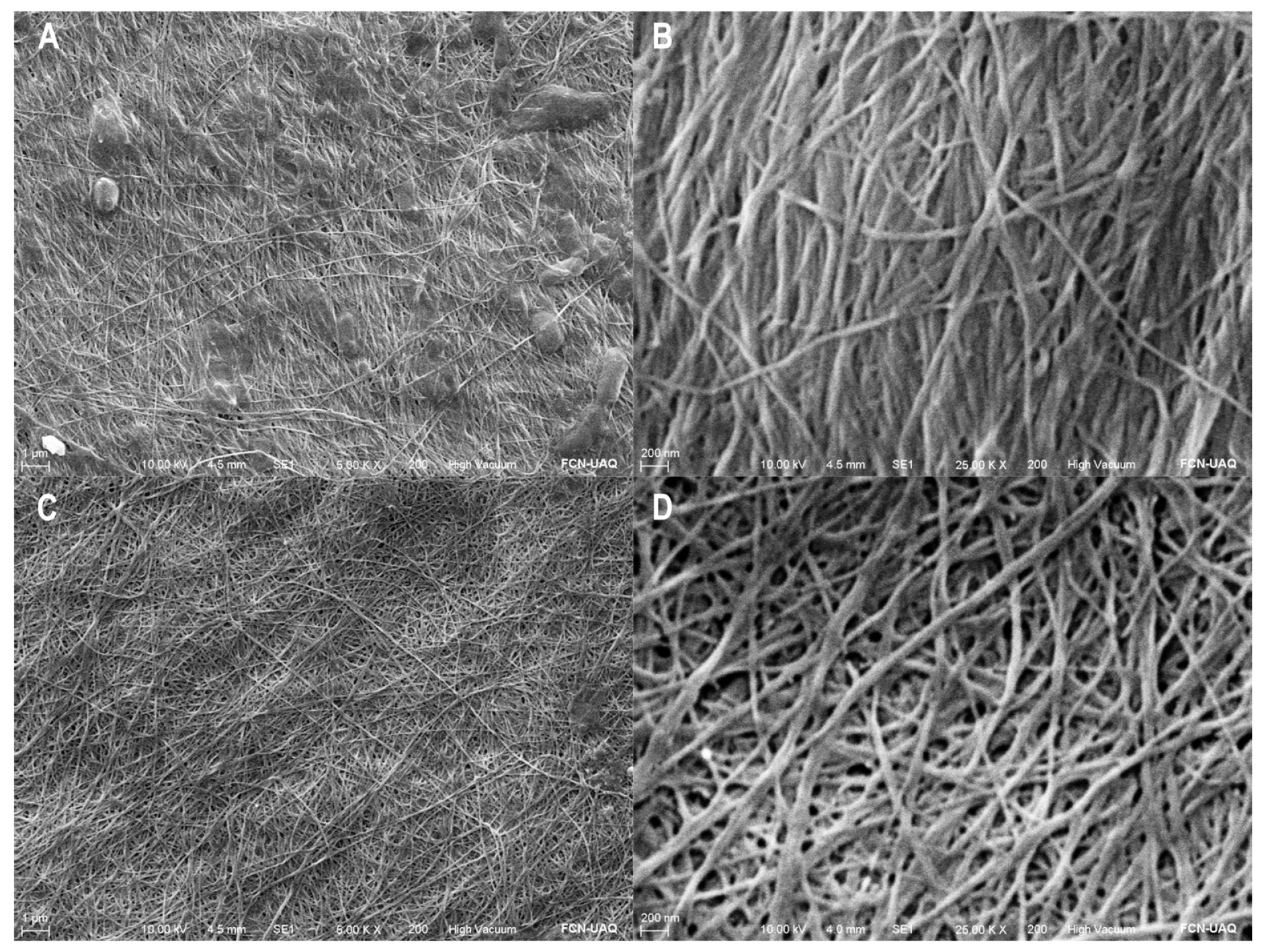
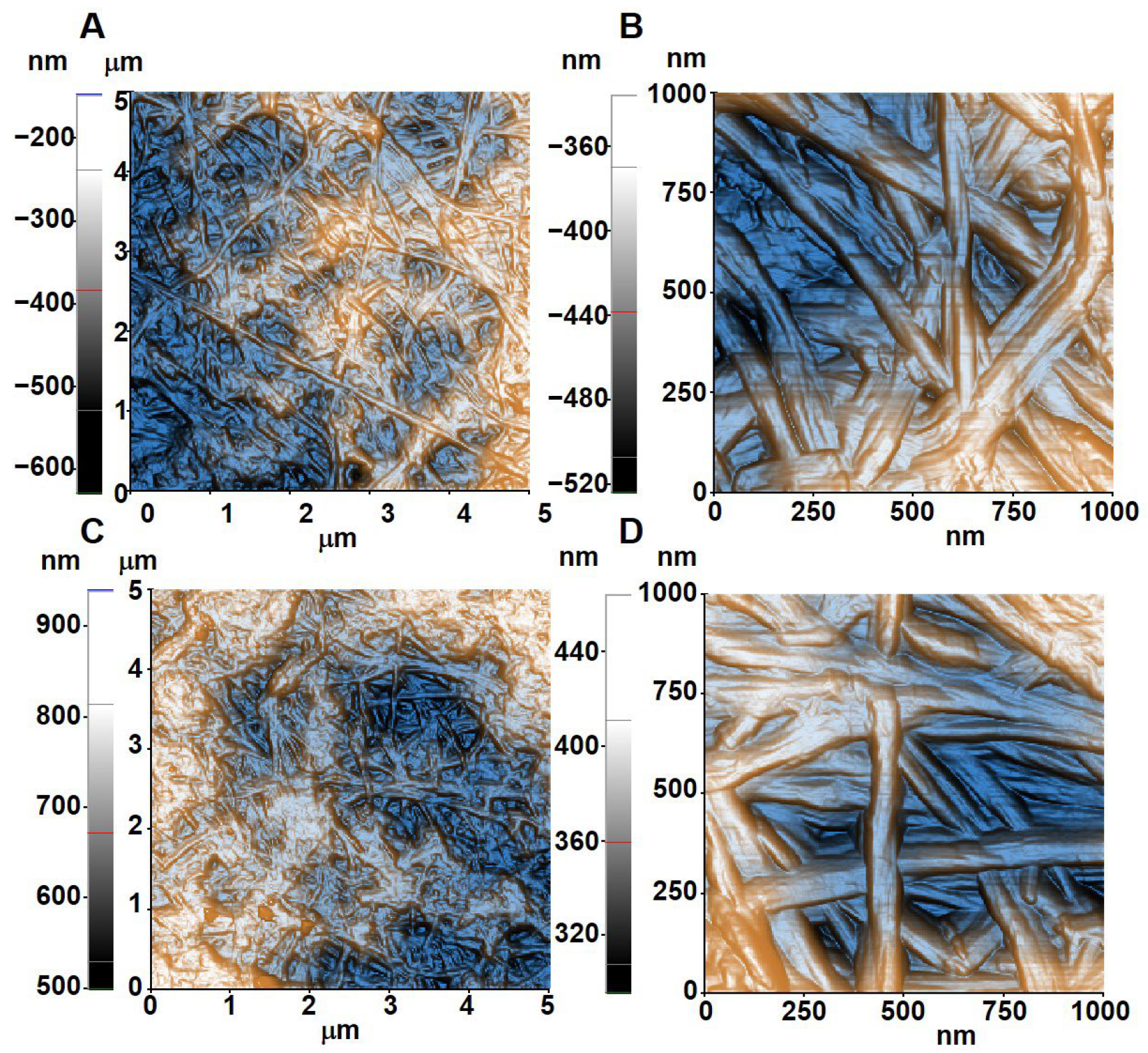
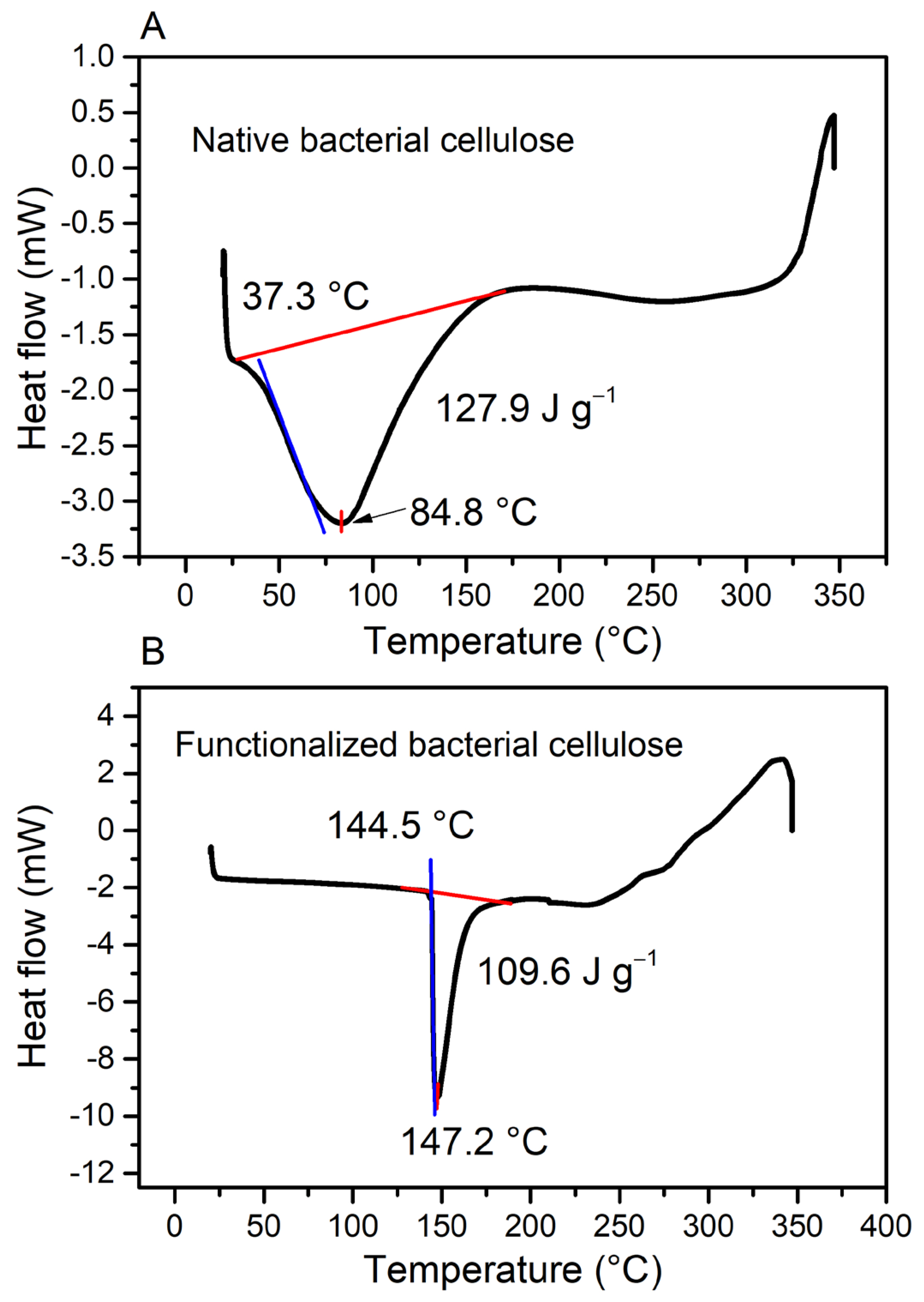
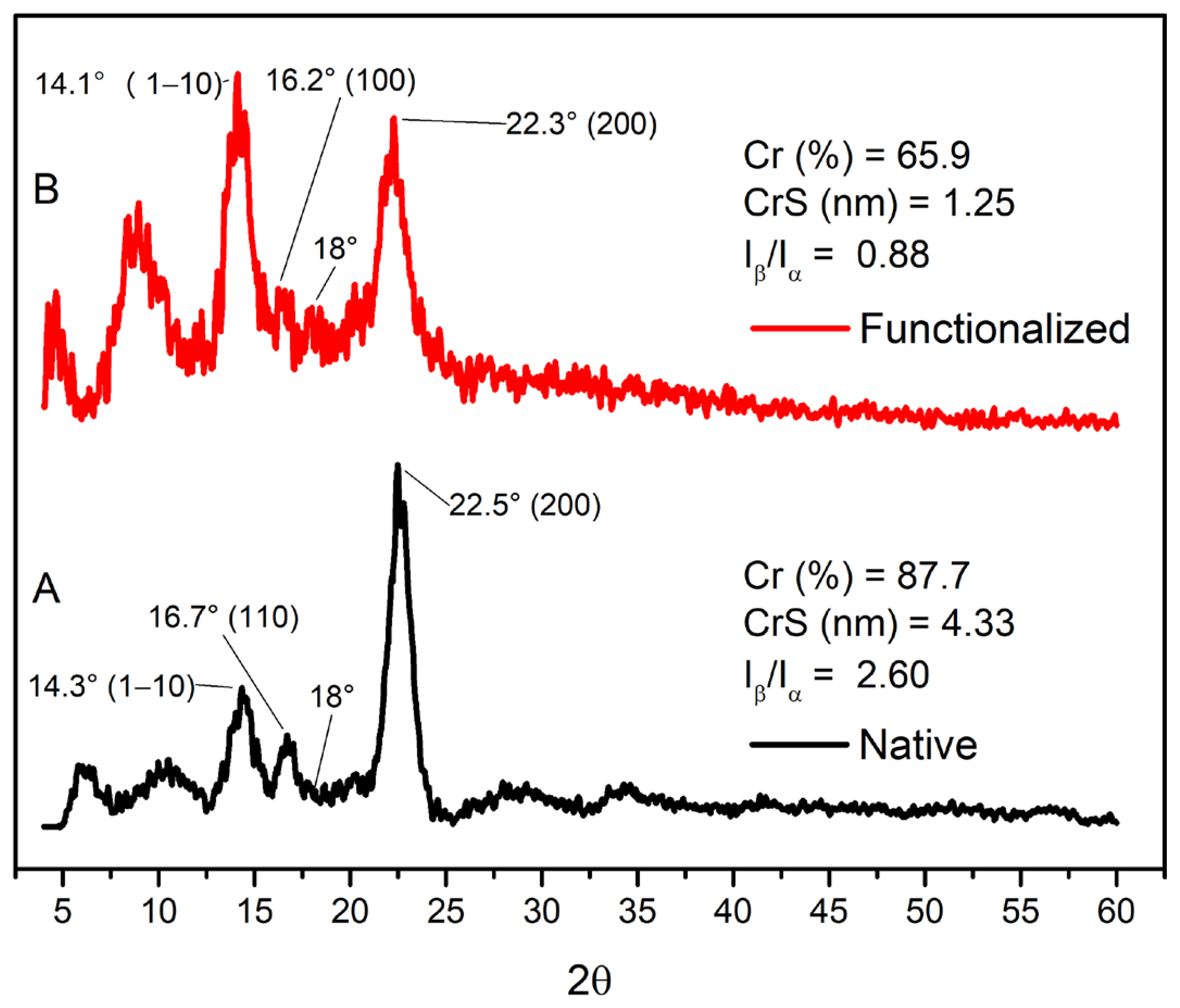
3.2. Characteristics of Adsorbent
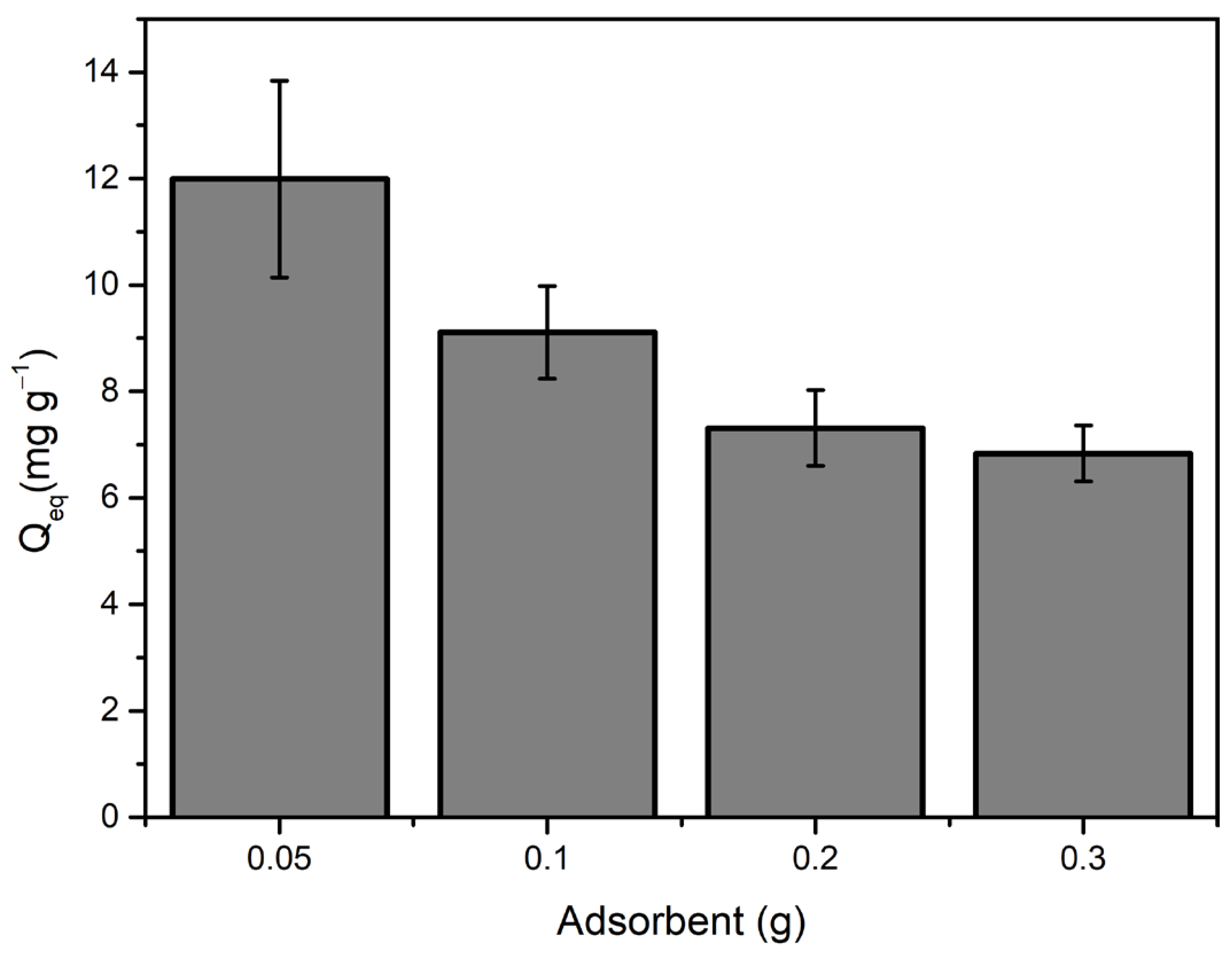
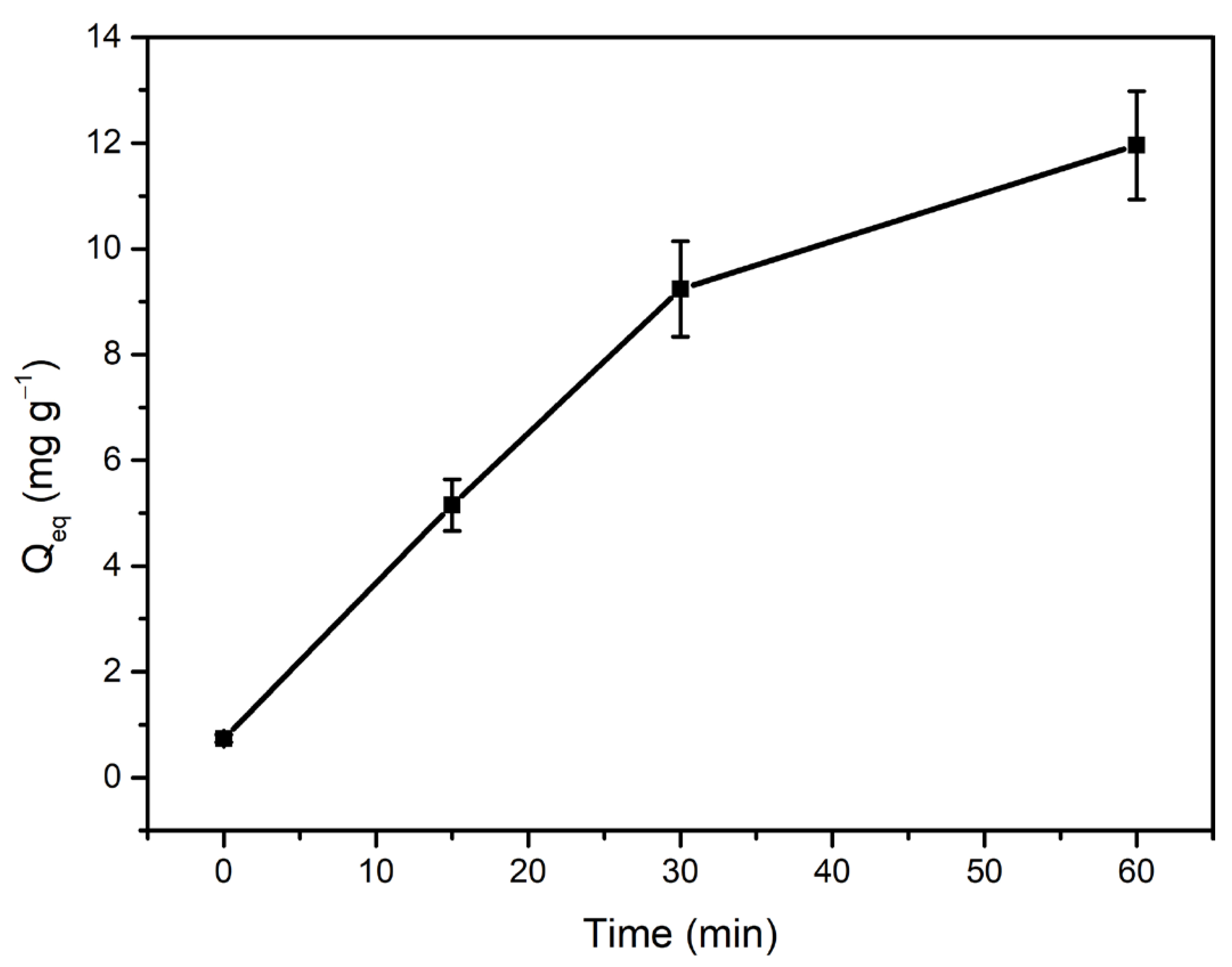
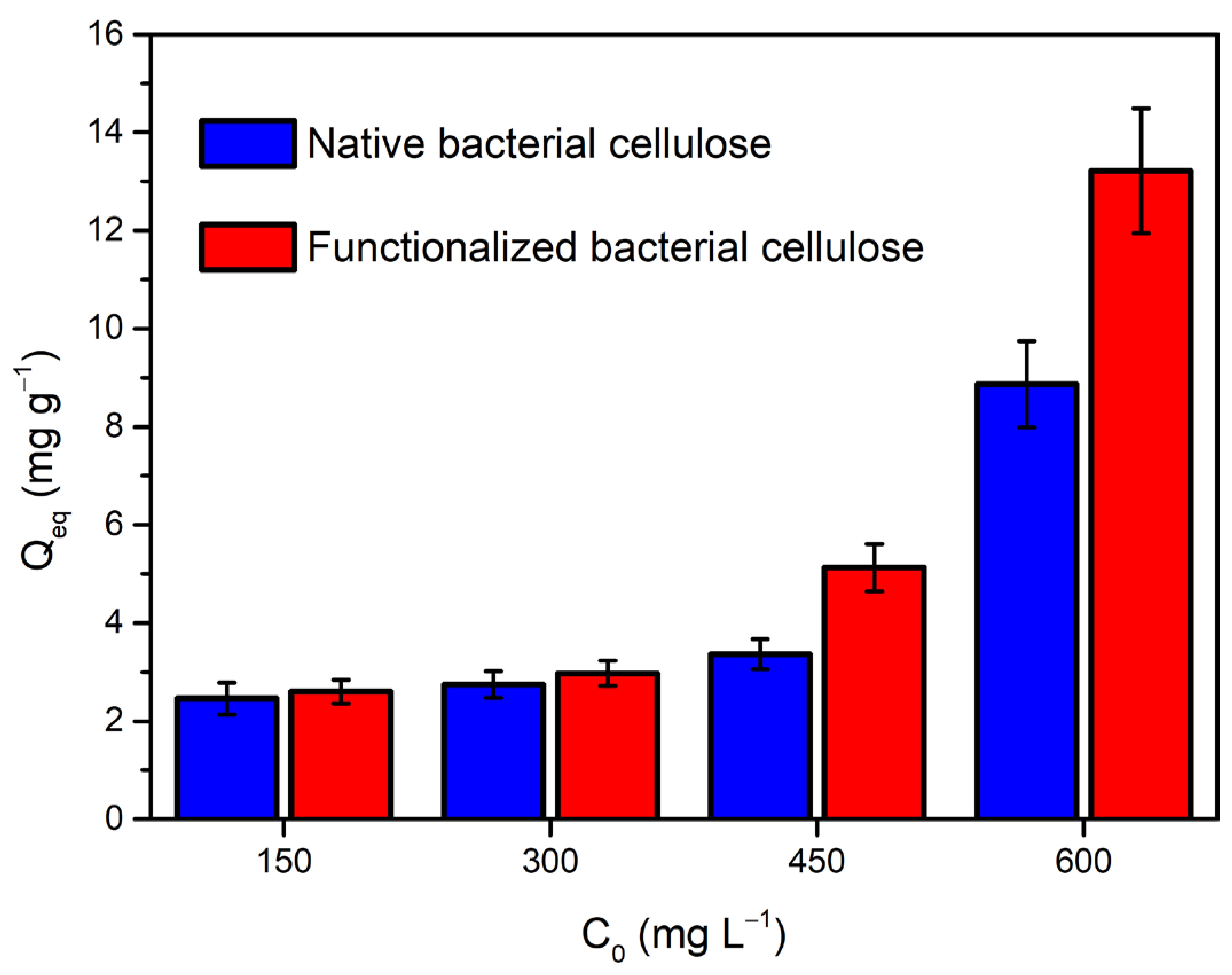
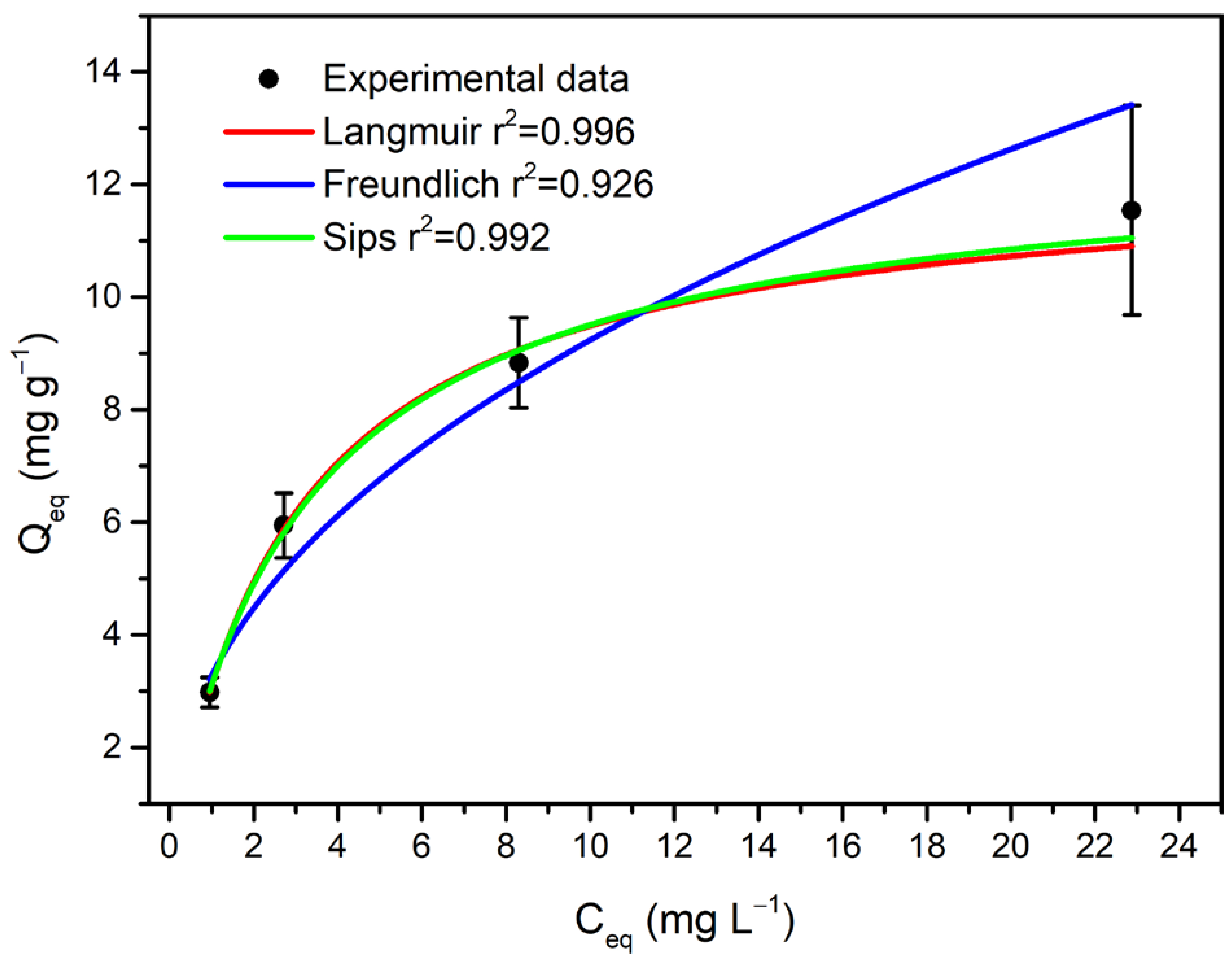
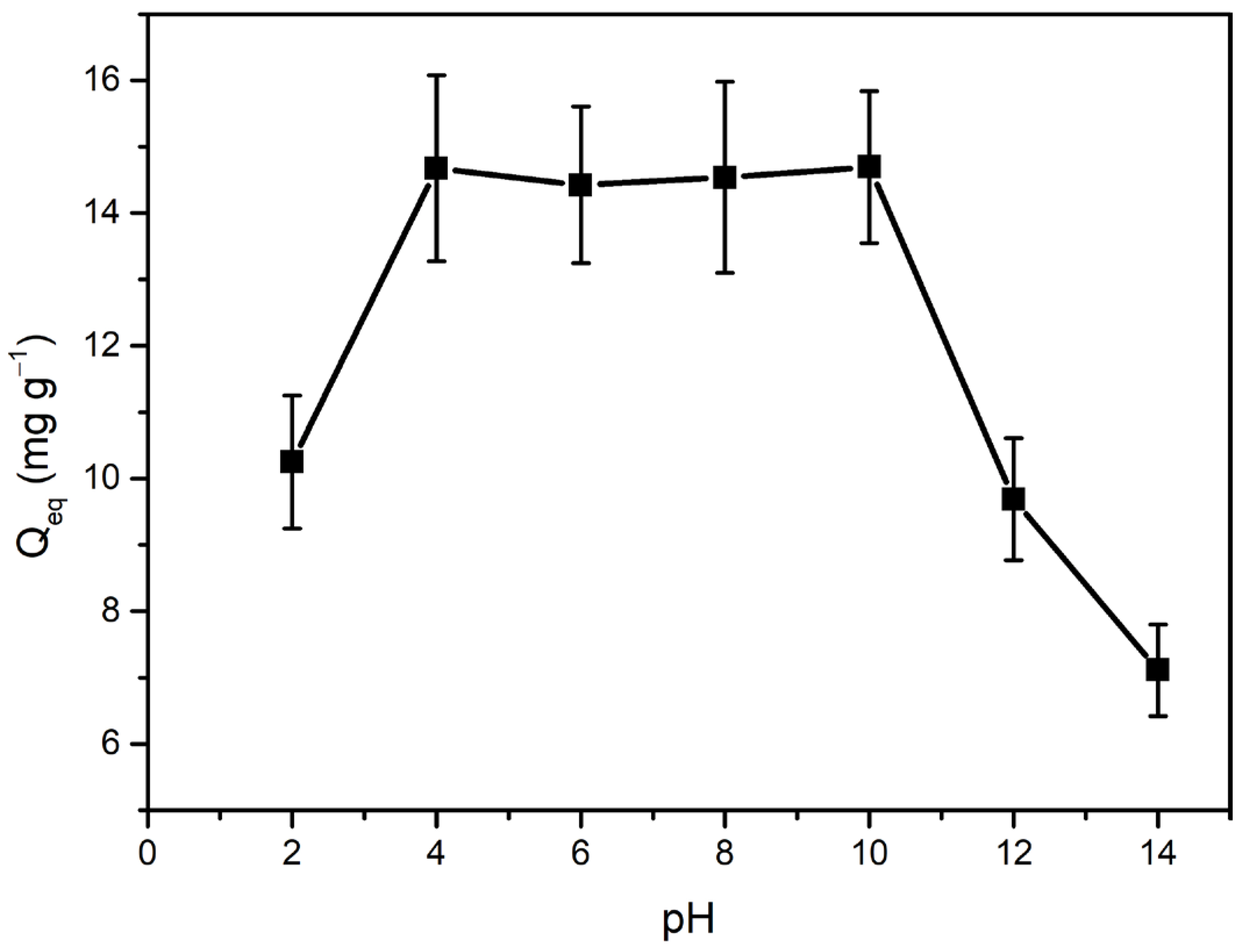
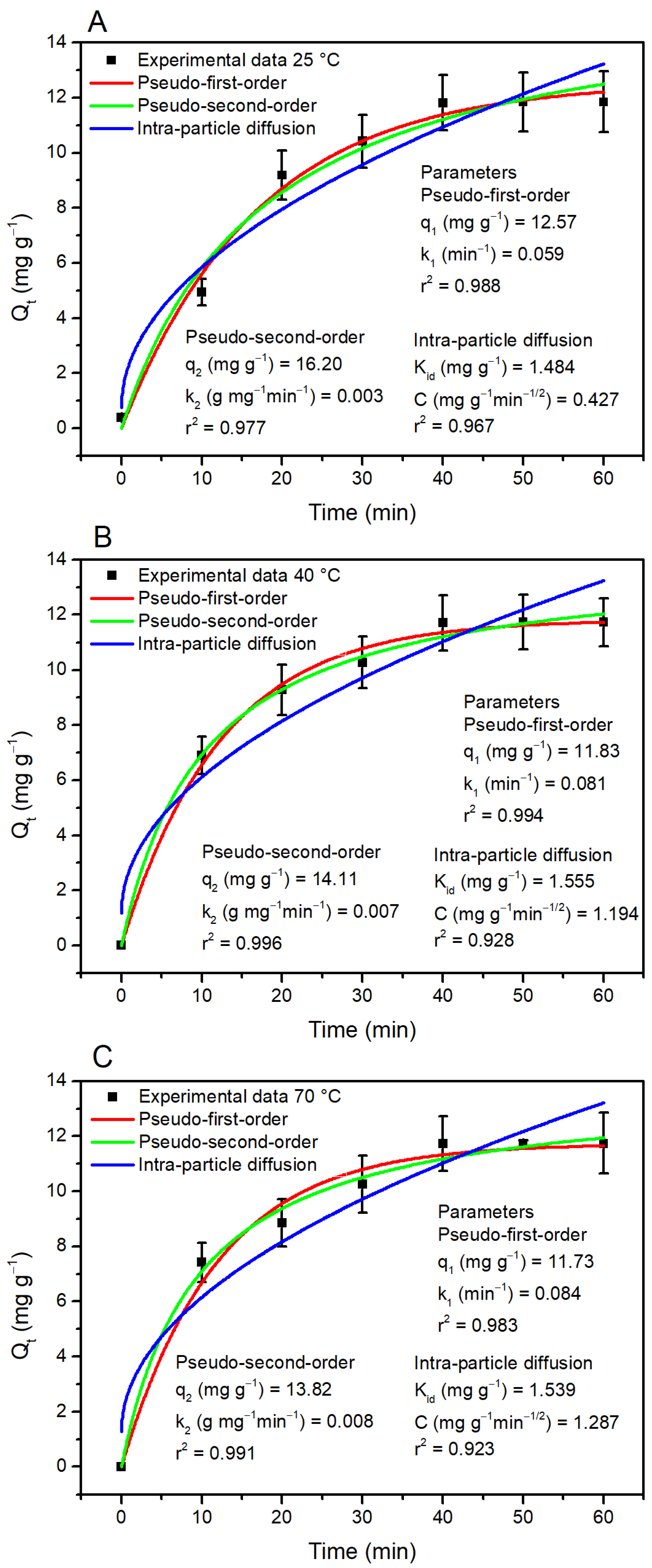
3.3. Adsorption Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omer, A.M.; Sadik, W.A.; Abbas, R.; Tamer, T.M.; Abd-Ellatif, M.M.; Mohy-Eldin, M.S. Novel Amino-Ethyl Carboxymethyl Cellulose Crosslinked Ampholyte Hydrogel Development for Methyl Orange Removal from Waste Water. Sci. Rep. 2024, 14, 14701. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ha, L.Q.; Nguyen, T.D.L.; Ly, P.H.; Nguyen, D.M.; Hoang, D. Nanocellulose and Graphene Oxide Aerogels for Adsorption and Removal Methylene Blue from an Aqueous Environment. ACS Omega 2022, 7, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Advancements in Cellulose-Based Superabsorbent Hydrogels: Sustainable Solutions across Industries. Gels 2024, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; El Makhfouk, M. Dried Prickly Pear Cactus (Opuntia ficus indica) Cladodes as a Low-Cost and Eco-Friendly Biosorbent for Dyes Removal from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2013, 44, 52–60. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.I.; Dotto, G.L.; Tanabe, E.H.; Sellaoui, L.; Carvalho, S.H.V.; et al. Adsorption of Methylene Blue on Agroindustrial Wastes: Experimental Investigation and Phenomenological Modelling. Prog. Biophys. Mol. Biol. 2019, 141, 60–71. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-Based Films and Membranes: A Comprehensive Review on Preparation and Applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Dang, X.; Li, N.; Yu, Z.; Ji, X.; Yang, M.; Wang, X. Advances in the Preparation and Application of Cellulose-Based Antimicrobial Materials: A Review. Carbohydr. Polym. 2024, 342, 122385. [Google Scholar] [CrossRef]
- Candra, A.; Ahmed, Y.W.; Kitaw, S.L.; Anley, B.E.; Chen, K.-J.; Tsai, H.-C. A Green Method for Fabrication of a Biocompatible Gold-Decorated-Bacterial Cellulose Nanocomposite in Spent Coffee Grounds Kombucha: A Sustainable Approach for Augmented Wound Healing. J. Drug Deliv. Sci. Technol. 2024, 94, 105477. [Google Scholar] [CrossRef]
- Quijano, L.; Rodrigues, R.; Fischer, D.; Tovar-Castro, J.D.; Payne, A.; Navone, L.; Hu, Y.; Yan, H.; Pinmanee, P.; Poon, E.; et al. Bacterial Cellulose Cookbook: A Systematic Review on Sustainable and Cost-Effective Substrates. J. Bioresour. Bioprod. 2024, 9, 379–409. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Tang, Y. Constructing Bacterial Cellulose and Its Composites: Regulating Treatments towards Applications. Cellulose 2024, 31, 7793–7817. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Xue, L.; Sun, H.; Guo, Z.; Zhang, Y.; Yang, L. Carboxylic Acid Functionalized Sesame Straw: A Sustainable Cost-Effective Bioadsorbent with Superior Dye Adsorption Capacity. Bioresour. Technol. 2017, 238, 675–683. [Google Scholar] [CrossRef]
- Villabona-Ortíz, Á.; Figueroa-Lopez, K.J.; Ortega-Toro, R. Kinetics and Adsorption Equilibrium in the Removal of Azo-Anionic Dyes by Modified Cellulose. Sustainability 2022, 14, 3640. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Zhang, Z.; Li, P.; He, C.; Zhong, M. Pb(II) Adsorption Properties of a Three-Dimensional Porous Bacterial Cellulose/Graphene Oxide Composite Hydrogel Subjected to Ultrasonic Treatment. Materials 2024, 17, 3053. [Google Scholar] [CrossRef] [PubMed]
- Agüero, Á.; Corral Perianes, E.; Abarca de las Muelas, S.S.; Lascano, D.; de la Fuente García-Soto, M.d.M.; Peltzer, M.A.; Balart, R.; Arrieta, M.P. Plasticized Mechanical Recycled PLA Films Reinforced with Microbial Cellulose Particles Obtained from Kombucha Fermented in Yerba Mate Waste. Polymers 2023, 15, 285. [Google Scholar] [CrossRef]
- De Melo, L.M.; Soares, M.G.; Bevilaqua, G.C.; Schmidt, V.C.R.; de Lima, M. Historical Overview and Current Perspectives on Kombucha and SCOBY: A Literature Review and Bibliometrics. Food Biosci. 2024, 59, 104081. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Sarikaya Aydin, S.; Gültekin Subasi, B.; Erskine, E.; Gök, R.; Ibrahim, S.A.; Yilmaz, B.; Özogul, F.; Capanoglu, E. Additional Advances Related to the Health Benefits Associated with Kombucha Consumption. Crit. Rev. Food Sci. Nutr. 2024, 64, 6102–6119. [Google Scholar] [CrossRef]
- Prajapati, K.; Prajapati, J.; Patel, D.; Patel, R.; Varshnei, A.; Saraf, M.; Goswami, D. Multidisciplinary Advances in Kombucha Fermentation, Health Efficacy, and Market Evolution. Arch. Microbiol. 2024, 206, 366. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Olivito, F.; Algieri, V.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Costanzo, P.; Maiuolo, L.; De Nino, A. Cellulose Citrate: A Convenient and Reusable Bio-Adsorbent for Effective Removal of Methylene Blue Dye from Artificially Contaminated Water. RSC Adv. 2021, 11, 34309–34318. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter xylinum. II. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Majamo, S.L.; Amibo, T.A.; Mekonnen, D.T. Expermental Investigation on Adsorption of Methylene Blue Dye from Waste Water Using Corncob Cellulose-Based Hydrogel. Sci. Rep. 2024, 14, 4540. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.D.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical Structure Variations of Bacterial Cellulose Produced by Different Komagataeibacter xylinus Strains and Carbon Sources in Static and Agitated Conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Chen, Y.; Jun, W. Enhanced Bacterial Cellulose Production in Gluconacetobacter xylinus by Overexpression of Two Genes (BscC and BcsD) and a Modified Static Culture. Int. J. Biol. Macromol. 2024, 260, 129552. [Google Scholar] [CrossRef]
- Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Modelling PH Dynamics, SCOBY Biomass Formation, and Acetic Acid Production of Kombucha Fermentation Using Black, Green, and Oolong Teas. Processes 2024, 12, 1301. [Google Scholar] [CrossRef]
- Sevcik, C. Caveat on the Boltzmann Distribution Function Use in Biology. Prog. Biophys. Mol. Biol. 2017, 127, 33–42. [Google Scholar] [CrossRef]
- El Aferni, A.; Guettari, M.; Tajouri, T. Mathematical Model of Boltzmann’s Sigmoidal Equation Applicable to the Spreading of the Coronavirus (Covid-19) Waves. Environ. Sci. Pollut. Res. 2021, 28, 40400–40408. [Google Scholar] [CrossRef]
- Aung, T.; Kim, M.J. A Comprehensive Review on Kombucha Biofilms: A Promising Candidate for Sustainable Food Product Development. Trends Food Sci. Technol. 2024, 144, 104325. [Google Scholar] [CrossRef]
- Tarhan Kuzu, K.; Aykut, G.; Tek, S.; Yatmaz, E.; Germec, M.; Yavuz, I.; Turhan, I. Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes 2023, 11, 2100. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, A.; Sarangi, P.K.; Chawade, A.; Pareek, N.; Tripathi, D.; Vivekanand, V. Machine Learning Approach for Microbial Growth Kinetics Analysis of Acetic Acid-Producing Bacteria Isolated from Organic Waste. Biochem. Eng. J. 2024, 202, 109164. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy; Springer: Dordrecht, The Netherlands, 2019; Volume 26, ISBN 1057001821. [Google Scholar]
- Ibrahim, M.A.; Salama, A.; Zahran, F.; Abdelfattah, M.S.; Alsalme, A.; Bechelany, M.; Barhoum, A. Fabrication of Cellulose Nanocrystals/Carboxymethyl Cellulose/Zeolite Membranes for Methylene Blue Dye Removal: Understanding Factors, Adsorption Kinetics, and Thermodynamic Isotherms. Front. Chem. 2024, 12, 1330810. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Han, Y.H.; Ye, Y.X.; Shi, X.X.; Xiang, P.; Chen, D.L.; Li, M. Physicochemical Characterization of High-Quality Bacterial Cellulose Produced by Komagataeibacter Sp. Strain W1 and Identification of the Associated Genes in Bacterial Cellulose Production. RSC Adv. 2017, 7, 45145–45155. [Google Scholar] [CrossRef]
- Hajialigol, S.; Masoum, S. Optimization of Biosorption Potential of Nano Biomass Derived from Walnut Shell for the Removal of Malachite Green from Liquids Solution: Experimental Design Approaches. J. Mol. Liq. 2019, 286, 110904. [Google Scholar] [CrossRef]
- Poornachandhra, C.; Jayabalakrishnan, R.M.; Prasanthrajan, M.; Balasubramanian, G.; Lakshmanan, A.; Selvakumar, S.; John, J.E. Cellulose-Based Hydrogel for Adsorptive Removal of Cationic Dyes from Aqueous Solution: Isotherms and Kinetics. RSC Adv. 2023, 13, 4757–4774. [Google Scholar] [CrossRef]
- Le, H.V.; Dao, N.T.; Bui, H.T.; Kim Le, P.T.; Le, K.A.; Tuong Tran, A.T.; Nguyen, K.D.; Mai Nguyen, H.H.; Ho, P.H. Bacterial Cellulose Aerogels Derived from Pineapple Peel Waste for the Adsorption of Dyes. ACS Omega 2023, 8, 33412–33425. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Jongsomjit, B.; Robison, J.; Phisalaphong, M. Activated Carbon from Bacterial Cellulose as an Effective Adsorbent for Removing Dye from Aqueous Solution. Sep. Sci. Technol. 2019, 54, 2180–2193. [Google Scholar] [CrossRef]
- Quiñones-Cerna, C.; Rodríguez-Soto, J.C.; Barraza-Jáuregui, G.; Huanes-Carranza, J.; Cruz-Monzón, J.A.; Ugarte-López, W.; Hurtado-Butrón, F.; Samanamud-Moreno, F.; Haro-Carranza, D.; Valdivieso-Moreno, S.; et al. Bioconversion of Agroindustrial Asparagus Waste into Bacterial Cellulose by Komagataeibacter rhaeticus. Sustainability 2024, 16, 736. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; El-Maghraby, A.; Sadik, W.A.A.; El-Demerdash, A.G.M.; Fadl, E.A. Biodegradable Cellulose Nanocrystals Hydrogels for Removal of Acid Red 8 Dye from Aqueous Solutions. Sci. Rep. 2022, 12, 6424. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial Cellulose/Attapulgite Magnetic Composites as an Efficient Adsorbent for Heavy Metal Ions and Dye Treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.N.; Abd-Elhamid, A.I.; El-Bardan, A.A.; Soliman, H.M.A.; Mohy-Eldin, M.S. Development of Carboxymethyl Cellulose-Graphene Oxide Biobased Composite for the Removal of Methylene Blue Cationic Dye Model Contaminate from Wastewater. Sci. Rep. 2023, 13, 14265. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, T.W.; Yang, Y.L.; Bai, Z.C.; Xia, L.R.; Wang, M.; Lv, X.L.; Li, L. Removal of Heavy Metal Ions and Anionic Dyes from Aqueous Solutions Using Amide-Functionalized Cellulose-Based Adsorbents. Carbohydr. Polym. 2020, 230, 115619. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro-Reyes, A.; Marín-Medina, K.; Escamilla-García, M.; Favela-Camacho, S.E.; Barrón-García, O.Y.; Campos-Guillén, J.; Ramos-López, M.A.; Pool, H.; Rodríguez-de León, E.; Rodríguez Morales, J.A. Functionalized Bacterial Cellulose: A Potential Sustainable Adsorbent for Methylene Blue Removal. Polysaccharides 2025, 6, 8. https://doi.org/10.3390/polysaccharides6010008
Amaro-Reyes A, Marín-Medina K, Escamilla-García M, Favela-Camacho SE, Barrón-García OY, Campos-Guillén J, Ramos-López MA, Pool H, Rodríguez-de León E, Rodríguez Morales JA. Functionalized Bacterial Cellulose: A Potential Sustainable Adsorbent for Methylene Blue Removal. Polysaccharides. 2025; 6(1):8. https://doi.org/10.3390/polysaccharides6010008
Chicago/Turabian StyleAmaro-Reyes, Aldo, Karina Marín-Medina, Monserrat Escamilla-García, Sarai E. Favela-Camacho, Oscar Yael Barrón-García, Juan Campos-Guillén, Miguel Angel Ramos-López, Héctor Pool, Eloy Rodríguez-de León, and José Alberto Rodríguez Morales. 2025. "Functionalized Bacterial Cellulose: A Potential Sustainable Adsorbent for Methylene Blue Removal" Polysaccharides 6, no. 1: 8. https://doi.org/10.3390/polysaccharides6010008
APA StyleAmaro-Reyes, A., Marín-Medina, K., Escamilla-García, M., Favela-Camacho, S. E., Barrón-García, O. Y., Campos-Guillén, J., Ramos-López, M. A., Pool, H., Rodríguez-de León, E., & Rodríguez Morales, J. A. (2025). Functionalized Bacterial Cellulose: A Potential Sustainable Adsorbent for Methylene Blue Removal. Polysaccharides, 6(1), 8. https://doi.org/10.3390/polysaccharides6010008












