Granulometry and Functional Properties of Yuca Flour (Yucca decipiens Trel.) for Food Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Properties
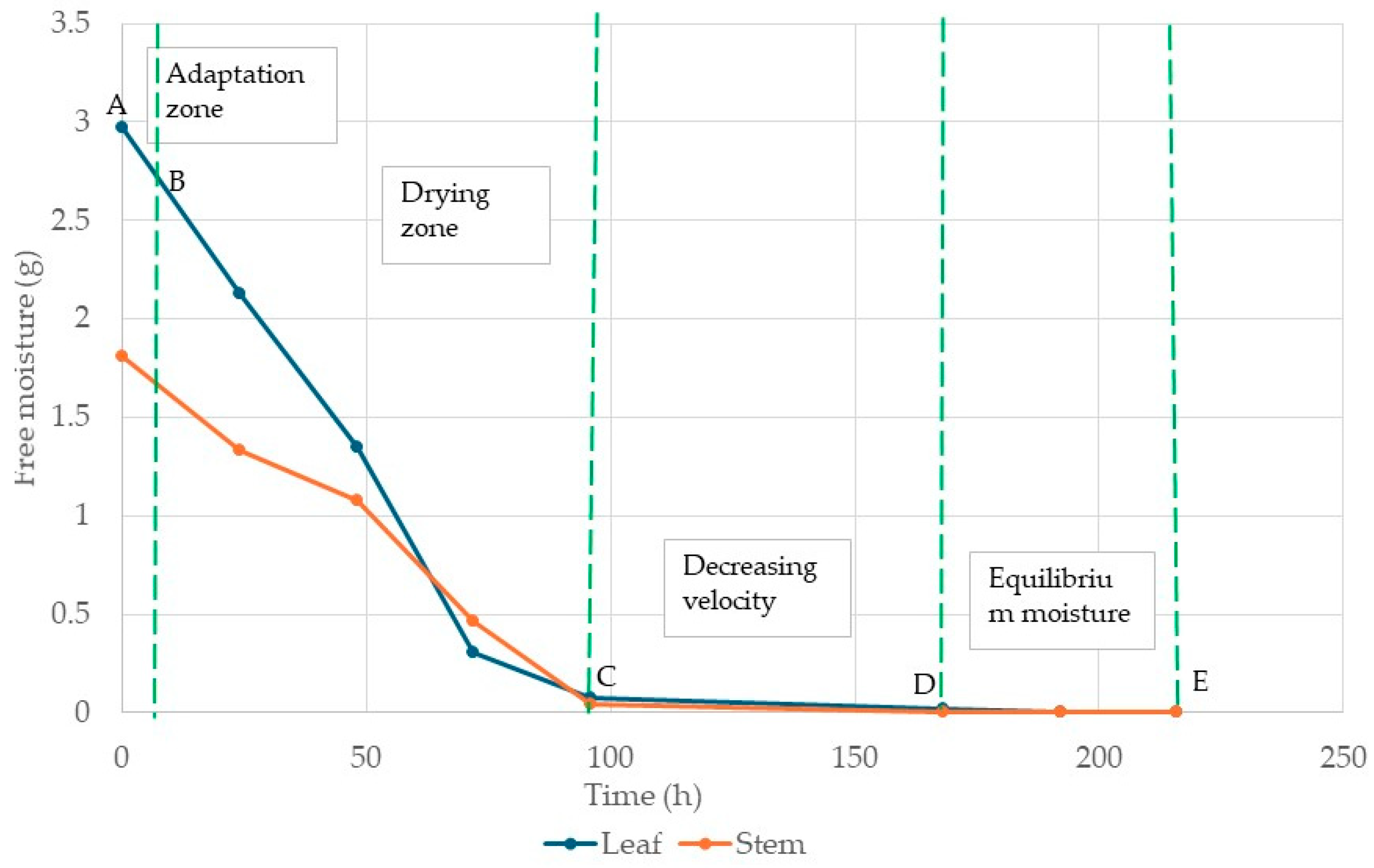
2.1.1. Analysis of Drying Kinetics and Uncertainty of Yucca decipiens Leaf and Stem Flour
2.1.2. Milling
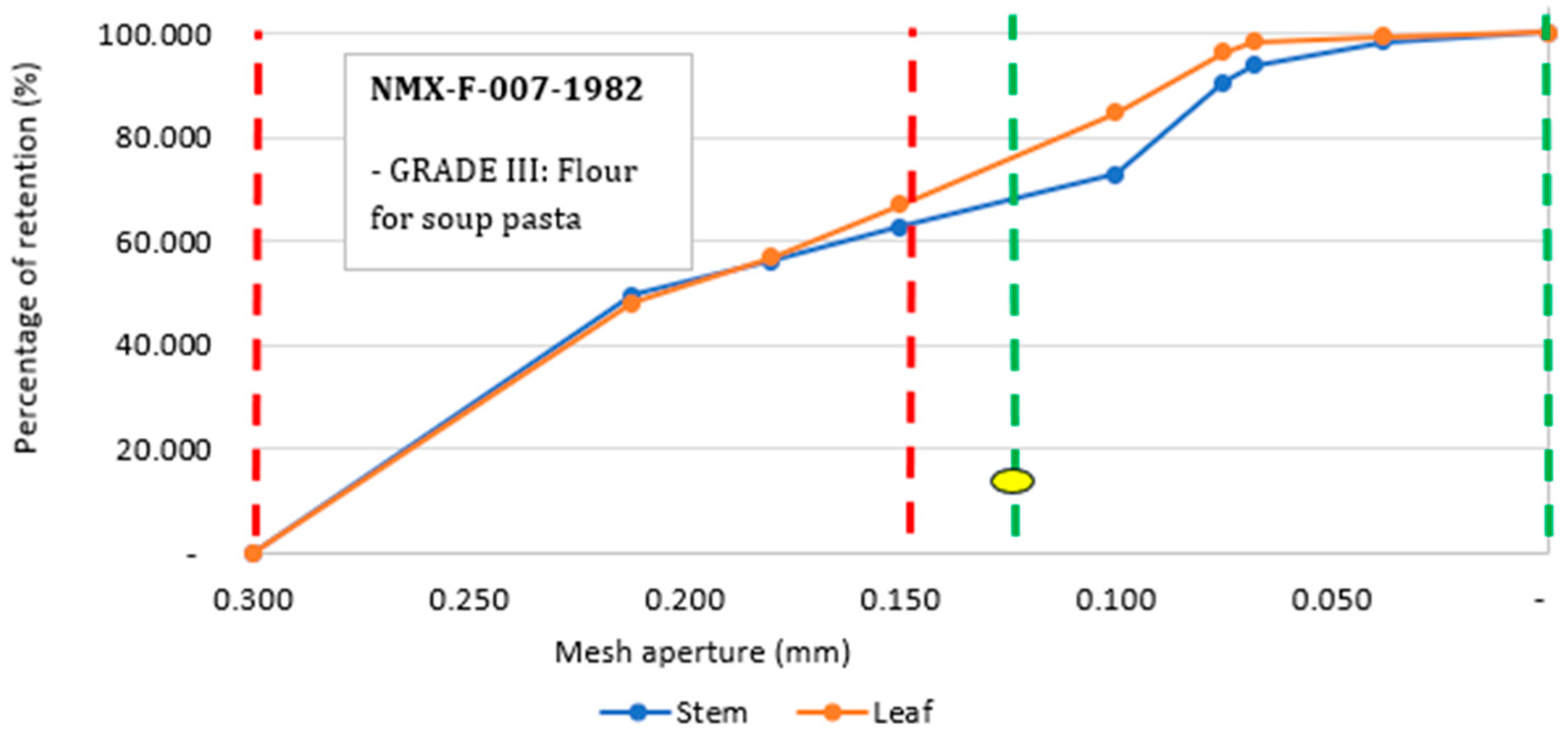
2.1.3. Granulometric Analysis
2.1.4. Particle Size Index
2.1.5. Particle Size Distribution
2.2. Functional Properties
2.2.1. Water Absorption Index (WAI)
2.2.2. Oil Absorption Index (FAI)
2.2.3. Swelling Capacity
2.2.4. Foam Formation and Stability
2.2.5. Expansion Test
2.2.6. Pelshenke Value
2.3. Gravimetric Properties
2.3.1. Bulk Density
2.3.2. Particle Density
Porosity
2.4. Frictional Properties
2.4.1. Internal Friction
2.4.2. External Friction
2.4.3. Texture
2.5. Rheological Properties
2.5.1. Viscosity
2.5.2. Electrical Conductivity
2.5.3. Potential of Hydrogen (pH)
2.6. Statistical Analysis
3. Results
3.1. Physical Properties
3.1.1. Analysis of Drying Kinetics and Uncertainty of Yucca decipiens Leaf and Stem Flour
3.1.2. Granulometric Analysis
3.1.3. Particle Size Index (PSI)
3.1.4. Particle Size
3.2. Functional Properties
3.2.1. Pelshenke Value
3.2.2. Gravimetric Properties
3.3. Frictional Properties
3.3.1. Internal Friction
3.3.2. External Friction
3.3.3. Texture Properties
3.4. Rheological Properties
3.4.1. Viscosity
3.4.2. Electrical Conductivity and pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearlstein, T.; Steiner, M. Premenstrual dysphoric disorder: Burden of illness and treatment update. J. Psychiatry Neurosci. 2008, 33, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Fu, Y.J.; Zu, Y.G.; Schwarz, G.; Konkimalla, V.S.; Wink, M. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr. Med. Chem. 2007, 14, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- DOF. Diario Oficial de la Federación. NOM-059-SEMARNAT-2010 (NORMA Oficial Mexicana NOM-059-SEMARNAT-2010). Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. [Environmental Protection—Native Species of Wild Flora and Fauna in Mexico—Risk Categories and Specifications for Their Inclusion, Exclusion or Change—List of Species at Risk]. 2010. Available online: https://www.dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm (accessed on 12 September 2024).
- Coffman, C.; García, V. Functional properties and amino acid content of a protein isolate from mung bean flour. J. Food Technol. 1977, 12, 473–487. [Google Scholar] [CrossRef]
- 56–60 Physicochemical Test Methods of the Cereal & Grains Association. 1976. Available online: https://www.cerealsgrains.org/resources/Methods/Pages/56PhysicochemicalTests.aspx (accessed on 24 April 2024).
- Martínez-Betancourt, S.R.; Rössel-Kipping, E.D.; López-Martínez, L.A.; Ortiz-Laurel, H.; Loera-Alvarado, G.; Amante-Orozco, A.; Ruiz-Vera, V.M. Potential use of physical characteristics of squash seeds (Cucurbita moschata), pea pods (Pisum sativum) and green bean (Phaseolus vulgaris) in Agroindustry 4.0. Agrociencia 2022, 56, 11–20. [Google Scholar] [CrossRef]
- Serna, G.H. Gerencia Estratégica. Teoría, Metodología, Alineamiento, Implementación y Mapas Estratégicos. Índices de Gestión [Strategic Management. Theory, Methodology, Alignment, Implementation and Strategic Maps. Management Índices], 9th ed.; 3R Editores: Bogotá, Colombia, 2003. [Google Scholar]
- AACC (American Association of Cereal Chemists). Method 55-10.01. Approved Methods of the AACC. 2000. Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 12 April 2024).
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials: Structure, Physical Characteristics, and Mechanical Properties, 2nd ed.; Gordon and Breach Science Publishers: New York, NY, USA, 1986; p. 891. Available online: https://www.taylorfrancis.com/books/mono/10.4324/9781003062325/physical-properties-plant-animal-materials-1-physical-characteristics-mechanical-properties-nuri-mohsenin (accessed on 11 August 2024).
- Dobarganes, C.; Márquez-Ruiz, G.; Velasco, J. Interactions between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Dutta, S.K.; Nema, V.K.; Bharddwaj, R.K. Physical properties of gram. J. Agric. Eng. Res. 1988, 39, 259–268. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of A.O.A.C. Internacional, 18th ed.; AOAC: Rockville, MD, USA, 2005; Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 22 May 2024).
- AOAC (Association of Official Analytical Chemists). Association of Official Analytical Chemists International Official Methods of Analysis, 16th ed.; AOAC: Rockville, MD, USA, 1997; Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 22 May 2024).
- Bressani, R.; Turcios, J.C.; Reyes, L.; Mérida, R. Caracterización física y química de harinas industriales nixtamalizadas de maíz de consumo humano en América Central [Physical and chemical characterization of nixtamalized industrial corn flours for human consumption in Central America]. Arch. Latinoam. Nutr. 2001, 51, 309–313. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222001000300015 (accessed on 18 August 2024).
- Beuchat, L. Functional and electrophoretic characteristics of succynalated peanut flour proteins. J. Agric. Food Chem. 1977, 25, 258–263. [Google Scholar] [CrossRef]
- Robertson, J.A.; Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibaukt, F. Hydration properties of dietary fibre and resistant starch: A European collaborative study. Lebensm. Wiss. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Pineda-Castro, M.L.; Chacón-Villalobos, A.; Cordero-Gamboa, G. Efecto de las condiciones de secado sobre la cinética de deshidratación de las hojas de morera (Morus alba). Agron. Mesoam. 2008, 20, 275–283. [Google Scholar] [CrossRef][Green Version]
- SCFI (Secretaría de Comercio y Fomento Industrial). Norma Mexicana NMX-F-007-1982. Harina de Trigo [Wheat Flour]. 1982. Available online: www.economia-nmx.gob.mx/normas/nmx/1977/nmx-z-013-1-1977.pdf (accessed on 12 June 2024).
- Castaño, M.N.; Ferrari, E.D.; Picca, A.T.; Curti, M.I.; Ribotta, P.D.; León, A.E.; Paccapelo, H.A. Caracterización de harinas de tritíceas híbridas [Characterization of hybrid triticum flours]. Agriscientia 2017, 34, 15–25. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Figueroa, J.D.C. Efecto del tamaño de partícula y temperatura en la viscosidad de botanas extrudidas nixtamalizadas de maíz azul integral [Effect of particle size and temperature on the viscosity of extruded nixtamalized snacks made from whole blue corn]. Investig. Desarro. Cienc. Tecnol. Aliment. 2019, 4, 56–65. Available online: http://www.fcb.uanl.mx/IDCyTA/files/volume4/4/1/8.pdf (accessed on 15 June 2024).
- Budâcan, I.; Pop, D.; Drocaş, I. Size distribution of maize milled particles Obtained by using a hammer mill. Acta Tech. Napoc. 2013, 56, 631–636. Available online: https://atna-mam.utcluj.ro/index.php/Acta/article/view/108 (accessed on 17 June 2024).
- Hernández, A. Microbiología Industrial, 1st ed.; Editorial Universidad Estatal a Distancia: San José, Costa Rica, 2003; Available online: https://www.academia.edu/6057657/microbiologia_industrial (accessed on 22 June 2024).
- González, M.C. Use of Sweet Potato (Ipomoea batatas) for the Development of Functional Flours and Their Application in the Production of Gluten-Reduced Muffins. Bachelor’s Thesis, Universidad Autónoma de Puebla, Puebla, Mexico, 2016. Available online: https://repositorioinstitucional.buap.mx/items/a6624583-fd60-427e-b7e5-52360d2ce18e (accessed on 27 August 2024).
- Niba, L.L.; Bokonga, M.M.; Jackson, E.L.; Schlimme, D.S.; Li, B.W. Physicochemical properties and starch granular characteristics of flour from various Manihot esculenta (cassava) genotypes. J. Food Sci. 2001, 67, 1701–1705. [Google Scholar] [CrossRef]
- Kinsella, J.E. Propiedades funcionales de las proteínas en los alimentos: Un estudio. Reseñas Críticas Cienc. Aliment. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. El impacto de la germinación y el descascarado en los nutrientes, antinutrientes, biodisponibilidad in vitro de hierro y calcio, y digestibilidad in vitro de almidón y proteína de algunas semillas de leguminosas. LWT-Cienc. Tecnol. Aliment. 2006, 39, 548–557. [Google Scholar] [CrossRef]
- Miquilena, D.M.; Higuera, N. Efecto del remojo, cocción y germinación sobre las propiedades funcionales de la harina de frijol gandul (Cajanus cajan). Rev. Científica UDO Agrícola 2012, 12, 147–155. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=6104328 (accessed on 16 September 2024).
- Kaushal, P.; Kumar, V.; Sharma, H.K. Estudio comparativo de las propiedades fisicoquímicas, funcionales, antinutricionales y de empastado de las harinas de taro (Colocasia esculenta), arroz (Oryza sativa), frijol gandul (Cajanus cajan) y frijol rojo (Phaseolus vulgaris). LWT-Cienc. Tecnol. Aliment. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Kuntz, I.D. Hidratación de macromoléculas. III. Hidratación de polipéptidos. Rev. Soc. Química Estadounidense 1971, 93, 514–516. [Google Scholar] [CrossRef]
- Granito, M.; Torres, A.; Pérez, S. Mejora de la calidad nutricional de judías (Phaseolus vulgaris) y garbanzos (Cicer arietinum): Fermentación con Lactobacillus casei. Arch. Latinoam. Nutr. 2004, 54, 428–433. Available online: https://www.alanrevista.org (accessed on 23 September 2024).
- Sangronis, E.; Machado, C.J.; Contreras, A. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. Food Sci. Technology 2007, 40, 116–120. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.; Tapia-Blácido, D.R.; Menegalli, F.C. Physical–chemical, thermal, and functional properties of achira (Canna indica L.) flour and starch from different geographical origin. Starch Starke 2012, 64, 348–358. [Google Scholar] [CrossRef]
- Badui-Dergal, S. Química de los Alimentos [Food Chemistry], 4th ed.; Editorial Pearson Educación: Mexico City, Mexico, 2006; Available online: https://www.academia.edu/28233446/qu%c3%admica_de_los_alimentos_badui_4edi (accessed on 18 June 2024).
- Miquilena, E.; Higuera, A.; Rodríguez, B. Evaluación de propiedades funcionales de cuatro harinas de semillas de leguminosas comestibles cultivadas en Venezuela [Evaluation of functional properties of four seed flours from edible legumes grown in Venezuela]. Rev. Fac. Agron. (Univ. Zulia) 2016, 33, 58–75. Available online: https://produccioncientificaluz.org/index.php/agronomia/article/view/27193 (accessed on 21 June 2024).
- Cheftel, J.C.; Thiebaud, M.; Dumay, E. High Pressure—Low Temperature Processing of Foods: A Review; Advances in High Pressure Bioscience and Biotechnology II; Winter, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- AACC (American Association of Cereal Chemists). Approved Methods of the AACC. 1976. Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 12 April 2024).
- Bedolla, S.; Rooney, L.W. Characteristics of US and Mexican instant maize flours for tortilla and snack preparation. Cereals Food World 1984, 29, 732–735. Available online: https://europepmc.org/article/AGR/IND85022082 (accessed on 20 August 2024).
- Elías-Silupu, J.W.; García-Rivas, P.C.E.; Pérez-Salcedo, R.; Yauris-Silvera, C.R. Caracterización Fisicoquímica de Pan con Sustitución Parcial de Harina de Trigo por Harina de Quinua (Chenopodium quinoa willd) y Kiwicha (Amaranthus caudatus L.) Germinadas [Physicochemical Characterization of Bread with Partial Sustitución of wheat flour for Quinoa flour (Chenopodium quinoa willd) and Kiwicha (Amaranthus caudatus L.)] Germinated. SENDAS 2021, 2, 69–83. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Álvarez-Castillo, M.J.; Rössel-Kipping, E.D.; Ortiz-Laurel, H.; López-Martínez, L.A.; Amante-Orozco, A. Potential of the physical and chemical characteristics of prickly pear (Opuntia albicarpa Seheinvar var. villanueva) seeds in agroindustrial processes: Frutos de las cactáceas. Agro Product. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Rössel-Kipping, E.D.; Ortiz-Laurel, H.; Amante-Orozco, A.; Durán-García, H.M.; López- Martínez, L.A. Características físicas y químicas de la semilla de calabaza para mecanización y procesamiento [Physical and chemical characteristics of pumpkin seeds for machining and processing]. Nova Sci. 2018, 10, 61–77. [Google Scholar] [CrossRef]
- Ospina, M.J. Características Físico-Mecánicas y Análisis de Calidad de Granos [Physical-Mechanical Characteristics and Quality Analysis of Grains]; Departamento de Ingeniería Agrícola, Universidad Nacional de Colombia: Bogotá, Colombia, 2001; p. 225. Available online: https://books.google.com.ec/books?id=2DWmqb6xP3wC&printsec=frontcover&hl=es#v=onepage&q&f=false (accessed on 22 August 2024).
- Sologubik, C.A.; Campañone, L.A.; Pagano, A.M.; Gely, M.C. Effect of moisture content on some physical properties of barley. Ind. Crops Prod. 2013, 43, 762–767. [Google Scholar] [CrossRef]
- Domínguez-Zarate, P.A.; García-Martínez, I.; Güemes-Vera, N.; Totosaus, A. Textura, color y aceptación sensorial de tortillas y pan producidos con harina de ramón (Brosimum alicastrum) para incrementar la fibra dietética total [Texture, colour and sensory acceptance of tortillas and bread produced with ramon flour (Brosimum alicastrum) to increase total dietary fibre]. Cienc. Tecnol. Agropecu. 2019, 20, 699–719. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Morales-Sánchez, E.; Reyes-Vega, M.L.; Gaytán-Martínez, M. Propiedades funcionales de harinas de maíz nixtamalizado obtenidas por extrusión a baja temperatura [Functional properties of nixtamalized corn flours obtained by low-temperature extrusion]. CyTA J. Food 2013, 12, 263–270. [Google Scholar] [CrossRef]
- Roddick, J.G. Steroidal Glycoalkaloid Alpha-Tomatine. Phytochemistry 1974, 13, 9–25. [Google Scholar] [CrossRef]
- Convention on Biological Diversity (CDB). Elías-Silupu on Biological Diversity. United Nations Environment Programme. 1992. Available online: https://www.biodiversidad.gob.mx/planeta/internacional/cbd (accessed on 12 April 2024).
| Organ | Area (mm2) | Perimeter (mm) |
|---|---|---|
| Stem | 0.02 ± 0.01 | 0.71 ± 0.34 |
| Leaf | 0.03 ± 0.02 | 0.79 ± 0.36 |
| Organ | Water Absorption (mL g−1) | Oil Absorption (mL g−1) | Expansion (%) | Swelling Capacity (mL g−1) | Foaming Capacity (mL g−1) | Foaming Stability (%) |
|---|---|---|---|---|---|---|
| Stem | 0.11 ± 0.05 | 0.41 ± 0.11 | 45.5 ± 6.36 | 0.65 ± 0.36 | 3.57 ± 1.41 | 100 ± - |
| Leaf | 0.11 ± 0.03 | 0.39 ± 0.05 | 35.73 ± 6.86 | 0.62 ± 0.54 | 3.47 ± 1.96 | 100 ± - |
| Variable | Floating Time (min) | Disintegration Time (min) |
|---|---|---|
| Stem | 0.50 ± 0.28 | 19.77 ± 0.44 |
| Leaf | 1.12 ± 0.32 | 19.68 ± 1.28 |
| Organ | Apparent Density (g mL−1) | Particle Density (g mL−1) | Porosity (%) |
|---|---|---|---|
| Stem | 0.24 ± 0.07 | 0.42 ± 0.09 | 44 ± 0.07 |
| Leaf | 0.25 ± 0.05 | 0.29 ± 0.06 | 38 ± 0.10 |
| Organ | Material | Angle (°) | µe (-) |
|---|---|---|---|
| Leaf | Stainless steel | 30.65 | 0.59 ± 0.03 |
| Wood | 35.25 | 0.76 ± 0.18 | |
| Polyethylene plastic | 34.90 | 0.70 ± 0.03 | |
| G.L.-A.ss | 32.20 | 0.63 ± 0.02 | |
| Ceramic floor | 27.60 | 0.52 ± 0.04 | |
| Stem | Stainless steel | 31.50 | 0.61 ± 0.03 |
| Wood | 35.15 | 0.76 ± 0.18 | |
| Polyethylene plastic | 35.00 | 0.70 ± 0.04 | |
| G.L.-A.ss | 31.45 | 0.61 ± 0.03 | |
| Ceramic tiles | 28.75 | 0.55 ± 0.03 |
| Variable | Leaf | Stem |
|---|---|---|
| Hardness (g) | 621.83 ± 21.70 | 3135.48 ± 91.38 |
| Deformation according to hardness (mm) | 7.64 ± 0.53 | 12.42 ± 2.41 |
| Deformation according to hardness (%) | 38.26 ± 2.61 | 63.13 ± 10.57 |
| Recoverable deformation (mm) | 1.15 ± 0.08 | 18.82 ± 2.27 |
| Recoverable work (mJ) | 1.42 ± 0.14 | 0.94 ± 0.55 |
| Total work (mJ) beginning of form | 27.60 ± 3.38 | 63.86 ± 23.34 |
| Peak pressure (N m−2) | 12,040.87 ± 5.20 | 73,834.67 ± 5.72 |
| Deformation at load peak | 0.31 ± 0.09 | 0.61 ± 0.14 |
| Adhesive force (g) | 26.21 ± 8.86 | 41.35 ± 2.87 |
| Adhesiveness (mJ) | 0.46 ± 0.32 | 0.72 ± 0.40 |
| Resilience | 0.06 ± 0.02 | 0.05 ± 0.01 |
| Sample length (mm) | 20.00 ± 0.00 | 20.00 ± 0.00 |
| Organ | Viscosity (Cp) | Shear Force (Dyne cm−2) | Temperature (°C) | Torque (%) | Cutting Range (s−1) | Speed (RPM) |
|---|---|---|---|---|---|---|
| Stem | 3.33 ± 0.34 | 5.69 ± 0.28 | 21.85 ± 0.35 | 1.25 ± 0.07 | 165 ± - | 22 ± - |
| Leaf | 5.97 ± 0.87 | 8.905 ± 0.10 | 22.05 ± 0.21 | 1.95 ± 0.21 | 165 ± - | 22 ± - |
| Electrical Conductivity (µS cm−1) | ||||
|---|---|---|---|---|
| Organ | 15 °C | 25 °C | 35 °C | pH |
| Stem | 2303.5 ± 24.32 | 2371.5 ± 35.35 | 2424.50 ± 26.87 | 5.53 ± 0.39 |
| Leaf | 2996.0 ± 16.49 | 3039.0 ± 26.97 | 3074.75 ± 36.06 | 9.38 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Betancourt, S.R.; Cadena-Iñiguez, J.; Loera-Alvarado, G.; Ruiz-Vera, V.M.; Soto-Hernández, R.M.; López-Padilla, C.; García-Flores, D.A. Granulometry and Functional Properties of Yuca Flour (Yucca decipiens Trel.) for Food Purposes. Polysaccharides 2025, 6, 16. https://doi.org/10.3390/polysaccharides6010016
Martínez-Betancourt SR, Cadena-Iñiguez J, Loera-Alvarado G, Ruiz-Vera VM, Soto-Hernández RM, López-Padilla C, García-Flores DA. Granulometry and Functional Properties of Yuca Flour (Yucca decipiens Trel.) for Food Purposes. Polysaccharides. 2025; 6(1):16. https://doi.org/10.3390/polysaccharides6010016
Chicago/Turabian StyleMartínez-Betancourt, Selena R., Jorge Cadena-Iñiguez, Gerardo Loera-Alvarado, Víctor M. Ruiz-Vera, Ramón Marcos Soto-Hernández, Concepción López-Padilla, and Dalia Abigail García-Flores. 2025. "Granulometry and Functional Properties of Yuca Flour (Yucca decipiens Trel.) for Food Purposes" Polysaccharides 6, no. 1: 16. https://doi.org/10.3390/polysaccharides6010016
APA StyleMartínez-Betancourt, S. R., Cadena-Iñiguez, J., Loera-Alvarado, G., Ruiz-Vera, V. M., Soto-Hernández, R. M., López-Padilla, C., & García-Flores, D. A. (2025). Granulometry and Functional Properties of Yuca Flour (Yucca decipiens Trel.) for Food Purposes. Polysaccharides, 6(1), 16. https://doi.org/10.3390/polysaccharides6010016






