Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
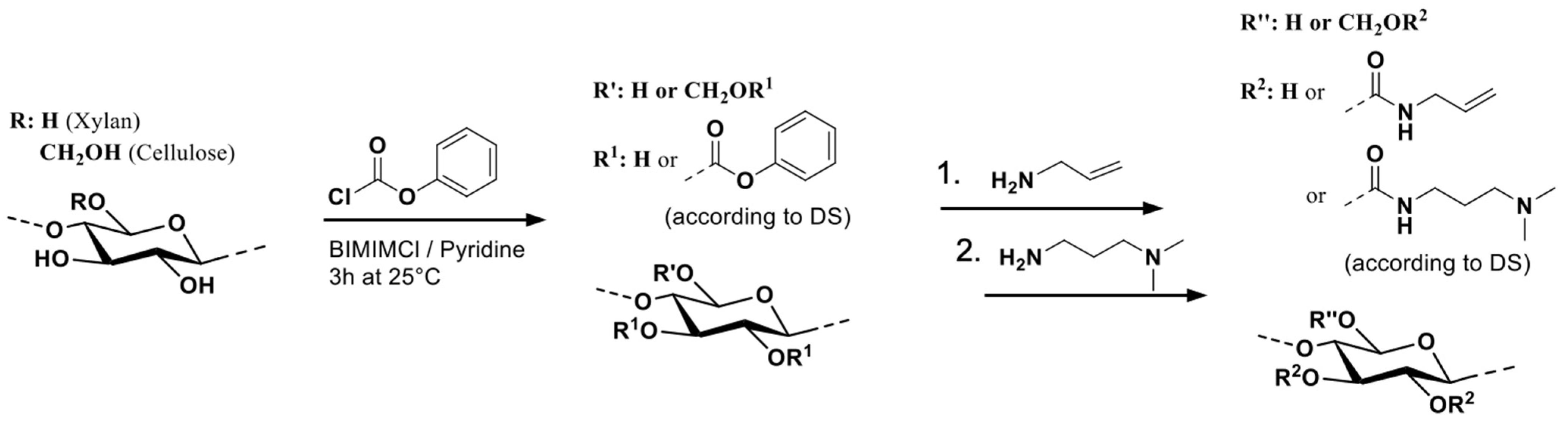
2.3. Synthesis of Allyl-Functionalized Polysaccharide Carbamate (Typical Example, AFC 02)
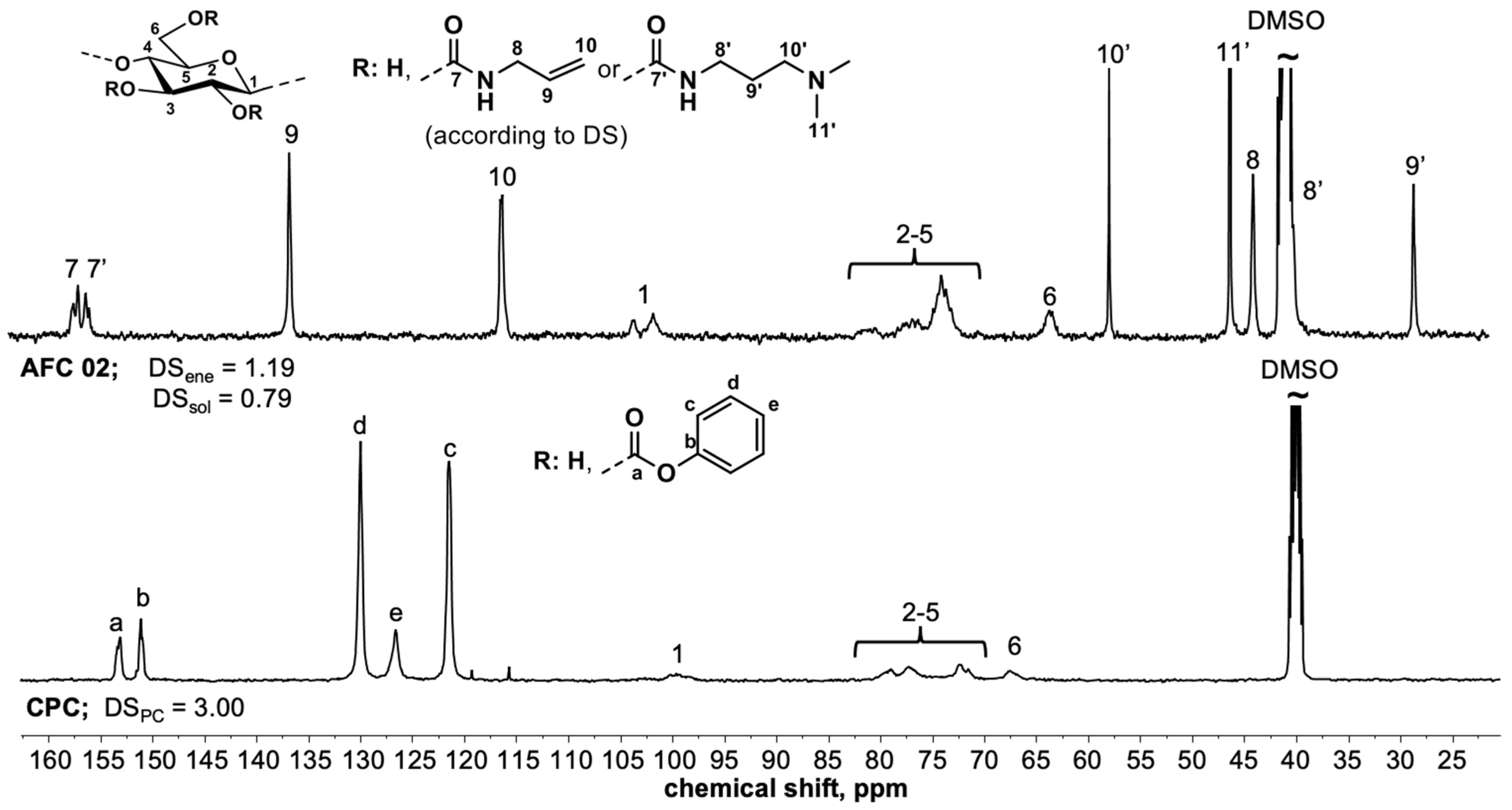
- DSene = 1.19; DSsol = 0.79.
- Yield: 47 g (129.82 mmol; 75% molar yield).
- 13C NMR (125 MHz, DMSO-d6): (δ, ppm) 156.1, 155.3, 135.8, 115.4, 102,7, 100.9, 73.1, 62.6, 56.9, 45.4, 43.1, 39.1, 27.6.
- Elemental analysis: theoretical: C% 51.41, H% 7.01, and N% 10.72; experimental: C% 50.21, H% 6.82, and N% 10.69.
2.4. Preparation of Polysaccharide Hydrogels Through Thiol–Ene Click Reaction (General Procedure)
2.5. Hydrogel Characterization
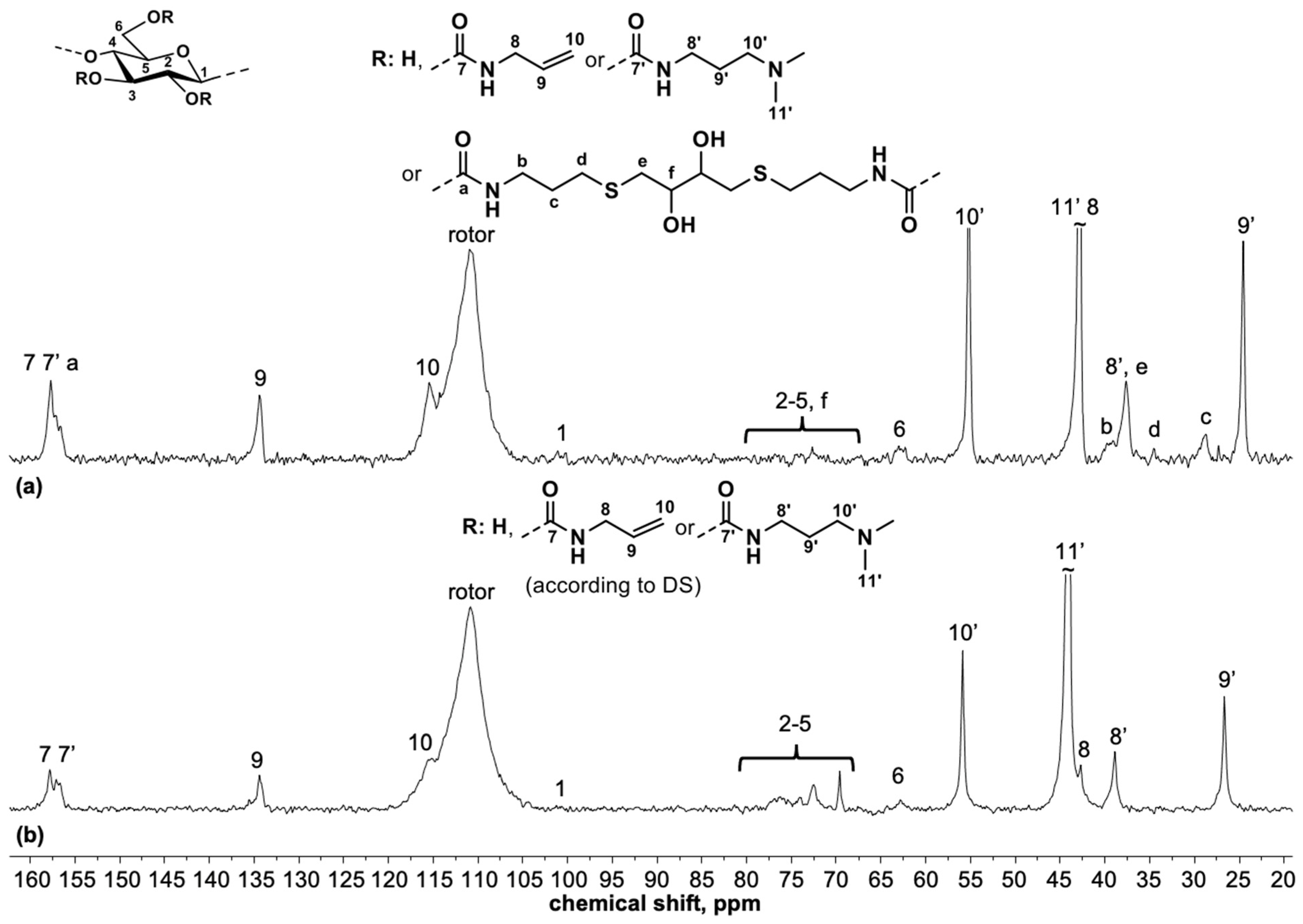
2.5.1. High-Resolution Magic-Angle Spinning NMR Spectroscopy (HR-MAS NMR)
2.5.2. Swelling Behavior
2.5.3. Mechanical Testing
2.6. Preliminary Printing Trials
2.7. Three-Dimensional Printing Experiments
3. Results and Discussion
3.1. Synthesis of Allyl-Functionalized Polysaccharide Carbamates
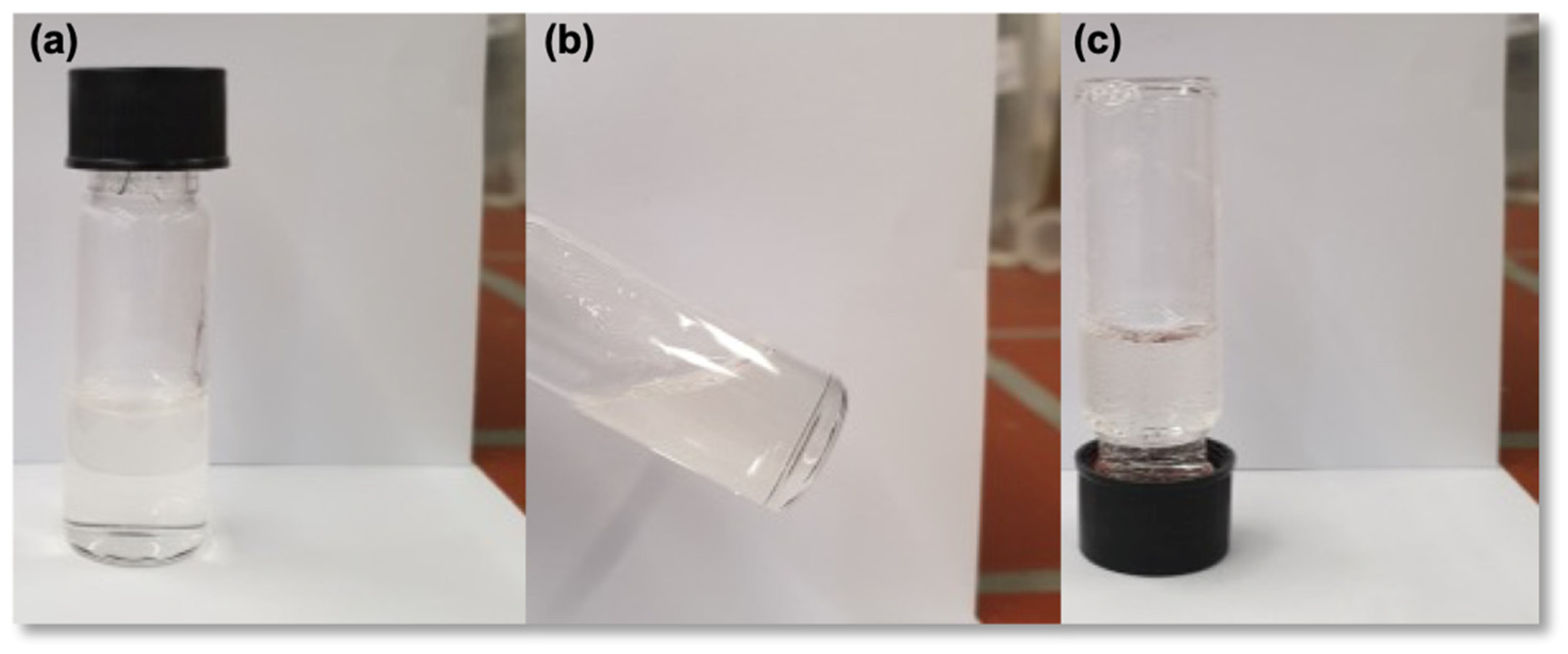
3.2. Preparation of Hydrogels Through Thiol–Ene Click Reaction
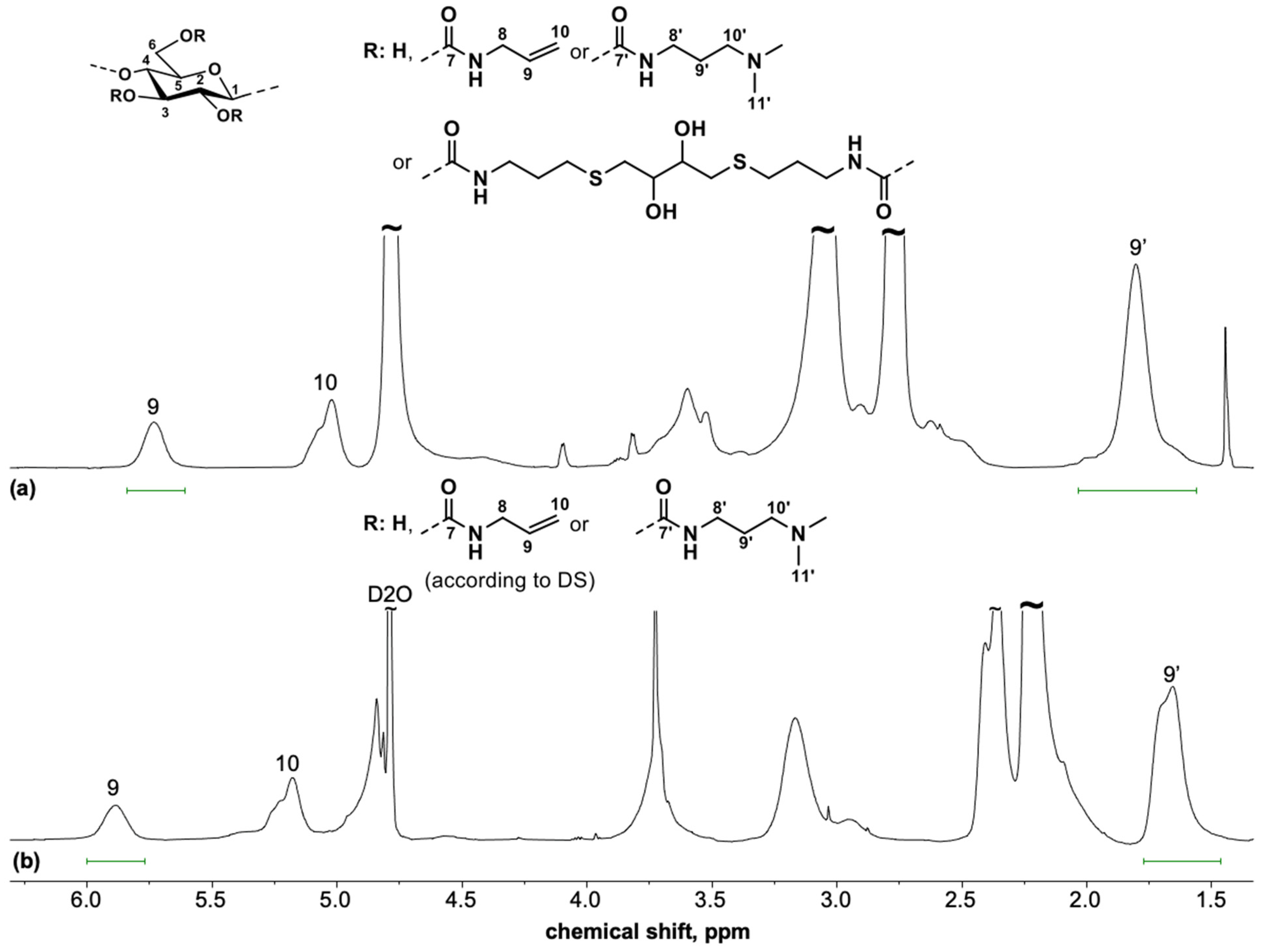
3.3. Spectroscopic Characterization of Polysaccharide Gels
3.4. Swelling Behavior of Thiol–Ene Crosslinked Gels
3.5. Morphology and Pore Distribution of Thiol–Ene Crosslinked Gels
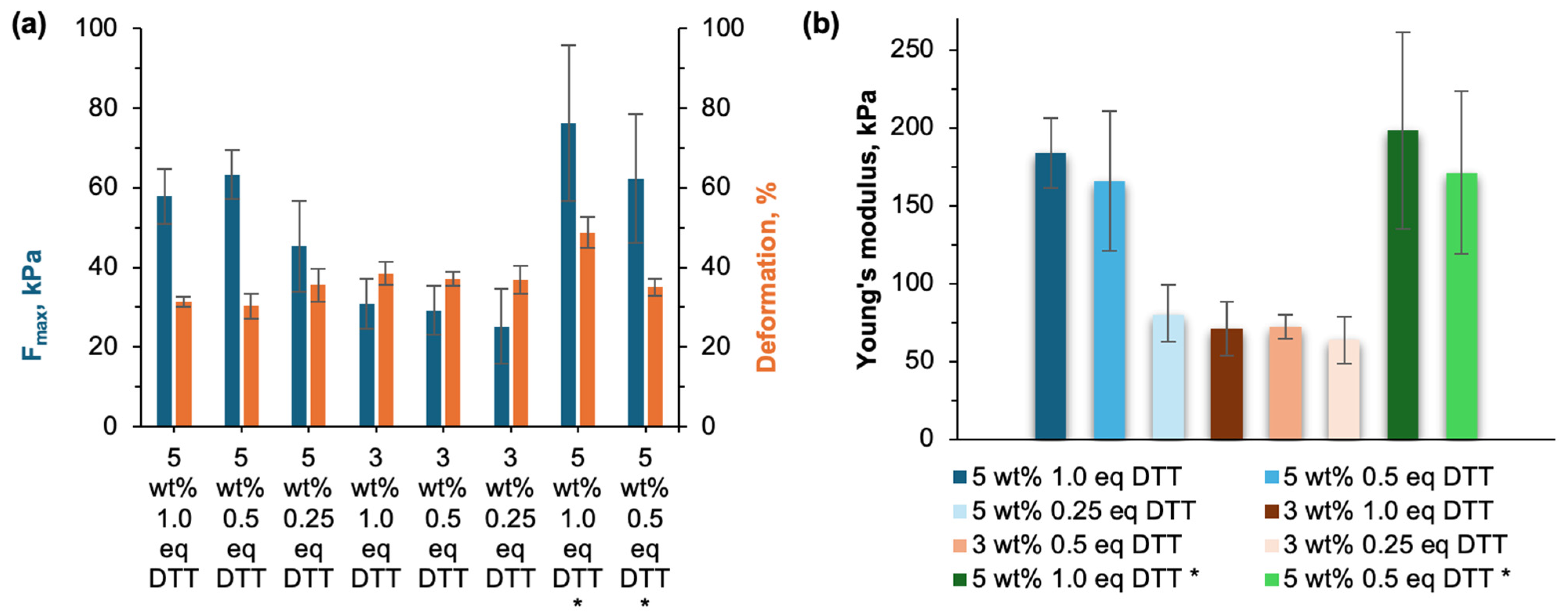
3.6. Mechanical Properties of Thiol–Ene Crosslinked Gels
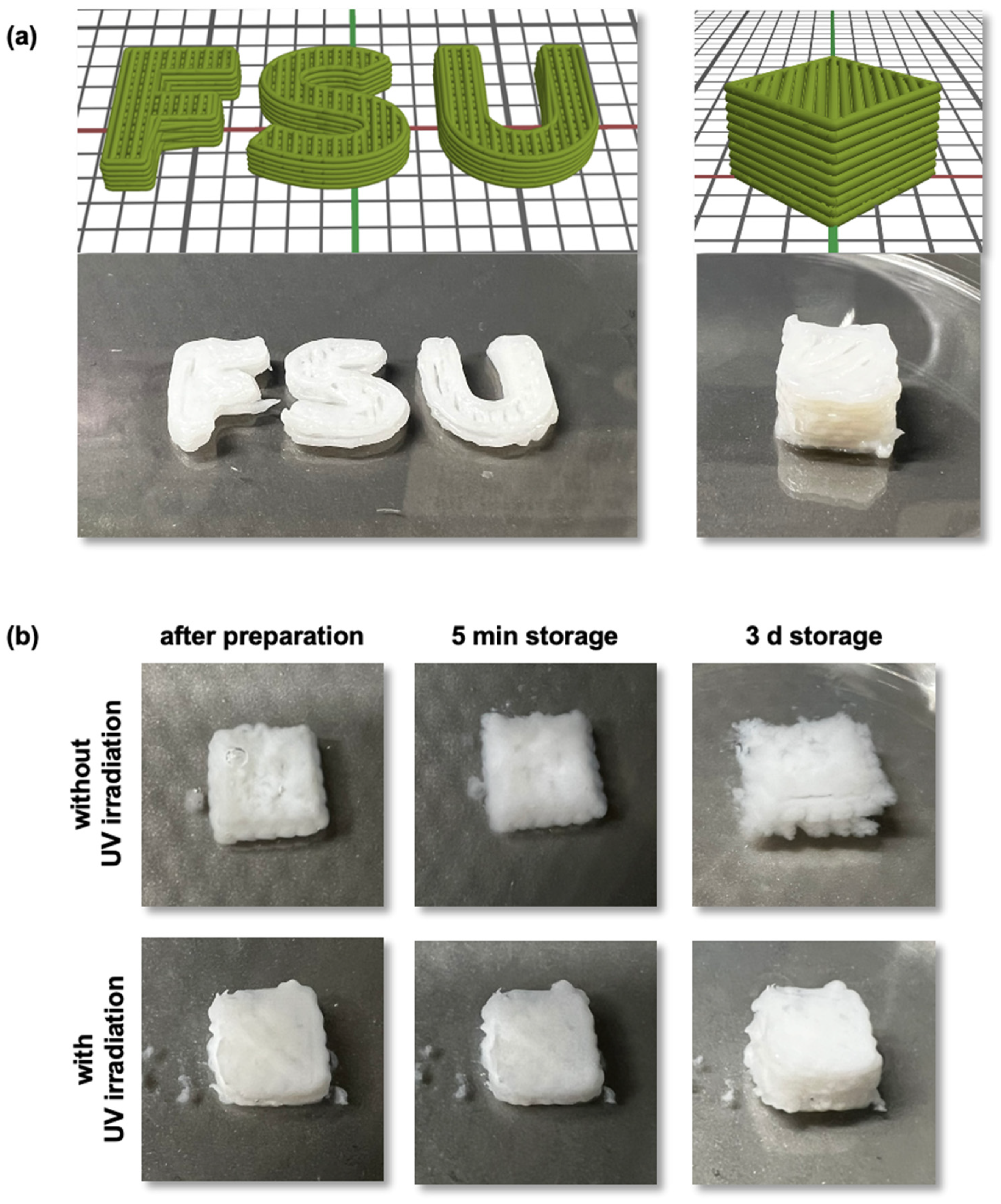
3.7. Three-Dimensional Printing and Injectable Hydrogels Based on Polysaccharide Carbamates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burdick, J.A.; Murphy, W.L. Moving from Static to Dynamic Complexity in Hydrogel Design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive Hydrogels: Fabrication and Biomedical Applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(vinyl alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Oliver-Urrutia, C.; Rosales Ibanez, R.; Flores-Merino, M.V.; Vojtova, L.; Salplachta, J.; Celko, L.; Kaiser, J.; Montufar, E.B. Lyophilized Polyvinylpyrrolidone Hydrogel for Culture of Human Oral Mucosa Stem Cells. Materials 2021, 14, 227. [Google Scholar] [CrossRef]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and Swelling of Poly(acrylic acid) Hydrogels: Effect of pH, Ionic Strength, and Dilution on the Crosslinked Polymer Structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.K.; Stevens, M.M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent Advances in Polysaccharide-based Hydrogels for Synthesis and Applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Zhao, J.; Marczynski, M.; Henkel, M.; Lieleg, O. Agarose-based Hydrogels with Tunable, Charge-Selective Permeability Properties. J. Appl. Polym. Sci. 2023, 140, e54303. [Google Scholar] [CrossRef]
- Koschella, A.; Hartlieb, M.; Heinze, T. A “Click-Chemistry” Approach to Cellulose-based Hydrogels. Carbohydr. Polym. 2011, 86, 154–161. [Google Scholar] [CrossRef]
- Gericke, M.; Skodda, L.H.; Heinze, T. Reactive Xylan Derivatives for Azid-/Alkyne-Click-Chemistry Approaches—From Modular Synthesis to Gel-Formation. Carbohydr. Polym. 2023, 300, 120251. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, H. Biomedical Applications of Copper-free Click Chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [PubMed]
- Atmani, Z.; Heinze, T.; Gericke, M. Development of Tailored Polysaccharide Gels Through Selective Diels–Alder Crosslinking. Cellulose 2025, 32, 187–209. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–Enes: Chemistry of the Past with Promise for the Future. J. Polym. Sci. A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol–ene Click Hydrogels for Therapeutic Delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click Chemistry in Materials Science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, C.; Li, L. Review of 3D Printable Hydrogels and Constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- Malafaia, A.P.; Sobreiro-Almeida, R.; Rodrigues, J.M.M.; Mano, J.F. Thiol-Ene Click Chemistry: Enabling 3D Printing of Natural-based Inks for Biomedical Applications. Biomater. Adv. 2025, 167, 214105. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef]
- Hu, H.; You, J.; Gan, W.; Zhou, J.; Zhang, L. Synthesis of Allyl Cellulose in NaOH/Urea Aqueous Solutions and its Thiol–Ene Click Reactions. Polym. Chem. 2015, 6, 3543–3548. [Google Scholar] [CrossRef]
- Gabriel, L.; Gericke, M.; Heinze, T. Modular Synthesis of Non-Charged and Ionic Xylan Carbamate Derivatives from Xylan Carbonates. Carbohydr. Polym. 2019, 207, 782–790. [Google Scholar] [CrossRef]
- Lindemann, H.; Heinze, T. Cellulose Allylcarbamate with High Content of Reactive Double Bonds for Thiol-Ene Reaction. React. Funct. Polym. 2022, 176, 105306. [Google Scholar] [CrossRef]
- Gericke, M.; Atmani, Z.; Skodda, L.H.; Heinze, T. Synthesis and Characterization of Polysaccharide Carbamates and Mixed Carbamates with Tunable Water Solubility. Carbohydr. Polym. Technol. Appl. 2024, 7, 100479. [Google Scholar] [CrossRef]
- Gericke, M.; Gabriel, L.; Geitel, K.; Benndorf, S.; Trivedi, P.; Fardim, P.; Heinze, T. Synthesis of Xylan Carbonates—An Approach Towards Reactive Polysaccharide Derivatives Showing Self-Assembling into Nanoparticles. Carbohydr. Polym. 2018, 193, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Fritzinger, B.; Martins, J.C.; Dubruel, P. Hydrogel Network Formation Revised: High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance as a Powerful Tool for Measuring Absolute Hydrogel Cross-Link Efficiencies. Appl. Spectrosc. 2010, 64, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Love, D.M.; Kim, K.; Goodrich, J.T.; Fairbanks, B.D.; Worrell, B.T.; Stoykovich, M.P.; Musgrave, C.B.; Bowman, C.N. Amine Induced Retardation of the Radical-mediated Thiol-Ene Reaction via the Formation of Metastable Disulfide Radical Anions. J. Org. Chem. 2018, 83, 2912–2919. [Google Scholar] [CrossRef]
- Akil, Y.; Castellani, R.; Lehnen, R.; Budtova, T.; Saake, B. Hydroxyalkylation of Xylan Using Propylene Carbonate: Comparison of Products from Homo- and Heterogeneous Synthesis by HRMAS NMR and Rheology. Cellulose 2018, 25, 217–231. [Google Scholar] [CrossRef]
- Koivunotko, E.; Merivaara, A.; Niemelä, A.; Valkonen, S.; Manninen, K.; Mäkinen, H.; Viljanen, M.; Svedström, K.; Diaz, A.; Holler, M.; et al. Molecular Insights on Successful Reconstitution of Freeze-dried Nanofibrillated Cellulose Hydrogel. ACS Appl. Bio Mater. 2021, 4, 7157–7167. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Omidian, H.; Dey Chowdhury, S.; Babanejad, N. Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics 2023, 15, 1836. [Google Scholar] [CrossRef]
- Alam, M.N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Feng, W.J.; Wang, Z.K. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Liao, Q.; Meldrum, O.W.; Guo, L.; Wang, K.; Zhang, S.; Liu, Y.; Chen, X.; Zhu, J.; Li, L. Mechanical Properties and Wound Healing Potential of Bacterial Cellulose-Xyloglucan-Dextran Hydrogels. Carbohydr. Polym. 2023, 321, 121268. [Google Scholar] [CrossRef]
- Mietner, J.B.; Jiang, X.H.; Edlund, U.; Saake, B.; Navarro, J.R.G. 3D Printing of a Bio-based Ink Made of Cross-linked Cellulose Nanofibrils with Various Metal Cations. Sci. Rep. 2021, 11, 6461. [Google Scholar] [CrossRef]
- Lackner, F.; Liu, H.; Stiglic, A.D.; Bracic, M.; Kargl, R.; Nidetzky, B.; Mohan, T.; Kleinschek, K.S. 3D Printed Porous Nanocellulose-based Scaffolds As Carriers for Immobilization of Glycosyltransferases. ACS Appl. Bio Mater. 2022, 5, 5728–5740. [Google Scholar] [CrossRef]
- Lackner, F.; Knechtl, I.; Novak, M.; Nagaraj, C.; Stiglic, A.D.; Kargl, R.; Olschewski, A.; Kleinschek, K.S.; Mohan, T. 3D-Printed Anisotropic Nanofiber Composites with Gradual Mechanical Properties. Adv. Mater. Technol. 2023, 8, 2201708. [Google Scholar] [CrossRef]


| Reaction Conditions | Sample ID | Results | |||||
|---|---|---|---|---|---|---|---|
| Polysaccharide Carbonate (a) | DSPC (b) | Molar Ratio (c) | DSene (b) | DSsol (b) | Solubility (d) | ||
| Buffer pH 5 | EtOH/H2O | ||||||
| CPC | 3.00 | 1.00 + 4.00 | AFC 01 | 0.84 | 1.55 | + | + |
| CPC | 3.00 | 0.80 + 4.20 | AFC 02 | 1.19 | 0.79 | + | + |
| CPC | 3.00 | 0.55 + 4.45 | AFC 03 | 0.78 | 1.18 | + | + |
| XPC | 2.00 | 0.55 + 3.45 | AFC 04 | 0.49 | 1.20 | + | + |
| XPC | 2.00 | 0.30 + 3.70 | AFC 05 | 0.22 | 1.19 | + | + |
| Polymer | Crosslinking Conditions | Gelt Time | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | DSene (a) | Polymer Concentration [wt%] | Linker | eq. of Linker [mol] (b) | <10 s | <20 s | <30 s | <40 s |
| AFC 02 | 1.19 | 7.5 | DTT | 1.00 | + | |||
| AFC 02 | 1.19 | 7.5 | DTT | 0.50 | + | |||
| AFC 02 | 1.19 | 7.5 | DTT | 0.25 | + | |||
| AFC 02 | 1.19 | 7.5 | DTT | 0.10 | + | |||
| AFC 02 | 1.19 | 5 | DTT | 1.00 | + | |||
| AFC 02 | 1.19 | 5 | DTT | 0.50 | + | |||
| AFC 02 | 1.19 | 5 | DTT | 0.25 | + | |||
| AFC 02 | 1.19 | 5 | DTT | 0.10 | + | |||
| AFC 02 | 1.19 | 3 | DTT | 1.00 | − | + | ||
| AFC 02 | 1.19 | 3 | DTT | 0.50 | − | + | ||
| AFC 02 | 1.19 | 3 | DTT | 0.25 | − | + | ||
| AFC 02 | 1.19 | 3 | DTT | 0.10 | − | − | + | |
| AFC 02 | 1.19 | 1.5 | DTT | 1.00 | − | + | ||
| AFC 02 | 1.19 | 1.5 | DTT | 0.50 | − | − | + | |
| AFC 02 | 1.19 | 1.5 | DTT | 0.25 | − | − | + | |
| AFC 02 | 1.19 | 1.5 | DTT | 0.10 | − | − | − | + |
| AFC 02 | 1.19 | 1 | DTT | 1.00 | − | + | ||
| AFC 02 | 1.19 | 1 | DTT | 0.50 | − | − | + | |
| AFC 02 | 1.19 | 1 | DTT | 0.25 | − | − | + | |
| AFC 02 | 1.19 | 1 | DTT | 0.10 | − | − | − | + |
| AFC 02 | 1.19 | 1 | EDT | 0.25 | − | − | + | |
| AFC 02 | 1.19 | 1 | EDT | 0.10 | − | − | − | + |
| AFC 02 | 1.19 | 0.5 | DTT | 1.00 | − | − | − | − |
| AFC 03 | 0.78 | 3 | DTT | 1.00 | − | + | ||
| AFC 03 | 0.78 | 3 | DTT | 0.50 | − | + | ||
| AFC 03 | 0.78 | 3 | DTT | 0.25 | − | + | ||
| AFC 03 | 0.78 | 3 | DTT | 0.10 | − | + | ||
| AFC 04 | 0.49 | 5 | DTT | 1.00 | + | |||
| AFC 04 | 0.49 | 3 | DTT | 1.00 | + | |||
| AFC 04 | 0.49 | 2.5 | DTT | 1.00 | − | − | − | − |
| AFC 05 | 0.22 | 5 | DTT | 1.00 | + | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atmani, Z.; Steindorfer, T.; Kargl, R.; Kleinschek, K.S.; Heinze, T.; Gericke, M. Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry. Polysaccharides 2025, 6, 13. https://doi.org/10.3390/polysaccharides6010013
Atmani Z, Steindorfer T, Kargl R, Kleinschek KS, Heinze T, Gericke M. Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry. Polysaccharides. 2025; 6(1):13. https://doi.org/10.3390/polysaccharides6010013
Chicago/Turabian StyleAtmani, Zakaria, Tobias Steindorfer, Rupert Kargl, Karin Stana Kleinschek, Thomas Heinze, and Martin Gericke. 2025. "Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry" Polysaccharides 6, no. 1: 13. https://doi.org/10.3390/polysaccharides6010013
APA StyleAtmani, Z., Steindorfer, T., Kargl, R., Kleinschek, K. S., Heinze, T., & Gericke, M. (2025). Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry. Polysaccharides, 6(1), 13. https://doi.org/10.3390/polysaccharides6010013









