Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities
Abstract
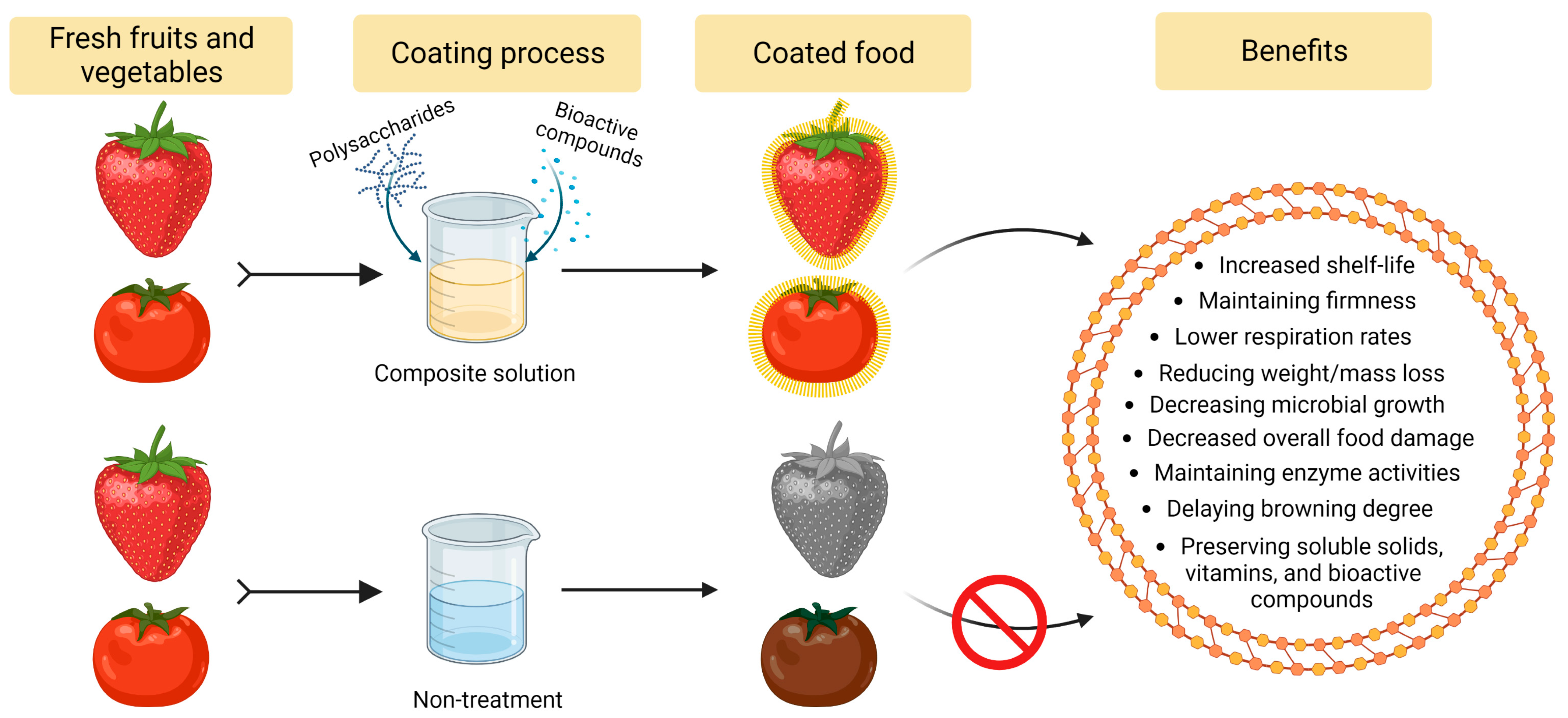
1. Introduction
2. Improvement of Quality Parameters of Foods by Polysaccharide-Based Edible Coating
3. Bioactivities Exhibited by Edible Coatings
4. Conclusions and Future Prospects in Polysaccharide-Based Edible Coatings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT-Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Tripathi, A.; Singh, D.; Pandey, K.; Singh, J. Postharvest diseases of leguminous vegetable crops and their management. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 387–396. [Google Scholar]
- Stoops, J.; Ruyters, S.; Busschaert, P.; Spaepen, R.; Verreth, C.; Claes, J.; Lievens, B.; Van Campenhout, L. Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol. 2015, 48, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2020, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.K.; Uddin, M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Masarirambi, M.T.; Nxumalo, K.; Earnshaw, D.M.; Musi, P.J.; Dlamini, B. Genetic manipulation and product shelf life: Is there a connection? A developing world perspective. J. Exp. Agric. Int. 2019, 41, 1–7. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef]
- Sharma, P.; Shehin, V.P.; Kaur, N.; Vyas, P. Application of edible coatings on fresh and minimally processed vegetables: A review. Int. J. Veg. Sci. 2019, 25, 295–314. [Google Scholar] [CrossRef]
- Millican, J.M.; Agarwal, S. Plastic pollution: A material problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Monteiro Fritz, A.R.; de Matos Fonseca, J.; Trevisol, T.C.; Fagundes, C.; Valencia, G.A. Active, eco-friendly and edible coatings in the post-harvest—A critical discussion. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 433–463. [Google Scholar]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M.; Martin-Gonzalez, F.S.; Jones, O.G.; Garcia-Bravo, J.M.; Kaplan, I. Development of chitosan films from edible crickets and their performance as a bio-based food packaging material. Polysaccharides 2021, 2, 744–758. [Google Scholar] [CrossRef]
- FDA. SCOGS (Select Committee on GRAS Substances). 2022. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 3 January 2023).
- Díaz-Montes, E. Polysaccharide-based biodegradable films: An alternative in food packaging. Polysaccharides 2022, 3, 761–775. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. An innovative single step of cross-linked alginate-based edible coating for maintaining postharvest quality and reducing chilling injury in rose apple cv. ‘Tabtimchan’ (Syzygium samarangenese). Sci. Hortic. 2021, 292, 110648. [Google Scholar] [CrossRef]
- Aziz, M.S.A.; Salama, H.E. Developing multifunctional edible coatings based on alginate for active food packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef]
- Emragi, E.; Kalita, D.; Jayanty, S.S. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions. LWT 2021, 153, 112580. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; de Lucena, F.A.; da Silva, K.G.; Martins, L.P.; de Castro, R.J.S.; Sato, H.H. Influence of edible coatings composed of alginate, galactomannans, cashew gum, and gelatin on the shelf- life of grape cultivar ‘Italia’: Physicochemical and bioactive properties. LWT-Food Sci. Technol. 2021, 152, 112315. [Google Scholar] [CrossRef]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT-Food Sci. Technol. 2021, 148, 111770. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Garcia, S. Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf life of fresh-cut yacon (Smallanthus sonchifolius). Food Bioprocess Technol. 2018, 11, 1605–1614. [Google Scholar] [CrossRef]
- Li, X.-Y.; Du, X.-L.; Liu, Y.; Tong, L.-J.; Wang, Q.; Li, J.-L. Rhubarb extract incorporated into an alginate-based edible coating for peach preservation. Sci. Hortic. 2019, 257, 108685. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Thyme oil alginate-based edible coatings inhibit growth of pathogenic microorganisms spoiling fresh-cut cantaloupe. Food Biosci. 2019, 32, 100467. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, R.; Li, W.; Song, J.; Cheng, F. Improved postharvest preservation effects of Pholiota nameko mushroom by sodium alginate-based edible composite coating. Food Bioprocess Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of chia seed mucilage/bacterial cellulose edible coating on bioactive compounds and antioxidant activity of strawberries during cold storage. Int. J. Biol. Macromol. 2021, 190, 618–623. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit. Postharvest Biol. Technol. 2019, 157, 110960. [Google Scholar] [CrossRef]
- Parven, A.; Sarker, R.; Megharaj, M.; Meftaul, I.M. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Optimized alginate and Aloe vera gel edible coating reinforced with nTiO2 for the shelf life extension of tomatoes. Int. J. Biol. Macromol. 2020, 165, 2693–2701. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2019, 262, 109041. [Google Scholar] [CrossRef]
- Jiwanit, P.; Pitakpornpreecha, T.; Pisuchpen, S.; Leelasuphakul, W. The use of Aloe vera gel coating supplemented with Pichia guilliermondii BCC5389 for enhancement of defense-related gene expression and secondary metabolism in mandarins to prevent postharvest losses from green mold rot. Biol. Control 2018, 117, 43–51. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Prudencio, S.H.; Mali, S.; Celligoi, M.A.P.C. Assessment of a new edible film biodegradable based on starch-nystose to increase quality and the shelf life of blackberries. Food Biosci. 2021, 42, 101173. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Ejaz, S.; Hussain, S.; Ercisli, S.; Saleem, M.S.; Sardar, H. Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 2021, 168, 77–85. [Google Scholar] [CrossRef]
- Sousa, F.F.; Junior, J.S.P.; Oliveira, K.T.; Rodrigues, E.C.; Andrade, J.P.; Mattiuz, B.-H. Conservation of ‘Palmer’ mango with an edible coating of hydroxypropyl methylcellulose and beeswax. Food Chem. 2020, 346, 128925. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ribeiro, C.P.P.; Uliana, N.R.; Rodrigues, M.B.C.; da Rosa, C.G.; Ferrareze, J.P.; Veeck, A.P.D.L.; Nunes, M.R. Bioactive and pH-sensitive films based on carboxymethyl cellulose and blackberry (Morus nigra L.) anthocyanin-rich extract: A perspective coating material to improve the shelf life of cherry tomato (Solanum lycopersicum L. var. cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Leyva-Gómez, G.; Urbán-Morlán, Z. Effects of UV-C and edible nano-coating as a combined strategy to preserve fresh-cut cucumber. Polymers 2021, 13, 3705. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.B.; Silva, G.M.C.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Wan, C.; Chen, C.; Chen, M.; Chen, J. Quality and biochemical changes of navel orange fruits during storage as affected by cinnamaldehyde -chitosan coating. Sci. Hortic. 2018, 239, 80–86. [Google Scholar] [CrossRef]
- Li, Y.-N.; Ye, Q.-Q.; Hou, W.-F.; Zhang, G.-Q. Development of antibacterial ε-polylysine/chitosan hybrid films and the effect on citrus. Int. J. Biol. Macromol. 2018, 118 Pt B, 2051–2056. [Google Scholar] [CrossRef]
- Xu, D.; Qin, H.; Ren, D. Prolonged preservation of tangerine fruits using chitosan/montmorillonite composite coating. Postharvest Biol. Technol. 2018, 143, 50–57. [Google Scholar] [CrossRef]
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria × ananassa) under commercial storage environment. Food Bioprocess Technol. 2018, 11, 1566–1574. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing α-tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Rodrigues, F.A.M.; dos Santos, S.B.F.; Lopes, M.M.D.A.; Guimarães, D.J.S.; Silva, E.D.O.; Filho, M.D.S.M.D.S.; Mattos, A.L.A.; da Silva, L.M.R.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. Antioxidant films and coatings based on starch and phenolics from Spondias purpurea L. Int. J. Biol. Macromol. 2021, 182, 354–365. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of mango fruit with guar-based edible coatings enriched with Spirulina platensis and Aloe vera extract during storage at ambient temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Ahad, T.; Malik, A.R.; Ganaie, T.A.; Masoodi, F.A.; Gani, A. Effect of gum arabic, xanthan and carrageenan coatings containing antimicrobial agent on postharvest quality of strawberry: Assessing the physicochemical, enzyme activity and bioactive properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, W.; Meng, F.; Wang, L. Fabricating an anti-shrinking κ-carrageenan/sodium carboxymethyl starch film by incorporating carboxylated cellulose nanofibrils for fruit preservation. Int. J. Biol. Macromol. 2021, 191, 706–713. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Effect of basil leaves extract on modified moth bean starch active film for eggplant surface coating. LWT 2021, 145, 111380. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Sucheta; Chaturvedi, K.; Sharma, N.; Yadav, S.K. Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. Int. J. Biol. Macromol. 2019, 133, 284–293. [Google Scholar] [CrossRef]
- Oyom, W.; Xu, H.; Liu, Z.; Long, H.; Li, Y.; Zhang, Z.; Bi, Y.; Tahergorabi, R.; Prusky, D. Effects of modified sweet potato starch edible coating incorporated with cumin essential oil on storage quality of ‘early crisp’. LWT-Food Sci. Technol. 2022, 153, 112475. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E. Effect of nano-edible coating based on beeswax solid lipid nanoparticles on strawberry’s preservation. Coatings 2020, 10, 253. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef]
- Paniagua, A.; East, A.; Hindmarsh, J.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Qamer, Z.; Chaudhary, M.T.; DU, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. rongrien and see-chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure—Activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P. Crop-based biodegradable packaging and its environmental implications. CABI Rev. 2009, 2008, 1–25. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the most important methods of improving the processing properties of starch toward non-food applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Microbial exopolysaccharides as drug carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr. 2006, 46, 1–22. [Google Scholar] [CrossRef]
- Divya, K.; Smitha, V.; Jisha, M. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018, 114, 572–577. [Google Scholar] [CrossRef]
- Islam, M.Z.; Saha, T.; Monalisa, K.; Hoque, M.M. Effect of starch edible coating on drying characteristics and antioxidant properties of papaya. J. Food Meas. Charact. 2019, 13, 2951–2960. [Google Scholar] [CrossRef]
| Coating Material | Food Coated | Principal Effect of Coating | References |
|---|---|---|---|
| Alginate | Rose apple (Syzygium samarangenese) cv. Tabtimchan | ↓ weight loss, respiration rate, MDA and H2O2 content ↓ LOX activity ↑ CAT and APX | Duong, et al. [21] |
| Alginate, Aloe vera, garlic oil | Tomatoes (Solanum lycopersicum L.) | ↑ shelf life period ↑ UV-shielding, barrier, thermal, and mechanical properties | Abdel Aziz and Salama [22] |
| Alginate, chitosan, zein, potato starch, essential oil (oregano and cinnamon) | Potatoes (Solanum tuberosum L.) cultivars (Rio Grande Russet, Yukon Gold, and Purple Majesty) | ↓ weight loss ≈ firmness ↑ sensory properties, particularly with colored skin potatoes ↑ shelf life period by sprout inhibition | Emragi, et al. [23] |
| Alginate, galactomannans, cashew gum, and gelatin | Table grapes (Vitis vinifera) cv. Italia | ↓ weight loss ≈ firmness and color ↑ phenolic compound content and antioxidant potential | de Souza, et al. [24] |
| Alginate, hydroxyethyl cellulose, asparagus (Asparagus officinalis L.) waste extract | Strawberry (Fragaria × ananassa) | ↓ color change, weight loss ≈ total phenolic and flavonoid contents | Liu, et al. [25] |
| Alginate, linseed mucilage, probiotic bacteria (Lactobacillus casei LC-01), fructooligosacharides | Fresh-cut yacon (Smallanthus sonchifolius) | ↓ weight loss and browning | Rodrigues, et al. [26] |
| Alginate, rhubarb (Rheum rhaponticum L.) extract | Peaches (Prunus persica) | ↓ weight loss, respiration rate, MDA content, and PPO activity ↑ firmness and the TSS content | Li, et al. [27] |
| Alginate, thyme oil | Fresh-cut cantaloupe (Cucumis melo L.) | ≈ respiration, color, and sensory characteristics | Sarengaowa, et al. [28] |
| Alginate, thyme essential oil, nisin, and L-cysteine | * Mushroom (Pholiota nameko) | ↓ weight loss, degree of browning, and MDA content ↓ PPO and POD activities ≈ soluble sugar, ascorbic acid, and soluble protein contents | Zhu, et al. [29] |
| Bacterial cellulose nano-fiber (Gluconacetobacter xylinu), chia (Salvia hispanica) seed mucilage | Strawberry (Fragaria × ananassa) | ≈ the phenolic, flavonoids, and ascorbic acid ↓ PPO and POD activities | Mousavi, et al. [30] |
| Aloe vera gel | Lychee (Litchi chinensis Sonn. cv. Gola) | ↓ browning index, weight loss, superoxide anion, relative electrolyte leakage, H2O2 and MDA content ≈ total anthocyanin, total phenolic and ascorbic acid, CAT, SOD, and APX activities | Ali, et al. [31] |
| Aloe vera gel | Papaya (Carica papaya L.) | ↑ shelf life period ↓ weight loss and disease severity | Parven, et al. [32] |
| Aloe vera gel, alginate, titanium oxide nanoparticles (nTiO2) | Tomatoes (Solanum lycopersicum L.) | ↑ shelf life period ↓ weight loss | Salama and Abdel Aziz [33] |
| Aloe vera gel, basil (Ocimum basilicum L.) seed mucilage | Apricot (Prunus armeniaca L. cv. Nouri) | ≈ firmness, TA, total phenolic and ascorbic acid contents | Nourozi and Sayyari [34] |
| Aloe vera gel, Pichia guilliermondii BCC5389 | Shogun mandarins (Citrus reticulate Blanco cv. Shogun) | ↓ weight loss ≈ shikimic acid, total phenolics, and lignin content | Jiwanit, et al. [35] |
| Cassava starch, nystose | Blackberries (Rubus spp. cv. Tupy) | ↓ increase in pH ≈ firmness and anthocyanin content ↓ counts of psychrotrophic microorganisms, molds, and yeast | Bersaneti, et al. [36] |
| Cassava starch, starch nanocrystals | Pear (Pyrus pyrifolia Nakai) | ≈ color, texture, cell membrane permeability, total phenolic, TSS, and TA contents ↓ POD and PPO activities | Dai, et al. [37] |
| Cellulose | Kinnow mandarin fruit (Citrus nobilis L. × Citrus deliciosa T.) | ↓ chilling injury symptoms, disease incidence, weight loss, MDA content, H2O2 and electrolyte leakage ≈ APX, POD, SOD, and CAT activities | Ali, et al. [38] |
| Cellulose, beeswax | Mango (Mangifera indica L. cv. Palmer) | ↓ fruit ripening ≈ peel and pulp color and firmness ≈ TA, and TSS ↓ weight loss and the disease incidence | Sousa, et al. [39] |
| Cellulose, blackberry (Morus nigra L.) anthocyanin rich-extract | Cherry tomato (Solanum lycopersicum L. var. Cerasiforme) | ≈ constant weight and firmness | Sganzerla, et al. [40] |
| Cellulose, lemon essential oil, alginate, pectin | Cucumber (Cucumis sativus L.) | ↓ weight loss ↑ shelf life period | Zambrano-Zaragoza, et al. [41] |
| Cellulose nanofiber, iron, chitosan, curcumin | Kiwifruits (Actinidia deliciosa cv. Hayward) | ↓ mass loss, firmness loss, respiration rate, and microbial count | Ghosh, et al. [42] |
| Chitosan | Guava (Psidium guajava L.) | ↓ weight loss, browning index, and respiration rate ≈ firmness and skin color were maintained ↓ PAL activity ↑ POD activity | Batista Silva, et al. [43] |
| Chitosan, cinnamaldehyde | Orange (Citrus sinensis L., Osbeck) | ≈ SOD, CAT, POD, and PPO activities ↓ postharvest decay and mass loss ≈ vitamin C and TSS | Gao, et al. [44] |
| Chitosan and ε-polylysine | Satsuma mandarin (Citrus unshiu Marc.) | ≈ TSS, ascorbic acid content ↓ disease incidence | Li, et al. [45] |
| Chitosan and montmorillonite | Tangerine (Citrus tangerine Hort. ex Tanaka) | ↓ weight loss and decay rate ≈ TSS and TA contents | Xu, et al. [46] |
| Chitosan, thymol and quinoa protein | Strawberries (Fragaria × ananassa Duch. cv. Albion) | ↓ mass loss and fungal decay ≈ flavor and aroma | Robledo, et al. [47] |
| Chitosan, zein and tocopherol | * Mushroom (Agaricus bisporus) | ↓ weight loss, browning index, and respiration rate ↓ POD and PPO activities ≈ firmness, CAT, SOD activities, total phenolic content and DPPH radical scavenging activity | Zhang, et al. [48] |
| Fruit starch and phenolic stem bark extract (both from Spondias purpurea L.) | Mango (Mangifera indica L. cv. Tommy Atkins) | ↓ browning index and reduced fungus attack | Rodrigues, et al. [49] |
| Guar gum, Aloe vera gel, and extracts of Spirulina platensis | Mango (Mangifera indica L.) | ↓ weight loss ≈ ascorbic acid, the total phenol content, and firmness | Ebrahimi and Rastegar [50] |
| Gum arabic, carrageenan, xanthan gum, lemon grass essential oil | Strawberry (Fragaria × ananassa) | ↓ weight loss ≈ ascorbic acid, anthocyanin, phenolic compound contents, and firmness | Wani, et al. [51] |
| κ-carrageenan, starch, cellulose nanofibrils | Strawberry (Fragaria × ananassa) | ↓ weight loss ≈ vitamin C, TSS, hardness, TA and pH | Zhang, et al. [52] |
| Moth bean starch, basil leaves extract | Eggplant (Solanum melongena) | ↓ moisture loss and firmness ↓ increase in TSS and color changes ↑ shelf life period | Kumar, et al. [53] |
| Pectin, crude mulberry (Morus alba) leaf extract (deoxynojirimycin and chlorogenic acid) | Capsicum annum L. | ↑ shelf life period ↓ mass loss | Shivangi, et al. [54] |
| Pectin, corn flour, and beetroot powder | Tomatoes (Solanum lycopersicum L.) | ↓ weight loss and respiration | Sucheta, et al. [55] |
| Sweet potato starch and cumin essential oil | Pear (Pyrus bretchneideri Rehd.) | ↓ rot lesion on infected pear caused by Alternaria alternata ↓ changes in color, firmness, and chlorophyll degradation | Oyom, et al. [56] |
| Xanthan gum, beeswax | Strawberry (Fragaria × ananassa cv. Camarosa) | ↓ weight loss, firmness loss, and decay index | Zambrano-Zaragoza, et al. [57] |
| Polysaccharides | Characteristics | FDA 21 CFR Regulation 1 | References | |
|---|---|---|---|---|
| Advantages | Disadvantages | |||
| Chitosan (N-acetyl-D-glucosamine) |
|
| Not currently available 2 | Dutta, et al. [62], Sahariah and Másson [63], Garg, et al. [64] |
| Alginate |
|
| 184.1187 | Campos, et al. [65], Pawar and Edgar [66], Gheorghita Puscaselu, et al. [67] |
| Cellulose |
|
| 182.90 | Hassan, et al. [68], Nešić, Cabrera-Barjas, Dimitrijević-Branković, Davidović, Radovanović, and Delattre [10] |
| Starch |
|
| 172.892, 182.70 | Campos, Gerschenson, and Flores [65], Cruz-Romero and Kerry [69], Zarski, et al. [70] |
| Carrageenan |
|
| 172.620 | Campos, Gerschenson, and Flores [65], Necas and Bartosikova [71], Tabernero and Cardea [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities. Polysaccharides 2023, 4, 99-115. https://doi.org/10.3390/polysaccharides4020008
Cruz-Monterrosa RG, Rayas-Amor AA, González-Reza RM, Zambrano-Zaragoza ML, Aguilar-Toalá JE, Liceaga AM. Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities. Polysaccharides. 2023; 4(2):99-115. https://doi.org/10.3390/polysaccharides4020008
Chicago/Turabian StyleCruz-Monterrosa, Rosy G., Adolfo A. Rayas-Amor, Ricardo M. González-Reza, María L. Zambrano-Zaragoza, José E. Aguilar-Toalá, and Andrea M. Liceaga. 2023. "Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities" Polysaccharides 4, no. 2: 99-115. https://doi.org/10.3390/polysaccharides4020008
APA StyleCruz-Monterrosa, R. G., Rayas-Amor, A. A., González-Reza, R. M., Zambrano-Zaragoza, M. L., Aguilar-Toalá, J. E., & Liceaga, A. M. (2023). Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities. Polysaccharides, 4(2), 99-115. https://doi.org/10.3390/polysaccharides4020008








