Abstract
Lignocellulosic residues have been receiving growing interest as a promising source of polysaccharides, which can be converted into a variety of compounds, ranging from biofuels to bioplastics. Most of these can replace equivalent products traditionally originated from petroleum, hence representing an important environmental advantage. Lignocellulosic materials are theoretically unlimited, cheaper and may not compete with food crops. However, the conversion of these materials to simpler sugars usually requires cellulolytic enzymes. Being still associated with a high cost of production, cellulases are commonly considered as one of the main obstacles in the economic valorization of lignocellulosics. This work provides a brief overview of some of the most studied strategies that can allow an important reduction of cellulases consumption, hence improving the economy of lignocellulosics conversion. Cellulases recycling is initially discussed regarding the main processes to recover active enzymes and the most important factors that may affect enzyme recyclability. Similarly, the potential of enzyme immobilization is analyzed with a special focus on the contributions that some elements of the process can offer for prolonged times of operation and improved enzyme stability and robustness. Finally, the emergent concept of consolidated bioprocessing (CBP) is also described in the particular context of a potential reduction of cellulases consumption.
1. Introduction
Important changes throughout the world’s economy and population have been imposing, in recent years, new significant challenges to energy supply, resulting from a substantial growth in global energy needs [1]. Additionally, petroleum, which has been for decades a key element in energy supply chains, faces a gradual decrease on its world-wide reserves [2] but also a rising exposure to geo-political instability in some regions of the globe; all combined, this led to increasingly more frequent fluctuations of its market price [3]. In addition, the combined growth of energy demand but also of other common necessities (e.g., plastics) that are usually produced by the petrochemical industry is creating alarming levels of greenhouse gas (GHG) emissions, which result in more and more environmental concerns due to a growing depletion of the ozone layer [4]. This problem demands immediate action, which may involve a transition to a bio-based economy. Such a transition actually began several decades ago, namely with the production of bioethanol from energy crops. As this bio-industry has grown, new concerns have been raised regarding the potential of energy crops competing with arable land for food crops, which could affect food prices.
In contrast to that, increased attention has been given to a new promising source of sugars that could also be biologically converted into biofuels or other chemicals [5,6], designated as lignocellulosic materials (LCMs). Lignocellulosic materials are theoretically unlimited, cheaper and may not compete with food crops [7]. However, because of their recalcitrant structure, they also require more complex processes and present more operational constraints (e.g., mass transfer limitations, high viscosities, etc.) that can affect process productivities. A major aspect of converting lignocellulosic materials relates to the need for cellulolytic enzymes (Figure 1), required to convert complex sugars to simpler monomers, which still represents one of the main costs of the process.
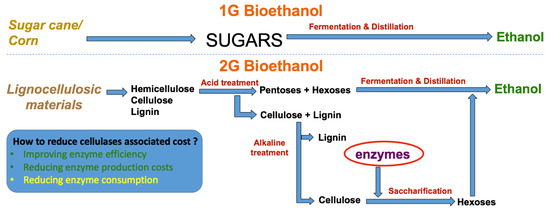
Figure 1.
Simplified schematic on the production of bioethanol from energy crops vs lignocellulosic materials.
1.1. Lignocellulosic Materials
Lignocellulosic materials present a complex structure, constituted mostly by complex polysaccharides, namely cellulose, hemicellulose and lignin (Figure 2). Cellulose is composed of long, linear chains of glucose molecules linked by β(1-4) glycosidic bonds. A high crystallinity degree, resulting from hydrogen bonds between different cellulose chains, makes it very robust and hard to digest. In comparison, hemicellulose is constituted by shorter chains, not strictly linear, of different monomers such as xylose, glucose, mannose, galactose, rhamnose and arabinose. Due to its amorphous structure, it also presents a less robust structure, thus being more easily digested. Another major component of LCMs is lignin, which is commonly associated with the rigidity and impermeability of these materials. In contrast, lignin is not a polysaccharide but a polymer of different aromatic compounds, such as p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol [8]. Cellulose typically holds the biggest share of potential to produce high-value compounds since its hydrolysis results only in glucose, which is easily used by traditional fermentation organisms, such as the yeast Saccharomyces cerevisiae. In addition, hemicellulose can be found at interesting levels, however, its hydrolysis produces different monomeric sugars, some of which are still not efficiently metabolized by important fermentation organisms (e.g., xylose).
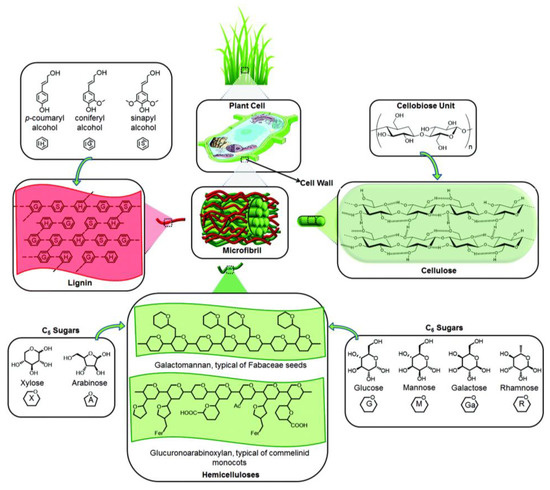
Figure 2.
Main structural components of a lignocellulosic material (reproduced from Isikgor and Becer [14] with permission from the Royal Society of Chemistry, 2015).
Lignocellulosic materials can refer to a broad range of potential sources, the most common of which consists of agro-forestry residues (e.g., sub-products of agriculture, lignocellulosic parts of energy crops, residues from forest cleaning, etc.). Furthermore, they can also consist of common wastes generated by industry, such as brewer’s spent grains and paper sludge, or even from human daily activity as is the case with food wastes and municipal solid waste [9].
1.2. The High Cost of Enzymes
Adding to the cost of LCMs, the cost of cellulolytic enzymes (cellulases) corresponds to one of the other main challenges for the economic conversion of these residues. While an exact cost estimation is difficult to find, with industrial partners frequently negotiating individually the price of enzymes with their producers, it is commonly regarded as very high. In an early study by Klein-Marcusschamer et al. [10], the authors reported an enzyme cost around $0.68 per gallon of ethanol produced. Smaller value estimations have also been reported, with $0.32 pointed to by Dutta et al. [11], $0.3 by Lynd et al. [12] and even $0.1 by Aden and Foust [13].
Regardless of the exact prices, it is well recognized that cellulases still contribute to an important part in the cost of lignocellulosic conversion. Reducing this cost has been pursued using three main approaches: an increase of enzyme efficiency, which would lead to less enzymes required; a reduction of the production cost of enzymes, namely by employing more efficient cellulolytic organisms and/or less expensive feedstocks; and the utilization of enzymes over prolonged periods [15]. Most efforts have been focused on the first two, not only by industry (e.g., Novozymes, DSM, Dupont, etc.) but also academia. However, the extent to which this cost can be pushed further down has a limit, which could mean that pursuing potential ways of better enzyme usage, by re-using enzymes several times, may be the next step on this quest.
In the context of achieving a reduction of the overall consumption of cellulases, two main strategies have been particularly considered: the recovery of enzymes after hydrolysis and their reutilization in a new round of hydrolysis, also known as cellulases recycling, the immobilization of enzymes as a way for their easy recovery and/or for their prolonged application over a continuous operation. A more recent approach that can also enable interesting savings is the utilization of efficient fermentation organisms producing cellulases, under the emergent concept of consolidated bioprocessing (CBP). While cellulases recycling and enzyme immobilization can somehow seem very similar, it should be noted that neither is enzyme immobilization always conducted for enzyme reutilization purposes nor cellulases recycling always mediated by immobilization. Regarding CBP, its contribution can be framed under two perspectives: the utilization of efficient fermentation organisms capable of producing cellulases and the adoption of cell recycling schemes for organisms with cell wall-anchored cellulases, hence also allowing the recycling of their cellulases.
2. Fundamentals of Enzymatic Conversion of LCMs
After pretreatment of the lignocellulosic material, the obtained solid is incubated with a commercial cellulases cocktail under specific conditions. Typically, process requirements are dependent of the source of cellulases and their optimum hydrolysis conditions. For the case of fungal cellulases, the most employed class by industry, the temperature is usually in the range of 45–55 °C and the pH between 4.5 and 5.5 [16]. An efficient enzymatic conversion of LCMs involves multiple classes of enzymes, such as cellulases and hemicellulases, associated with the hydrolysis of cellulose and hemicellulose, respectively. As cellulose hydrolysis represents a superior bio-production potential, we will especially address this fraction in this work. The hydrolysis of cellulose chains is rather complex as distinct types of enzymes are involved [10]. In addition to that, the same enzyme from different origins can have distinct hydrolysis mechanisms [17]. Among some of the most common cellulase producers are the fungus Aspergillus niger and Trichoderma reesei, but also the bacteria Clostridium thermocellum [18].
Generally speaking, a cellulolytic organism usually secrets a significant number of cellulases, among which are two classes especially relevant for cellulose hydrolysis: endoglucanases (EGs), which randomly break internal β-1,4-glycosidic bonds, and exoglucanases, such as cellobiohydrolases (CBHs), which act on the reducing/non-reducing ends of cellulose chains. These are complemented by a final class of β-glucosidases, catalyzing the hydrolysis of cellobiose into glucose [19]. In an efficient hydrolysis, these enzymes act synergistically in a concerted action that involves multiple steps: cellulases adsorption onto the substrate, formation of the complex cellulase-substrate, cleavage of the glycosidic bond, displacement of the enzyme over the cellulose chain and cellulases desorption (Figure 3).
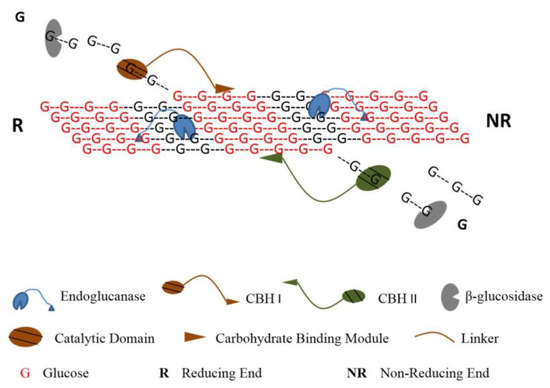
Figure 3.
Simplified schematic of the action of different cellulases on cellulose chains (reproduced from Kumar and Murthy [20] with permission from BioMed Central Ltd., 2013).
Currently regarded as the world biggest cellulases producer [8,21], T. reesei has possibly the best studied cellulolytic system so far. Nearly 80% of the total secreted proteins correspond to cellobiohydrolases, 60% being Cel7A and 20% Cel6A [22]. Even though the two act processively on the cellulose chains, Cel6A attacks non-reducing ends while Cel7A acts on reducing ends. Furthermore, both types conduct a reversible binding to the substrate, which is especially relevant in a context of enzyme recycling. Adding to these, a wide range of different EGs are also secreted, such as Cel5A, Cel5B and Cel7B, among others; these, however, are produced in much inferior amounts [23]. Finally, T. reesei still secrets seven β-glucosidases, although these are typically produced under very low levels; for an efficient cellulose hydrolysis this is commonly solved with the addition of an “external” enzyme from another organism such as Aspergillus niger [24].
3. Cellulases Recycling
Recycling cellulases implies that active enzymes at the end of hydrolysis/fermentation are efficiently recovered and posteriorly reutilized in a new round of hydrolysis. Alternatively, it can also rely on a direct recycling of the final hydrolyzate with no previous separation process. According to the scheme of the base process on which enzyme recycling would be applied, this step could be implemented at different points of the process: after enzymatic hydrolysis, after fermentation and after final product recovery [25]. The latter option brings an immediate advantage since enzymes would be recycled with low amounts of sugars and/or final products. However, that would require that downstream processing would not involve thermal-abrasive processes, such as distillation, a common operation in industrial downstream processing.
Determining the most suitable point and approach for efficient cellulases recycling is case-dependent and should consider distinct aspects: what is the percentage of final enzymes that remain active after hydrolysis? What is the fraction of active enzymes that are free and bound to the final solid? Can solid-bound enzymes be easily desorbed from the solid? What kind of inhibitory compounds (e.g., sugars, ethanol, pretreatment inhibitors) are present with the enzymes?
3.1. The Influence of Solid-Enzyme Interactions and the Role of Substrate Composition
A key aspect determining the potential and expected complexity for a possible cellulases recycling scheme relates to the interaction between the LCM and the employed cellulolytic enzymes. This is a rather complex aspect since different enzymes normally present distinct affinities to a solid [15] and the same enzyme can also have different affinities to different solids [17], inclusively to different components of the same solid (e.g., cellulose and lignin). How efficiently cellulases will adsorb and later desorb onto the different components of the solid will ultimately dictate their distribution after hydrolysis and the complexity of the recovery/recycling system. Hence, while free enzymes can be more easily/directly recovered from the final medium, solid-bound enzymes will require a previous step of desorption from the solid, unless recovered directly with the final solid.
In relation to the affinity of a given cellulase towards the LCM, a key element to consider is the presence of cellulose-binding domains (CBDs). These are a particular class of carbohydrate-binding modules (CBMs) consisting of small amino acid sequences with a carbohydrate binding activity [26]. These modules, with no direct catalytic activity, have been reported to improve the adsorption to carbohydrates and, in the specific case of CBDs, to cellulose. Hence, the presence of these structures would not only allow an increase of enzymes adsorbed to cellulose, but also a superior enzyme selectivity. In addition, CBMs have been also reported to promote the disruption of crystalline regions of the substrate [27], which would enhance enzymes’ access to cellulose. In fact, a recombinantly produced CBM (CBM3) was recently proposed as additive for enhanced enzymatic hydrolysis of the whole slurry from autohydrolyzed Eucalyptus globulus wood [28]. In the particular context of cellulases recycling, the role of CBDs is equally important since it will critically influence the distribution of free and solid-bound enzymes and, consequently, how easily the different types will be recovered. As an example, the recovery of free enzymes through fresh substrate addition and subsequent solid-liquid separation depends on an efficient binding of enzymes in the beginning of hydrolysis. On the other hand, for the recovery of solid-bound enzymes, these would ideally have a low affinity to non-hydrolyzed components of the LCM (e.g., lignin). While excepting for Cel12A, all major cellulases of T. reesei have a CBD [29], this component is typically not found in β-glucosidases, which will likely reduce its ability to bind cellulose. For example, in an early study from Tu et al. [30], the authors reported that commercial cellulases from T. reesei (Celluclast and Spezyme CP) presented higher substrate affinities compared to those produced from Penicillium sp., which according to the authors can be explained by the fact that two of the main cellulases secreted by this organism (EG and EG b1) do not present a CBD.
In relation to the other element of this interaction, the effect of the LCM composition can be found equally determinant for a suitable enzyme distribution. Hence, even though enzymes have a high affinity not only for cellulose but also lignin, their behavior after hydrolysis is very distinct. In the case of cellulose, they are usually released into the liquid fraction once the cellulose chains are hydrolyzed. In contrast to that, cellulases adsorbed onto lignin do not naturally desorb from it, a mechanism usually designated as non-productive binding [31]. In a previous study by Qi et al. [32], the authors observed that, after 48 h of hydrolysis of a residue containing 20% of lignin, nearly 30% of the enzymes were in the supernatant, but when the lignin content of the initial solid was only 3.6% the amount of free enzymes increased to 65%. According to Kumar et al. [33], lignin not only competitively binds onto cellulases, decreasing their availability to adsorb on cellulose, but also blocks their access to cellulose with the formation of a physical barrier. In this sense, different physico-chemical treatments have already been used to decrease this lignin barrier [34]. Regarding its effect due to competitive binding with cellulases, the extent of it will depend on the lignin chemical structure since the interactions between lignin and cellulases are mostly hydrophobic [35,36]; as a consequence, different types of lignin will also bind to cellulases with different affinities. In this sense, the utilization of different compounds such as soy protein [37], BSA [31,38] and surfactants [39], among others, have already been successfully used to attenuate this effect. These compounds usually bind to lignin, and by competing with cellulases, they consequently reduce cellulases adsorption onto lignin.
Another important aspect related to the lignocellulosic material points to the cellulose content of the final hydrolysis residue. In a previous work from Rodrigues et al. [40], the authors observed that the efficiency of cellulases recovery from spent solids applying a process of alkaline elution was significantly inferior when the solid consisted of cellulose, as oppsed to lignin: an enzyme desorption test conducted over pure lignin allowed adsorbed Cel7A enzymes to be fully recovered; when the same procedure was conducted over Cellulose CF11, a maximum of 59% and 62% of enzymes were recovered from the solid hydrolyzed for a period of 24 and 48 h, respectively. This particular aspect raises a new discussion over the selection of hydrolysis conditions, since reaching high cellulose conversions seems to get an even higher importance. In fact, the same authors also observed that the efficiency of Cel7A recovery from the spent solid obtained after the hydrolysis of wheat straw had a direct relation with the extension of hydrolysis, which itself showed a high dependence on the hydrolysis temperature. According to the authors, high temperatures resulted in an increase of enzyme denaturation, directly resulting in a superior amount of unconverted cellulose.
3.2. Recycling Free Cellulases in the Liquid Fraction
In general terms, free cellulases refers to those that after hydrolysis, or at the moment of the recycling process, are desorbed from LCM components and are free on the liquid fraction, being more easily recovered [41]. It is worth noting that even the solid fraction can comprise ranging levels of free enzymes since the spent solid also contains some liquid retained inside its matrix. The recycling of free enzymes present in the liquid fraction (obtained after a solid-liquid separation step) has been mostly studied following two main approaches: re-adsorption onto a fresh substrate [17,42,43,44,45] and recovery using membranes [46,47,48].
Cellulases re-adsorption onto fresh substrate consists of exposing free cellulases to a new batch of fresh substrate for a limited period [30] under conditions promoting enzyme adsorption [43], specifically in terms of temperature and agitation. Based on their retained high affinity for the lignocellulosic solid [40], partially due to the presence of CBMs, most of the enzymes are expected to be bound to the solid after this short period, and thus separated from the previous product of hydrolysis/fermentation [49]. A prolonged contact period would be undesirable, especially if the enzymes to be recovered are present in a final medium after fermentation, with high concentrations of final product and minimum levels of residual sugars. In these circumstances, allowing an extended contact of enzymes will result in a considerable amount of sugars in the final product stream, which if not further converted/recovered would not have a value. Furthermore, as hydrolysis proceeds it also increases the probability of enzymes being released into the liquid fraction, thus not being recovered.
After the incubation period, the solid (together with, expectedly, a considerable amount of adsorbed enzymes) is separated from the liquid, ideally only containing the product of hydrolysis/fermentation [45,50]. This solid is finally resuspended in fresh buffer/water for the desirable level of solids consistency and a new round of hydrolysis begins. A particular disadvantage of this technique is that it usually requires the addition of fresh β-glucosidases; this occurs because, as discussed above, this class of enzymes typically has a very low binding affinity to the solid, hindering their recovery via re-adsorption onto fresh substrate [51]. This approach, which has already been studied in several works, has been showing some interesting results. In an early study from Tu et al. [17] the authors observed that 88% of the free cellulases were recovered via re-adsorption onto a fresh solid. Using the same method, Shang et al. [45] verified that a second round of hydrolysis still presented nearly 46.7% of the hydrolysis yield achieved on the initial round. Mesa et al. [52] reported that after the hydrolysis of sugarcane bagasse, approximately 77% of the enzymes were recovered via re-adsorption onto fresh substrate. Qi et al. [32] conducted four consecutive rounds of hydrolysis of alkaline pretreated wheat straw, and after hydrolysis, free enzymes were recovered via adsorption onto fresh substrate; after four rounds with only the addition of fresh β-glucosidase and solid substrate, a hydrolysis yield of 75% was still achieved.
Concerning the potential need for β-glucosidases supplementation, some attempts have been made to reduce the levels of freshly added enzymes. In a recent work from Guo et al. [53] the authors were able to considerably reduce the levels of added enzymes using a β-glucosidase secreting yeast. In a different approach, Waeonukul et al. [54] fused the initial β-glucosidases with a CBM3, enabling them to bind to the new solid and be recovered in a similar way to what occurs to the other enzymes.
A possible alternative to re-adsorption onto fresh substrate may include the utilization of membranes that could enable the separation of enzymes (larger and more complex molecules) from fermentable sugars/fermentation products. This is commonly conducted with an ultrafiltration process, employing a membrane with a small cutoff, usually around 5–10 kDa [32,47]; it is worth mentioning that the dimensions of common enzymes found on cellulase cocktails are typically superior to 20 kDa [15]. Thus, this process would generate a permeate stream with the final product, and ideally no enzymes, and a concentrate/retentate stream with most of the enzymes, which can then be added to fresh solid in a new round of hydrolysis. In a previous work from Lu et al. [46] free cellulases from the liquid fraction were recovered using ultrafiltration and subsequently used in a new round of hydrolysis. According to the authors, saccharification efficiency only decreased 25% after the third round of hydrolysis (hence, two recycling rounds). In another study Rodrigues et al. [55] conducted three consecutive rounds of hydrolysis and fermentation of pretreated wheat straw, where free cellulases at the end of each round were recovered via ultrafiltration and re-used in the subsequent round, together with 20% of fresh enzymes. Using this recycling strategy and specific operation conditions consisting of a hydrolysis temperature of 37 °C and an enzyme dosage of 40 FPU/gcellulose, the authors observed that cellulose hydrolysis after 48 h decreased from 100% in the first round to 89% in the second round, and still 81% in the 3rd round. Similar results were previously obtained by our research when four consecutive rounds of hydrolysis and fermentation were successively conducted with the addition of only 20–30% of fresh enzymes [24]. Differently, in this case, also enzymes that were desorbed from the spent solid were recovered via ultrafiltration. The addition of 30% of fresh enzymes was shown to be efficient to compensate moderate activity losses during hydrolysis and fermentation, but also during the ultrafiltration process. Comparatively to the first round of hydrolysis, with 94% of glucan conversion, the second and third rounds still allowed glucan conversions of 92 and 83%, respectively.
Even though this may bring an immediate economic advantage when comparing to the previous approach, since β-glucosidase supplementation is no longer required, the additional cost from a more complex process involving industrial-scale ultrafiltration should also be considered. In a recent study from our group, an economic analysis was performed to assess the viability of a cellulases recycling system employing an ultrafiltration process to recover free enzymes. Even though the overall recycling process was shown to be economically attractive, a sensitive analysis suggested that its economic output is exposed in some extent to some variables, namely the cost of ultrafiltration membrane and the price of cellulases cocktail [56].
3.3. Recycling Cellulases Adsorbed to the Spent Solid
Depending on the specific cellulases-solid interactions of each case, a significant fraction of initial enzymes can still be found bound to the solid after hydrolysis, especially when this is incomplete, and thus should also be considered for recovery. Indeed, in a recent study from our group, Gomes et al. [57] observed that after the hydrolysis of an 18% (w/v) suspension of recycled paper sludge using Cellic CTec2, nearly 60% of final Cel7A activity was on the solid fraction. As for the free enzymes on the liquid fraction, these enzymes still retain their capacity to bind and hydrolyze fresh substrate [40].
A possible and very simple way to recover this fraction would be by recycling the final solid itself with the adsorbed enzymes. While this could represent an interesting option in some cases, especially when considerable fractions of unconverted cellulose would thus be recycled for a new batch of hydrolysis (somehow working as an alternative fed-batch), one should also consider the accumulation of other components that could interfere with the process. The most relevant of these would be lignin, which would be gradually accumulating (as an inert element) and affecting the hydrolysis of cellulose, either because of its non-productive binding or its barrier effect [32,51]. In a previous work from Weiss et al. [58] the authors reported that by recycling 50% of the residual solids, together with the addition of 67% of new enzymes, it was possible to obtain a hydrolysis yield above 60%. However, and as the authors pointed out, there was a clear increase of lignin content as the operation runs increased.
A more viable option to recover this fraction of enzymes may require their initial desorption from the solid, being posteriorly recycled employing the same methods used for free enzymes fraction. When referring to the desorption of cellulases from the spent solid one should consider two very distinct types of interactions. On the one hand there is a strong binding of cellulases to cellulose, which is typically mediated by cellulose-binding domains. On the other hand, cellulases usually bind to lignin in a non-specific way, mostly depending on hydrophobic interactions between specific regions of enzymes and lignin. One common method to desorb enzymes relies on a change of the medium pH [25]. This is based on the fact that, as for other types of proteins, cellulases present many amino acids with a side chain with a pH titratable group. Hence, a pH change may result in modifications of their structure, which itself can result in alterations in the way they interact with other elements, such as lignin and cellulose. In fact, this inclusively explains the need for a highly controlled pH during enzymatic hydrolysis, typically conducted in a very narrow range of pH values (4.5–5).
Indeed, early studies from Otter et al. [59,60] showed that a change to an alkaline pH allowed a partial recovery of solid-bound cellulases; however, activity levels decreased beyond specific pH values. According to Otter et al. [59], it was possible to desorb nearly 40–45% of solid-bound Avicelase when the pH was raised to 10, while a further increase could even result in a higher desorption but, on the other hand, also a drastic reduction in enzyme activity. In a previous study by Rodrigues et al. [40] the authors conducted an analysis of Intrinsic Tryptophan Fluorescence (ITF) and Circular Dichroism (CD) over Cel7A when subjected to a pH increase from 4.8 to 9–10. The results suggested that while significant conformational changes seemed to occur over the Cel7A structure, these were reversed when the pH was restored to 4.8. Still according to the authors, the activity towards the hydrolysis of low-molecular weight substrates, such as MUC (4-methylumbelliferyl-β-D-cellobioside), was not affected. Similar indications were obtained from a previous work from our group, where solid-bound enzymes were desorbed applying an alkaline elution step [24]. The activity of Cel7A reported on the solid fraction after hydrolysis was 0.79 ± 0.01 IU/mL and after alkaline elution 0.76 ± 0.06 IU/mL, which does not constitute a statistically significant change. Additionally, it was also observed that approximately 82% of bound enzymes were desorbed from the solid, becoming available to an easy recycling.
Another common approach to facilitate the recovery of this fraction of enzymes relies on the action of some compounds widely recognized to interfere and affect the binding of enzymes to the solid. While they cannot be directly associated to the recycling step itself, they will promote the desorption of enzymes from the solid. In the early studies from Otter et al. [60], the authors reported that the utilization of detergents generally improved the desorption of Avicelase from Avicel, with the only exception being sodium dodecyl sulfate. The best option consisted of Tween 80, for which 67% of bound cellulases were desorbed. In a previous study from Tu et al. [30], the authors also found that the addition of Tween 80 in the hydrolysis medium resulted in a higher amount of free enzymes during hydrolysis, but also in the protein fraction desorbed after hydrolysis. The effects from this class of compounds can be attributed to a possible competition with cellulases for lignin-binding sites, which would reduce the extent of non-productive enzymes binding [39]. Another relevant class of compounds may correspond to polyhydric alcohols. An early study from Zhu et al. [61] showed that both glycerol and ethylene glycol had a superior effect on cellulases desorption compared to surfactants (e.g., Triton X-100), where the utilization of 72% ethylene glycol allowed 76% of enzymes adsorbed on a corn stover solid to be recovered.
3.4. Whole Slurry Recycling
As an alternative to exclusively recycle a specific fraction of final active enzymes (free or solid-bound), a much simpler option is the recycling of the whole solids slurry, which would enable both fractions to be recovered. Consequently, and since this does not require any separation of the different fractions, it represents the simplest recycling method [25]. Nonetheless, and recalling many of the previously described limitations of solid-bound enzymes recycling, this technique has a very strict range of applicability. Even not considering the gradual accumulation of final product (sugars/fermentation product), which can be critical or not depending on the production levels and the rate of slurry recycling, a relevant issue to consider is the accumulation of insoluble solids over operational rounds. The consequences of that would not only be an increase of rheological issues and mass transfer limitations but also a superior inhibition of cellulases due to non-productive binding. Because of that, whole slurry recycling can be considered an option with limited applicability, which would depend on the operation conditions (e.g., level of solids consistency) and intrinsic rheological properties of the lignocellulosic material. On the other hand, recycling the whole slurry would imply that a fraction of the final product is also transferred to the new production cycle, hence resulting in a superior concentration of final product, which would equally allow it to operate with inferior solid loadings [25]. Possibly because many of the technical constraints mentioned above, and even despite its low cost and easy implementation, whole slurry recycling has barely been reported so far. One of the few examples is the work reported by Haven et al. [62] relatively to a demonstration-scale study performed by Inbicon consisting of the hydrolysis of pretreated wheat straw. According to the authors, by recycling 20% of the fermentation broth, it was possible to reduce the amount of fresh enzymes required by 5%, which although may seem very low, on an industrial level can represent a significant saving.
4. Cellulases Immobilization
4.1. Immobilization Fundamentals
Enzyme immobilization is a procedure widely used in a variety of industrial, medical and analytical processes [63]. The use of immobilized enzymes in any bioprocess leads to an easy handling, separation and reuse of the enzyme. In addition, the storage and operational stability of the enzymes is usually increased, enabling their use in different operation modes such as semi-continuous or continuous operations [64]. Enzyme immobilization can be divided into three main categories: entrapment/encapsulation, cross-linking and binding to a support (Figure 4). Entrapment and encapsulation are low-cost techniques (mainly due to the use of polymers) in which enzymes are either trapped or retained inside a capsule, separated from the reaction medium [65]. Cross-linking enzyme (CLE) technology is a simple carrier-free technique performed with the addition of a cross-linking agent to an enzyme preparation, usually producing a rigid three-dimensional complex of enzymes [66]. Finally, binding to a support is based on the simple attachment of the enzyme to a solid support mediated by different possible chemical interactions (either covalent or non-covalent) [65]. In the particular case of cellulases, immobilization onto a support is one of the most interesting techniques since some strategic advantages can be gained after immobilization, which will be featured below.
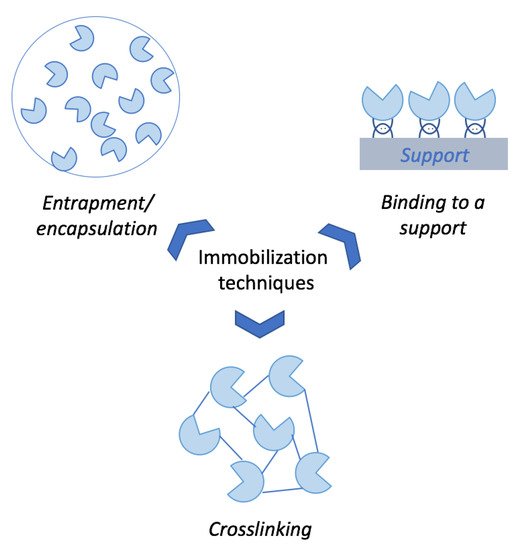
Figure 4.
Main techniques employed for enzymes immobilization.
4.2. Supports for Enzyme Immobilization
One of the most important aspects of an immobilization process is the type of support used. This is attributed to the many advantages a particular support can offer to the enzyme such as protection against inhibitors, ease of recovery, mechanical strength and the possibility of use in continuous operations, all important contributors to maximize the operational time of the enzyme [67]. The selection of the right support will hence impact the resulting properties of the immobilized enzyme. According to Kołodziejczak-Radzimska and Jesionowski [68], the immobilization support must either have sufficient reactive functional groups or must be easily modified to introduce chemical groups for a successful bonding. Additionally, the support should ideally have a large surface area, specific and stable reactive groups to achieve a multipoint enzyme-support attachment and also high chemical, thermal and mechanical stability [69,70].
4.2.1. Chitosan
Chitosan, a biopolymer obtained from chitin, has been widely used as support for cellulases immobilization due to its large possible configurations, high affinity to proteins and multiple other properties such as biodegradability, nontoxicity, hydrophilicity and biocompatibility [71,72]. Dinçer and Telefoncu [73] have previously immobilized cellulases from Aspergillus niger using chitosan beads coated with maleic anhydride-modified polyvinyl alcohol (PVA). When cellulases are immobilized inside beads, a common limitation is a poor enzyme-substrate diffusion, especially when LCMs are used. Therefore, the authors immobilized the cellulases on the surface of chitosan beads where the PVA coating shifted the optimum pH of the enzyme to neutral, by providing a polyanionic micro-environment to the enzyme. Another reported use of chitosan for cellulases immobilization was in combination with magnetic coal fly ash under two approaches: as microcomposites and porous gel beads [74]. According to the authors, both supports (microcomposites and porous gel beads) led to similar glucose production yields (using CMC as substrate) and kept up to 69.9% of the original production yield after reusing the immobilized cellulases for 10 cycles. Chitosan can also be used to modify magnetic nanoparticles, such as Fe3O4, thus gaining their magnetic properties leading to an easy handling of the enzyme [71,75,76].
4.2.2. Magnetic Nanoparticle
Magnetic nanoparticles gained a considerable interest as immobilization support since they can be easily manipulated by applying magnetic fields. This is especially relevant because on the hydrolysis of a lignocellulosic material, even when 100% of cellulose is converted, lignin and ashes still remain as final solid residue. Therefore, an easy way to recover the immobilized enzymes is by applying an external magnetic field, thus separating the enzymes from the solids and the medium [77]. Some authors have already tested the hydrolysis of different lignocellulosic materials using immobilized cellulases on magnetic nanoparticles, but further research is required since the materials used usually have a small particle size, thus the diffusion problem is not really faced. For example, Jia et al. [78] immobilized cellulases on a magnetic support and evaluated the hydrolysis yield using powdered bamboo (80 mesh) at different concentrations (0.075%, 0.15%, 0.30% (w/v)), obtaining the highest hydrolysis yield at 0.075% (w/v) load. When higher concentrations were tested, the diffusion limitations increased, leading to a low activity of the immobilized enzymes. Tan et al. [79] recently proposed an attractive immobilization procedure using chitosan and Fe3O4 nanoparticles. Before immobilizing the enzymes on the support (magnetic nanoparticles coated with chitosan), this was mixed with cellulose powder in order to avoid bonding on the active site of the enzyme. This created a core-shell structure with the active cellulases retained inside the shell and where the chitosan acted as an outside shell preventing the enzymes from escaping while maintaining magnetic properties and allowing the substrate to reach cellulases.
4.3. Enzymatic Hydrolysis of Pretreated Lignocellulosic Materials Using Immobilized Enzymes
Enzyme stability after immobilization provides a convenient advantage for lignocellulosics hydrolysis. Since lignocellulosic materials usually require a pretreatment operation, aimed to enhance cellulose accessibility by cellulases, some enzyme inhibitors might be released which may affect the saccharification yield [80]. An example of a possible solid pretreatment refers to the utilization of ionic liquids (IL), which can cause further inactivation of enzymes during the saccharification step. In this case, it would be desirable to have a stable enzyme against residual ionic liquids, avoiding the solid washing step or even enabling an in situ saccharification. Grewald et al. [81] studied the performance of immobilized cellulases from Trichoderma reesei on the hydrolysis of sugarcane bagasse and wheat straw pretreated with 1-ethyl-3-methylimidazoliumacetate [EMIM][Ac] ionic liquid. According to the authors, cellulases immobilized on magnetic nanoparticles had a 3-fold higher hydrolysis yield compared with the free enzymes. Furthermore, when the stability of the immobilized enzymes were tested under concentrations of [EMIM][Ac] in the range of 10–50%, it was found that the immobilized enzymes exhibited higher endoglucanase activity compared to the free enzymes. In another study, Zhou et al. [82] immobilized cellulases from Trichoderma viride on a metal-organic framework support, testing their tolerance towards different concentrations of [Emim]DEP ionic liquid. According to the authors, for the highest concentration of 50% (v/v) of [Emim]DEP, immobilized cellulases had an 8.5-fold higher FPase activity compared to free enzymes. The stability gain might be associated to an increased enzyme rigidity after immobilization, but more interestingly, these results suggest that the pretreatment and saccharification could be carried out as a one-pot process. In this scope, Qi et al. [83] immobilized cellulases on a metal-organic framework and compared free and immobilized enzymes against inhibitors usually found in the pretreatment slurry. When exposed to 5 g/L of formic acid and vanillin, immobilized enzymes obtained an increase in the hydrolysis yield of 18.7% and 19.6%, respectively. According to the authors, this improvement can be attributed to the covalent bonding between the enzyme and the support, which partially block the inhibition site on the surface of the enzyme.
4.4. Recent Advances on Cellulases Immobilization
Different approaches for enzymes immobilization have shown promising results with the use of novel technologies. An example is 3D printing, where the structure of the support can be conveniently defined, leading to a support material that can be manipulated in terms of a specific surface area and surface-reactive groups for a better immobilization [84]. Additionally, with 3D technology the shape of the support can be manipulated to match a desired configuration, for example to match the shape of a reactor [85]. Another interesting proposal is the use of lignocellulosic materials as immobilization support for an integrated biorefinery. Lignocellulosic materials fulfill some properties required in a promising support, such as large availability and low cost [86]. Finally, relevant progress in new innovative supports should also be noted such as the cases of biomimetic films [87], nanomaterials [88] and stimuli-responsive smart materials [89].
5. Consolidated Bioprocessing
Consolidated bioprocessing (CBP), which aims to combine cellulases production, hydrolysis and fermentation in a single step, appeared as a solution to decrease costs related with hydrolytic enzymes [90]. In the particular context of cellulosic ethanol production, the establishment of CBP requires the development of microorganism(s) with both fermentative and cellulolytic capacities, allowing for the direct conversion of lignocellulosic biomass (with or without pretreatment) into bioethanol, without the addition of external enzymes. The development of these CBP microorganisms has followed two main strategies: (1) modifying a cellulolytic microorganism to improve its fermentative capacities or (2) modifying an ethanologenic microorganism to produce cellulolytic enzymes. These CBP microorganisms should still present the essential traits to perform an efficient fermentation of lignocellulose: broad substrate utilization, high ethanol yields and productivities with minimal by-product formation, high ethanol tolerance and increased tolerance towards lignocellulosic-derived inhibitors and process hardiness (e.g., variations in pH and temperature) [91]. In fact, thermotolerance is a highly desirable trait for a CBP microorganism, as most cellulases present a high optimal temperature (>40 °C) [90].
5.1. Improvement of Ethanol Production in Cellulolytic Microorganisms
The approaches for genetic engineering of cellulolytic microorganisms aiming to improve its fermentative capacity have been mainly focused on anaerobic bacteria from the Clostridium genus (Table 1). The cellulose-degrading ability of these bacteria arises from its capacity to display a cellulosome at their cell surface [92]. A cellulosome is a multienzyme complex composed of a non-catalytic protein (scaffoldin) where a variety of polysaccharide-degrading enzymes (e.g., cellulases, hemicellulases) are assembled. This scaffolding can also contain one or more cellulose binding domains (CBD) to target its substrate. Clostridium thermocellum has been one of the most studied cellulosome-producing bacteria and is a promising CBP microorganism. Different approaches have been used to improve its capacity to produce ethanol [93,94]. In one of these, C. thermocellum was initially modified to block acetate, lactate, H2 and formate production, and after two rounds of adaptive evolution, the evolved strain was able to produce 22 g/L of ethanol from Avicel [95]. Genetic modification of Clostridium cellulovorans strains by expressing heterologous aldehyde/alcohol dehydrogenases resulted in improved ethanol production from cellulose, however with low ethanol yields and titers due to the simultaneous production of butanol [96,97]. Clostridium cellulolyticum was also modified via inactivation of lactate dehydrogenase and malate dehydrogenase to shift the metabolism towards ethanol fermentation, decreasing accumulation of organic acids, which resulted in 8.5 times more ethanol than with the wild-type strain [98].
Caldicellulosiruptor bescii is a hyperthermophilic anaerobic bacterium capable of secreting extremely active hyperthermostable cellulases and has been engineered for improved ethanol production: either via inactivation of lactate dehydrogenase, heterologous expression of acetaldehyde/alcohol dehydrogenase genes and/or of rnf genes [99,100,101]. Nonetheless, and despite its high cellulolytic capacity, the ethanol titers obtained were low (≤3.5 g/L) with yields very far from the theoretical (Table 1). In an unprecedented report, one of the engineered C. bescii was able to produce cellulosic ethanol at the high temperature of 75 °C [100]. Despite the very low amount of ethanol produced (0.10 g/L), the fermentation temperature near the boiling point of ethanol raises the possibility of in situ product removal, a beneficial process optimization due to the low tolerance of these bacteria towards ethanol.
As previously discussed, the filamentous fungus Trichoderma reesei has been widely employed to produce cellulases. In order to use it as a CBP microorganism, T. reesei was submitted to genome shuffling with S. cerevisiae genomic DNA in order to improve its tolerance towards ethanol, and the resultant tolerant strain was used to produce 3.1 g/L of ethanol from unpretreated sugarcane bagasse [102]. The plant pathogenic fungus Fusarium oxysporum, which presents cellulase secreting capacities, was also modified to enhance ethanol production from lignocellulose through overexpression of a hexose transporter gene to improve glucose and xylose uptake [103]. Both these fungi are mesophilic, which could be a disadvantage when comparing with the themophilic bacteria, as they require mild temperature fermentations (Table 1).
5.2. Engineering Ethanologenic Microorganisms for Cellulase Production
The yeast S. cerevisiae, being the preferred microorganism for the production of bioethanol, has been the most explored microorganism for application in CBP processes. In addition, this yeast plays a central role in lignocellulosic valorization processes not only to bioethanol [104] but also to top chemicals [105] due to its tolerance to adverse lignocellulose-based process conditions [106]. Taking advantage of the extensive genetic toolbox available, a wide variety of modifications has been applied to this yeast in order to provide it with efficient cellulolytic activity, following three distinct approaches: enzyme secretion, cellulosomes and cell surface display [104].
Table 2 lists the works with higher ethanol titers for each of the different approaches. A strain constructed to secrete BGL1, EG and CBHI from T. reesei allowed the production of 24 g/L of ethanol from alkaline peroxide pretreated wheat straw [107]. In the cellulosome approach, S. cerevisiae strains are normally modified to display a scaffolding from Clostridium bacteria, which is then assembled with a variety of hydrolytic enzymes [104]. Following this strategy, a strain displaying a scaffolding from Clostridium cellulovorans with a BGL from Saccharomycopsis fibuligera and a chimeric EG from Clostridium thermocellum produced 3.4 g/L of ethanol from carboxymethyl cellulose (CMC) [108]. The highest reported ethanol titer with a CBP S. cerevisiae was obtained using a strain displaying cellulases on its cell surface, anchored by the glycosylphosphatidylinositol (GPI)-anchoring system [109]. This strain was also modified for improved thermotolerance via transformation with an artificial zinc finger protein library, resulting in the production of 28 g/L of ethanol in a CBP of NaOH-pretreated Jerusalem artichoke stalk at 42 °C. More recently, lytic polysaccharide monooxygenases (LPMOs) were reported to play an important role in the hydrolysis of recalcitrant cellulose, making the polymer more accessible to the activities of endo and exo-glucanases in the presence of an electron donor [110]. Considering this, Liang and collaborators [111] engineered an S. cerevisiae strain to display a cellulosome with LPMO and CDH (cellobiose dehydrogenase, as an electron donor), improving the ethanol titers and yields by more than 1.7 fold, in comparison with a cellulosome presenting only β-glucosidase, endoglucanase and cellobiohydrolase.
One limitation of the use of S. cerevisiae as CBP host is its inability to natively consume xylose, a major constituent of lignocellulosic materials. In this sense, efforts have been made to develop strains capable of utilizing the hemicellulosic fraction of lignocellulose, arming S. cerevisiae with hemicellulolytic enzymes (xylanases and β-xylosidases) and xylose consumption pathways [112], reaching ethanol titers as high as 11 g/L [113,114]. In fact, combination of both cellulolytic and hemicellulotyic abilities, as well as capacity to ferment xylose, has been attempted in order to more efficiently utilize the carbohydrates present in lignocellulose. Nevertheless, and despite the development of a strain capable of secreting seven active (hemi)cellulases, its utilization to ferment a mixture of xylan, cellulose and cellobiose resulted in ethanol titers below 2 g/L (Table 2).
Other non-Saccharomyces yeasts were also modified for CBP processes (Table 2). The thermotolerant Kluyveromyces marxianus was modified to secrete cellulases or to display a cellulosome [115,116,117], however, even with higher temperature processes (37 and 40 °C) the maximum ethanol titer attained with these strains was 3.1 g/L. Pichia pastoris, the most frequently used yeast for heterologous production of proteins, was also modified to display a cellulosome, resulting in the production of 2.5 g/L of ethanol from Avicel [118]. The ethanologenic bacteria Zymomonas mobilis, which is extensively studied for second generation ethanol, was engineered to secrete hydrolytic enzymes and was able to produce 43 g/L and 32 g/L of ethanol from CMC and NaOH-pretreated sugar cane bagasse, respectively [119]. In a combination of both strategies used for the development of CBP microorganisms, the bacteria Escherichia coli and Geobacillus thermoglucosidasius were heavily engineered in order to simultaneously enable them with cellulase-secreting abilities and improved capacity to produce ethanol [120,121]. These resulted in 0.19 g/L of ethanol from nitric acid/ammonia pretreated wheat straw using the modified G. thermoglucosidasius strain and 7.6 g/L of ethanol from acid pretreated Arundo donax cane with the E. coli strain.

Table 1.
Different strategies and outcomes of modified cellulolytic microorganisms for consolidated bioprocessing. Ethanol yield was calculated (when data was available) as the ratio of grams of ethanol produced by the total of potential fermentable sugars in the medium and presented as percentage of the theoretical yield 0.511 g/g. N.D.: not determined/described.
Table 1.
Different strategies and outcomes of modified cellulolytic microorganisms for consolidated bioprocessing. Ethanol yield was calculated (when data was available) as the ratio of grams of ethanol produced by the total of potential fermentable sugars in the medium and presented as percentage of the theoretical yield 0.511 g/g. N.D.: not determined/described.
| Microorganism | Modification | Substrate | Ethanol Yield (% of Theoretical) | Ethanol Titer/Time | Temperature | Reference |
|---|---|---|---|---|---|---|
| Cellulolytic Bacteria | ||||||
| Clostridium cellulolyticum | Deletion of L-lactate dehydrogenase (ldh) and L-malate dehydrogenase (mdh) genes | 10 g/L Avicel | 48% | 2.7 g/L | 34 °C | [98] |
| 10 g/L acid-pretreated switchgrass | n.d. | 1.3 g/L | ||||
| Clostridium cellulovorans | Expression of aldehyde/alcohol dehydrogenase gene (adhE2) from Clostridium acetobutylicum | 14 g/L Avicel | 20% | 1.6 g/L in 204 h | 37 °C | [96] |
| C. cellulovorans | Expression of aldehyde/alcohol dehydrogenase gene (adhE2) from Clostridium acetobutylicum | 25 g/L cellulose | 14% | 2.0 g/L in 288 h | 37 °C | [97] |
| Clostridium thermocellum | Deletion of hpt, hydG, ldh, pfl, and pta-ack to block acetate, lactate, H2 and formate production. Adaptive evolution. | 60 g/L Avicel | 66% | 22 g/L in 122 h | 55 °C | [95] |
| C. thermocellum | Expression of pdc from Acetobacter pasteurianus and the adhA from Thermoanaerobacterium saccharolyticum | 60 g/L Avicel | 63% | 21 g/L in 100 h | 55 °C | [94] |
| C. thermocellum | Overexpression of rnf genes. Deletion of hydG | 50 g/L Avicel | 18% | 5.1 g/L | 55 °C | [93] |
| Caldicellulosiruptor bescii | Deletion of lactate dehydrogenase gene (ldh) and heterologous expression of a Clostridium thermocellum bifunctional acetaldehyde/alcohol dehydrogenase gene (adhE) | 20 g/L Avicel | 5.70% | 0.64 g/L | 65 °C | [99] |
| 10 g/L unpretreated switchgrass | n.d. | 0.59 g/L | ||||
| C. bescii | Deletion of lactate dehydrogenase gene (ldh) and heterologous expression of acetaldehyde/alcohol dehydrogenase genes from Thermoanaerobacter pseudethanolicus (adhB and adhE) | 20 g/L Avicel | 0.92% | 0.10 g/L in 42 h | 75 °C | [100] |
| 20 g/L unpretreated switchgrass | n.d. | 0.073 g/L in 16 h | ||||
| C. bescii | Expression of adhE from Clostridium thermocellum and rnf genes from Thermoanaerobacter sp. | 20 g/L Avicel | 31% | 3.5 g/L in 200 h | 60 °C | [101] |
| Cellulolytic Fungi | ||||||
| Fusarium oxysporum | Overexpression of Hxt | Alkali pretreated wheat straw | 78% | 0.32 g/g of alkali-treated straw | 30 °C | [103] |
| Trichoderma reesei | Genome shuffling with Saccharomyces cerevisiae gDNA | 50 g/L unpretreated sugarcane bagasse | 17% | 3.1 g/L in 120 h | 30 °C | [102] |

Table 2.
Different strategies and outcomes of modified ethanologenic microorganisms for consolidated bioprocessing. Ethanol yield was calculated (when data was available) as the ratio of grams of ethanol produced by the total of potential fermentable sugars in the medium and presented as percentage of the theoretical yield 0.511 g/g. N.D.: not determined/described. β-glucosidase (BGL), endoglucanase (EG), cellobiohydrolase I (CBHI), cellobiohydrolase II (CBHII), β-xylosidase (XylA); xylanase (Xyn) LPMO: lytic polysaccharide monooxygenases. CDH: cellobiose dehydrogenase. CMC: carboxymethyl cellulose. PASC: phosphoric acid swollen cellulose.
Table 2.
Different strategies and outcomes of modified ethanologenic microorganisms for consolidated bioprocessing. Ethanol yield was calculated (when data was available) as the ratio of grams of ethanol produced by the total of potential fermentable sugars in the medium and presented as percentage of the theoretical yield 0.511 g/g. N.D.: not determined/described. β-glucosidase (BGL), endoglucanase (EG), cellobiohydrolase I (CBHI), cellobiohydrolase II (CBHII), β-xylosidase (XylA); xylanase (Xyn) LPMO: lytic polysaccharide monooxygenases. CDH: cellobiose dehydrogenase. CMC: carboxymethyl cellulose. PASC: phosphoric acid swollen cellulose.
| Microorganism | Modification | Substrate | Ethanol Yield (% of Theoretical) | Ethanol Titer/Time | Temperature | Reference |
|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae | ||||||
| Secretion. BGL, EG and CBHI from T. reesei | Alkaline peroxide pretreated wheat straw | 70% | 24 g/L in 80 h | 30 °C | [107] | |
| Cellulosome. Scaffoldin from Clostridium cellulovorans. BGL from Saccharomycopsis fibuligera and chimeric EG from Clostridium thermocellum. Produced in separate strains | 10 g/L CMC | 60% | 3.4 g/L in 16 h | 30 °C | [108] | |
| Cellulosome. Scaffoldin from C. thermocellum. BGL from Aspergillus aculeatus, EG and CBHII from T. reesei, LPMO from Thermoascus aurantiacus and CDH from Humicola insolens | 10 g/L PASC | 47% | 2.7 g/L in 96 h | 30 °C | [111] | |
| 10 g/L Avicel | 31% | 1.8 g/L in 96 h | ||||
| Cell-surface display. Delta cocktail integration. BGL from Aspergillus aculeatus, EG and CBHII from T. reesei. Expression of Artificial Zinc Finger Protein-AZFP library for thermotolerance. | 20 g/L PASC | 76% | 8.7 g/L in 48 h | 42 °C | [109] | |
| 200 g/L of NaOH pretreated Jerusalem artichoke stalk | 43% | 28 g/L in 60 h | ||||
| Industrial-derived host | Secretion. BGL from T. reesei), EG from A. oryzae, CBHI from Talaromyces emersonii, CBHII from Chrysosporium lucknowense, XylA and Xyn from A. niger and Xyn from C. cellulovorans. Isomerase xylose consumption pathway. | 20 g/L cellobiose, 20 g/L corn cob xylan and 20 g/L CMC. | <5.9% | <2.0 g/L in 96 h | [122] | |
| Non-Saccharomyces ethanologenic microorganisms | ||||||
| Zymomonas mobilis | Secretion. EG from Enterobacter cloacae | CMC | n.d. | 5.5% (v/v) ~43 g/L in 72 h | 30 °C | [119] |
| NaOH pretreated sugar cane bagasse | n.d. | 4% (v/v) ~32 g/L in 72 h | ||||
| Z. mobilis | Secretion. EG from Z. mobilis ZM4 (ATCC 31821) and Xyn from uncultured bacterium | 1% pretreated (liquid hot water) rice straw | n.d. | 2.6 g/L in 72 h | 30 °C | [123] |
| Kluyveromyces marxianus | Secretion. BGL from Neocallimastix patriciarum and EG and CBHI from T. reesei | 2% CMC | 10% | 1.2 g/L in 24 h | 37 °C | [115] |
| K. marxianus | Secretion. CBHI and II and EGIII from T. reesei, EG A from Aspergillus niger, BGL from Neocallimastix patriciarum and a cellodextrin transporter from Neurospora crassa | 100 g/L Avicel | 1% | 0.6 g/L at 120 h | 40 °C | [116] |
| K. marxianus | Cellulosome. Scaffoldin and the anchoring protein from Clostridium thermocellum. BGL from Neocallimastix patriciarum, EGIII and CBHI from T. reesei, LPMO from Thermoascus aurantiacus and CDH from Thermothelomyces thermophila | 10 g/L Avicel | 54% | 3.1 g/L in 120 h | 37 °C | [117] |
| Pichia pastoris | Cellulosome. IM7/CL7 system as scaffolding. CBH from Yarrowia lipolytica, EG from C. thermocellum DSM1237, BGL from Thermoanaerobacterium thermosaccharolyticum DSM 571, CBM from Thermobifida fusca (recombinantly expressed and purified from E. coli) | 10 g/L CMC | 90% | 5.1 g/L | 30 °C | [118] |
| 10 g/L Avicel | 44% | 2.5 g/L | ||||
| Escherichia coli | ΔpflB, ΔadhE, ΔfrdA, ΔxylFGH, ΔldhA, PpflB:pdcZm-adhBZm, evolved. Expressing EndoG, a multifunctional glucanase and xylanase, from bovine rumen microbiota | Dilute acid pretreated Arundo donax | n.d. | 7.6 g/L | 39 °C | [120] |
| Geobacillus thermoglucosidasius | Deletion of ldh gene (encoding lactate dehydrogenase) and pfl gene (coding for pyruvate formate lyase) and upregulation of the pdh gene (encoding pyruvate dehydrogenase). BGL from Thermoanaerobacter brockii and a multidomain cellulase from Caldicellulosiruptor bescii | 1% nitric acid/ ammonia pretreated wheat straw | n.d. | 0.19 g/L in 24 h | 55 °C | [121] |
5.3. CBP Industrial Applications
Despite the several attempts and studies to develop a CBP microorganism, the resulting strains are still far from reaching the requirements for a feasible production of lignocellulosic ethanol: titers higher than 4% (w/v) with yields superior to 90% of theoretical maximum and a productivity of at least 1 g/L/h [91,124]. Nonetheless, these strains may still be applied in bioethanol plants to decrease the requirements of external enzymes, a strategy already used in the production of starch-derived bioethanol where amylase-producing S. cerevisiae strains are used [125]. In fact, the importance of developing CBP technology is already beyond academic interest, with industries such as Mascoma (now owned by Lallemand) and Qteros being founded based on this concept. Also interesting from an industrial point of view is the possible advantages of using cell-surface engineered CBP microorganisms in cell recycle batch fermentations, allowing the recycling of the enzymes attached to their cell surface. In fact, this strategy was already reported for the fermentation of hydrothermally-pretreated rice straw, where a cellulase-displaying S. cerevisiae strain was used in five consecutive cycles of fermentation, maintaining productivity values and with an average ethanol titer of 42.2 g/L [126].
6. Conclusions
Reducing the amounts of cellulases required in the industrial conversion of lignocellulosic materials is an efficient way to improve the economy of these processes. Different approaches have been studied in this context, with enzyme recycling and immobilization assuming the biggest role for several decades. Cellulases recycling has been widely studied among academia with a special emphasis on the mechanisms governing enzymes adsorption/desorption onto the solid and possible ways for their recovery after hydrolysis. Still, there is lack of practical applications in the conversion of real lignocellulosic materials for several rounds of hydrolysis, where the potential enzyme savings can be estimated. In addition, immobilization may confer critical beneficial features to the enzymatic system, not only due to improved stability and robustness, but also facilitated recovery. Despite this, the application of immobilization cellulases in the conversion of real lignocellulosics materials have been barely studied so far and should deserve a growing interest in the upcoming years. A final aspect to consider is the potential hold by CBP processes where the in situ production of cellulases in an integrated fashion will definitely contribute to reducing commercial enzymes consumption.
Author Contributions
Conceptualization, D.G. and L.D.; writing—original draft preparation, D.G., J.C. and E.Z.; writing—review and editing, D.G., J.C., E.Z., J.T. and L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was c arried out at the Biomass and Bioenergy Research Infrastructure (BBRI)- LISBOA-01-0145-FEDER-022059, supported by Operational Programme for Competitiveness and Internationalization (PORTUGAL2020), by Lisbon Portugal Regional Operational Programme (Lisboa 2020) and by North Portugal Regional Operational Program (Norte 2020) under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF) and has been supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020, the MIT-Portugal Program (Ph.D. Grant PD/BD/128247/2016 to Joana Cunha) and through Project EcoTech (POCI-01-0145- FEDER-032206/ FAPESP 2018/07522-6) funded by the European Regional Development Fund under the scope of Norte2020—Programa Operacional Regional do Norte. The authors also acknowledge the Ph.D. fellowship of Elisa Zanuso supported by the Mexican Science and Technology Council—Energy sustainability fund of Secretary of Energy Mexico (CONACY-SENER) (CONACYT ID 639021/495314).
Conflicts of Interest
The authors declare no conflict of interest.
References
- U.S. Energy Information Administration. International Energy Outlook; DOE/EIA-0484; Office of Energy Analysis U.S. Department of Energy: Washington, DC, USA, 2016.
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Mashima, H.; Tanaka, H. Scale up of fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour. Technol. 2001, 76, 1–8. [Google Scholar] [CrossRef]
- Singh, A.; Pant, D.; Korres, N.E.; Nizami, A.S.; Prasad, S.; Murphy, J.D. Key issues in life cycle assessment of ethanol production from lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2010, 101, 5003–5012. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Gomes, D.; Cruz, M.; de Resende, M.; Ribeiro, E.; Teixeira, J.; Domingues, L. Very High Gravity Bioethanol Revisited: Main Challenges and Advances. Fermentation 2021, 7, 38. [Google Scholar] [CrossRef]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef]
- Hayes, D.J.M. Second-generation biofuels: Why they are taking so long. WIREs Energy Environ. 2012, 2, 304–334. [Google Scholar] [CrossRef]
- Klein-Marcusschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, W.H. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1089. [Google Scholar] [CrossRef]
- Dutta, A.; Dowe, N.; Ibsen, K.N.; Schell, D.J.; Aden, A. An economic comparison of different fermentation configurations to convert corn stover to ethanol using Z. mobilis and Saccharomyces. Biotechnol. Prog. 2010, 26, 64–72. [Google Scholar]
- Lynd, L.R.; Laser, M.S.; Bransby, D.; Dale, B.E.; Davison, B.; Hamilton, R.; Himmel, M.; Keller, M.; McMillan, J.D.; Sheehan, J.; et al. How biotech can transform biofuels. Nat. Biotechnol. 2008, 26, 169–172. [Google Scholar] [CrossRef]
- Aden, A.; Foust, T. Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of corn stover to ethanol. Cellulose 2009, 16, 535–545. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497. [Google Scholar] [CrossRef]
- Pribowo, A.; Arantes, V.; Saddler, J.N. The adsorption and enzyme activity profiles of specific Trichoderma reesei cellulose/xylanase components when hydrolyzing steam pretreated corn stover. Enzym. Microb. Technol. 2012, 50, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T. Fungal Cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Tu, M.; Chandra, R.P.; Saddler, J.N. Evaluating the distribution of cellulases and the recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnol. Prog. 2007, 23, 398–406. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Deswal, D.; Sharma, S.; Bhattacharya, A.; Jain, K.K.; Kaur, A.; Pletschke, B.I.; Singh, A.; Karp, M. Revisiting cellulase production and redefining current strategies based on major challenges. Renew. Sustain. Energy Rev. 2016, 55, 249–272. [Google Scholar] [CrossRef]
- Segato, F.; Damásio, A.R.L.; de Lucas, R.C.; Squina, F.M.; Prade, R.A. Genomics Review of Holocellulose Deconstruction by Aspergilli. Microbiol. Mol. Biol. Rev. 2014, 78, 588–613. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Stochastic molecular model of enzymatic hydrolysis of cellulose for ethanol production. Biotechnol. Biofuels 2013, 6, 63. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef]
- Suominen, P.L.; Mantyla, A.L.; Karhunen, T.; Hakola, S.; Nevalainen, K.M.H. High frequency one-step gene replacement in Trichoderma reesei. II. Effects of deletions of individual cellulase genes. Mol. Gen. Genet. 1993, 241, 523–530. [Google Scholar] [CrossRef]
- Seiboth, B.; Ivanova, C.; Seidl-Seiboth, V. Trichoderma reesei: A Fungal Enzyme Producer for Cellulosic Biofuels. In Biofuel Production—Recent Developments and Prospects; Bernardes, M.A.S., Ed.; InTech: London, UK, 2011; pp. 309–340. [Google Scholar]
- Gomes, D.; Domingues, L.; Gama, M. Valorizing recycled paper sludge by a bioethanol production process with cellulase recycling. Bioresour. Technol. 2016, 216, 637–644. [Google Scholar] [CrossRef]
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Biorefin. 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Boraston, A.; Bray, M.; Burn, E.; Creagh, A.L.; Gilkes, N.; Guarna, M.; Jervis, E.; Johnson, P.; Kormos, J.; McIntosh, L.; et al. The structure and function of cellulose binding domains. In Carbohydrate from Trichoderma Reesei and Other Microorganisms; Claeyssen, M., Nerinkx, W., Piens, K., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 139–146. [Google Scholar]
- Arantes, V.; Saddler, J.N. Access to cellulose limits the efficiency of enzymatic hydrolysis: The role of amorphogenesis. Biotechnol. Biofuels 2010, 3, 4. [Google Scholar] [CrossRef]
- Oliveira, C.; Romani, A.; Gomes, D.; Cunha, J.T.; Gama, F.M.; Domingues, L. Recombinant family 3 carbohydrate-binding module as a new additive for enhanced enzymatic saccharification of whole slurry from autohydrolyzed Eucalyptus globulus wood. Cellulose 2018, 25, 2505–2514. [Google Scholar] [CrossRef]
- Viikari, L.; Alapuranen, M.; Puranen, T.; Vehmaanperä, J.; Siika-Aho, M. Thermostable enzymes in lignocellulose hydrolysis. Adv. Biochem. Eng. Biotechnol. 2007, 108, 121–145. [Google Scholar]
- Tu, M.; Chandra, R.P.; Saddler, J.N. Recycling cellulases during the hydrolysis of steam exploded and ethanol pretreated Lodgepole pine. Biotechnol. Prog. 2007, 23, 1130–1137. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol. Bioeng. 2006, 94, 611–617. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Su, Y.; Wan, Y. Enzyme adsorption and recycling during hydrolysis of wheat straw lignocelluloses. Bioresour. Technol. 2011, 102, 2881–2889. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef]
- Barsberg, S.; Selig, M.J.; Felby, C. Impact of lignins isolated from pretreated lignocelluloses on enzymatic cellulose saccharification. Biotechnol. Lett. 2013, 35, 189–195. [Google Scholar] [CrossRef]
- Schmaier, A.H.; Silver, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C.; Vroman, L.; Colman, R.W. The effect of high molecular weight kininogen on surface-adsorbed fibrinogen. Thromb. Res. 1984, 33, 51–67. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Du, J.; Yang, Y.; Jin, Y. Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresour. Technol. 2015, 181, 7–12. [Google Scholar] [CrossRef]
- Luo, X.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Chen, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51. [Google Scholar] [CrossRef]
- Arias, J.M.; Moraes, A.D.; Modesto, L.F.A.; de Castro, A.M.; Pereira, N. Addition of Surfactants and Non-Hydrolytic Proteins and Their Influence on Enzymatic Hydrolysis of Pretreated Sugarcane Bagasse. Appl. Biochem. Biotechnol. 2017, 181, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Leitão, A.F.; Moreira, S.; Felby, C.; Gama, M. Recycling of cellulases in lignocellulosic hydrolysates using alkaline elution. Bioresour. Technol. 2012, 110, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Rodrigues, A.C.; Domingues, L.; Gama, M. Cellulase recycling in biorefineries–is it possible? Appl. Microbiol. Biotechnol. 2015, 99, 4131–4143. [Google Scholar] [CrossRef]
- Tu, M.; Saddler, J.N. Potential enzyme cost reduction with the addition of surfactant during the hydrolysis of pretreated softwood. Appl. Biochem. Biotechnol. 2010, 161, 274–287. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, B.; Zhang, M.; Zheng, Z.; Yu, H. Enzymatic hydrolysis, adsorption, and recycling during hydrolysis of bagasse sulfite pulp. Bioresour. Technol. 2013, 146, 288–293. [Google Scholar] [CrossRef]
- Eckard, A.D.; Muthukumarappan, K.; Gibbons, W. Enhanced bioethanol production from pretreated corn stover via multi-positive effect of casein micelles. Bioresour. Technol. 2013, 135, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Su, R.; Huang, R.; Yang, Y.; Qi, W.; Li, Q.; He, Z. Recycling cellulases by pH-triggered adsorption-desorption during the enzymatic hydrolysis of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 5765–5774. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Yang, B.; Gregg, D.; Saddler, J.N.; Mansfield, S.D. Cellulase adsorption and an evaluation of enzyme recycle during hydrolysis of steam-exploded softwood residues. Appl. Biochem. Biotechnol. 2002, 98, 641–654. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Yong, Q.; Yu, S. Three-stage hydrolysis to enhance enzymatic saccharification of steam-exploded corn stover. Bioresour. Technol. 2010, 101, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, W.; Qi, B.; Lu, J.; Wan, Y. Recycling cellulase from enzymatic hydrolyzate of acid treated wheat straw by electroultrafiltration. Bioresour. Technol. 2013, 144, 186–193. [Google Scholar] [CrossRef]
- Emert, G.; Blotkamp, P.J. Method for Enzyme Reutilization. U.S. Patent No. US4220721, 02 September 1980. [Google Scholar]
- Tu, M.; Zhang, X.; Paice, M.; MacFarlane, P.; Saddler, J.N. The potential of enzyme recycling during the hydrolysis of a mixed softwood feedstock. Bioresour. Technol. 2009, 100, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, A.H.C.; Saddler, J.N. Evaluation of cellulase recycling strategies for the hydrolysis of lignocellulosic substrates. Biotechnol. Bioeng. 1995, 45, 328–336. [Google Scholar] [CrossRef]
- Mesa, L.; González, E.; Cara, C.; Castro, E.; Mussatto, S.I. An approach to cellulase recovery from enzymatic hydrolysis of pretreated sugarcane bagasse with high lignin content. Biocatal. Biotransform. 2015, 33, 287–297. [Google Scholar] [CrossRef]
- Guo, H.; Zou, S.; Liu, B.; Su, R.; Huang, R.; Qi, W.; Zhang, M.; He, Z. Reducing β-glucosidase supplementation during cellulase recovery using engineered strain for successive lignocellulose bioconversion. Bioresour. Technol. 2015, 187, 362–368. [Google Scholar] [CrossRef]
- Waeonukul, R.; Kosugi, A.; Prawitwong, P.; Deng, L.; Tachaapaikoon, C.; Pason, P.; Ratanakhanokchai, K.; Saito, M.; Mori, Y. Novel cellulase recycling method using a combination of Clostridium thermocellum cellulosomes and Thermoanaerobacter brockii β-glucosidase. Bioresour. Technol. 2013, 130, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Felby, C.; Gama, M. Cellulase stability, adsorption/desorption profiles and recycling during successive cycles of hydrolysis and fermentation of wheat straw. Bioresour. Technol. 2014, 156, 163–169. [Google Scholar] [CrossRef]
- Gomes, D.G.; Serna-Loaiza, S.; Cardona, C.A.; Gama, M.; Domingues, L. Insights into the economic viability of cellulases recycling on bioethanol production from recycled paper sludge. Bioresour. Technol. 2018, 267, 347–355. [Google Scholar] [CrossRef]
- Gomes, D.G.; Gama, F.M.; Domingues, L. Determinants on an efficient cellulase recycling process for the production of bioethanol from recycled paper sludge under high solid loadings. Biotechnol. Biofuels 2018, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Börjesson, J.; Pedersen, L.S.; Meyer, A.S. Enzymatic lignocellulose hydrolysis: Improved cellulase productivity by insoluble solids recycling. Biotechnol. Biofuels 2013, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Otter, D.E.; Munro, P.A.; Scott, G.K.; Geddes, R. Elution of Trichoderma reesei cellulase from cellulose by pH adjustment with sodium hydroxide. Biotechnol. Lett. 1984, 6, 369–374. [Google Scholar] [CrossRef]
- Otter, D.E.; Munro, P.A.; Scott, G.K.; Geddes, R. Desorption of Trichoderma reesei cellulose from cellulose by a range of desorbents. Biotechnol. Bioeng. 1989, 34, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H.P. Direct quantitative determination of adsorbed cellulase on lignocellulosic biomass with its application to study cellulose desorption for potential recycling. Analyst 2009, 134, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Haven, M.Ø.; Lindedam, J.; Jeppesen, M.D.; Elleskov, M.; Rodrigues, A.C.; Gama, M.; Jørgensen, H.; Felby, C. Continuous recycling of enzymes during production of lignocellulosic bioethanol in demonstration scale. Appl. Energy 2015, 159, 188–195. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Paradisi, F. General overview on immobilization techniques of enzymes for biocatalysis. In Catalyst Immobilization; Benaglia, M., Puglisi, A., Eds.; John Wiley & Sons, Ltd.: Weinheim, Germany, 2020; pp. 409–435. [Google Scholar]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Stephanopoulos, G. Enzymes in biotechnology: Critical platform technologies for bioprocess development. Curr. Opin. Biotechnol. 2021, 69, 91–102. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Engineering of immobilized enzymes: pH, thermal stability and kinetic aspects. In Practical Aspects of Chemical Engineering; Ochowiak, M., Woziwodzki, S., Mitkowski, P., Doligalski, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 161–170. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.-C.; Negra, M.D.; Kaiser, A.; Pinelo, M. Enzyme immobilization on inorganic surfaces for membrane reactor applications: Mass transfer challenges, enzyme leakage and reuse of materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Asar, M.F.; Ahmad, N.; Husain, Q. Chitosan modified Fe3O4/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: Its application in the saccharification of microcrystalline cellulose. Prep. Biochem. Biotechnol. 2020, 50, 460–467. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Dinçer, A.; Telefoncu, A. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B Enzym. 2007, 45, 10–14. [Google Scholar] [CrossRef]
- Zang, L.; Qiao, X.; Hu, L.; Yang, C.; Liu, Q.; Wei, C.; Qiu, J.; Mo, H.; Song, G.; Yang, J.; et al. Preparation and evaluation of coal fly ash/chitosan composites as magnetic supports for highly efficient cellulase immobilization and cellulose bioconversion. Polymers 2018, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Pi, L.; Fang, L.; Wu, R.; Xiong, C. Application and characterization of magnetic chitosan microspheres for enhanced immobilization of cellulase. Biocatal. Biotransform. 2016, 34, 272–282. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Xing, Z.; Geng, Y.; Wilson, J. Preparation and characterization of magnetic Fe3O4—chitosan nanoparticles for cellulase immobilization. Cellulose 2017, 24, 5541–5550. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 2017, 22, 269. [Google Scholar] [CrossRef]
- Tan, L.; Tan, Z.; Feng, H.; Qiu, J. Cellulose as a template to fabricate a cellulase-immobilized composite with high bioactivity and reusability. New J. Chem. 2018, 42, 1665–1672. [Google Scholar] [CrossRef]
- Tantayotai, P.; Rattanaporn, K.; Tepaamorndech, S.; Cheenkachorn, K.; Sriariyanun, M. Analysis of an ionic liquid and salt tolerant microbial consortium which is useful for enhancement of enzymatic hydrolysis and biogas production. Waste Biomass Valoriz. 2019, 10, 1481–1491. [Google Scholar] [CrossRef]
- Grewal, J.; Ahmad, R.; Khare, S.K. Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour. Technol. 2017, 242, 236–243. [Google Scholar] [CrossRef]
- Zhou, M.; Ju, X.; Zhou, Z.; Yan, L.; Chen, J.; Yao, X.; Xu, X.; Li, L.Z. Development of an immobilized cellulase system based on metal-organic frameworks for improving ionic liquid tolerance and in situ saccharification of bagasse. ACS Sustain. Chem. Eng. 2019, 7, 19185–19193. [Google Scholar] [CrossRef]
- Qi, B.; Luo, J.; Wan, Y. Immobilization of cellulase on a core-shell structured metal-organic framework composites: Better inhibitors tolerance and easier recycling. Bioresour. Technol. 2018, 268, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A versatile approach for enzyme immobilization using chemically modified 3D-printed scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- Jiang, W.; Pei, R.; Zhou, S.F. 3D-printed xylanase within biocompatible polymers as excellent catalyst for lignocellulose degradation. Chem. Eng. J. 2020, 400, 125920. [Google Scholar] [CrossRef]
- Rodríguez-Restrepo, Y.A.; Orrego, C.E. Immobilization of enzymes and cells on lignocellulosic materials. Environ. Chem. Lett. 2020, 18, 787–806. [Google Scholar] [CrossRef]
- He, B.; Chang, P.; Zhu, X.; Zhang, S. Anemone-inspired enzymatic film for cellulose heterogeneous catalysis. Carbohydr. Polym. 2021, 260, 117795. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dhanya, B.S.; Verma, M.L. Nano-immobilized biocatalysts and their potential biotechnological applications in bioenergy production. Mater. Sci. Energy Technol. 2020, 3, 808–824. [Google Scholar] [CrossRef]
- Han, J.; Wan, J.; Wang, Y.; Wang, L.; Li, C.; Mao, Y.; Ni, L. Recyclable soluble-insoluble upper critical solution temperature-type poly(methacrylamide- co-acrylic acid)-cellulase biocatalyst for hydrolysis of cellulose into glucose. ACS Sustain. Chem. Eng. 2018, 6, 7779–7788. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kondo, A. Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem. 2012, 47, 1287–1294. [Google Scholar] [CrossRef]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Newcomb, M.; Wu, J.H.D. Cellulase, Clostridia, and Ethanol. Microbiol. Mol. Biol. Rev. 2005, 69, 124–154. [Google Scholar] [CrossRef]
- Lo, J.; Olson, D.G.; Murphy, S.J.L.; Tian, L.; Hon, S.; Lanahan, A.; Guss, A.M.; Lynd, L.R. Engineering electron metabolism to increase ethanol production in Clostridium thermocellum. Metab. Eng. 2017, 39, 71–79. [Google Scholar] [CrossRef]
- Tian, L.; Perot, S.J.; Hon, S.; Zhou, J.; Liang, X.; Bouvier, J.T.; Guss, A.M.; Olson, D.G.; Lynd, L.R. Enhanced ethanol formation by Clostridium thermocellum via pyruvate decarboxylase. Microb. Cell Fact. 2017, 16, 171. [Google Scholar] [CrossRef]
- Tian, L.; Papanek, B.; Olson, D.G.; Rydzak, T.; Holwerda, E.K.; Zheng, T.; Zhou, J.; Maloney, M.; Jiang, N.; Giannone, R.J.; et al. Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum. Biotechnol. Biofuels 2016, 9, 116. [Google Scholar] [CrossRef]
- Yang, X.; Xu, M.; Yang, S.T. Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab. Eng. 2015, 32, 39–48. [Google Scholar] [CrossRef]
- Bao, T.; Zhao, J.; Li, J.; Liu, X.; Yang, S.T. n-Butanol and ethanol production from cellulose by Clostridium cellulovorans overexpressing heterologous aldehyde/alcohol dehydrogenases. Bioresour. Technol. 2019, 285, 121316. [Google Scholar] [CrossRef]
- Li, Y.; Tschaplinski, T.J.; Engle, N.L.; Hamilton, C.Y.; Rodriguez, M.; Liao, J.C.; Schadt, C.W.; Guss, A.M.; Yang, Y.; Graham, D.E. Combined inactivation of the Clostridium cellulolyticum lactate and malate dehydrogenase genes substantially increases ethanol yield from cellulose and switchgrass fermentations. Biotechnol. Biofuels 2012, 5, 2. [Google Scholar] [CrossRef]
- Chung, D.; Cha, M.; Guss, A.M.; Westpheling, J. Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc. Natl. Acad. Sci. USA 2014, 111, 8931–8936. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Cha, M.; Snyder, E.N.; Elkins, J.G.; Guss, A.M.; Westpheling, J. Cellulosic ethanol production via consolidated bioprocessing at 75 °C by engineered Caldicellulosiruptor bescii. Biotechnol. Biofuels 2015, 8, 163. [Google Scholar] [CrossRef]
- Williams-Rhaesa, A.M.; Rubinstein, G.M.; Scott, I.M.; Lipscomb, G.L.; Poole, I.I.F.L.; Kelly, R.M.; Adams, M.W.W. Engineering redox-balanced ethanol production in the cellulolytic and extremely thermophilic bacterium, Caldicellulosiruptor bescii. Metab. Eng. Commun. 2018, 7, 00073. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, D.; Wei, Y.; Wang, Q.; Li, Z.; Chen, Y.; Huang, R. Direct Ethanol Production from Lignocellulosic Sugars and Sugarcane Bagasse by a Recombinant Trichoderma reesei Strain HJ48. Sci. World J. 2014, 798683. [Google Scholar] [CrossRef]
- Ali, S.S.; Nugent, B.; Mullins, E.; Doohan, F.M. Insights from the fungus Fusarium oxysporum point to high affinity glucose transporters as targets for enhancing ethanol production from lignocellulose. PLoS ONE 2013, 8, 54701. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Romani, A.; Costa, C.E.; Sa-Correia, I.; Domingues, L. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, L.; Yang, R.; Hou, P.; Liu, J. Direct bioethanol production from wheat straw using xylose/glucose co-fermentation by co-culture of two recombinant yeasts. J. Ind. Microbiol. Biotechnol. 2017, 44, 453–464. [Google Scholar] [CrossRef]
- Hyeon, J.-E.; Yu, K.O.; Suh, D.J.; Suh, Y.W.; Lee, S.E.; Lee, J.; Han, S.O. Production of minicellulosomes from Clostridium cellulovorans for the fermentation of cellulosic ethanol using engineered recombinant Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2010, 310, 39–47. [Google Scholar] [CrossRef]
- Khatun, M.M.; Yu, X.; Kondo, A.; Bai, F.; Zhao, X. Improved ethanol production at high temperature by consolidated bioprocessing using Saccharomyces cerevisiae strain engineered with artificial zinc finger protein. Bioresour. Technol. 2017, 245, 1447–1454. [Google Scholar] [CrossRef]
- Phillips, C.M.; Beeson, W.T.; Cate, J.H.; Marletta, M.A. Cellobiose Dehydrogenase and a Copper-Dependent Polysaccharide Monooxygenase Potentiate Cellulose Degradation by Neurospora crassa. ACS Chem. Biol. 2011, 6, 1399–1406. [Google Scholar] [CrossRef]
- Liang, Y.; Si, T.; Ang, E.L.; Zhao, H. Engineered Pentafunctional Minicellulosome for Simultaneous Saccharification and Ethanol Fermentation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 6677–6684. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Soares, P.O.; Romaní, A.; Thevelein, J.M.; Domingues, L. Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways. Biotechnol. Biofuels 2019, 12, 20. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hasunuma, T.; Hori, Y.; Yamada, R.; Kondo, A. Direct ethanol production from hemicellulosic materials of rice straw by use of an engineered yeast strain codisplaying three types of hemicellulolytic enzymes on the surface of xylose-utilizing Saccharomyces cerevisiae cells. J. Biotechnol. 2012, 158, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Romaní, A.; Inokuma, K.; Johansson, B.; Hasunuma, T.; Kondo, A.; Domingues, L. Consolidated bioprocessing of corn cob-derived hemicellulose: Engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol. Biofuels 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Ho, C.Y.; Ho, F.J.; Tsai, T.Y.; Ke, H.M.; Wang, C.H.T.; Chen, H.L.; Shih, M.C.; Huang, C.C.; Li, W.H. PGASO: A synthetic biology tool for engineering a cellulolytic yeast. Biotechnol. Biofuels 2012, 5, 53. [Google Scholar] [CrossRef]
- Chang, J.J.; Ho, F.J.; Ho, C.Y.; Wu, Y.C.; Hou, Y.H.; Huang, C.C.; Shih, M.C.; Li, W.H. Assembling a cellulase cocktail and a cellodextrin transporter into a yeast host for CBP ethanol production. Biotechnol. Biofuels 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Anandharaj, M.; Lin, Y.J.; Rani, R.P.; Nadendla, E.K.; Ho, M.C.; Huang, C.C.; Cheng, J.F.; Chang, J.J.; Li, W.H. Constructing a yeast to express the largest cellulosome complex on the cell surface. Proc. Natl. Acad. Sci. USA 2020, 117, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qiao, J.; Wang, X.; Sun, W.; Chen, L.; Li, S.; Wu, K.; Ma, L.; Liu, Y. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production. Biotechnol. Biofuels 2020, 13, 108. [Google Scholar] [CrossRef]
- Thirumalai Vasan, P.; Sobana Piriya, P.; Immanual Gilwax Prabhu, D.; John Vennison, S. Cellulosic ethanol production by Zymomonas mobilis harboring an endoglucanase gene from Enterobacter cloacae. Bioresour. Technol. 2011, 102, 2585–2589. [Google Scholar] [CrossRef]
- Loaces, I.; Schein, S.; Noya, F. Ethanol production by Escherichia coli from Arundo donax biomass under SSF, SHF or CBP process configurations and in situ production of a multifunctional glucanase and xylanase. Bioresour. Technol. 2017, 224, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Bashir, Z.; Sheng, L.; Anil, A.; Lali, A.; Minton, N.P.; Zhang, Y. Engineering Geobacillus thermoglucosidasius for direct utilisation of holocellulose from wheat straw. Biotechnol. Biofuels 2019, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.; Deparis, Q.; Foulquié-Moreno, M.R.; Thevelein, J.M. Simultaneous secretion of seven lignocellulolytic enzymes by an industrial second-generation yeast strain enables efficient ethanol production from multiple polymeric substrates. Metab. Eng. 2020, 59, 131–141. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Sowatad, A.; Kanokratana, P.; Havanapan, P.-O.; Champreda, V. Expression and Extracellular Secretion of Endo-glucanase and Xylanase by Zymomonas mobilis. Appl. Biochem. Biotechnol. 2019, 187, 239–252. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Stark, B.C. Recent trends in bioethanol production from food processing byproducts. J. Ind. Microbiol. Biotechnol. 2016, 43, 1593–1609. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V. Chapter 22—Bioethanol Production from Corn. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 615–631. [Google Scholar]
- Matano, Y.; Hasunuma, T.; Kondo, A. Cell recycle batch fermentation of high-solid lignocellulose using a recombinant cellulase-displaying yeast strain for high yield ethanol production in consolidated bioprocessing. Bioresour. Technol. 2013, 135, 403–409. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).