Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts
Abstract
1. Introduction
2. Effect of Culture Conditions on Gellan Production by Sphingomonas elodea
2.1. Effect of Carbon Source
2.2. Influence of Nitrogen Source
2.3. Effect of Aeration and Surfactants
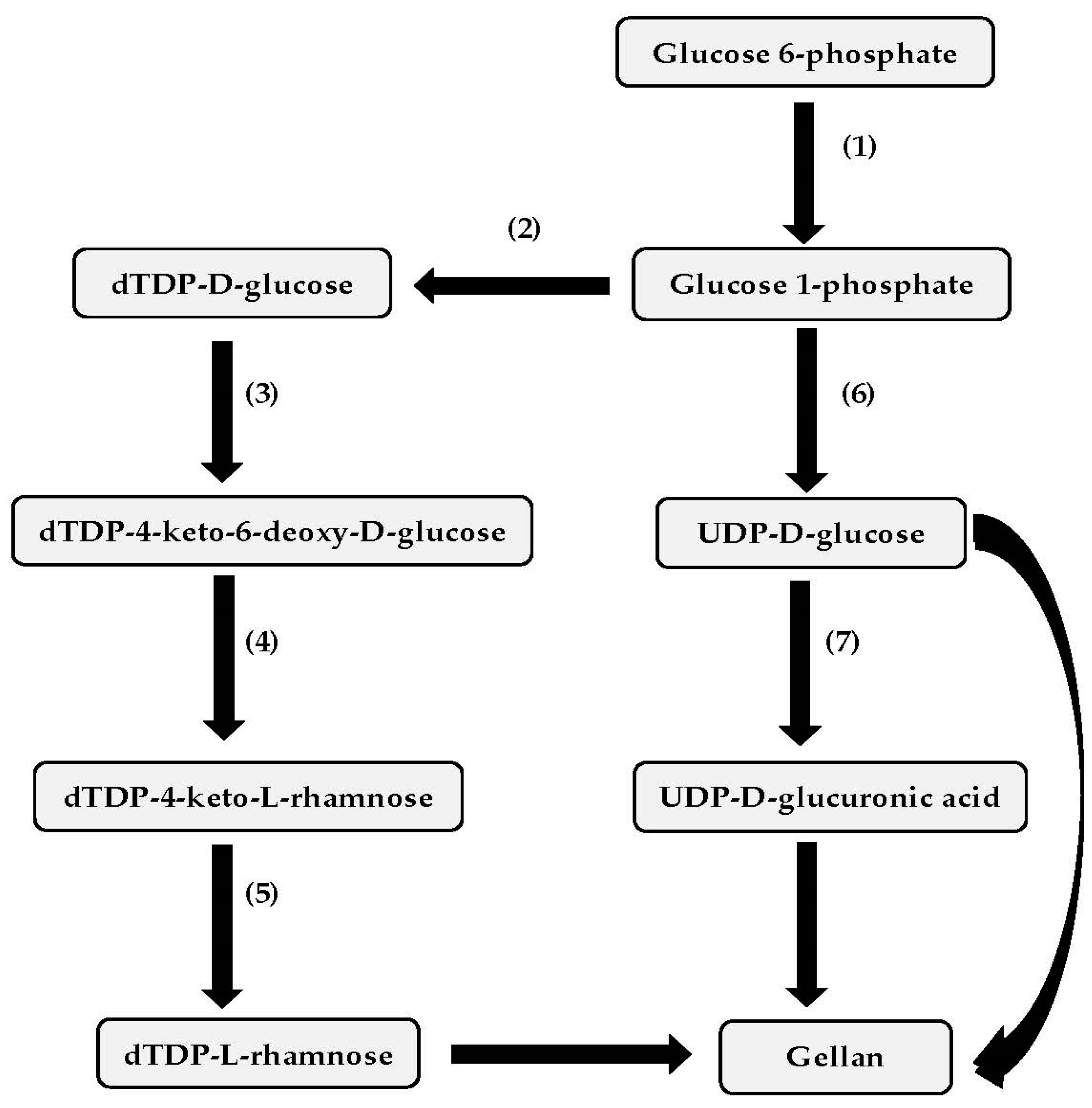
3. Genetics of Gellan Biosynthesis and Mutant Isolation
3.1. Genetics of Gellan Biosynthesis
3.2. Mutant Isolation Related to Gellan Production
4. Gellan Synthesis on Processing Coproducts
4.1. Dairy Processing Coproduct
4.2. Corn Processing Coproducts
4.3. Soluble Starch as a Carbon Source for Gellan Production
4.4. Other Processing Coproducts
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a Pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091. [Google Scholar] [CrossRef]
- Anson, A.; Fisher, P.J.; Kennedy, A.F.D.; Sutherland, I.W. A bacterium yielding a polysaccharide with unusual properties. J. Appl. Bacteriol. 1987, 62, 147–150. [Google Scholar] [CrossRef]
- Pollock, T.J. Gellan-related polysaccharides and the genus Sphingomonas. J. Gen. Microbiol. 1993, 139, 1939–1945. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine nucleoside catabolism in Sphingomonas paucimobilis: Role of cytidine deaminase and uridine phosphorylase. Microbiol. Res. 1995, 150, 149–152. [Google Scholar] [CrossRef]
- O’Neil, M.A.; Silvendran, R.R.; Morris, J. Structure of extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 123–133. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Millane, R.P.; Arnott, S.; Atkins, E.D.T. The crystal structure of gellan. Carbohydr. Res. 1988, 175, 1–15. [Google Scholar] [CrossRef]
- Campana, S.; Ganter, J.; Milas, M.; Rinaudo, M. On the solution properties of bacterial polysaccharides of the gellan family. Carbohydr. Res. 1992, 231, 31–38. [Google Scholar] [CrossRef]
- Crescenzi, V.; Dentini, M.; Dea, I.C.M. The influence of side-chains on the dilute-solution properties of three structurally related bacterial anionic polysaccharides. Carbohydr. Res. 1987, 160, 283–302. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Puigjaner, L.C.; Joyce, K.L.; Arnott, S. Cation interactions in gellan: An X-ray study of the potassium salt. Carbohydr. Res. 1988, 181, 23–40. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Radha, A.; Thailambal, V.G. Roles of potassium ions, acetyl and l-glyceryl groups in native gellan double helix: An X-ray study. Carbohydr. Res. 1992, 224, 1–17. [Google Scholar] [CrossRef]
- Tang, J.; Lelievre, J.; Tung, M.A.; Zeng, Y. Polymer and ion concentration effects on gellan strength and strain. J. Food Sci. 1994, 59, 216–220. [Google Scholar] [CrossRef]
- Tang, J.; Tung, M.A.; Zeng, Y. Mechanical properties of gellan gels in relation to divalent cations. J. Food Sci. 1995, 60, 748–752. [Google Scholar] [CrossRef]
- Manna, B.; Gambhir, A.; Ghosh, P. Production and reheological characteristics of the microbial gum gellan. Lett. Appl. Microbiol. 1996, 23, 141–145. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, S. Flow behaviour of gellan sol with selected cations. J. Food Sci. Technol. 2015, 52, 1233–1237. [Google Scholar] [CrossRef][Green Version]
- Vilela, J.A.P.; da Cunha, R.L. Emulsions stabilized by high acyl gellan and KCl. Food Res. Int. 2017, 91, 47–54. [Google Scholar] [CrossRef]
- West, T.P.; Strohfus, B.; Santiago, M.F. A colorimetric assay for gellan elaborated by Sphingomonas paucimobilis ATCC 31461. World J. Microbiol. Biotechnol. 2000, 16, 529–531. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Danalache, F.; Carvalho, C.; Alves, V.D.; Moldão-Martins, M.; Mata, P. Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int. J. Biol. Macromol. 2016, 84, 43–53. [Google Scholar] [CrossRef]
- Tomadonia, B.; Moreira, M.R.; Pereda, M.; Ponce, A.G. Gellan-based coatings incorporated with natural antimicrobials in fresh-cut strawberries: Microbiological and sensory evaluation through refrigerated storage. LWT-Food Sci. Technol. 2018, 97, 384–389. [Google Scholar] [CrossRef]
- Torresa, O.; Yamada, A.; Rigby, N.M.; Hanawa, T.; Kawano, Y.; Sarkar, A. Gellan gum: A new member in the dysphagia thickener family. Biotribiology 2019, 17, 8–18. [Google Scholar] [CrossRef]
- Shinsho, A.; Brenner, T.; Descallar, F.B.; Tashiro, Y.; Ando, N.; Zhou, Y.; Ogawa, H.; Matsukawa, S. The thickening properties of native gellan gum are due to freeze drying–induced aggregation. Food Hydrocoll. 2020, 109, 105997. [Google Scholar] [CrossRef]
- Linn, C.C.; Cassida, L.E. Gelrite as a gelling agent in media for the growth of thermophilic microorganisms. Appl. Environ. Microbiol. 1984, 47, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Rygaard, A.M.; Thøgersen, M.S.; Nielsen, K.F.; Gram, L.; Bentzon-Tilia, M. Effects of gelling agent and extracellular signaling molecules on the culturability of marine bacteria. Appl. Environ. Microbiol. 2017, 83, e00243-17. [Google Scholar] [CrossRef] [PubMed]
- McGuffey, J.C.; Leon, D.; Dhanji, E.Z.; Mishler, D.M.; Barrick, J.E. Bacterial production of gellan gum as a do-it-yourself alternative to agar. J. Microbiol. Biol. Educ. 2018, 19, 1530. [Google Scholar] [CrossRef]
- Crescenzi, V.; Dentini, M.; Segatori, M.; Tiblandi, C.; Callegaro, L.; Benedetti, L. Synthesis and preliminary characterisation of new esters of the bacterial polysaccharide gellan. Carbohydr. Res. 1992, 231, 73–81. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Development of mucoadhesive sprayable gellan gum fluid gels. Int. J. Pharm. 2015, 488, 12–19. [Google Scholar] [CrossRef]
- Wahba, M.I. Processed gellan gum beads as covalent immobilization carriers. Biocatal. Agric. Biotechnol. 2018, 14, 270–278. [Google Scholar] [CrossRef]
- Zia, Z.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Kuhn, K.R.; e Silva, F.G.D.; Netto, F.M.; da Cunha, R.L. Production of whey protein isolate—Gellan microbeads for encapsulation and release of flaxseed bioactive compounds. J. Food Eng. 2019, 247, 104–114. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Gellan gum-based delivery systems of therapeutic agents and cells. Carbohydr. Polym. 2020, 229, 115430. [Google Scholar] [CrossRef]
- Monier, M.; Shafik, A.L.; El-Mekabaty, A. Designing and investigation of photo-active gellan gum for the efficient immobilization of catalase by entrapment. Int. J. Biol. Macromol. 2020, 161, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef]
- Moxon, S.R.; Smith, A.M. Controlling the rheology of gellan gum hydrogels in cell culture conditions. Int. J. Biol. Macromol. 2016, 84, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sebri, N.J.M.; Amin, K.A.M. Gellan gum/ibuprofen hydrogel for dressing application: Mechanical properties, release activity and biocompatibility studies. Int. J. Appl. Chem. 2016, 12, 483–498. [Google Scholar]
- Lago, M.E.L.; da Silva, L.P.; Henriques, C.; Carvalho, A.F.; Reis, R.L.; Marques, A.P. Generation of gellan gum-based adipose-like microtissues. Bioengineering 2018, 5, 52. [Google Scholar] [CrossRef]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106A, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Yuan, Y.; Liu, C.; Zhang, D.; Yang, Z.; Yang, C.; Ma, C. Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Appl. Environ. Microbiol. 2006, 72, 3367–3374. [Google Scholar] [CrossRef]
- Lim, S.-M.; Wu, J.R.; Lee, J.-W.; Kim, S.-K. Optimization of culture condition for gellan production by Pseudomonas elodea ATCC 31461. Korean J. Life Sci. 2003, 13, 705–711. [Google Scholar]
- West, T.P.; Strohfus, B. Effect of complex nitrogen sources upon gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 1998, 94, 145–152. [Google Scholar]
- Kanari, B.; Banik, R.R.; Upadhyay, S.N. Effect of environmental factors and carbohydrate on gellan gum production. Appl. Biochem. Biotechnol. 2002, 102–103, 129–139. [Google Scholar] [CrossRef]
- West, T.P. Effect of temperature on bacterial gellan production. World J. Microbiol. Biotechnol. 2003, 19, 649–652. [Google Scholar] [CrossRef]
- Zhu, G.; Sheng, L.; Tong, Q. A new strategy to enhance gellan production by two-stage culture in Sphingomonas paucimobilis. Carbohydr. Polym. 2013, 98, 829–834. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; Fullenkamp, N.A. Effect of culture medium pH on bacterial gellan production. Microbios 2001, 105, 133–140. [Google Scholar]
- Zhu, G.; Guo, N.; Yong, Y.; Xiong, Y.; Tong, Q. Effect of 2-deoxy-d-glucose on gellan gum biosynthesis by Sphingomonas paucimobilis. Bioprocess Biosyst. Eng. 2019, 42, 897–900. [Google Scholar] [CrossRef]
- West, T.P.; Strohfus, B. Effect of yeast extract on gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 1999, 97, 85–93. [Google Scholar]
- Banik, R.M.; Santhiagu, A. Improvement in production and quality of gellan gum by Sphingomonas paucimobilis under high dissolved oxygen tension levels. Biotechnol. Lett. 2006, 28, 1347–1350. [Google Scholar] [CrossRef]
- Giaviasis, I.; Robertson, I.; McNeil, B.; Harvey, L.M. Simultaneous and rapid monitoring of biomass and biopolymer production by Sphingomonas paucimobilis using Fourier transform-near infrared spectroscopy. Biotechnol. Lett. 2003, 25, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sheng, L.; Tong, Q. Enhanced gellan gum production by hydrogen peroxide (H2O2) induced oxidative stresses in Sphingomonas paucimobilis. Bioprocess Biosyst. Eng. 2014, 37, 743–748. [Google Scholar] [CrossRef]
- Arockiasamy, S.; Banik, R.M. Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J. Biosci. Bioeng. 2008, 105, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Harding, N.E.; Patel, Y.N.; Coleman, R.J. Organization of genes required for gellan polysaccharide biosynthesis in Sphingomonas elodea ATCC 31461. J. Ind. Microbiol. Biotechnol. 2004, 31, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Marques, A.R.; Fialho, A.M.; Granja, A.T.; Sa´-Correia, I. Proteins encoded by Sphingomonas elodea ATCC 31461 rmlA and ugpG genes, involved in gellan gum biosynthesis, exhibit both dTDP- and UDP-glucose pyrophosphorylase activities. Appl. Environ. Microbiol. 2005, 71, 4703–4712. [Google Scholar] [CrossRef]
- Attallah, A.G.; Abd-El-Aal, S.K.; Ibrahim, S.A.; El-Sayd, M.A. Improvement the efficiency of Sphingomonas paucimobilis to produce gellan gum by genetically approach. Int. J. ChemTech Res. 2014, 6, 64–79. [Google Scholar]
- Marques, A.R.; Ferreira, P.B.; Sa´-Correia, I.; Fialho, A.M. Characterization of the ugpG gene encoding a UDP-glucose pyrophosphorylase from the gellan gum producer Sphingomonas paucimobilis ATCC 31461. Mol. Gen. Genom. 2003, 268, 816–824. [Google Scholar] [CrossRef]
- Aragáo, D.; Fialho, A.M.; Marques, A.R.; Mitchell, E.P.; Sa´-Correia, I.; Frazáo, C. The complex of Sphingomonas elodea ATCC 31461 glucose-1-phosphate uridylyltransferase with glucose-1-phosphate reveals a novel quaternary structure, unique among nucleoside diphosphate-sugar pyrophosphorylase members. J. Bacteriol. 2007, 189, 4520–4528. [Google Scholar] [CrossRef]
- Granja, A.T.; Popescu, A.; Marques, A.R.; Sá-Correia, I.; Fialho, A.M. Biochemical characterization and phylogenetic analysis of UDP-glucose dehydrogenase from the gellan gum producer Sphingomonas elodea ATCC 31461. Appl. Microbiol. Biotechnol. 2007, 76, 1319–1327. [Google Scholar] [CrossRef]
- Vartak, N.B.; Lin, C.C.; Cleary, J.M.; Fagan, M.J.; Saier, M.H., Jr. Glucose metabolism in ‘Sphingomonas elodea’: Pathway engineering via construction of a glucose-6-phosphate dehydrogenase insertion mutant. Microbiology 1995, 141, 2339–2350. [Google Scholar] [CrossRef]
- West, T.P. Isolation of a mutant strain of Pseudomonas sp. ATCC 31461 exhibiting elevated polysaccharide production. J. Ind. Microbiol. Biotechnol. 2002, 29, 185–188. [Google Scholar] [CrossRef]
- West, T.P. Improved polysaccharide production using strain improvement. In Microbial Processes and Products; Barredo, J.-L., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2005; Chapter 17; pp. 301–312. [Google Scholar]
- Li, A.; Hu, T.; Luo, H.; Alam, N.-U.; Xin, J.; Li, H.; Lin, Y.; Huang, J.; Meng, Y.; Meng, F.; et al. A carotenoid- and poly-β-hydroxybutyrate-free mutant strain of Sphingomonas elodea ATCC 31461 for the commercial production of gellan. mSphere 2019, 4, e00668-19. [Google Scholar] [CrossRef]
- Dlamini, A.M.; Peiris, P.S. Production of exopolysaccharide by Pseudomonas sp. ATCC 31461 (Pseudomonas elodea) using whey as fermentation substrate. Appl. Microbiol. Biotechnol. 1997, 47, 52–57. [Google Scholar] [CrossRef]
- Fialho, A.M.; Martins, L.O.; Donval, M.-L.; Leitao, J.H.; Ridout, M.J.; Jay, A.J.; Morris, V.J.; Sá-Correia, I. Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl. Environ. Microbiol. 1999, 65, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- West, T.P.; Strohfus, B. Effect of carbon source on exopolysaccharide production by Sphingomonas paucimobilis ATCC 31461. Microbiol. Res. 1998, 153, 327–329. [Google Scholar] [CrossRef]
- West, T.P.; Fullenkamp, N.A. Ability of casamino acids to support gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 2000, 102, 89–101. [Google Scholar]
- Huang, J.; Zhu, S.; Li, C.; Zhang, C.; Ji, Y. Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep. Biochem. Biotechnol. 2020, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoff, A.; Gibbons, W.R.; Bauer, N.; West, T.P. Development of a low-cost medium for producing gellan from Sphingomonas paucimobilis. J. Biotech Res. 2010, 2, 67–78. [Google Scholar]
- Nampoothiri, K.M.; Singhania, R.R.; Sabarinath, C.; Pandey, A. Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem. 2003, 38, 1513–1519. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Saudagar, P.S.; Singhal, R.S.; Pandey, A. Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J. Biosci. Bioeng. 2006, 102, 150–156. [Google Scholar] [CrossRef]
- Banik, R.R.; Santhiagu, A.; Upadhyay, S.N. Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour. Technol. 2007, 98, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, N.-K.; Shin, M.-K.; Kim, S.-K.; Kaplan, D.L.; Lee, J.-W. Production of gellan gum by Sphingomonas paucimobilis NK2000 with soybean pomace. Biochem. Eng. J. 2003, 16, 357–360. [Google Scholar] [CrossRef]
- Giavasis, I.; Petrotos, K. Biovalorization of olive mill waste water for the production of gellan gum from Sphingomonas paucimobilis. Br. Biotechnol. J. 2016, 11, 1–15. [Google Scholar] [CrossRef]

| Sphingomonas elodea Strain | Carbon Source | Nitrogen Source | Growth Conditions | Gellan (G/L) | Reference |
|---|---|---|---|---|---|
| ATCC 31461 | Maltose corn syrup | Corn steep solids | 72 h, 30 °C | 2.71 | [43] |
| ATCC 31461 | Maltose corn syrup | Corn steep solids | 72 h, 30 °C | 2.98 | [43] |
| ATCC 31461 | Maltose corn syrup | Tryptone | 72 h, 30 °C | 1.93 | [43] |
| ATCC 31461 | Maltose corn syrup | Peptone | 72 h, 30 °C | 1.80 | [43] |
| ATCC 31461 | Soluble starch | Peptone | 72 h, 30 °C | 5.73 | [44] |
| ATCC 31461 | Maltose corn syrup | Hydrolyzed soy protein | 72 h, 30 °C | 2.24 | [60] |
| EGP-1 | Maltose corn syrup | Hydrolyzed soy protein | 72 h, 30 °C | 3.20 | [60] |
| ATCC 31461 | Whey | Tryptone | 64 h, 28 °C | 3.10 | [64] |
| ATCC 31461 | Whey | Peptone | 72 h, 30 °C | 7.90 | [65] |
| ZJUT 1008 | Glucose | Corn Steep liquor | 72 h, 30 °C | 14.41 | [68] |
| ATCC 31461 | Glucose | Condensed corn solubles | 82 h, 30 °C | 13.40 | [69] |
| ATCC 31461 | Soluble starch | Tryptone | 48 h, 30 °C | 24.32 | [70] |
| ATCC 31461 | Soluble starch | Yeast extract/tryptophan | 48 h, 30 °C | 43.60 | [71] |
| ATCC 31461 | Molasses | Tryptone | 48 h, 30 °C | 13.81 | [72] |
| NK 2000 | Glucose | Soybean pomace | 72 h, 30 °C | 7.50 | [73] |
| ATCC 31461 | Olive oil waste water | Yeast extract | 63 h, 30 °C | 6.80 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts. Polysaccharides 2021, 2, 234-244. https://doi.org/10.3390/polysaccharides2020016
West TP. Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts. Polysaccharides. 2021; 2(2):234-244. https://doi.org/10.3390/polysaccharides2020016
Chicago/Turabian StyleWest, Thomas P. 2021. "Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts" Polysaccharides 2, no. 2: 234-244. https://doi.org/10.3390/polysaccharides2020016
APA StyleWest, T. P. (2021). Synthesis of the Microbial Polysaccharide Gellan from Dairy and Plant-Based Processing Coproducts. Polysaccharides, 2(2), 234-244. https://doi.org/10.3390/polysaccharides2020016





