Impacts of Glucagon-like Peptide-1 Receptor-Agonist (GLP-1 RA) Treatment for Metabolic Disturbances and Weight Gain in Patients on Clozapine/Olanzapine: A Systematic Review
Abstract
1. Introduction
1.1. Schizophrenia and Its Health Burden
1.2. Management of Schizophrenia
1.3. Olanzapine and Clozapine
1.4. Metabolic Side Effects
1.5. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs)
1.6. GLP-1 RAs with Olanzapine/Clozapine
1.7. Objectives
2. Materials and Methods
2.1. Guidelines and Registration
2.2. Search Terms
2.3. Screening Process
- (a)
- Available in English;
- (b)
- Original research that has a peer-reviewed full-text manuscript or conference abstract;
- (c)
- Has a quantitative methodology;
- (d)
- Includes outcome data on metabolic parameters and/or weight changes;
- (e)
- Includes stratified data for at least one patient on a GLP-1 RA and at least one of either clozapine or olanzapine.
2.4. Data Extraction
2.5. Data Synthesis and Analysis
2.6. Quality Assessment
3. Results
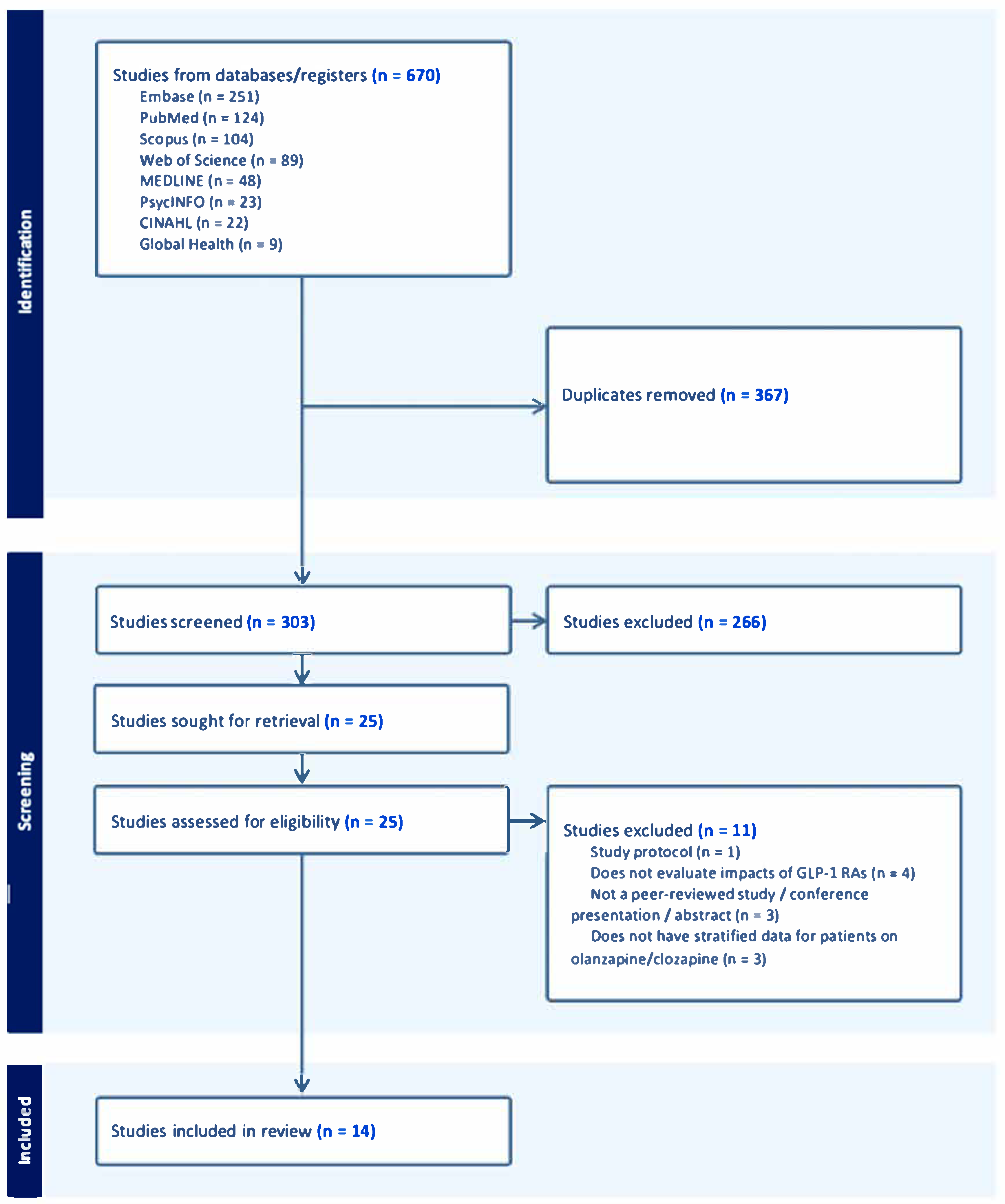
3.1. Screening
3.2. Characteristics of Included Studies
3.3. Critical Appraisal
3.4. Patient Characteristics
3.5. Impacts on Weight
3.6. Impacts on Metabolic Parameters
3.7. Other Benefits/Trends
3.8. Adverse Effects and Tolerability
4. Discussion
4.1. Key Findings and Implications
4.2. Need for Further Research
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; Tuominen, L.; Dargél, A.; Carvalho, A.F.; Fornaro, M.; et al. Incidence, prevalence, and global burden of schizophrenia—Data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol. Psychiatry 2023, 28, 5319–5327. [Google Scholar] [CrossRef] [PubMed]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301, Erratum in Lancet Psychiatry 2017, 4, e19. https://doi.org/10.1016/S2215-0366(17)30326-7. [Google Scholar] [CrossRef] [PubMed]
- Rössler, W.; Salize, H.J.; van Os, J.; Riecher-Rössler, A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005, 15, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojtabai, R.; Servis, M.; Walaszek, A.; Buckley, P.; Lenzenweger, M.F.; et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Am. J. Psychiatry 2020, 177, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 2021; ISBN 978-0-89042-469-8. [Google Scholar]
- Stroup, T.S.; Marder, S.R. Schizophrenia in Adults: Maintenance Therapy and Side Effect Management. Available online: https://www.uptodate.com/contents/schizophrenia-in-adults-maintenance-therapy-and-side-effect-management?search=schizophrenia%20treatment&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2 (accessed on 10 September 2025).
- Agid, O.; Crespo-Facorro, B.; de Bartolomeis, A.; Fagiolini, A.; Howes, O.D.; Seppälä, N.; Correll, C.U. Overcoming the barriers to identifying and managing treatment-resistant schizophrenia and to improving access to clozapine: A narrative review and recommendation for clinical practice. Eur. Neuropsychopharmacol. 2024, 84, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.; Cheng, C.; Volkovinskaia, A.; Baker, G.B.; Dursun, S.M. Clozapine: Why Is It So Uniquely Effective in the Treatment of a Range of Neuropsychiatric Disorders? Biomolecules 2021, 11, 1030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, S.; Schneider-Thoma, J.; Bighelli, I.; Siafis, S.; Wang, D.; Burschinski, A.; Schestag, K.; Samara, M.; Leucht, S. A network meta-analysis of efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 917–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, E.; Siafis, S.; Fernando, P.; Falkai, P.; Honer, W.G.; Röh, A.; Siskind, D.; Leucht, S.; Hasan, A. Efficacy and safety of clozapine in psychotic disorders—A systematic quantitative meta-review. Transl. Psychiatry 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Legge, S.E.; Hamshere, M.; Hayes, R.D.; Downs, J.; O’Donovan, M.C.; Owen, M.J.; Walters, J.T.R.; MacCabe, J.H. Reasons for discontinuing clozapine: A cohort study of patients commencing treatment. Schizophr. Res. 2016, 174, 113–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salviato Balbão, M.; Cecílio Hallak, J.E.; Arcoverde Nunes, E.; Homem de Mello, M.; Triffoni-Melo Ade, T.; Ferreira, F.I.; Chaves, C.; Durão, A.M.; Ramos, A.P.; de Souza Crippa, J.A.; et al. Olanzapine, weight change and metabolic effects: A naturalistic 12-month follow up. Ther. Adv. Psychopharmacol. 2014, 4, 30–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuen, J.W.Y.; Kim, D.D.; Procyshyn, R.M.; Panenka, W.J.; Honer, W.G.; Barr, A.M. A Focused Review of the Metabolic Side-Effects of Clozapine. Front. Endocrinol. 2021, 12, 609240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siskind, D.J.; Leung, J.; Russell, A.W.; Wysoczanski, D.; Kisely, S. Metformin for Clozapine Associated Obesity: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0156208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.K.; Wang, C.K.; Bai, Y.M.; Huang, C.Y.; Lee, S.D. Outcomes of obese, clozapine-treated inpatients with schizophrenia placed on a six-month diet and physical activity program. Psychiatr. Serv. 2007, 58, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Zimbron, J.; Khandaker, G.M.; Toschi, C.; Jones, P.B.; Fernandez-Egea, E. A systematic review and meta-analysis of randomised controlled trials of treatments for clozapine-induced obesity and metabolic syndrome. Eur. Neuropsychopharmacol. 2016, 26, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Lu, M.L.; Huang, M.C.; Chen, P.Y.; Lin, Y.K.; Lin, S.K.; Chen, C.H. Effects of Low Dose Metformin on Metabolic Traits in Clozapine-Treated Schizophrenia Patients: An Exploratory Twelve-Week Randomized, Double-Blind, Placebo-Controlled Study. PLoS ONE 2016, 11, e0168347, Erratum in: PLoS ONE 2018, 13, e0193315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhuo, C.; Xu, Y.; Wang, H.; Zhou, C.; Liu, J.; Yu, X.; Shao, H.; Tian, H.; Fang, T.; Li, Q.; et al. Clozapine induces metformin-resistant prediabetes/diabetes that is associated with poor clinical efficacy in patients with early treatment-resistant schizophrenia. J. Affect. Disord. 2021, 295, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Horska, K.; Ruda-Kucerova, J.; Skrede, S. GLP-1 agonists: Superior for mind and body in antipsychotic-treated patients? Trends Endocrinol. Metab. 2022, 33, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Porras-Segovia, A.; Krivoy, A.; Horowitz, M.; Thomas, G.; Bolstridge, M.; Ion, D.; Shergill, S.S. Rapid-onset clozapine-induced loss of glycaemic control: Case report. BJPsych Open 2017, 3, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals with Obesity and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Hahn, M.; Correll, C.U.; Fink-Jensen, A.; Russell, A.W.; Bak, N.; Broberg, B.V.; Larsen, J.; Ishøy, P.L.; Vilsbøll, T.; et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: A systematic review and individual participant data meta-analysis. Diabetes Obes. Metab. 2019, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Michailidis, T.; Dimosiari, A.; Fragakis, N.; Tse, G.; Rizzo, M. Effect of Glucagon-like Peptide-1 Receptor Agonists on Cardio-Metabolic Risk Factors among Obese/Overweight Individuals Treated with Antipsychotic Drug Classes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomedicines 2023, 11, 669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: http://www.covidence.org (accessed on 6 October 2025).
- Joanna Briggs Institute. Critical Appraisal Tools. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 6 October 2025).
- Xu, Y.; Chen, C.; Wang, K. Global prevalence of hypertension among people living with HIV: A systematic review and meta-analysis. J. Am. Soc. Hypertens. 2017, 11, 530–540. [Google Scholar] [CrossRef]
- Bowring, A.L.; Veronese, V.; Doyle, J.S.; Stoove, M.; Hellard, M. HIV and sexual risk among men who have sex with men and women in Asia: A systematic review and meta-analysis. AIDS Behav. 2016, 20, 2243–2265. [Google Scholar] [CrossRef]
- Adalbert, J.R.; Varshney, K.; Tobin, R.; Pajaro, R. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: A scoping review. BMC Infect. Dis. 2021, 21, 985. [Google Scholar] [CrossRef]
- Ali, S.; Ghodsimaab, N.; Rusling, M.; Rashid, N. Effect of GLP-1 receptor agonists on obesity in patients with severe mental illness: A state hospital case series of clozapine-treated patients. Psychiatry Res. Case Rep. 2024, 3, 100231. [Google Scholar] [CrossRef]
- Ishøy, P.L.; Knop, F.K.; Vilsbøll, T.; Glenthøj, B.Y.; Ebdrup, B.H. Sustained weight loss after treatment with a glucagon-like peptide-1 receptor agonist in an obese patient with schizophrenia and type 2 diabetes. Am. J. Psychiatry 2013, 170, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Vedtofte, L.; Jakobsen, M.S.L.; Jespersen, H.R.; Jakobsen, M.I.; Svensson, C.K.; Koyuncu, K.; Schjerning, O.; Oturai, P.S.; Kjaer, A.; et al. Effect of Liraglutide Treatment on Prediabetes and Overweight or Obesity in Clozapine- or Olanzapine-Treated Patients with Schizophrenia Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 719–728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, C.; Maclaurin, S.; Donovan, A.L.; YS, C.; Freudenreich, O. GLP-1 Receptor Agonists for Clozapine-Induced Weight Gain: A Case Report with the Dual GLP-1/GIP Agonist Tirzepatide. Prim. Care Companion CNS Disord. 2025, 27, 25cr03952. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.R.; Strawn, J.R.; Adler, C.M.; Blom, T.J.; Welge, J.A.; DelBello, M.P. A double-blind, placebo-controlled trial of exenatide for the treatment of olanzapine-related weight gain in obese and overweight adults. J. Affect. Disord. 2025, 382, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Prasad, F.; De, R.; Korann, V.; Chintoh, A.F.; Remington, G.; Ebdrup, B.H.; Siskind, D.; Knop, F.K.; Vilsbøll, T.; Fink-Jensen, A.; et al. Semaglutide for the treatment of antipsychotic-associated weight gain in patients not responding to metformin—A case series. Ther. Adv. Psychopharmacol. 2023, 13, 20451253231165169, Erratum in Ther. Adv. Psychopharmacol. 2024, 14, 20451253241258536. https://doi.org/10.1177/20451253241258536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricciardi, F.L.; Melnitsky, J.L.; Peleg, S.B.; Govil, P.; Kantrowitz, J.T. Worth the weight? The challenges of administering the glucagon-like peptide 1 receptor agonist semaglutide with long-term olanzapine use in a patient with schizophrenia. J. Clin. Psychiatry 2025, 86, 25cr15857. [Google Scholar]
- Siskind, D.; Wysoczanski, D.; Russell, A.; Ashford, M. Weight loss associated with exenatide in an obese man with diabetes commenced on clozapine. Aust. N. Z. J. Psychiatry 2016, 50, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.J.; Russell, A.W.; Gamble, C.; Winckel, K.; Mayfield, K.; Hollingworth, S.; Hickman, I.; Siskind, V.; Kisely, S. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: A randomized controlled trial (CODEX). Diabetes Obes. Metab. 2017, 20, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Russell, A.; Gamble, C.; Baker, A.; Cosgrove, P.; Burton, L.; Kisely, S. Metabolic measures 12 months after a randomised controlled trial of treatment of clozapine associated obesity and diabetes with exenatide (CODEX). J. Psychiatr. Res. 2020, 124, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Baker, A.; Arnautovska, U.; Warren, N.; Russell, A.; DeMonte, V.; Halstead, S.; Iyer, R.; Korman, N.; McKeon, G.; et al. Efficacy and safety of semaglutide versus placebo for people with schizophrenia on clozapine with obesity (COaST): A phase 2, multi-centre, participant and investigator- blinded, randomised controlled trial in Australia. Lancet Psychiatry 2025, 12, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.K.; Larsen, J.R.; Vedtofte, L.; Jakobsen, M.S.L.; Jespersen, H.R.; Jakobsen, M.I.; Koyuncu, K.; Schjerning, O.; Nielsen, J.; Ekstrøm, C.T.; et al. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiatr. Scand. 2019, 139, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.; Thai, R.; Dike, D.; Sydner, B. Semaglutide Plus Metformin Versus Metformin Alone For Antipsychotic-Induced Weight Gain In Patients With Type 2 Diabetes Mellitus. Psychiatr. Q. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, W.J.; Zhu, H.; Li, H.F.; Qiao, J. Successful treatment of hyperglycemia with liraglutide in a hospitalized 27-year-old patient with schizophrenia: A case report. World J. Clin. Cases 2022, 10, 7495–7501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsen, J.R.; Vedtofte, L.; Holst, J.J.; Oturai, P.; Kjær, A.; Correll, C.U.; Vilsbøll, T.; Fink-Jensen, A. Does a GLP-1 receptor agonist change glucose tolerance in patients treated with antipsychotic medications? Design of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open 2014, 4, e004227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fink-Jensen, A. Does the Glucagon-like Peptide-1 Receptor Agonist Semaglutide Prevent Deterioration of Metabolic State in Prediabetic or Diabetic Patients with Schizophrenia Treated with the Antipsychotic Compounds Clozapine or Olanzapine? Available online: https://clinicaltrials.gov/study/NCT04892199 (accessed on 19 September 2025).
- Psychiatric Centre Rigshospitalet. Effect of Liraglutide Treatment on Prediabetes and Overweight or Obesity in Clozapine- or Olanzapine-Treated Patients with Schizophrenia Spectrum Disorder. Available online: https://clinicaltrials.gov/study/NCT01845259?term=Clozapine&intr=GLP1%20receptor%20agonist&rank=1#publications (accessed on 19 September 2025).
- University of Cincinnati. V/PDE-4 Inhibitor, Exenatide or Liraglutide in the Treatment of Patients with Depression. Available online: https://clinicaltrials.gov/study/NCT00845507?term=AREA%5BConditionSearch%5D(%22Depressive%20Disorder%22)%20AND%20AREA%5BInterventionSearch%5D(%22Incretins%22)&rank=2#publications (accessed on 19 September 2025).
- Ishøy, P.L.; Fagerlund, B.; Broberg, B.V.; Bak, N.; Knop, F.K.; Glenthøj, B.Y.; Ebdrup, B.H. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatr. Scand. 2017, 136, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Svensson, C.K.; Vedtofte, L.; Jakobsen, M.L.; Jespersen, H.S.; Jakobsen, M.I.; Koyuncu, K.; Schjerning, O.; Nielsen, J.; Ekstrøm, C.T.; et al. High prevalence of prediabetes and metabolic abnormalities in overweight or obese schizophrenia patients treated with clozapine or olanzapine. CNS Spectr. 2019, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, T.L.; Brennan, M.D. Glucagon-like peptide 1 receptor (GLP1R) haplotypes correlate with altered response to multiple antipsychotics in the CATIE trial. Schizophr. Res. 2014, 160, 73–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shukla, A.P.; Mandel, L.S.; Tchang, B.G.; Litman, E.; Cadwell, J.; Kumar, R.B.; Waitman, J.; Igel, L.I.; Christos, P.; Aronne, L.J. Medical Weight-Loss Outcomes in Patients Receiving Concomitant Psychotropic Medication: A Retrospective Cohort Study. Obesity 2020, 28, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, N.Y.; Kim, S.H.; Kim, K.A.; Kim, Y.S. Effect of liraglutide 3.0mg treatment on weight reduction in obese antipsychotic-treated patients. Psychiatry Res. 2021, 299, 113830. [Google Scholar] [CrossRef] [PubMed]
- Tempia Valenta, S.; Stecchi, M.; Perazza, F.; Nuccitelli, C.; Villanova, N.; Pironi, L.; Atti, A.R.; Petroni, M.L. Liraglutide 3.0 mg and mental health: Can psychiatric symptoms be associated to adherence to therapy? Insights from a clinical audit. Eat. Weight Disord. 2023, 28, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campforts, B.; Drukker, M.; van Amelsvoort, T.; Bak, M. Management of obesity with semaglutide or metformin in patients with antipsychotic-induced weight gain (MOSA): A non-randomised open-label pilot study. BMC Psychiatry 2024, 24, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, M.; Zhai, S.; Ouyang, X.; Liu, Z.; Ross, B. Factors influencing medication adherence among patients with severe mental disorders from the perspective of mental health professionals. BMC Psychiatry 2022, 22, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varshney, K.; Panda, S.; Fernando, H.; Sava, S.; Khan, T. Impacts of Glucagon-like Peptide-1 Receptor-Agonist (GLP-1 RA) Treatment for Metabolic Disturbances and Weight Gain in Patients on Clozapine/Olanzapine: A Systematic Review. Obesities 2025, 5, 72. https://doi.org/10.3390/obesities5040072
Varshney K, Panda S, Fernando H, Sava S, Khan T. Impacts of Glucagon-like Peptide-1 Receptor-Agonist (GLP-1 RA) Treatment for Metabolic Disturbances and Weight Gain in Patients on Clozapine/Olanzapine: A Systematic Review. Obesities. 2025; 5(4):72. https://doi.org/10.3390/obesities5040072
Chicago/Turabian StyleVarshney, Karan, Shivani Panda, Hilary Fernando, Sergiu Sava, and Taimur Khan. 2025. "Impacts of Glucagon-like Peptide-1 Receptor-Agonist (GLP-1 RA) Treatment for Metabolic Disturbances and Weight Gain in Patients on Clozapine/Olanzapine: A Systematic Review" Obesities 5, no. 4: 72. https://doi.org/10.3390/obesities5040072
APA StyleVarshney, K., Panda, S., Fernando, H., Sava, S., & Khan, T. (2025). Impacts of Glucagon-like Peptide-1 Receptor-Agonist (GLP-1 RA) Treatment for Metabolic Disturbances and Weight Gain in Patients on Clozapine/Olanzapine: A Systematic Review. Obesities, 5(4), 72. https://doi.org/10.3390/obesities5040072





