Nutritional Care Process in Hospitalized Patients with Obesity-Related Multimorbidity
Abstract
1. Introduction
2. Hospital Factors That Alter Nutritional Status in Patients with Obesity-Related Multimorbidity (ORM)
3. Nutritional Intervention in Hospitalized Adults with Obesity-Related Multimorbidity
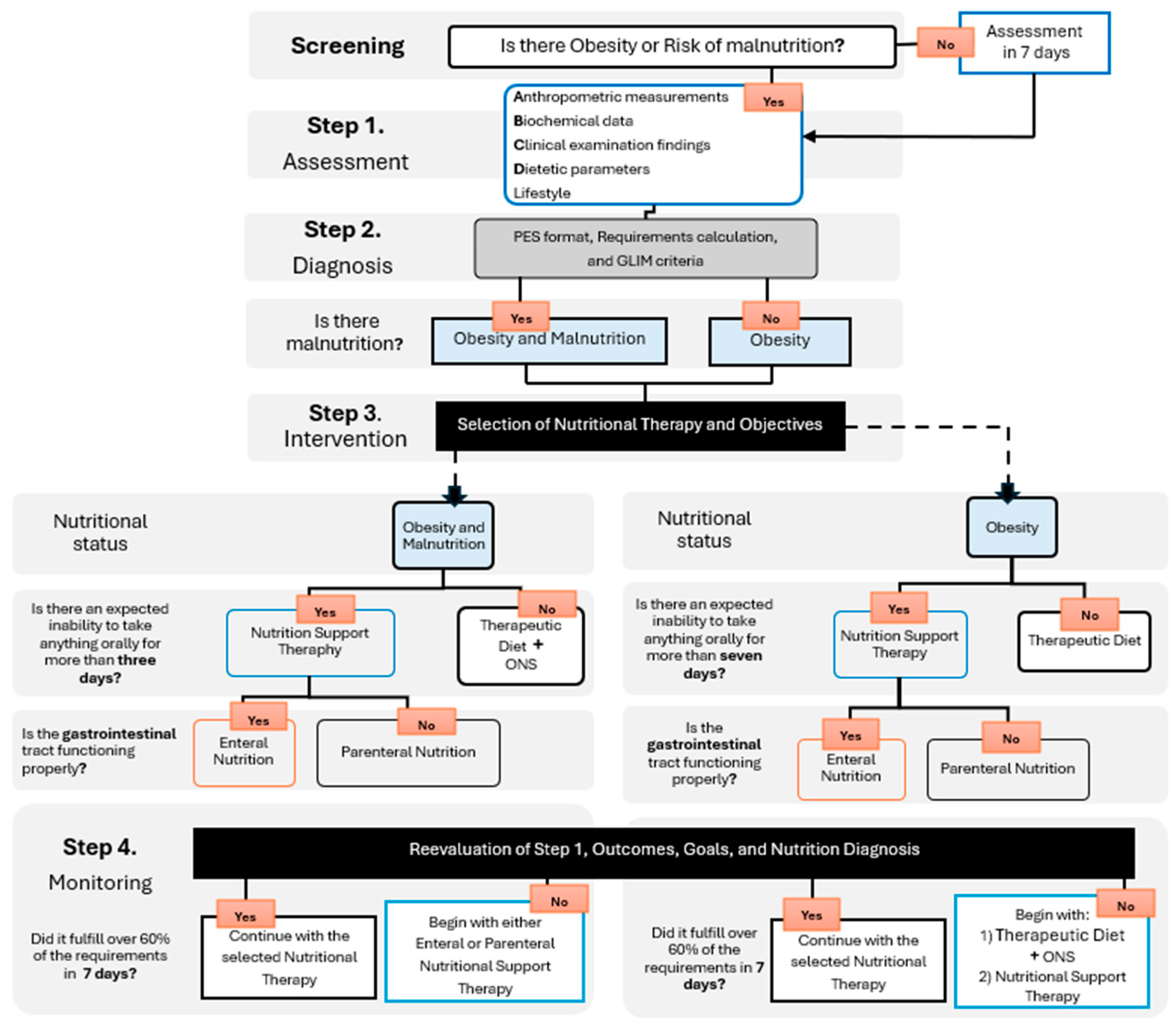
4. Nutritional Status Assessment
4.1. Anthropometric Measurements
4.2. Biochemical Data
4.3. Clinical Examination Findings
4.4. Dietary Parameters
4.5. Lifestyle
5. Nutritional Diagnosis
6. Estimation of Nutritional Requirements
7. Medical Nutrition Therapy Recommendations
- By consistency modification: clear liquids, full liquids, semi-liquid, blended, puréed, soft, finely chopped, and bland.
- By energy content: hypercaloric, isocaloric, and hypocaloric.
- By nutrient modification: modified in CHO, high-protein or low-protein, low-fat, lactose-free, high or low in fiber, dry, high or low in sodium, potassium, calcium, iron, vitamin K, etc.
7.1. Phenotype 1 “Hungry Brain”
7.2. Phenotype 2 “Emotional Hunger”
7.3. Phenotype 3 “Hungry Gut”
7.4. Phenotype 4 “Slow Burning”
8. Oral Nutritional Supplements
- Specialized polymeric formulas: Most frequently used are high-protein (Grade B strong consensus) [13] or modified in carbohydrate content, containing sources such as maltodextrins, monounsaturated fatty acids, omega-3 fatty acids, and soluble fiber to promote glycemic control.
- Formulas containing immunonutrients: Recommended for specific conditions such as systemic inflammation, perioperative support, trauma, burns, or gastrointestinal surgery.
- Oligomeric and elemental formulas: Indicated for conditions involving intestinal malabsorption.
- Fiber or protein modules: Used when the requirement for these nutrients is elevated in patients with ORM.
9. Recommendations on the Use of Enteral Nutrition
- The oral route fails to meet more than 60% of daily energy needs for 3 to 7 days.
- PO cannot or should not be used (due to invasive mechanical ventilation, sedation, coma, oral/esophageal/laryngeal/maxillofacial injury, obstruction, or surgery, etc.).
- Severe dysphagia contraindicates oral route. In individuals with severe malnutrition or in the ICU, EN should be initiated within the first 24 to 48 h of admission with trophic feeding providing 10 to 15 mL/hour or 500 kcal per day.
10. Recommendations on the Use of Parenteral Nutrition
- Gastrointestinal dysfunction
- Intolerance to EN for more than 3 to 7 days
- Intestinal obstruction
- Hollow viscus perforation
- Necrotizing pancreatitis with intolerance to EN
- Intractable vomiting and/or diarrhea
- Severe enteritis
- Intestinal ischemia
- High-output enteral fistula (greater than 500 mL per day)
- Need for bowel rest or fasting for more than 7 days
- Short bowel syndrome or type III intestinal failure.
11. Recommendations on the Use of Immunonutrients
11.1. Omega-3
11.2. Vitamin D3 (1.25 Dihydroxycholecalciferol)
11.3. Glutamine and/or Arginine
11.4. Antioxidant and Immunomodulatory Vitamins and Minerals (Vitamin C, E, B-Complex, Beta-Carotene, Selenium, and Zinc)
11.5. Prebiotics and Probiotics
12. Monitoring
13. Conclusions and Future Directions
- Malnutrition is underdiagnosed in individuals with obesity. This is because a specific nutritional screening program for people with obesity that considers metabolic morbidity, sarcopenia, symptoms of nutrient (antioxidant) deficiency, inability to feed orally, and infections has not been developed. This leads to delayed and inadequate medical and nutritional care.
- There are no specific guidelines for calculating total energy requirements (alternatives to IC), determining fluid, protein, specific amino acids, and immunonutrient requirements.
- In multimorbidity conditions, signs and symptoms are highly diverse, and if they are not integrated and analyzed as a whole, as established by the NCP, contradictory re-commendations could result. For example, for patients with sarcopenic obesity or critically ill patients, current guidelines recommend a high protein intake; however, those with coexisting kidney, liver, or heart failure may require fluid restrictions, which would prevent them from achieving the necessary protein intake. Also, the use of PN in ORM with kidney, liver, and heart failure and sarcopenic obesity should be addressed with caution. For now, it is recommended to prioritize the problem to be treated according to the maximum clinical benefit.
- Furthermore, the clinical, social, cultural, and psychological characteristics of each person influence dietary decisions to improve long-term adherence.
- For these reasons, it is necessary to create more lines of research that standardize nutritional recommendations by degree, phenotype, and causality of obesity and type of comorbidity. This way, health professionals who make up the multidisciplinary nutritional support teams will have better tools for decision-making when faced with the challenge of selecting and integrating the most appropriate nutritional approach for hospitalized patients with ORM.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABW | Adjusted body weight |
| AND | Academy of Nutrition and Dietetics |
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BMI | Body Mass Index |
| BSA | Body surface area |
| BUN | Blood Urea Nitrogen |
| CAT | Computed Axial Tomography |
| CHO | Carbohydrates |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| DASH | Dietary Approaches to Stop Hipertensión |
| DEXA | Dual-energy X-ray Absorptiometry |
| DRIs | Dietary Reference Intakes |
| eGFR | Estimated Glomerular Filtration Rate |
| EN | Enteral nutrition |
| EPA | Eicosapentaenoic Acid |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GLIM | Global Leadership Initiative on Malnutrition |
| GM | human gut microbiota |
| GPP | Good practice points or expert consensus |
| HDL | High-density lipoprotein colesterol |
| IBW | Ideal body weight. |
| IC | indirect calorimetry |
| ICU | Intensive care unit |
| LPL | Lipoprotein lipase |
| LPS | Bacterial lipopolysaccharides |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MCT | Medium Chain Triglycerides |
| mfBIA | Multi-frequency bioimpedance |
| mNUTRIC | Modified Nutrition Risk in Critically Ill |
| MRI | Magnetic Resonance Imaging |
| MUST | Malnutrition Universal Screening Tool |
| NB | Nitrogen Balance |
| NCP | Nutrition Care Process |
| NFPE | Nutrition-Focused Physical Exam |
| NRS | Nutritional Risk Screening |
| ONS | Oral Nutrition Support |
| ORM | Obesity-related multimorbidity |
| PES | Problem, Etiology and Signs and symptoms |
| PN | Parenteral Nutrition |
| PO | Post-operative |
| PVD | peripheral vascular disease |
| RDA | Recommended Dietary Allowances |
| REE | Resting energy expenditure |
| RFH-NPT | Royal Free Hospital-Nutritional Prioritizing Tool |
| RS | Refeeding syndrome |
| SAH | Systemic arterial hypertension |
| SIGN | Scottish Intercollegiate Guidelines Network |
| T2D | Type 2 diabetes |
| TEE | Total Energy Expenditure |
| Tmax | maximum temperature in degrees Celsius |
| UUN | Urine urea nitrogen |
| Vmin | minute volume of the respirator |
| VTA | Ventral tegmental area |
References
- Ezendu, K.; Pohl, G.; Lee, C.J.; Wang, H.; Li, X.; Dunn, J.P. Prevalence of obesity-related multimorbidity and its health care costs among adults in the United States. J. Manag. Care Spec. Pharm. 2025, 31, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development (OECD). The Heavy Burden of Obesity: The Economic Burden of Obesity 2019. Available online: https://www.oecd-ilibrary.org/sites/67450d67-en/1/2/3/index.html?itemId=/content/publication/67450d67-en&_csp_=77ac5dad9f2cb67b4d2e46c9fc814aa4&itemIGO=oecd&itemContentType=book#section-d1e8051 (accessed on 20 March 2025).
- Ostrominski, J.W.; Arnold, S.V.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’Connell, T.; Collins, E.B.; et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar] [CrossRef]
- Middeke, J.; Palmer, K.; Lövestam, E.; Vivanti, A.; Orrevall, Y.; Steiber, A.; Lyons-Wall, P.; Lo, J.; Devine, A.; Lieffers, J.; et al. Predictors of nutrition care process knowledge and use among dietitians internationally. J. Hum. Nutr. Diet. 2022, 35, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Academy of Nutrition and Dietetics (AND). International Dietetics and Nutrition Terminology (IDNT) Reference Manual: Standardized Language for the Nutrition Care Process, 4th ed.; Eat Right: Chicago, IL, USA, 2013; 427p. [Google Scholar]
- World Health Organization (WHO). Noncommunicable Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 March 2025).
- de Heredia, P.F.; Gómez-Martínez, S.; Marcos, A. Chronic and degenerative diseases: Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
- Gómez-Picard, P.; Fuster-Culebras, J. Atención a la cronicidad: Desafío estratégico, macrogestión y políticas de salud. Enferm. Clin. 2014, 24, 12–17. [Google Scholar] [CrossRef]
- Burgos, R.; Joaquín, C.; Blay, C.; Vaqué, C. Disease-related malnutrition in hospitalized chronic patients with complex needs. Clin. Nutr. 2020, 39, 1447–1453. [Google Scholar] [CrossRef]
- Briggs Early, K.; Stanley, K. Position of the Academy of Nutrition and Dietetics: The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes. J. Acad. Nutr. Diet. 2018, 118, 343–353. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Wunderle, C.; Gomes, F.; Schuetz, P.; Stumpf, F.; Austin, P.; Ballesteros-Pomar, M.D.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; et al. ESPEN guideline on nutritional support for polymorbid medical inpatients. Clin. Nutr. 2023, 42, 1545–1568. [Google Scholar] [CrossRef]
- Academy of Nutrition Dietetics (AND). Pocket Guide to Bariatric Surgery, Weight Management Dietetic Practice Group, 3rd ed.; Isom, K., Majumdar, M., Eds.; Eat Right: Chicago, IL, USA, 2021; 355p. [Google Scholar]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Abbasoglu, O.; Ioannou, E.; Meija, L.; Ottens-Oussoren, K.; Pichard, C.; Rothenberg, E.; Rubin, D.; Siljamäki-Ojansuu, U.; Vaillant, M.F.; et al. ESPEN guideline on hospital nutrition. Clin. Nutr. 2021, 40, 5684–5709. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The rate and assessment of muscle wasting during critical illness: A systematic review and meta-analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Ockenga, J.; Eshraghian, A.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Kani, H.T.; Khannoussi, W.; et al. Practical guideline on obesity care in patients with gastrointestinal and liver diseases–Joint ESPEN/UEG guideline. Clin. Nutr. 2023, 42, 987–1024. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.C.; et al. Guidelines for the provision of nutrition support therapy in the adult critically ill. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef]
- Shang, R.; Rodrigues, B. Lipoprotein lipase as a target for obesity/diabetes related cardiovascular disease. J. Pharm. Pharm. Sci. 2024, 27, 13199. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Sun, L.J.; Li, J.N.; Nie, Y.Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Londoño Pereira, M.; Estrada Restrepo, A.; Preciado Tamayo, Á.M.; Botero Bernal, M.; Germán Borda, M. Associations between nutritional status and abdominal adiposity with cognitive domains and depressive symptoms in older persons with multimorbidity: Understanding an understudied population. Rev. Esp. Geriatr. Gerontol. 2025, 60, 101558. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Ciaccio, M.; Stoian, A.P.; Salmen, T.; Bica, I.C.; Rangraze, I.; Tanani, M.E.; Rizzo, M.; Rizvi, A.A. New Frontiers in Nutritional and Therapeutic Interventions for Obesity Phenotypes. Medicina 2025, 61, 664. [Google Scholar] [CrossRef]
- Sanz-París, A.; Martín-Palmero, A.; Gomez-Candela, C.; García-Almeida, J.M.; Burgos-Pelaez, R.; Sanz-Arque, A.; Espina, S.; Arbones-Mainar, J.M.; Study VIDA group. GLIM Criteria at Hospital Admission Predict 8-Year All-Cause Mortality in Elderly Patients with Type 2 Diabetes Mellitus: Results from VIDA Study. JPEN J. Parenter. Enter. Nutr. 2020, 44, 1492–1500. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Guo, G.; Yang, W.; Zhang, X.; Yang, F.; Yang, J.; Hui, Y.; Wang, X.; Cui, B.; et al. Global Leadership Initiative on Malnutrition-defined malnutrition coexisting with visceral adiposity predicted worse long-term all-cause mortality among inpatients with decompensated cirrhosis. Nutr. Diabetes 2024, 14, 76. [Google Scholar] [CrossRef]
- Choban, P.; Dickerson, R.; Malone, A.; Worthington, P.; Compher, C.; American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. Clinical guidelines: Nutrition support of hospitalized adult patients with obesity. JPEN J. Parenter. Enter. Nutr. 2013, 37, 714–744. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Rafii, M.; Azcue, M.; Pencharz, P. Effect of Intravenous Infusion Solutions on Bioelectrical Impedance Spectroscopy. JPEN J. Parenter. Enter. Nutr. 2017, 41, 641–646. [Google Scholar] [CrossRef]
- Price, K.L.; Earthman, C.P. Update on body composition tools in clinical settings: Computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur. J. Clin. Nutr. 2019, 73, 187–193. [Google Scholar] [CrossRef]
- Alonso, A.L.; Munguía-Miranda, C.; Ramos-Ponce, D.; Hernandez-Saavedra, D.; Kumate, J.; Cruz, M. Waist perimeter cutoff points and prediction of metabolic syndrome risk. A study in a Mexican population. Arch. Med. Res. 2008, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Drover, J.W. Monitoring nutrition therapy in the critically ill patient with obesity. JPEN J. Parenter. Enter. Nutr. 2011, 35 (Suppl. S5), 44S–51S. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef]
- Matthews-Rensch, K.; Blackwood, K.; Lawlis, D.; Breik, L.; McLean, C.; Nguyen, T.; Phillips, S.; Small, K.; Stewart, T.; Thatcher, A.; et al. The Australasian Society of Parenteral and Enteral Nutrition: Consensus statements on refeeding syndrome. Nutr. Diet. J. Dietit. Assoc. Aust. 2025; Advance online publication. [Google Scholar] [CrossRef]
- Hummell, A.C.; Cummings, M. Role of the nutrition-focused physical examination in identifying malnutrition and its effectiveness. Nutr. Clin. Pract. 2022, 37, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ascencio-Peralta, C. Elementos Fundamentales en el Cálculo de Dietas, 3rd ed.; Manual Moderno: Bogotá, Colombia, 2024. [Google Scholar]
- Perez-Lizaur, A.B.; García-Campos, M. Dietas Normales y Terapéuticas. Los Alimentos en la Salud y la Enfermedad, 7th ed.; McGraw-Hill: Mexico City, Mexico, 2019. [Google Scholar]
- Perez-Lizaur, A.B.; Palacios, G.B. SMAE Sistema Mexicano de Alimentos Equivalentes 2.0, 5th ed.; Fondo de Nutrición y Salud: Mexico City, Mexico, 2024. [Google Scholar]
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef] [PubMed]
- Doley, J. Enteral Nutrition Overview. Nutrients 2022, 14, 2180. [Google Scholar] [CrossRef]
- Writing Group of the Nutrition Care Process/Standardized Language Committee. Nutrition care process and model part I: The 2008 update. J. Am. Diet. Assoc. 2008, 108, 1113–1117. [Google Scholar] [CrossRef]
- Writing Group of the Nutrition Care Process/Standardized Language Committee. Nutrition care process part II: Using the International Dietetics and Nutrition Terminology to document the nutrition care process. J. Am. Diet. Assoc. 2008, 108, 1287–1293. [Google Scholar] [CrossRef]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y.; González, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef]
- Tweel, L.E.; Compher, C.; Bear, D.E.; Gutierrez-Castrellon, P.; Leaver, S.K.; MacEachern, K.; Ortiz-Reyes, L.; Pooja, L.; León, A.; Wedemire, C.; et al. A Comparison of High and Usual Protein Dosing in Critically Ill Patients with Obesity: A Post Hoc Analysis of an International, Pragmatic, Single-Blinded, Randomized, Clinical Trial. Crit. Care Med. 2023, 52, 586–595. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures–2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity 2020, 28, O1–O58. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, M.F.; Alligier, M.; Baclet, N.; Capelle, J.; Dousseaux, M.P.; Eyraud, E.; Fayemendy, P.; Flori, N.; Guex, E.; Hennequin, V.; et al. Guidelines on Standard and Therapeutic Diets for Adults in Hospitals by the French Association of Nutritionist Dieticians (AFDN) and the French Speaking Society of Clinical Nutrition and Metabolism (SFNCM). Nutrients 2021, 13, 2434. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1046–e1081. [Google Scholar] [CrossRef]
- Le Goff, D.; Aerts, N.; Odorico, M.; Guillou-Landreat, M.; Perraud, G.; Bastiaens, H.; Musinguzi, G.; Le Reste, J.Y.; Barais, M. Practical dietary interventions to prevent cardiovascular disease suitable for implementation in primary care: An ADAPTE-guided systematic review of international clinical guidelines. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 93. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. J. Prev. Cardiol. 2025, 32, 184–220. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, R.; Rodriguez, V.; Quach, K.T.; Chen, X.; Sternson, S.M. Hedonic eating is controlled by dopamine neurons that oppose GLP-1R satiety. Science 2025, 387, eadt0773. [Google Scholar] [CrossRef]
- Escuro, A.A.; Hummell, A.C. Enteral Formulas in Nutrition Support Practice: Is There a Better Choice for Your Patient? Nutr. Clin. Pract. 2016, 31, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Church, A.; Zoeller, S. Enteral nutrition product formulations: A review of available products and indications for use. Nutr. Clin. Pract. 2023, 38, 277–300. [Google Scholar] [CrossRef]
- Corrigan, M.L.; Bobo, E.; Rollins, C.; Mogensen, K.M. Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Revised 2021 Standards of Practice and Standards of Professional Performance for Registered Dietitian Nutritionists (Competent, Proficient, and Expert) in Nutrition Support. J. Acad. Nutr. Diet. 2021, 121, 2071–2086.e59. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubeidi, D.; Davis, M.B.; Rahhal, R. Prevention of complications for hospitalized patients receiving parenteral nutrition: A narrative review. Nutr. Clin. Pract. 2024, 39, 1037–1053. [Google Scholar] [CrossRef]
- Bagheri, A.; Soltani, S.; Asoudeh, F.; Esmaillzadeh, A. Effects of omega-3 supplementation on serum albumin, pre-albumin and the CRP/albumin ratio in hospitalized patients: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 237–251. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhu, Y.; Liu, X.; Xia, H.; Yang, X.; Sun, G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: A randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2017, 56, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.; van den Helder, R.S.; Geenen, R.W.F.; Hunfeld, M.A.; Cense, H.A.; Demirkiran, A.; Houdijk, A.P.J. Four Weeks of Preoperative Omega-3 Fatty Acids Reduce Liver Volume: A Randomised Controlled Trial. Obes. Surg. 2019, 29, 2037–2044. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef]
- Raymond, J.L.; Morrow, K. Krause. Dietoterapia, 16th ed.; Elsevier: Barcelona, Spain, 2025. [Google Scholar]
- American Diabetes Association (ADA). 16. Diabetes Advocacy: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S182–S183. [Google Scholar] [CrossRef]
- Demetrowitsch, T.J.; Schlicht, K.; Knappe, C.; Zimmermann, J.; Jensen-Kroll, J.; Pisarevskaja, A.; Brix, F.; Brandes, J.; Geisler, C.; Marinos, G.; et al. Precision Nutrition in Chronic Inflammation. Front. Immunol. 2020, 11, 587895. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Reintam-Blaser, A.; Calder, P.C.; Casaer, M.; Hiesmayr, M.J.; Mayer, K.; Montejo, J.C.; Pichard, C.; Preiser, J.C.; van Zanten, A.R.H.; et al. Monitoring nutrition in the ICU. Clin. Nutr. 2019, 38, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; Limketkai, B.N.; Nelson, K.K.; Powers, J.; Ronan, A.; et al. When is enteral nutrition indicated? JPEN J. Parenter. Enter. Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef] [PubMed]
| Nutritional Problem (P) | Etiology (E) | Signs or Symptoms (S) | ||
|---|---|---|---|---|
| Increased energy expenditure. Deficient or excessive infusion of enteral or parenteral nutrition. Altered nutrition-related laboratory values. Increased nutrient needs (immunonutrients). | related to | Chronic-acute inflammation. Multimorbidity. Inadequate calculation of requirements due to obtaining weight. Malnutrition. Drug-nutrient interaction. Hypermetabolism. | as evidenced by | Weight loss. Increase in basal metabolic rate. Energy consumption percentage is less than 90% and greater than 110% per day. Alteration in blood of liver enzymes, pancreatic enzymes, lipids, proteins, electrolytes, glucose, nitrogen elements, vitamins, minerals, CO2 and pH. Clinical signs of specific nutrient deficiencies. |
| Inadequate protein, energy and fiber intake. Gastrointestinal function alteration. | Reduced appetite. Gastrointestinal symptoms. Inability or intolerance to oral or enteral feeding. Side effects of medical interventions or treatments. | Less than 90% of daily protein, energy, and fiber intake. Constipation, diarrhea, nausea, vomiting, abdominal bloating and/or slow gastric emptying. |
| Clinical Condition | Energy | Protein | CHO | Lipids | References |
|---|---|---|---|---|---|
| Obesity | ORM with metabolic dysregulation: Hypo-energetic diet in 20–25 kcal/kg of ABW/day (Recommendation). Avoid the prescription of a hypo-energetic diet for patients with acute conditions that do not lead to a metabolic response or surgical procedure, and sarcopenic obese elderly (Grade: GPP, consensus). Elderly patients: 27–30 kcal/kg of ABW/day (Grade GPP, Strong consensus). | 1.2–1.5 g/kg of ABW/day (Grade A, Strong consensus). or 1–1.1 g/kg of ABW/day (Recommendation) Impaired Kidney Function (eGFR <30 mL/min/1.73 m2): 0.8 g/kg of ABW/day (Grade B, Strong consensus). | 45% of TEE (Grade GPP, Majority agreement). | 20% of TEE (Grade GPP, Majority agreement). | [13,16,37] |
| T2D | 20% of TEE (Grade GPP, Strong consensus). 1–1.5 g/kg of ABW/day (Grade 0, Strong consensus). | <45% of TEE Avoided <40% of TEE in malnutrition (Grade GPP, Strong consensus). | 30–35% of TEE (Grade 0, Strong consensus). and Cardioprotective pattern. Saturated fatty acids: <7% of TEE. Monounsaturated fatty acids: 20% of TEE. Polyunsaturated fatty acids: 10% of TEE. Trans fatty acids: <1% of TEE. Cholesterol: <200 mg/day (Grade B, Strong consensus). | [16,51] | |
| Cardiovascular disease: SAH, AMI, and Stroke | 15–20% of TEE (Grade GPP, Strong consensus). | 45–60% of TEE (Grade GPP, Strong consensus). | [16,52,53] | ||
| MASLD | 1.2–1.5 g/kg of ABW/day (Grade GPP, strong consensus). and Low in aromatic amino acids (No consensus). | Glucose oxidation rate: ≤5 mg/kg/min. (Grade GPP, Strong consensus). | Mediterranean diet: 30–35% of TEE (Grade 0, Strong consensus). Omega-3 supplementation: 3–4 g/day (Grade GPP, Strong consensus). | [18,54,55] | |
| Obesity Post-bariatric Surgery | First 3 months of PO: 800 kcal/day 3 months to 1 year of PO: Do not exceed (Men: 1500 kcal/day and Women: 1200 kcal/day). 1 year of PO: 16 kcal/kg of ABW/day (Grade GPP, Strong consensus) | 10–35% of TEE First 3 months of PO, at least: Men: 56 g/day and Women: 46 g/day. 3 months to 1 year of PO: 0.8–1.2 g/kg IBW/day >1 year of PO: 1.1–1.2 g/kg IBW/day (Grade GPP, Strong consensus) | 50–130 g/day 0% added sugar (Grade GPP, Strong consensus). | 20–35% of TEE Monounsaturated fatty acids: 20% of TEE Saturated fatty acids: <10% of TEE (Grade GPP, Strong consensus). | [14,16,19,49] |
| Obesity in ICU | ESPEN Acute phase (First 3–7 days of ICU stay): <70% of TEE (Grade A, strong consensus). BMI > 30 kg/m2: 20–25 kcal/kg of ABW/day (Grade 0, Consensus). ASPEN Acute phase (First 7–10 days of ICU stay): 12–25 kcal/kg IBW/day (Grade Moderate, weak). BMI 30–35 kg/m2: 11–14 kcal/kg ABW/day (Expert consensus). BMI > 50 kg/m2: 22–25 kcal/kg IBW/day (Expert consensus). Over 60 years old: Penn State University: TEE = (REE with Mifflin × 0.96) + (Tmax × 167) + (Vmin × 31) − 6212 (Recommendation). | ESPEN 1.3 g/kg of ABW/day (Grade GPP, Consensus). ASPEN Acute phase: 0.8–1.2 g/kg IBW/day (Recommendation). BMI 30–39.9 kg/m2: ≥2.0 g/kg IBW/day (Expert consensus). | At least 130 g/day Glucose oxidation rate: <5 mg/kg/min (Grade GPP, Strong consensus). | Dose: <1.5 g/kg of ABW/day (Grade 0, Strong consensus). Type of mixed oil in Respiratory Distress Syndrome, Acute Lung Injury, and Sepsis: LCT + MCT and omega-3 (Grade 0, Strong consensus). | [15,18,19,20] |
| Clinical Condition | Fiber | Micronutrients | Therapeutic Diet | References |
|---|---|---|---|---|
| Obesity | 25–35 g/day Older patients: 30 g/day (Grade 0, Strong consensus). | DRIs. Vitamin D: 4000–5000 IU/day (100–125 mg/day) should be administered for 2 months in patients with recurrent deficiency (Grade B, Strong consensus). | Isocaloric in acute care (Grade B, Strong consensus). Hypocaloric diets could improve metabolic outcomes in patients with severe insulin resistance, and in rehabilitation units for obesity (Grade 0, Strong consensus). Behavioral lifestyle changes in relation to the type of obesity phenotype (Grade B, Strong consensus). Cardioprotective pattern controlled in CHO: Mediterranean, DASH, vegetarian or vegan style diet (Grade A, Strong consensus). Cardioprotective pattern features: (1) Decrease salt intake (<6 g/day). In arterial hypertension or acute decompensated heart Failure at least 2.8 g (Grade B, Strong consensus). (2) Eat two portions of oily fisheach week. (3) Choose whole grains instead of refined grain. (4) Eat vegetables every day at least 300 g, fruit and berries at least 200 g. (5) Eat nuts and legumes 3 times per week. (6) Consume less red and processed meat, refined CHO, and sugar-sweetened beverages. (7) Replace saturated fats with unsaturated fats (Grade GPP, Strong consensus). T2D–Quantify CHO (Grade A, Strong consensus). Low-carbohydrate enteral formulas in patients with severe insulin resistance (Grade GPP, No Consensus). EN in MASLD: standard formulas, soy-free, with branched-chain amino acids (Recommendation, no consensus). PN in MASLD: omega-3, long-chain triglycerides with minimal soy content (Grade 0, Strong consensus). | [12,13,16,18,50] |
| T2D | 20–40 g/day (Grade 0, Strong consensus). | DRIs. Vitamin C. 200–500 mg/day in patients with chronic oxidative stress (T2D, smoking, heart failure, alcoholism, COPD, and dialysis) or malabsorption (Grade GPP, Strong Consensus). Chromium: 200 –250 µg/day for 2 weeks for patients with PN who are suspected to be deficient due to insulin resistance (Grade 0, Strong consensus). | [12,16,18,28,50] | |
| Cardiovascular disease: SAH, AMI, and Stroke | DRIs. Sodium: 1–3 g/day; <200 mg/day (in uncontrolled SAH or renal failure) Potassium: 2000–3700 mg/day. Calcium: 800–1500 mg/day. Magnesium: 240–1000 mg/day Vitamin C: 200–500 mg/day in patients with chronic oxidative stress (diabetes mellitus, smoking, heart failure, alcoholism, severe COPD, and chronic dialysis or malabsorption (Grade GPP, Strong consensus). | [12,16,18,50,56] | ||
| MASLD | 25–35 g/day (Grade 0, Strong consensus). | DRIs. Sodium: 2 g/day (in ascites, edema) Calcium: 800–1200 mg/day. B1: 100 mg/day D3: At least 3000 IU/day. Vitamin E: 800 IU/day Choline: 400–550 mg/day (Grade 0, Strong consensus). | [12,18,50,55,57] | |
| Obesity Post-bariatric Surgery | First 3 months of Post: Low fiber diet (Grade GPP, Strong consensus). | B12: 350–1000 μg/day. Iron: 45–60 mg/day Folic acid: 400–1000 μg/day (Grade A, Strong consensus). D3: 3000–6000 IU/day (Grade B, Strong consensus). Calcium: 1200–1500 mg/day. B1: ≥12 mg/day. Zinc: 8–22 mg/day (Grade 0, Strong consensus). Cooper: 1–2 mg/day. Vitamin A: 5000 UI/day. Vitamin E: 15 mg/day. Vitamin K: 90–120 μg/day (Grade GPP, Strong consensus). | Interventions should first include dietary change (Grade B, Strong consensus) First 2 days: Clear liquids Days 10 to 14: Full liquids After 14 days: Mechanically and chemically soft After 3 months: Hypocaloric diet (Grade GPP, Strong consensus) | [12,14,16,49,50] |
| Obesity in ICU | Acute phase: Do not use fiber (Grade 0, Strong consensus). Recovery phase: 10–20 g/day (Grade GPP, Consensus). | DRIs in EN or PN. B1: 100–300 mg/day IV from admission for 3–4 days (Grade B, Consensus). Vitamin C: 2–3 g/day IV repletion dose, during the acute phase of inflammation (Grade B, Consensus). Measure 25(OH) D in all patients considered at risk (Grade GPP, Strong consensus). Chromium: Insulin resistant patients, 3–20 μg/hour IV for 10 h and up to 4 days, may be required (Grade 0, Strong consensus). | EN or PN Provide less than 70% of requirements during the first week of ICU stay (Grade B, strong consensus). | [18,19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Carranza, T.; León-Téllez Girón, A.; Mimiaga-Hernádez, C.; Aguilar-Vargas, A. Nutritional Care Process in Hospitalized Patients with Obesity-Related Multimorbidity. Obesities 2025, 5, 39. https://doi.org/10.3390/obesities5020039
Rivera-Carranza T, León-Téllez Girón A, Mimiaga-Hernádez C, Aguilar-Vargas A. Nutritional Care Process in Hospitalized Patients with Obesity-Related Multimorbidity. Obesities. 2025; 5(2):39. https://doi.org/10.3390/obesities5020039
Chicago/Turabian StyleRivera-Carranza, Tania, Angélica León-Téllez Girón, Claudia Mimiaga-Hernádez, and Adriana Aguilar-Vargas. 2025. "Nutritional Care Process in Hospitalized Patients with Obesity-Related Multimorbidity" Obesities 5, no. 2: 39. https://doi.org/10.3390/obesities5020039
APA StyleRivera-Carranza, T., León-Téllez Girón, A., Mimiaga-Hernádez, C., & Aguilar-Vargas, A. (2025). Nutritional Care Process in Hospitalized Patients with Obesity-Related Multimorbidity. Obesities, 5(2), 39. https://doi.org/10.3390/obesities5020039





