The Effects of High-Intensity Interval Training (HIIT) on Sleep Quality in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria and Study Selection
- Population/participants: Obese patients aged 18–75 years with a body mass index (BMI) ≥ 30 kg/m2, without restrictions on comorbidities.
- Intervention: HIIT with no restrictions on type, frequency, intensity, or duration of training.
- Comparison/control group: Participants who did not engage in high-intensity interval training. They may have performed stretching exercises or received general recommendations.
- Outcomes: Sleep quality was assessed through both subjective and objective methods. Subjective assessments included tools such as the Pittsburgh Sleep Quality Index (PSQI) [25] and Epworth Sleepiness Scale (ESS) [26]. Objective assessments involved the use of sleep monitoring devices, such as polysomnography (PSG) [27].
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
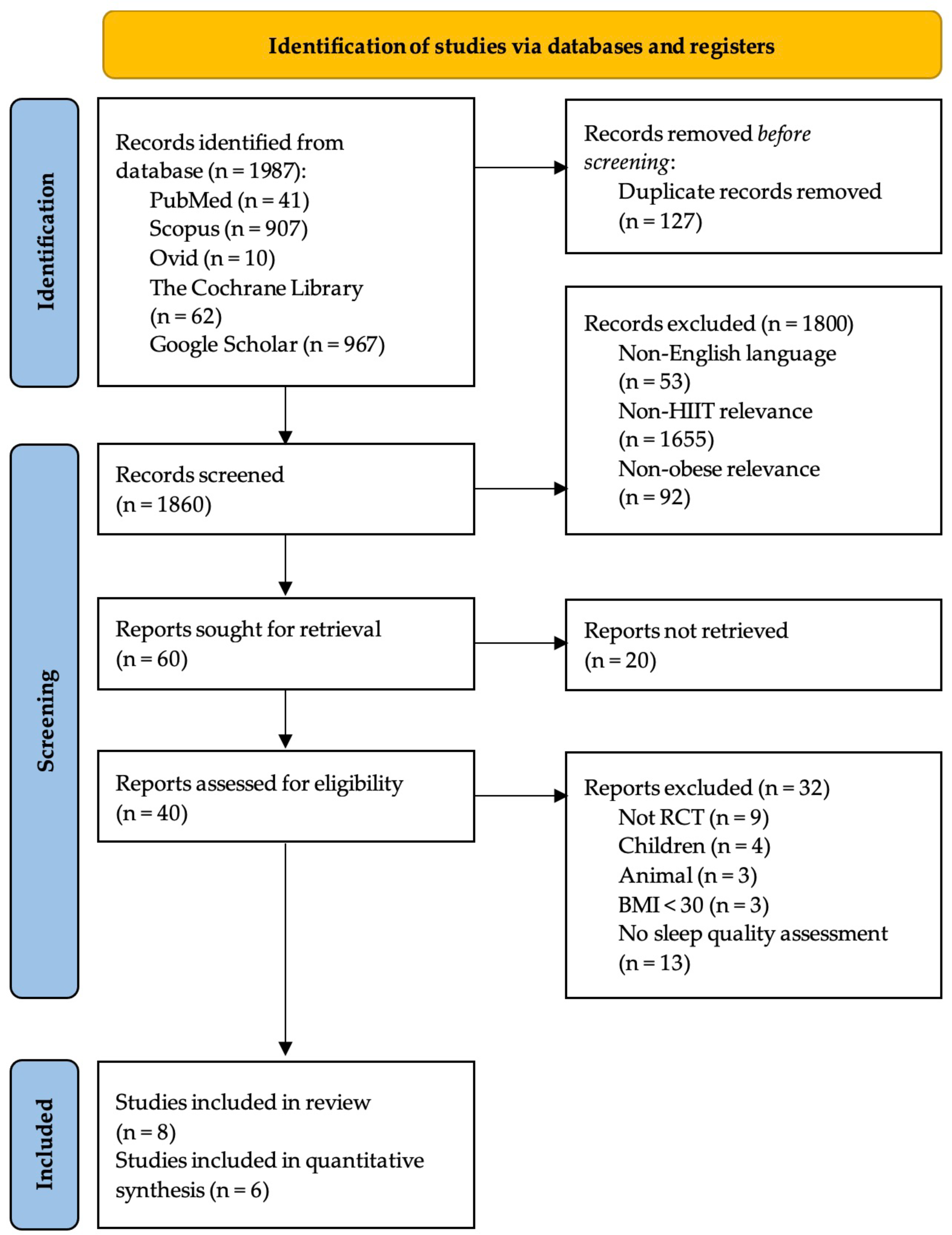
3.1. Search Results
3.2. Study Characteristics
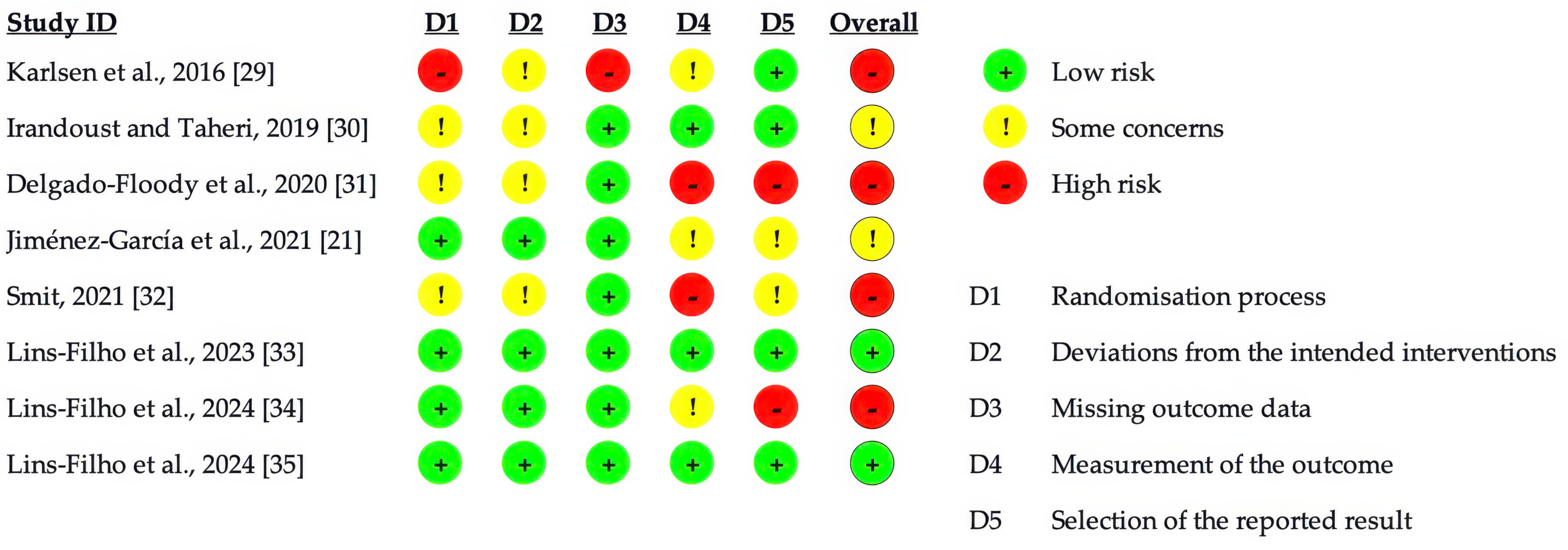
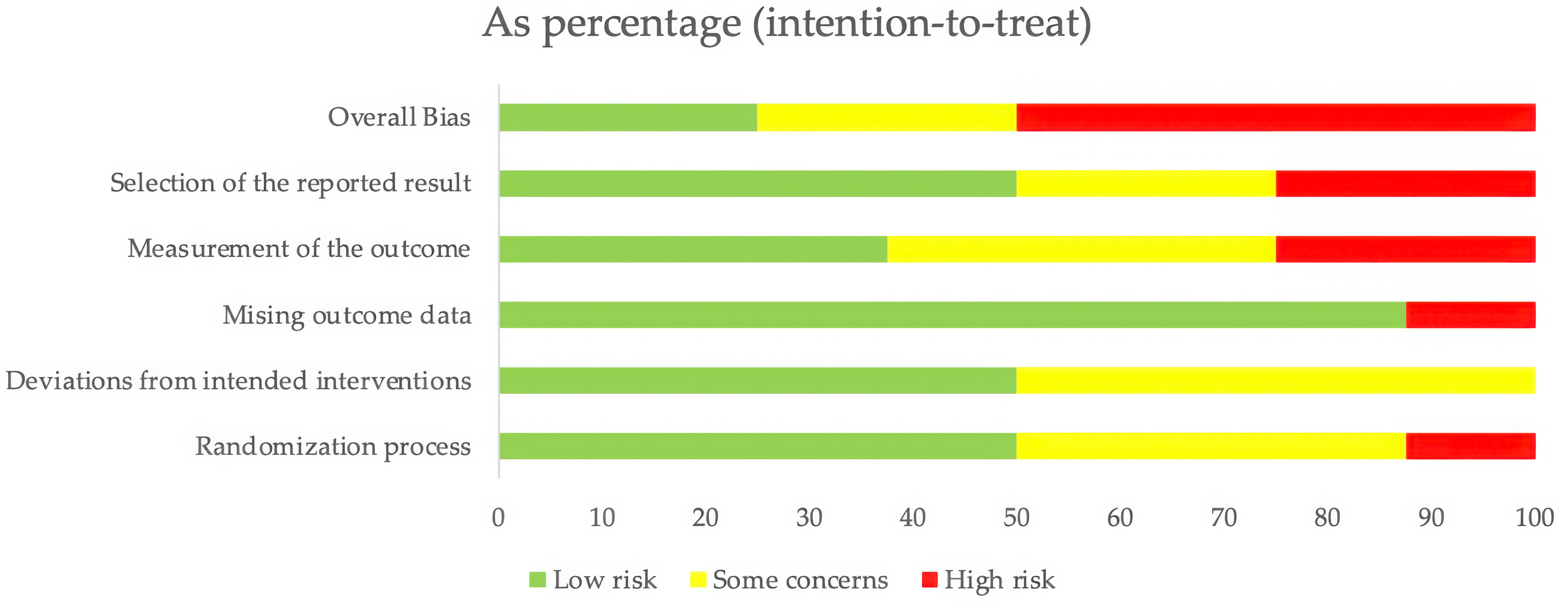
3.3. Quality Assessment of Included Studies
3.4. Meta-Analysis
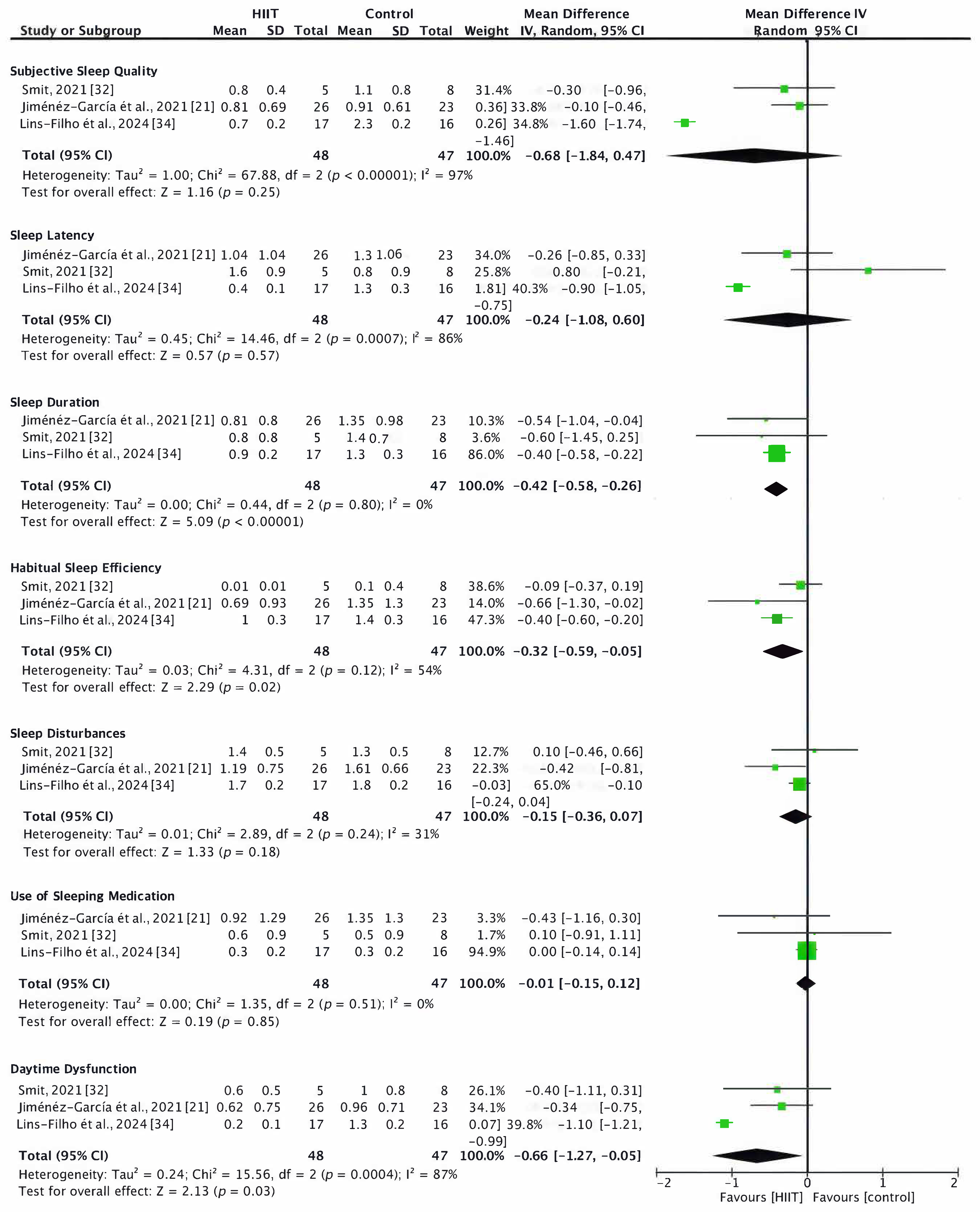
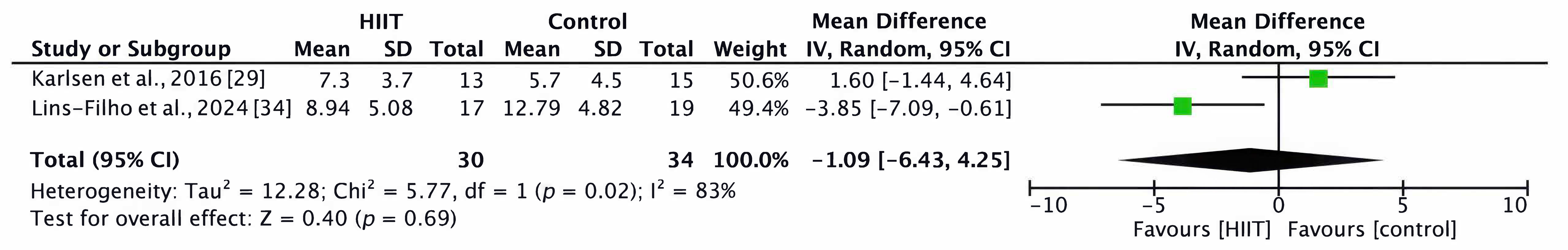
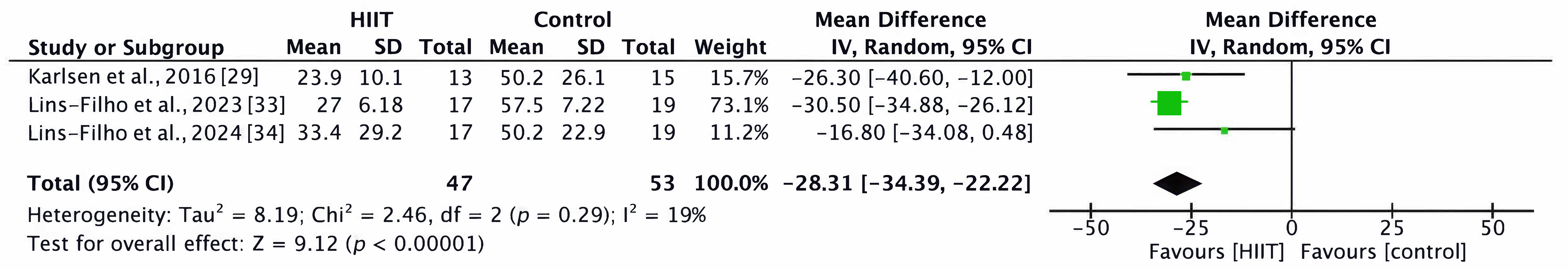
3.4.1. Sleep Quality
- Total PSQI Score
- PSQI Subscales
- Subgroup Analysis
3.4.2. Daytime Sleepiness
3.4.3. Sleep Apnea and Hypopnea Episodes
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | one-repetition maximum |
| AHI | Apnea–Hypopnea Index |
| BMI | body mass index |
| CI | confidence interval |
| ESS | Epworth Sleepiness Scale |
| GSQ | good sleep quality |
| H-HIIT | healthy high-intensity interval training |
| H-nonHIIT | healthy non-high-intensity interval training |
| HIIT | high-intensity interval training |
| HRmax | maximum heart rate |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| MICT | moderate-intensity continuous training |
| MIIT | moderate-intensity interval training |
| MD | mean difference |
| NCDs | noncommunicable diseases |
| NS | not significant |
| O-HIIT | overweight high-intensity interval training |
| O-nonHIIT | overweight non-high-intensity interval training |
| ODI | Oxygen Desaturation Index |
| OSA | obstructive sleep apnea |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSG | polysomnography |
| PSQ | poor sleep quality |
| PSQI | Pittsburgh Sleep Quality Index |
| PTSD | post-traumatic stress disorder |
| RAST | Running-Based Anaerobic Sprint Test |
| REM | rapid eye movement |
| REVMAN | Review Manager |
| RT | resistance training |
| RoB 2 | Risk of Bias 2 Tool |
| TNF-α | tumor necrosis factor alpha |
| TRX | total resistance exercises |
References
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Roblin, L. Childhood obesity: Food, nutrient, and eating-habit trends and influences. Appl. Physiol. Nutr. Metab. 2007, 32, 635–645. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Léger, D.; Bayon, V. Societal costs of insomnia. Sleep Med. Rev. 2010, 14, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef]
- Potter, G.D.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Obesity and psychiatric disorders: Commonalities in dysregulated biological pathways and their implications for treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A.; et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase-response curves for exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef]
- Wolff, C.A.; Esser, K.A. Exercise Timing and Circadian Rhythms. Curr. Opin. Physiol. 2019, 10, 64–69. [Google Scholar] [CrossRef]
- Kessler, H.S.; Sisson, S.B.; Short, K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef]
- Jiménez-García, J.D.; Hita-Contreras, F.; de la Torre-Cruz, M.J.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Fábrega-Cuadros, R.; Martínez-Amat, A. Effects of HIIT and MIIT Suspension Training Programs on Sleep Quality and Fatigue in Older Adults: Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 1211. [Google Scholar] [CrossRef]
- Min, L.; Wang, D.; You, Y.; Fu, Y.; Ma, X. Effects of High-Intensity Interval Training on Sleep: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10973. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Jafari, B.; Mohsenin, V. Polysomnography. Clin. Chest Med. 2010, 31, 287–297. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5, Updated August 2024 ed.; Cochrane: London, UK, 2024. [Google Scholar]
- Karlsen, T.; Nes, B.M.; Tjønna, A.E.; Engstrøm, M.; Støylen, A.; Steinshamn, S. High-intensity interval training improves obstructive sleep apnoea. BMJ Open Sport Exerc. Med. 2016, 2, e000155. [Google Scholar] [CrossRef]
- Irandoust, K.; Taheri, M. Effect of a high intensity interval training (HIIT) on serotonin and cortisol levels in obese women with sleep disorders. Women’s Health Bull. 2019, 6, e83303. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Latorre-Román, P.; Jerez-Mayorga, D.; Caamaño-Navarrete, F.; Cano-Montoya, J.; Laredo-Aguilera, J.A.; Carmona-Torres, J.M.; Cobo-Cuenca, A.I.; Pozuelo-Carrascosa, D.P.; Álvarez, C. Poor Sleep Quality Decreases Concurrent Training Benefits in Markers of Metabolic Syndrome and Quality of Life of Morbidly Obese Patients. Int. J. Environ. Res. Public Health 2020, 17, 6804. [Google Scholar] [CrossRef]
- Smit, Z. The Effects of High-Intensity Interval Training on Pain Parameters and Sleep Quality in Overweight and Healthy-Weight Women. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2021. [Google Scholar]
- Lins-Filho, O.; Germano-Soares, A.H.; Aguiar, J.L.P.; de Almedia, J.R.V.; Felinto, E.C.; Lyra, M.J.; Leite, D.B.; Moura, M.A.S.; Kline, C.E.; Pedrosa, R.P. Effect of high-intensity interval training on obstructive sleep apnea severity: A randomized controlled trial. Sleep Med. 2023, 112, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lins-Filho, O.; Aguiar, J.L.P.; Soares Germano, A.H.; Vieira de Almeida, J.R.; Felinto Dos Santos, E.C.; Lyra, M.J.; Farah, B.Q.; Pedrosa, R.P. Effects of high-intensity interval training on subjective sleep quality and daytime sleepiness in patients with obstructive sleep apnea: A secondary analysis from a randomized controlled trial. Sleep Med. 2024, 121, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Lins-Filho, O.; Germano-Soares, A.H.; Aguiar, J.L.P.; de Almedia, J.R.V.; Felinto, E.C.; Lyra, M.J.; Leite, D.B.; Drager, L.F.; Farah, B.Q.; Pedrosa, R.P. Effect of 12-week high-intensity interval training on hemodynamic variables at rest and during exercise in patients with obstructive sleep apnoea. J. Hypertens. 2024, 42, 742–745. [Google Scholar] [CrossRef]
- McGranahan, M.J.; O’Connor, P.J. Effect of high-intensity interval training exercise on sleep quality in women with probable post-traumatic stress disorder: A pilot randomized controlled trial. Sleep Med. 2025, 129, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- de Freitas, F.; Antônio, M.; Zago, M.R.; Zambon, M.; Videira-Silva, A. Utility of a high-intensity interval training app as a remote exercise support strategy in children with obesity: An exploratory study of adherence, effect, and perceptions of its use. Digit. Health 2024, 10, 20552076241291386. [Google Scholar] [CrossRef]


| Author, Year | Main Characteristic of Participants | Sample Size | Sleep Outcomes Measurement | Outcomes | |
|---|---|---|---|---|---|
| Subjective | Objective | ||||
| Karlsen et al., 2016 [29] | Obese patients with moderate-to-severe OSA
| HIIT = 13 Control = 15 Total = 28 | ESS | AHI ODI Oxygen saturation Self-reported sleep patterns | HIIT vs. control (post-intervention comparison)
|
| Irandoust and Taheri, 2019 [30] | Obese middle-aged women with sleep disorders
| HIIT = 15 Control = 14 Total = 29 | PSQI | HIIT vs. Control (post-intervention comparison)
| |
| Delgado-Floody et al., 2020 [31] | Morbidly obese patients
| GSQ = 15 PSQ = 14 Total = 29 | PSQI | Pre-post comparisons within-group
| |
| Jiménez-García et al., 2021 [21] | Older adults with sleep disturbances and fatigue
| HIIT = 26 MIIT = 24 Control = 23 Total = 73 | PSQI | HIIT vs. Control (post-intervention comparison)
| |
| Smit, 2021 [32] | Overweight and healthy-weight women
| O-HIIT = 5 O-nonHIIT = 8 H-HIIT = 8 H-nonHIIT = 6 Total = 27 | PSQI | O-HIIT vs. O-nonHIIT (post-intervention comparison)
| |
| Lins-Filho et al., 2023 [33] | Adults with moderate-to-severe OSA
| HIIT = 17 Control = 19 Total = 36 | PSQI | PSG Pulse oximetry AHI | HIIT vs. control (post-intervention comparison)
|
| Lins-Filho et al., 2024 [34] | Adults with moderate-to-severe OSA
| HIIT = 17 Control = 19 Total = 36 | PSQI ESS | AHI | HIIT vs. control (post-intervention comparison)
|
| Lins-Filho et al., 2024 [35] | Adults with moderate-to-severe OSA
| HIIT = 13 Control = 13 Total = 26 | AHI | HIIT vs. control (post-intervention comparison)
| |
| Author (Year) | HIIT | ||||
|---|---|---|---|---|---|
| Type | Intensity | Duration | Frequency (Sessions per Week) | Length (Weeks) | |
| Karlsen et al., 2016 [29] | Treadmill walking/running | 90–95% HRmax (exercise), 70% HRmax (rest) | ~45 min (10 min warm-up at ~70% HRmax, 4 intervals × 4 min at 90–95% HRmax, separated by 3 min at ~70% HRmax) | 2 | 12 |
| Irandoust and Taheri, 2019 [30] | 35 m sprints (RAST protocol) | High intensity (near maximum effort) | ~30 min (RAST protocol: 3 sets × 6 sprints of 35 m with 10 s of rest between sprints, and 4 min rest between sets) | 3 | 1 |
| Delgado-Floody et al., 2020 [31] | Concurrent training (HIIT + RT) | HIIT: 6–9 Borg Scale (perceived exertion) RT: 40–60% 1RM (progressive load) | 45 min (HIIT: 60 s cycling at max effort + 60–120 s rest) (RT: 3 sets, 60 s per exercise with 60–120 s rest) | 2 | 20 |
| Jiménez-García et al., 2021 [21] | Suspension training (TRX) focusing on squat exercises | 90–95% HRmax (exercise), 50–70% HRmax (rest) | 45 min (TRX suspension training: 4 intervals × 4 min at 90–95% HRmax for HIIT group, or 70% HRmax for MIIT group, with recovery in between intervals) | 2 (Tuesdays and Thursdays) | 12 |
| Smit, 2021 [32] | Home-based, dynamic resistance exercises | Perceived exertion level at ~16–18 (Borg scale) | ~20–30 min (home-based dynamic resistance exercises: combinations of 4 exercises, each performed for 30 s, repeated multiple times for ~20–30 min) | 6 (Participants allowed flexibility in scheduling sessions, up to twice a day) | 14 |
| Lins-Filho et al., 2023 [33] | Treadmill walking/running | 90–95% HRmax (exercise), 50–55% HRmax (rest) | ~45 min (5 intervals × 4 min of walking/running at 90–95% HRmax, interspersed with 3 min of walking at 50–55% HRmax, plus warm-up and cool-down periods) | 3 | 12 |
| Lins-Filho et al., 2024 [34] | Treadmill walking/running | 90–95% HRmax (exercise), 50–55% HRmax (rest) | ~45 min (5 intervals × 4 min of walking/running at 90–95% HRmax, interspersed with 3 min of recovery walking at 50–55% HRmax) | 3 | 12 |
| Lins-Filho et al., 2024 [35] | Treadmill walking/running | 90–95% HRmax (exercise), 50–55% HRmax (rest) | ~45 min (5 intervals × 4 min of walking/running at 90–95% HRmax, interspersed with 3 min of recovery walking at 50–55% HRmax) | 3 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawinchotpaisan, K.; Segsarnviriya, C.; Norchai, P. The Effects of High-Intensity Interval Training (HIIT) on Sleep Quality in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obesities 2025, 5, 32. https://doi.org/10.3390/obesities5020032
Kawinchotpaisan K, Segsarnviriya C, Norchai P. The Effects of High-Intensity Interval Training (HIIT) on Sleep Quality in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obesities. 2025; 5(2):32. https://doi.org/10.3390/obesities5020032
Chicago/Turabian StyleKawinchotpaisan, Kittibhum, Charnsiri Segsarnviriya, and Phawit Norchai. 2025. "The Effects of High-Intensity Interval Training (HIIT) on Sleep Quality in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Obesities 5, no. 2: 32. https://doi.org/10.3390/obesities5020032
APA StyleKawinchotpaisan, K., Segsarnviriya, C., & Norchai, P. (2025). The Effects of High-Intensity Interval Training (HIIT) on Sleep Quality in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obesities, 5(2), 32. https://doi.org/10.3390/obesities5020032





