Lycium barbarum for Health and Longevity: A Review of Its Biological Significance
Abstract
1. Introduction
2. Methodology
3. Phytochemistry
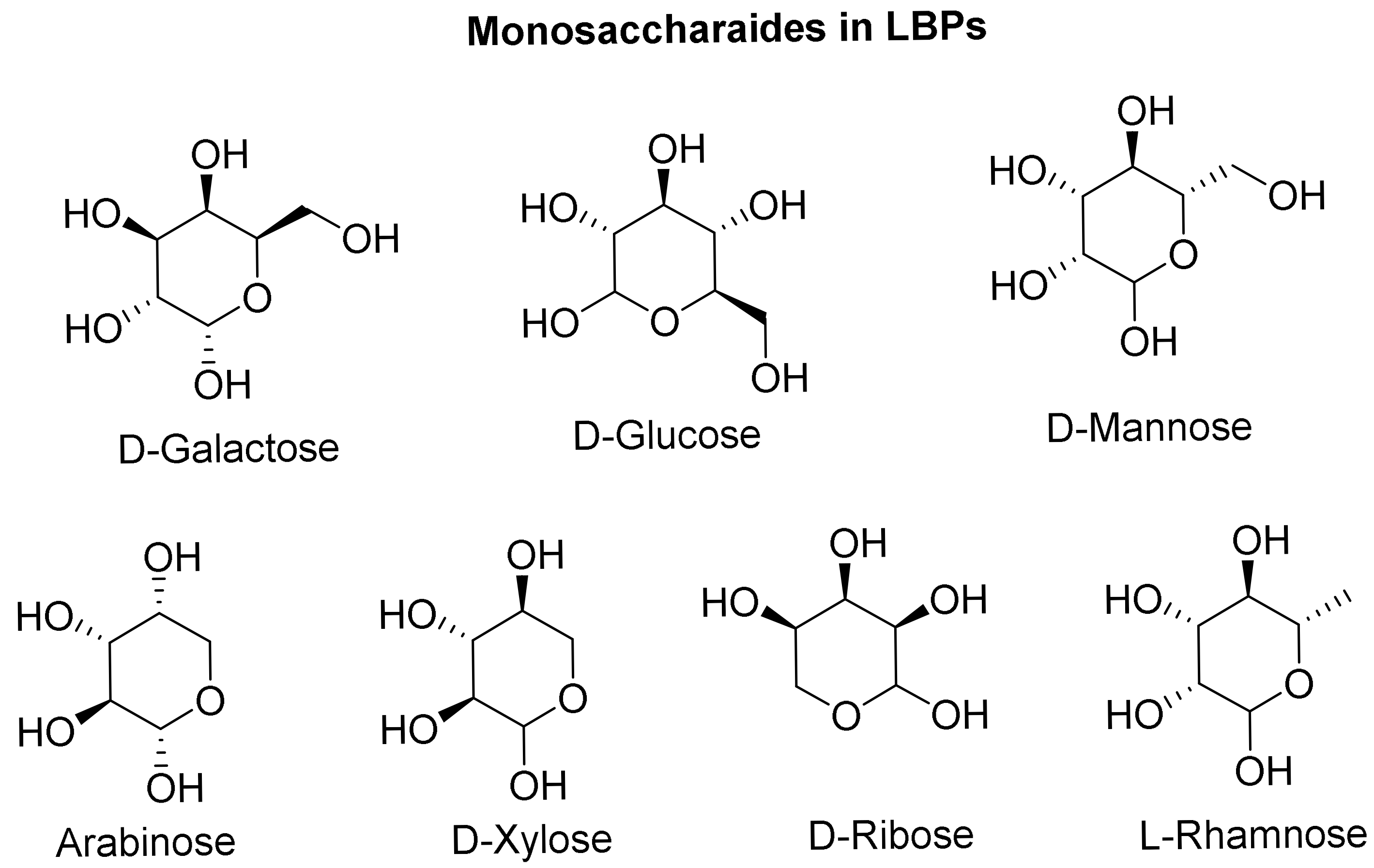
3.1. Polysaccharides
3.2. Polyphenols
3.3. Alkaloids
3.4. Carotenoids
4. Biological Properties
4.1. Cardiovascular Benefits
4.2. Glycaemic Control and Anti-Diabetic Activity
4.2.1. Obesity and Insulin Sensitivity
4.2.2. Glycaemic Control and Diabetes Management
4.2.3. Effects on Lipid Metabolism
4.2.4. Obesity-Related Bone Health
4.3. Antioxidant Activity
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vitro | Flavonoids, Zeaxanthin, LBP | Effective free radical scavenging | H-donation, metal chelation | [103] |
| In vitro | Phenolics, Flavonoids, Anthocyanins | Strong correlation with antioxidant capacity | Assay-based correlation | [104] |
| In vitro | Polyphenols, Organic acids | High TPC, FRAP, TBCC levels | Direct ROS scavenging | [51] |
| In vitro | Zeaxanthin, Carotenoids | ABTS/FRAP correlations | Antioxidant capacity | [74] |
| In vitro | Aqueous extract | ↑ GSH, ↓ lipid peroxidation and protein carbonyls | Antioxidant enzyme activation | [105] |
| In vitro | LBP | ↓ Apoptosis, ↑ cell protection against H2O2 | Nrf2 pathway | [106] |
| In vivo | LBP | ↑ SOD, ↑ CAT, ↑ GPx, ↓ MDA | Enzyme-mediated ROS defence | [107] |
| In vivo | LBP | Protected liver/kidney from ROS damage | Antioxidant defence | [108] |
4.4. Anti-Inflammatory Activity
4.5. Immunomodulatory Effects
4.6. Anticancer Activity
4.6.1. Inhibition of Cancer Cell Proliferation
4.6.2. Anti-Metastatic Properties
4.6.3. Synergistic Effects with Chemotherapy
4.6.4. Mitigation of Chemotherapy-Induced Toxicity
4.7. Hepatoprotective Activity
4.8. Antimicrobial Effect
4.9. Prebiotic Activity
4.10. Neuroprotective Effects
4.11. Anti-Aging Activity
4.11.1. Glycation and Oxidative Stress Reduction
4.11.2. Stem Cell Promotion and Tissue Regeneration
4.11.3. Anti-Apoptotic Effects
4.12. Ocular Health
4.12.1. Glaucoma
4.12.2. Retinitis Pigmentosa
4.12.3. Age-Related Macular Degeneration
4.12.4. Ocular Hypertension
4.12.5. Transient Retinal Ischaemia
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | Achyranthes bidentata polysaccharide |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ALT | Alanine aminotransferase |
| AMD | Age-related macular degeneration |
| AOH | Acute ocular hypertension |
| ARE | Antioxidant response element |
| ARPE | Arising retinal pigment epithelia |
| AST | Aspartate aminotransferase |
| ATF6 | Activating transcription factor 6 |
| ATGL | Adipose triglyceride lipase |
| ATK | Protein kinase B (also known as Akt) |
| B-ALP | Bone-alkaline phosphatase |
| Bax | Bcl-2-associated X protein |
| BclxL | B-cell lymphoma-extra large |
| CAT | Catalase |
| CDK | Cyclin-dependent kinase |
| Chrdl1 | Chordin-like 1 |
| CIH | Chronic intermittent hypoxia |
| CONT | Complete optic nerve transection |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CVD | Cardiovascular disease |
| CypD | Cyclophilin D |
| CRP | C-reactive protein |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| eNOS | Endothelial nitric oxide synthase |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| FAS | Fatty acid synthase |
| FC | Folin–Ciocalteu |
| FGFR1 | Fibroblast growth factor receptor 1 |
| Fsp27 | Fat-specific protein 27 |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalents |
| GC | Gas chromatography |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| GRK2 | G protein-coupled receptor kinase 2 |
| HO-1 | Haem oxygenase 1 |
| hPDLSCs | Human periodontal ligament stem cells |
| HPLC | High-performance liquid chromatography |
| IFN | Interferon |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| I/R | Ischaemia/reperfusion |
| JNK | c-Jun N-terminal kinase |
| L. Barbarum | Lycium Barbarum |
| LBLF | L. Barbarum leaf flavonoids |
| LBP | L. Barbarum polysaccharide |
| LC | Liquid chromatography |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MDSCs | Myeloid-derived suppressor cells |
| mfERG | Multifocal electroretinography |
| MIC | Minimum inhibitory concentration |
| MMP-9 | Matrix metalloproteinase 9 |
| MS/MS | Tandem mass spectrometry |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor protein 3 |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PI3K | Phosphoinositide 3-kinase |
| PONT | Partial optic nerve transection |
| PPAR-α | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RCC | Renal cell carcinoma |
| RGC | Retinal ganglion cell |
| ROS | Reactive oxygen species |
| RSM | Response surface methodology |
| RP | Retinitis pigmentosa |
| SCFAs | Short-chain fatty acids |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| sONE | An antisense mRNA |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TBCC | Total bioactive compound content |
| TFC | Total flavonoid content |
| TLR | Toll-like receptor |
| TNF | Tumour necrosis factor |
| TOR | Target of rapamycin |
| TPC | Total phenolic content |
| TQ | Triple quadrupole |
| TXNIP | Thioredoxin-interacting protein |
| UPLC | Ultra performance liquid chromatography |
| UVB | Ultraviolet B radiation |
References
- Matos, L.C.; Machado, J.P.; Monteiro, F.J.; Greten, H.J. Understanding traditional chinese medicine therapeutics: An overview of the basics and clinical applications. Healthcare 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.F.; Laitman, J.T. The yin, yang, and anatomy of traditional Chinese medicine in The Anatomical Record. Anat. Rec. 2023, 306, 2915–2919. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fang, M.; Hu, Y.; Wang, X. Four types of traditional Chinese medicine inducing epileptic seizures. Seizure 2012, 21, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- German Federal Institute for Risk Assessment. Superfoods—Super Good? German Federal Institute for Risk Assessment: Berlin, Germany, 2020. [Google Scholar]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality variation of Goji (fruits of Lycium spp.) in China: A comparative morphological and metabolomic analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.-A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Rubio, R.M.; García, M.R.; Barroso, N.N.; Iñiguez, F.M.S.; Gómez, M.J.R.; Magro, P.C. Classification of goji berry (Lycium barbarum L.) varieties according to physicochemical and bioactive signature. Eur. Food Res. Technol. 2024, 251, 355–365. [Google Scholar] [CrossRef]
- Ju, Y.; Liu, H.; Niu, S.; Kang, L.; Ma, L.; Li, A.; Zhao, Y.; Yuan, Y.; Zhao, D. Optimizing geographical traceability models of Chinese Lycium barbarum: Investigating effects of region, cultivar, and harvest year on nutrients, bioactives, elements and stable isotope composition. Food Chem. 2025, 467, 142286. [Google Scholar] [CrossRef]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, Y.; Ma, L.; Kang, L.; Liu, H.; Ma, X.; Zhao, D. Comparative analysis of polyphenols in Lycium barbarum fruits using UPLC-IM-QTOF-MS. Molecules 2023, 28, 4930. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical characterization, antioxidant and antimicrobial properties of goji berries cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.-W.; Wang, H.-Q.; Jin, L.; Duan, J.-A. Comparison of functional components and antioxidant activity of Lycium barbarum L. fruits from different regions in China. Molecules 2019, 24, 2228. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Lin, H.; He, C.; Li, Q.; Shen, G.; Feng, J. Geographical origin identification and quality comparison of Ningxia goji berries (Lycium barbarum L.) by NMR-based techniques. J. Food Compos. Anal. 2023, 119, 105258. [Google Scholar] [CrossRef]
- Berisha, A.; Alexa, E.-A.; Kelleher, R.; Zhang, T. From botany to bedside: A review of the health benefits of Lycium barbarum as a functional food. Explor. Foods Foodomics 2025, 3, 101070. [Google Scholar] [CrossRef]
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Sicari, V.; Romeo, R.; Mincione, A.; Santacaterina, S.; Tundis, R.; Loizzo, M.R. Ciabatta bread incorporating goji (Lycium barbarum L.): A new potential functional product with impact on human health. Foods 2023, 12, 566. [Google Scholar] [CrossRef]
- Blasi, F.; Rocchetti, G.; Montesano, D.; Lucini, L.; Chiodelli, G.; Ghisoni, S.; Baccolo, G.; Simonetti, M.S.; Cossignani, L. Changes in extra-virgin olive oil added with Lycium barbarum L. carotenoids during frying: Chemical analyses and metabolomic approach. Food Res. Int. 2018, 105, 507–516. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum berries (Solanaceae) as source of bioactive compounds for healthy purposes: A review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef] [PubMed]
- Rjeibi, I.; Feriani, A.; Ben Saad, A.; Ncib, S.; Sdayria, J.; Saidi, I.; Souid, S.; Hfaiedh, N.; Allagui, M.S. Phytochemical characterization and bioactivity of Lycium europaeum: A focus on antioxidant, antinociceptive, hepatoprotective and nephroprotective effects. Biomed. Pharmacother. 2017, 95, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Long, L.N.; Kang, B.J.; Jiang, Q.; Chen, J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, G.; Sun, X.; He, S.; Dou, G. Distinct role of Lycium barbarum L. polysaccharides in oxidative stress-related ocular diseases. Pharmaceuticals 2023, 16, 215. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Lopatriello, A.; Previtera, R.; Pace, S.; Werner, M.; Rubino, L.; Werz, O.; Taglialatela-Scafati, O.; Forino, M. NMR-based identification of the major bioactive molecules from an Italian cultivar of Lycium barbarum. Phytochemistry 2017, 144, 52–57. [Google Scholar] [CrossRef]
- Chao, S.; Schreuder, M.; Young, G.; Nakaoka, K.; Moyes, L.; Oberg, C. Preclinical study: Antioxidant levels and immunomodulatory effects of wolfberry juice and other juice mixtures in mice. Jana 2004, 7, 2–8. [Google Scholar]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Yu, M.-S.; Lai, C.S.-W.; So, K.-F.; Yuen, W.-H.; Chang, R.C.-C. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on β-amyloid peptide neurotoxicity. Brain Res. 2007, 1158, 123–134. [Google Scholar] [CrossRef]
- Wawruszak, A.; Czerwonka, A.; Okła, K.; Rzeski, W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extract on human breast cancer T47D cell line. Nat. Prod. Res. 2016, 30, 1993–1996. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, M.; Lan, J.; Yang, L.; Wang, Z.; Wang, R.; Tao, H.; Shi, Y. A comprehensive review on the ethnobotany, phytochemistry, pharmacology and quality control of the genus Lycium in China. Food Funct. 2023, 14, 2998–3025. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium barbarum (Goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Magiera, S.; Zaręba, M. Chromatographic Determination of Phenolic Acids and Flavonoids in Lycium barbarum L. and Evaluation of Antioxidant Activity. Food Anal. Methods 2015, 8, 2665–2674. [Google Scholar] [CrossRef]
- Qiang, X.; Xia, T.; Geng, B.; Zhao, M.; Li, X.; Zheng, Y.; Wang, M. Bioactive components of Lycium barbarum and deep-processing fermentation products. Molecules 2023, 28, 8044. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, H.; Xu, J.; Chang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Duan, C.; Huang, J.; Fu, Y. A sustainable and integrated natural surfactant mediated microwave-assisted extraction technique enhances the extraction of phytochemicals from plants. Ind. Crops Prod. 2022, 184, 115043. [Google Scholar] [CrossRef]
- Perera, C.O.; Alzahrani, M.A.J. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: A review. LWT 2021, 142, 111114. [Google Scholar] [CrossRef]
- Song, Y.-R.; Han, A.-R.; Park, S.-G.; Cho, C.-W.; Rhee, Y.-K.; Hong, H.-D. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Gao, S.; Yan, S.; Zhou, Y.; Feng, Y.; Xie, X.; Guo, W.; Shen, Q.; Chen, C. Optimisation of enzyme-assisted extraction of Erythronium sibiricum bulb polysaccharide and its effects on immunomodulation. Glycoconj. J. 2022, 39, 357–368. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zhou, S.; Sun, D. Surface optimization method to optimize the enzymatic extraction of Lycium barbarum polysaccharides. Chin. Wild Plant Resour. 2017, 36, 27–33. [Google Scholar]
- Kou, R.; Zuo, G.; Liu, J.; Di, D.; Guo, M. Structural properties and hypoglycaemic activity of polysaccharides extracted from the fruits of Lycium barbarum L. using various extraction media. Ind. Crops Prod. 2022, 188, 115725. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S. Effects of extraction methods on extraction ratio and antioxidant activity of polysaccharide from Lycium barbarum. Agricul. Sci. 2019, 47, 169–173. [Google Scholar]
- Quan, N.; Wang, Y.-D.; Li, G.-R.; Liu, Z.-Q.; Feng, J.; Qiao, C.-L.; Zhang, H.-F. Ultrasound–microwave combined extraction of novel polysaccharide fractions from Lycium barbarum leaves and their in vitro hypoglycemic and antioxidant activities. Molecules 2023, 28, 3880. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, Z.; Zhang, X.; Yang, J.; Lai, X.; Yang, G. Determination of polyphenols in Lycium barbarum leaves by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2017, 50, 761–776. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Ebeydulla, R.; Wang, X.; Zhao, L.; Xu, D.; Sun, H.; Ji, B.; Zhou, F. Nutritional and functional components of Chinese wolfberry dried by different drying methods. Food Sci. 2017, 38, 138–142. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Shi, Y.-P. Comprehensive analysis of phenolic compounds in four varieties of goji berries at different ripening stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Liu, G.-T.; Li, Y.-L.; Wang, J.; Dong, C.-Z.; Deng, M.; Tai, M.; Deng, L.; Che, B.; Lin, L.; Du, Z.-Y.; et al. Improvement of skin barrier dysfunction by phenolic-containing extracts of Lycium barbarum via Nrf2/HO-1 regulation. Photochem. Photobiol. 2022, 98, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Tartaglione, L.; Dell’Aversano, C.; Ciminiello, P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016, 194, 1254–1259. [Google Scholar] [CrossRef]
- Nardi, G.M.; Farias Januario, A.G.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.; Do Nascimento, S.R.; Gon, A.C.; Mariano, L.N.; Wagner, G.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacognosy. Res. 2016, 8, S42–S49. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, X.; Hu, Z.; Liu, J.; Ma, J.; Fan, Y.; Liu, D. iTRAQ-based proteomics identifies proteins associated with betaine accumulation in Lycium barbarum L. J. Proteom. 2024, 290, 105033. [Google Scholar] [CrossRef]
- Liu, W.; Xia, M.; Yang, L.; Wang, Z.; Wang, R.; Shi, Y. Development and optimization of a method for determining betaine and trigonelline in the fruits of Lycium species by using solid-phase extraction combined with high-performance liquid chromatography-diode array detector. J. Sep. Sci. 2020, 43, 2073–2078. [Google Scholar] [CrossRef]
- Yan, X.; Meng, X.; Pan, S.; Li, Z.; Song, Y. Determination of betaine in dried wolfberry by ion chromatography with pulsed amperometric detection. Food Sci. Technol. 2017, 42, 278–281. [Google Scholar]
- Chao, J.; Li, B.Y. Effects of Lycium barbarum polysaccharides extract and betaine on cell activation and liver fibrosis in rat hepatic stellate HSC-T6 cells. FASEB J. 2018, 31, 793–795. [Google Scholar] [CrossRef]
- Chen, D.; Guo, S.; Zhou, J.; Zhu, Y.; Zhang, F.; Zeng, F.; Duan, R.; Xu, M.; Duan, J.-A. Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 97, 104292. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J.-B.; Zhang, L.; Wang, H.-H.; Cao, Y.-G.; Zeng, M.-N.; Li, M.; Sun, Y.-J.; Du, K.; Xue, G.-M.; et al. Lycibarbarines A–C, three tetrahydroquinoline alkaloids possessing a spiro-heterocycle moiety from the fruits of Lycium barbarum. Org. Lett. 2021, 23, 858–862. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Migas, P.; Stefanowicz, J.; Luczkiewicz, M.; Krauze-Baranowska, M. Densitometric TLC analysis for the control of tropane and steroidal alkaloids in Lycium barbarum. Food Chem. 2017, 221, 535–540. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Böhm, V. Carotenoids. Antioxidants 2019, 8, 516. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In vitro protective effects of Lycium barbarum berries cultivated in Umbria (Italy) on human hepatocellular carcinoma cells. Biomed. Res. Int. 2016, 2016, 7529521. [Google Scholar] [CrossRef]
- Long, J.T.; Fan, H.X.; Zhou, Z.Q.; Sun, W.Y.; Li, Q.W.; Wang, Y.; Ma, M.; Gao, H.; Zhi, H. The major zeaxanthin dipalmitate derivatives from wolfberry. J. Asian Nat. Prod. Res. 2020, 22, 746–753. [Google Scholar] [CrossRef]
- Ren, R.; Li, Y.; Chen, H.; Wang, Y.; Yang, L.; Su, C.; Zhao, X.; Chen, J.; Ma, X. Carotenoid contents of Lycium barbarum: A novel QAMS analysis, geographical origins discriminant evaluation, and storage stability assessment. Molecules 2021, 26, 5374. [Google Scholar] [CrossRef]
- Yu, J.; Yan, Y.; Zhang, L.; Mi, J.; Yu, L.; Zhang, F.; Lu, L.; Luo, Q.; Li, X.; Zhou, X.; et al. A comprehensive review of goji berry processing and utilization. Food Sci. Nutr. 2023, 11, 7445–7457. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; pp. 57–71. [Google Scholar]
- Wu, H.; Liu, Y.; Hao, Y.; Hou, D.; Yang, R. Lycium barbarum polysaccharide protects cardiomyocytes from hypoxia/reoxygenation injury via activation of SIRT3/CypD signaling. Ann. Transl. Med. 2023, 11, 72. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, X.; Shen, X.; Ma, Y.; Jing, L. Lycium barbarum polysaccharide antagonizes cardiomyocyte apoptosis by inhibiting the upregulation of GRK2 induced by I/R injury, and salvage mitochondrial fission/fusion imbalance and AKT/eNOS signaling. Cell. Signal. 2022, 92, 110252. [Google Scholar] [CrossRef]
- Xue, S.; Hu, X.; Zhu, L.; Nie, L.; Li, G. Protective functions of Lycium barbarum polysaccharides in H(2)O(2)-injured vascular endothelial cells through anti-oxidation and anti-apoptosis effects. Biomed. Rep. 2019, 11, 207–214. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Lin, Y.; Suo, M.; Gong, L.; Chen, J.; Hui, R. Anti-hypertensive effect of Lycium barbarum L. with down-regulated expression of renal endothelial lncRNA sONE in a rat model of salt-sensitive hypertension. Int. J. Clin. Exp. Pathol. 2015, 8, 6981–6987. [Google Scholar]
- Huang, R.; Wu, E.; Deng, X. Potential of Lycium barbarum polysaccharide for the control of glucose and lipid metabolism disorders: A review. Int. J. Food Prop. 2022, 25, 673–680. [Google Scholar] [CrossRef]
- Liu, H.; Cui, B.; Zhang, Z. Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Res. Int. 2022, 159, 111408. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Hu, W.; Yang, J.; Lin, B.; Zhang, Z.; Chen, M.; Yi, J.; Zhu, C. Lycium barbarum polysaccharide ameliorates the accumulation of lipid droplets in adipose tissue via an ATF6/SIRT1-dependent mechanism. Acta Biochim. Biophys. Sin. 2024, 56, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Dai, Y.; Ma, L.; Di, D.; Liu, J. Screening, structural characterization and anti-adipogenesis effect of a water-soluble polysaccharide from Lycium barbarum L. by an activity-oriented approach. Food Biosci. 2023, 53, 102502. [Google Scholar] [CrossRef]
- Yang, F.L.; Wei, Y.X.; Liao, B.Y.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, J.L. Effects of Lycium barbarum polysaccharide on endoplasmic reticulum stress and oxidative stress in obese mice. Front. Pharmacol. 2020, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, L.; Ma, J.; Liu, S.; Fu, J.; Fan, Y.; Liu, Y. Lycium barbarum leaf flavonoids ameliorate high fructose induced insulin resistance in mice by regulating blood glucose and gut microbiota composition. Food Biosci. 2024, 62, 105087. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, K.; Wu, Y.; Li, J.; Ma, J.; Wang, L.; Zhang, C.; Li, J.; Wei, Y.; Yang, Y. Lycium barbarum polysaccharide mitigates high-fat-diet-induced skeletal muscle atrophy by promoting AMPK/PINK1/Parkin-mediated mitophagy. Int. J. Biol. Macromol. 2025, 301, 140488. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The Hypoglycemic, Hypolipidemic, and Anti-Diabetic Nephritic Activities of Zeaxanthin in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Fan, Y.; Zhang, Y.; Tao, X.; Yang, J. Lycium barbarum polysaccharides improved glucose metabolism in prediabetic mice by regulating duodenal contraction. Nutrients 2023, 15, 4437. [Google Scholar] [CrossRef]
- Wu, H.; Guo, H.; Zhao, R. Effect of Lycium barbarum polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM rats. Yakugaku Zasshi 2006, 126, 365–371. [Google Scholar] [CrossRef]
- Zhao, X.-q.; Guo, S.; Lu, Y.-y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.-x.; Wang, H.-q.; Zhang, W.-h.; Duan, J.-a. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, B.; Sui, J.; Ma, W.; Wang, S.; Yang, L.; Sun, G. Lycium barbarum polysaccharide regulates lipid metabolism and alters gut microbiota in high-fat diet-induced obese mice. Int. J. Environ. Res. Public Health 2022, 19, 12093. [Google Scholar] [CrossRef]
- Huang, Z.; Ye, Y.; Long, Z.; Qin, H.; Liu, L.; Xu, A.; Li, Z. Lycium barbarum polysaccharides improve lipid metabolism disorders of spotted sea bass Lateolabrax maculatus induced by high lipid diet. Int. J. Biol. Macromol. 2023, 242, 125122. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, J.; Fan, Z.; Sun, Z.; Zheng, X.; Zhang, H.; Xu, H.; Wang, L. Dietary Lycium barbarum polysaccharide modulates growth performance, antioxidant capacity, and lipid metabolism in common carp (Cyprinus carpio) fed with high-fat diet. Antioxidants 2024, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.N.; Chi, X.; Yan, L.; Pu, Z.Y.; Yang, J.J.; Zhang, Y.N. Lycium barbarum polysaccharides regulate the gut microbiota to modulate metabolites in high-fat diet-induced obese rats. J. Asian Nat. Prod. Res. 2024, 26, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Hu, B.; Song, Q.H. Lycium barbarum polysaccharide (LBP) inhibits palmitic acid (PA)-induced MC3T3-E1 cell apoptosis by regulating miR-200b-3p/Chrdl1/PPARγ. Food Nutr. Res. 2020, 64, 10-29219. [Google Scholar] [CrossRef]
- Li, Z.-X.; Zhuo, J.-L.; Yang, N.; Gao, M.-B.; Qu, Z.-H.; Han, T. Effect of Lycium barbarum polysaccharide on osteoblast proliferation and differentiation in postmenopausal osteoporosis. Int. J. Mol. Sci. 2024, 271, 132415. [Google Scholar] [CrossRef]
- Piñar-Gutierrez, A.; García-Fontana, C.; García-Fontana, B.; Muñoz-Torres, M. Obesity and bone health: A complex relationship. Int. J. Mol. Sci. 2022, 23, 8303. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- de Nijs, M.; Crews, C.; Dorgelo, F.; MacDonald, S.; Mulder, P.P.J. Emerging issues on tropane alkaloid contamination of food in Europe. Toxins 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhao, Q.; Zhu, Q.; He, X.; Gao, M.; Wang, Y. Lycium barbarum polysaccharide protects ARPE-19 cells against H2O2-induced oxidative stress via the Nrf2/HO-1 pathway. Mol. Med. Rep. 2021, 24, 769. [Google Scholar] [CrossRef]
- Pehlİvan KarakaŞ, F.; CoŞkun, H.; SoytÜrk, H.; Bozat, B.G. Anxiolytic, antioxidant, and neuroprotective effects of goji berry polysaccharides in ovariectomized rats: Experimental evidence from behavioral, biochemical, and immunohistochemical analyses. Turk. J. Biol. 2020, 44, 238–251. [Google Scholar] [CrossRef]
- Li, X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An insight into anti-inflammatory activities and inflammation related diseases of anthocyanins: A review of both in vivo and in vitro investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Park, Y.; Seo, H.; You, C.; Hwang, U.; Park, H.; Suh, H.-j. Supplementation of non-fermented and fermented goji berry (Lycium barbarum) improves hepatic function and corresponding lipid metabolism via their anti-inflammatory and antioxidant properties in high fat-fed rats. Appl. Biol. Chem. 2021, 64, 70. [Google Scholar] [CrossRef]
- Xiao, J.; Xing, F.; Huo, J.; Fung, M.L.; Liong, E.C.; Ching, Y.P.; Xu, A.; Chang, R.C.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci. Rep. 2014, 4, 5587. [Google Scholar] [CrossRef]
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium barbarum polysaccharide mediated the antidiabetic and antinephritic effects in diet-streptozotocin-induced diabetic Sprague Dawley rats via regulation of NF-κB. Biomed. Res. Int. 2016, 2016, 3140290. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, Y.Y.; Chen, H.G.; Zhou, X. Study on the efficacy and mechanism of Lycium barbarum polysaccharide against lead-induced renal Iinjury in mice. Nutrients 2021, 13, 2945. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Liu, Q.; Liu, Y.; Huang, D.; Pan, C.; Song, S.; Huang, K. Lycium barbarum polysaccharides improve CCl(4)-induced liver fibrosis, inflammatory response and TLRs/NF-kB signaling pathway expression in wistar rats. Life Sci. 2018, 192, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, J.; Yan, J.; Liu, S.; Guo, Y.; Chen, D.; Luo, Q. Lycium barbarum polysaccharides ameliorates renal injury and inflammatory reaction in alloxan-induced diabetic nephropathy rabbits. Life Sci. 2016, 157, 82–90. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H. Effect of Lycium barbarum polysaccharides on anti-tumor activity and immune function. Acta Nutr. Sin. 2003, 25, 200–202. [Google Scholar]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Lai, X.; Zhou, L. Polysaccharides from Chinese herbal Lycium barbarum induced systemic and local immune responses in H22 tumor-bearing Mice. J. Immunol. Res. 2018, 2018, 3431782. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yin, R.Q.; Liang, S.S.; Duan, Y.L.; Yao, J.H.; Duan, Y.L.; Yang, X.J. Effect of dietary Lycium barbarum polysaccharide on growth performance and immune function of broilers. J. Appl. Poult. Res. 2017, 26, 200–208. [Google Scholar] [CrossRef]
- Qian, L. Modulation of cytokine level and sperm quality of mice by Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 475–477. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Guo, M.; Jin, J.; Zhao, D.; Rong, Z.; Cao, L.-Q.; Li, A.-H.; Sun, X.-Y.; Jia, L.-Y.; Wang, Y.-D.; Huang, L.; et al. Research advances on anti-cancer natural products. Front. Oncol. 2022, 12, 866154. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor mechanisms of Lycium barbarum fruit: An overview of in vitro and in vivo potential. Life 2024, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Kwaśnik, P.; Lemieszek, M.K.; Rzeski, W. Impact of phytochemicals and plant extracts on viability and proliferation of NK cell line NK-92—A closer look at immunomodulatory properties of goji berries extract in human colon cancer cells. Ann. Agric. Environ. Med. 2021, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, K.D.; Slavov, I.J.; Iliev, I.A. Antioxidant activity and antiproliferative effects of Lycium barbarum’s (Goji berry) fractions on breast cancer cell lines. Folia Med. 2019, 61, 104–112. [Google Scholar] [CrossRef]
- Gong, G.; Liu, Q.; Deng, Y.; Dang, T.; Dai, W.; Liu, T.; Liu, Y.; Sun, J.; Wang, L.; Liu, Y.; et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2020, 149, 639–650. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of Lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Luo, S.; Zheng, Y.; Luo, X.; Zhou, L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: An in vitro and in vivo study. Food Nutr. Res. 2017, 61, 1399770. [Google Scholar] [CrossRef]
- Sanghavi, A.; Srivatsa, A.; Adiga, D.; Chopra, A.; Lobo, R.; Kabekkodu, S.P.; Gadag, S.; Nayak, U.; Sivaraman, K.; Shah, A. Goji berry (Lycium barbarum) inhibits the proliferation, adhesion, and migration of oral cancer cells by inhibiting the ERK, AKT, and CyclinD cell signaling pathways: An in-vitro study. F1000Research 2022, 11, 1563. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Gao, L.; Ma, L. Effect of Lycium barbarum polysaccharide combined with cisplatin on the proliferation of human lung cancer cells. Pharmacogn. Mag. 2022, 18, 378–385. [Google Scholar]
- Chen, S.; Liang, L.; Wang, Y.; Diao, J.; Zhao, C.; Chen, G.; He, Y.; Luo, C.; Wu, X.; Zhang, Y. Synergistic immunotherapeutic effects of Lycium barbarum polysaccharide and interferon-α2b on the murine Renca renal cell carcinoma cell line in vitro and in vivo. Mol. Med. Rep. 2015, 12, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-F.; Wan, L.-L.; Peng, J.-L.; Guo, C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem. Toxicol. 2011, 49, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Zhou, L.; Huang, R. Fraction from Lycium barbarum polysaccharides reduces immunotoxicity and enhances antitumor activity of doxorubicin in mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Li, Y.; Fan, Y.; Li, Y.; Cao, Y.; An, W.; Shi, Z.; Zhao, J.; Guo, S. Changes in metabolome and nutritional quality of Lycium barbarum fruits from three typical growing areas of China as revealed by widely targeted metabolomics. Metabolites 2020, 10, 46. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Liu, Y.; Tipoe, G.L.; Xing, F.; So, K.-F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef]
- Gündüz, E.; Dursun, R.; Zengin, Y.; İçer, M.; Durgun, H.M.; Kanıcı, A.; Kaplan, İ.; Alabalık, U.; Gürbüz, H.; Güloğlu, C. Lycium barbarum extract provides effective protection against paracetamol-induced acute hepatotoxicity in rats. Int. J. Clin. Exp. Med. 2015, 8, 7898–7905. [Google Scholar]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef]
- Sanghavi, A.D.; Chopra, A.; Shah, A.; Lobo, R.; Shenoy, P.A. Antimicrobial, anti-adhesion, anti-biofilm properties of goji berry (Lycium barbarum) against periodontal bacteria: Potential benefits for periodontal diseases. J. Complement. Integr. Med. 2023, 20, 129–136. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Guo, L.; Guan, Q.; Duan, W.; Ren, Y.; Zhang, X.J.; Xu, H.Y.; Shi, J.S.; Wang, F.Z.; Lu, R.; Zhang, H.L.; et al. Dietary goji shapes the gut microbiota to prevent liver injury induced by acute alcohol intake. Front. Nutr. 2022, 9, 929776. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Orsini, M.; Nascimento, O.J.; Matta, A.P.; Reis, C.H.; de Souza, O.G.; Bastos, V.H.; Moreira, R.; Ribeiro, P.; Fiorelli, S.; Novellino, P.; et al. Revisiting the term neuroprotection in chronic and degenerative diseases. Neurol. Int. 2016, 8, 6311. [Google Scholar] [CrossRef] [PubMed]
- Karvandi, M.S.; Sheikhzadeh Hesari, F.; Aref, A.R.; Mahdavi, M. The neuroprotective effects of targeting key factors of neuronal cell death in neurodegenerative diseases: The role of ER stress, oxidative stress, and neuroinflammation. Front. Cell. Neurosci. 2023, 17, 1105247. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Chen, L.; Ren, B.X.; Tang, F.R. Lycium barbarum ameliorates neural damage induced by experimental ischemic stroke and radiation exposure. Front. Biosci. 2023, 28, 38. [Google Scholar] [CrossRef]
- Lam, C.S.; Tipoe, G.L.; So, K.F.; Fung, M.L. Neuroprotective mechanism of Lycium barbarum polysaccharides against hippocampal-dependent spatial memory deficits in a rat model of obstructive sleep apnea. PLoS ONE 2015, 10, e0117990. [Google Scholar] [CrossRef]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Zhu, T.; Xu, S.; He, J.; Mao, N.; Liu, Z.; Wang, D. Neuroprotective effects of Lycium barbarum polysaccharide on light-induced oxidative stress and mitochondrial damage via the Nrf2/HO-1 pathway in mouse hippocampal neurons. Int. J. Biol. Macromol. 2023, 251, 126315. [Google Scholar] [CrossRef]
- Hu, X.; Qu, Y.; Chu, Q.; Li, W.; He, J. Investigation of the neuroprotective effects of Lycium barbarum water extract in apoptotic cells and Alzheimer’s disease mice. Mol. Med. Rep. 2018, 17, 3599–3606. [Google Scholar] [CrossRef]
- Yao, X.-l.; Wu, W.-l.; Zheng, M.-y.; Li, W.; Ye, C.-h.; Lu, X.-l. Protective effects of Lycium barbarum extract against MPP(+) -induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Zhong Yao Cai 2011, 34, 1241–1246. [Google Scholar]
- Lp, S.; Rr, U.; Mardhekar, V. Neuroprotective effects of Lycium barbarum in ischemic stroke: Current perspectives. Int. J. Pharmacogn. Chin. Med. 2019, 3, 126315. [Google Scholar] [CrossRef]
- Deng, H.B.; Cui, D.P.; Jiang, J.M.; Feng, Y.C.; Cai, N.S.; Li, D.D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on nonenzyme glycation in D-galactose induced mouse aging model. Biomed. Environ. Sci. 2003, 16, 267–275. [Google Scholar]
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Gong, G.; Song, S.; Ma, F.; Huang, L.; et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019, 10, 4231–4241. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Chen, X.; Sun, C.; Jin, C.; Ding, K.; Chen, C. LBP1C-2 from Lycium barbarum maintains skeletal muscle satellite cell pool by interaction with FGFR1. iScience 2023, 26, 106573. [Google Scholar] [CrossRef]
- Lai, S.; Liu, C.; Liu, C.; Fan, L.; Li, X.; Yang, Y.; Zhu, Y.; Deng, L.; Xiao, L.; Mu, Y. Lycium barbarum polysaccharide-glycoprotein promotes osteogenesis in hPDLSCs via ERK activation. Oral Dis. 2023, 29, 3503–3513. [Google Scholar] [CrossRef]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Sheng, X.; Gambino, P.E.; Costello, B.; Bojanowski, K. Protective effect of Fructus Lycii polysaccharides against time and hyperthermia-induced damage in cultured seminiferous epithelium. J. Ethnopharmacol. 2002, 82, 169–175. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Pu, J.; Luo, P.; Ma, W.; Wang, J.; Wei, J.; Wang, Y.; Fei, Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem. Biophys. Res. Commun. 2018, 495, 1187–1194. [Google Scholar] [CrossRef]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The role of SIRT3 in exercise and aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.; So, K.F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Chiu, K.; Yuan, Q.; Lin, B.; Chang, R.C.; So, K.F. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS ONE 2013, 8, e68881. [Google Scholar] [CrossRef]
- Chu, P.H.; Li, H.Y.; Chin, M.P.; So, K.F.; Chan, H.H. Effect of Lycium barbarum (wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS ONE 2013, 8, e81339. [Google Scholar] [CrossRef]
- Li, H.Y.; Huang, M.; Luo, Q.Y.; Hong, X.; Ramakrishna, S.; So, K.F. Lycium barbarum (wolfberry) increases retinal ganglion cell survival and affects both microglia/macrophage polarization and autophagy after rat partial optic nerve transection. Cell Transpl. 2019, 28, 607–618. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, J.; Peng, B.; Xing, F.; So, K.F.; Tipoe, G.L.; Lin, B. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci. Rep. 2014, 4, 7601. [Google Scholar] [CrossRef]
- Zheng, H.-l.; Li, M.-t.; Zhou, T.; Wang, Y.-y.; Shang, E.-X.; Hua, Y.-q.; Duan, J.-a.; Zhu, Y. Protective effects of Lycium barbarum L. berry extracts against oxidative stress-induced damage of the retina of aging mouse and ARPE-19 cells. Food Funct. 2023, 14, 399–412. [Google Scholar] [CrossRef]
- Mi, X.S.; Feng, Q.; Lo, A.C.; Chang, R.C.; Lin, B.; Chung, S.K.; So, K.F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef]
- Lakshmanan, Y.; Wong, F.S.-Y.; Yu, W.-Y.; Li, S.Z.-C.; Choi, K.-Y.; So, K.-F.; Chan, H.H.-L. Lycium brbarum polysaccharides rescue neurodegeneration in an acute ocular hypertension rat model under pre- and posttreatment conditions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2023–2033. [Google Scholar] [CrossRef]
- Mi, X.S.; Chiu, K.; Van, G.; Leung, J.W.; Lo, A.C.; Chung, S.K.; Chang, R.C.; So, K.F. Effect of Lycium barbarum Polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural. Regen. Res. 2012, 7, 645–651. [Google Scholar] [CrossRef]
- Mi, X.S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.; Chung, S.K.; So, K.F. Lycium barbarum polysaccharides related RAGE and Aβ levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural. Regen. Res. 2020, 15, 2344–2352. [Google Scholar] [CrossRef]
- Yang, D.; So, K.-F.; Lo, A.C. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin. Exp. Ophthalmol. 2017, 45, 717–729. [Google Scholar] [CrossRef]


| Model | Main Bioactives | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vitro | LBP | ↓ Myocardial damage, ↓ apoptosis, preserved mitochondrial function | SIRT3/CypD pathway | [77] |
| In vivo | LBP | ↓ Infarct size, prevented adverse cardiac remodelling, lowered oxidative stress, improved mitochondrial dynamics | GRK2 expression inhibition; restoration of mitochondrial fission/fusion balance; activation of AKT/eNOS signalling | [78] |
| In vitro | LBP | Enhanced antioxidant defences (↑ SOD, ↑ NO), ↓ oxidative damage (↓ MDA), anti-apoptotic effects | Oxidative stress regulation | [79] |
| In vivo | L. barbarum extract | Normalised blood pressure, ↑ eNOS, ↓ sONE expression | sONE/eNOS pathway modulation | [80] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. | |
|---|---|---|---|---|---|
| A | In vivo | LBP | ↓ Adipocyte lipid accumulation, ↑ insulin sensitivity | ATF6/SIRT1-dependent downregulation of Fsp27 | [83] |
| In vivo | LICP009-2-1 | ↓ Lipid accumulation and hyperlipidemia | Anti-adipogenic activity | [84] | |
| In vivo | LBP | ↓ Blood glucose, ↑ insulin sensitivity, ↑ testosterone | Anti-metabolic disturbance | [85] | |
| In vivo | LBLF | ↓ Oxidative stress, ↑ liver function Improved insulin resistance, optimised gut microbiota | MAPK and retinol metabolism pathways modulation | [86] | |
| In vitro In vivo | LBP | ↓ Obesity-related factors and ↑ muscle-related factors; ↑ glucose metabolism; mitigated ectopic fat and mitochondrial dysfunction | AMPK/PINK1/Parkin-mediated mitophagy; ↑ mitochondrial membrane potential and ATP, ↓ROS | [87] | |
| B | In vivo | Zeaxanthin | Improved blood glucose, lipid profile, nephroprotection | Modulation of inflammatory cytokines and antioxidant enzymes | [88] |
| In vivo | LBP | Improved gut motility, ↑ SCFA production | Neuronal regulation of duodenal contraction | [89] | |
| In vivo | LBP | ↓ Oxidative stress, protected DNA in lymphocytes | Antioxidant protection (↑ SOD, ↓ MDA/NO) | [90] | |
| In vivo | LBL | Restored organ function, improved lipid and glucose metabolism | Modulation of gut microbiota and metabolic disruption reversal | [91] | |
| C | In vivo | LBP | ↓ Cholesterol and triglycerides, promoted weight loss | Gut microbiota modulation (↑ Bacteroidetes, ↓ Firmicutes) | [92] |
| In vivo | LBP | Improved lipid metabolism, ↑ antioxidant capacity | Upregulation of lipid metabolism genes (FAS, PPAR-α, CPT1, ATGL) | [93] | |
| In vivo | LBP | Improved growth, lipid metabolism, antioxidant capacity | Downregulation of lipid metabolism genes (ACC1, PPAR-γ) | [94] | |
| In vivo | LBP | Improved lipid metabolism, alleviated metabolic disorder symptoms, ↓weight gain, ↑ microbial diversity | Modulation of gut microbiota (↑ Firmicutes, ↑ microbial diversity), regulation of >30 differential metabolites and 4 metabolic pathways | [95] | |
| D | In vitro | LBP | Mitigated osteoblast apoptosis | miR-200b-3p/Chrdl1/PPARγ pathway modulation | [96] |
| In vitro | LBP | Promoted osteoblast proliferation via SCFA production | Upregulation of B-ALP and osteocalcin expression | [97] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vivo | Methanol extract | ↓ Paw swelling, ↓ ROS, neutrophil migration | Myeloperoxidase inhibition | [56] |
| In vivo | Fermented/non-fermented extracts | Improved hepatic function, lipid metabolism | Anti-inflammatory + antioxidant | [111] |
| In vivo | LBP | ↓ Fat accumulation, fibrosis, inflammation | NF-κB, MAPK, autophagy | [112] |
| In vivo | LBP | ↓ Albuminuria, ↓ inflammatory, ↑ SOD ↓ Cytokines, ↓NF-κB | Renal protection | [113] |
| In vivo | LBP | Anti-inflammatory, anti-apoptotic | Nrf2 pathway | [114] |
| In vivo | LBP | ↓ Oxidative injury, ↓ inflammation markers | TLR/NF-κB inhibition | [115] |
| In vivo | LBP | ↓ ROS, 1CAM-1, improved kidney function | NF-κB and angiotensin downregulation | [116] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vivo | LBP | ↓ Tumour size, ↑ macrophage and lymphocyte activity | Immune activation, ↓ lipid peroxidation | [117] |
| In vivo | LBP | ↑ CD8+ T cell infiltration, ↓ T cell exhaustion | Enhanced systemic/local antitumour immunity | [118] |
| In vitro | LBP (>10 kDa) | ↑ Macrophage viability and NO, TNF-α, IL-6 | Cellular uptake via clathrin-mediated endocytosis | [119] |
| In vivo | LBP | ↑ Immune organ indices, IgG, CD4+/CD8+ ratio | Immune stimulation and improved feed efficiency | [24,120] |
| In vivo | LBP | ↑ Cytokines, improved sperm parameters | Immuno-protection in cyclophosphamide model | [121] |
| In vivo | LBP | ↓ Hepatotoxicity, ↑ SCFAs, modulated gut microbiota | Gut–immune axis modulation | [122] |
| In vivo | Juice blends | ↑ Splenic macrophages, spleen weight | Synergistic antioxidant-immune enhancement | [28] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vitro | Ethanol extract | ↑ NK cytotoxicity, ↓Proliferation | NK cell activation | [126] |
| In vitro | Ethanol extract | ↓ Proliferation, ↑ Apoptosis, ↑ Bax, ↓ BclxL | Mitochondrial pathway | [31] |
| In vitro | Polyphenol-rich extract | Inhibited proliferation in MCF-7 and MDA-MB-231 cancer cells | Dose-dependent inhibition | [127] |
| In vitro | LBP fractions | Induced G0/G1 arrest, apoptosis, ↓ mito. potential | ↑ Caspase, ↑ MAPK, ↓ Bcl-2 | [128] |
| In vitro | LBP | Induced S phase arrest and apoptosis, ↑ RNA/Ca2+ | Calcium-regulated apoptotic pathways | [129] |
| In vivo | LBP-3 (40–350 kDa) | Tumour suppression, S phase arrest immune support | Immune modulation | [130] |
| In vitro | Ethanol extract | ↓ Proliferation, ↓ migration | ERK1/2, AKT suppression | [131] |
| In vitro | LLB extract | No cytotoxicity or genotoxicity; ↓ DNA damage; ↓ pro-metastatic and ↑tumour suppressor genes | Modulation of oxidative stress, apoptosis, and cancer-related gene expression | [69] |
| In vitro | LBP | Combined LBP and cisplatin inhibited cell proliferation; ↑ apoptosis, ↓ ROS; modulated S/G2-M cell cycle phases | Enhanced apoptosis and cell cycle arrest via cyclin D1-CDK4-Rb pathway | [132] |
| In vitro In vivo | LBP | Combined LBP and IFN-α2b inhibited proliferation, induced apoptosis, ↓ tumour volume, and ↓ MDSC ratio | Synergistic regulation of apoptosis and immune suppression via cyclin D1/c-Myc/Bcl-2 pathway and MDSC modulation | [133] |
| In vitro In vivo | LBP | Reduced cardiac damage (↓ myofibrillar disarrangement), improved conduction abnormalities, and preserved anti-tumour activity of Doxorubicin | Suppression of oxidative stress (↑ SOD, ↑ GSH-Px, ↓ MDA) | [134] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. | |
|---|---|---|---|---|---|
| A | In vitro | LBP | ↓ Oxidative stress, ↓ apoptosis, ↓ inflammation | TXNIP-NLRP3 | [137] |
| In vivo | L. barbarum extract | ↓ ALT, ↓AST | Antioxidant enhancement | [138] | |
| In vivo | LBP | ↓ Fatty liver, ↑ liver enzymes, ↑ antioxidant activity | Hepatic protection and redox modulation | [139] | |
| B | In vitro | Yellow L. barbarum extract | Strong activity against Gram– and Candida | Dose-dependent | [14] |
| In vitro | Hydromethanolic extract | ↑ Antibacterial activity (Gram+ > Gram–) | Disruption of cell membrane integrity | [15] | |
| In vitro | Polyphenols (e.g., chlorogenic acid) | ↓ E. coli growth | Time/dose/temperature-dependent | [29] | |
| In vitro | Ethanolic extract | Effective against periodontal pathogens | Alternative to chlorhexidine | [140] | |
| C | In vivo | LBP | ↑ Firmicutes/Proteobacteria, ↓ Bacteroidetes ↑ Lactobacillus/Akkermansia/Prevotellaceae | Microbiota modulation Gut microbiota enrichment | [141] |
| In vitro | LBP | ↑ L. acidophilus, ↑B. longum (2.5–15% LBP) | Prebiotic-enhanced bacterial growth | [141] | |
| In vivo | L. barbarum | ↓ ALT/AST ↑ Lachnospiraceae, ↑ Ruminococcaceae | Gut–liver axis modulation | [142] | |
| In vitro | Aqueous extract | ↑ Lactobacillus, Bifidobacterium growth | Stimulated probiotic proliferation | [143] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vivo | L. barbarum extract | ↓ Oxidative stress and cytokines; ↑ hippocampal neuron survival | PI3K/Akt/GSK-3β, PKCε/Nrf2/HO-1, NR2A/NR2B | [146] |
| In vivo | LBP | ↑ Spatial memory, ↑ neurogenesis; ↓apoptosis and ER stress | Nrf2/HO-1 signalling | [147] |
| In vitro In vivo | LBP | ↓ ROS, ↓ mitochondrial damage; ↑ caspase-3/-9 activity | ↑ Nrf2 and HO-1 expression | [148] |
| In vivo In vitro | L. barbarum | Reversed cognitive impairment; ↓ apoptosis; ↑ antioxidant defence | Nrf2/HO-1 | [149] |
| In vitro In vivo | Water extract | ↑ Cell survival; ↓ROS; ↑ acetylcholine, choline acetyltransferase | Mitochondrial protection and neurotransmitter regulation | [150] |
| In vitro | Alkaline extract | ↓ Caspase-3 activity; ↑ Akt phosphorylation | Anti-apoptotic via Akt signalling | [30] |
| In vivo In vitro | L. barbarum extract | ↓ Dopaminergic neuron loss; ↓ROS; ↑ GSH | Mitochondrial stabilisation, antioxidative action | [151] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vivo | ABP and LBP | ↓ glycation end-products, ↑ IL-2, ↑ SOD, ↑ cognitive and motor function | Glycation inhibition; immune and oxidative modulation | [153] |
| In vivo | LBP, LBP-2 (arabinogalactan) | ↑ Lifespan, ↑ SOD/CAT, ↓ MDA | MAPK/TOR/S6K pathway; longevity gene upregulation | [154] |
| In vitro | LBP | ↓ UVB-induced DNA damage and ROS, ↑ Nrf2 activation, ↓ p38 MAPK | Nrf2/ARE; caspase-3, MMP-9 | [155] |
| In vivo In vitro | LBP1C-2 | ↑ Muscle stem cell self-renewal and repair | FGFR1 binding; Spry1 upregulation | [156] |
| In vivo In vitro | LBP | ↑ Osteogenic markers; ↓bone resorption and osteoclasts | ERK1/2 pathway activation | [157] |
| In vivo | LBP | ↓ Senescence, ↓ apoptosis | p53 signalling modulation | [158] |
| In vitro | LBP | ↓ Lipid peroxidation, delayed apoptosis | Oxidative stress reduction | [159] |
| In vitro | LBP | ↓ Oxidative stress, ↓ apoptosis and autophagy | PI3K/Akt/mTOR, Bcl-2/Bax, Caspase-3 | [160] |
| In vivo | LBP | ↑ SIRT3, ↓ CypD acetylation, ↑ mitochondrial protection | SIRT3/CypD pathway activation | [77] |
| Model | Main Bioactive(s) | Key Findings | Mechanism/Pathway | Refs. |
|---|---|---|---|---|
| In vivo | LBP | ↑ Nrf2 and HO-1; ↓ apoptosis; ↑ survival of ganglion cells | Nrf2/HO-1 antioxidant pathway | [162] |
| In vivo | LBP | ↓ oxidative stress; ↓ JNK; ↑ IGF-1; ↓ secondary RGC degeneration | JNK pathway, oxidative stress inhibition | [163] |
| In vivo | LBP | ↑ retinal function and visual signalling | Retinal functional recovery | [164] |
| In vivo | LBP | ↑ M2 microglia/macrophage polarisation; ↓ autophagy; ↑ RGC survival | Immune modulation | [165] |
| In vivo | LBP | Preserved photoreceptor morphology and visual behaviour | NF-κB and HIF-1α inhibition | [166] |
| In vivo | LBW-95E | ↑SOD, ↑GSH, ↑Nrf2; ↓ ROS, inflammation | Antioxidant and anti-inflammatory | [167] |
| In vivo | LBP | ↓ RGC loss; preserved blood-retinal barrier | Downregulation of inflammatory mediators | [168] |
| In vivo | LBP | Preserved inner retinal layer thickness; ↑retinal function | Protection from secondary degeneration | [169] |
| In vivo | LBP | ↓ ET-1 expression; ↑ ETA, ↓ ETB in RGCs | ET-1 signalling modulation | [170] |
| In vivo | LBP | ↓ astrocyte/microglia activation; preserved barrier integrity | Glial reactivity modulation | [171] |
| In vivo | LBP | ↑ viable retinal cells, ERG, ↓ glial activity | Neuroprotection | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Alexa, E.-A.; Liu, G.; Berisha, A.; Walsh, R.; Kelleher, R. Lycium barbarum for Health and Longevity: A Review of Its Biological Significance. Obesities 2025, 5, 35. https://doi.org/10.3390/obesities5020035
Zhang T, Alexa E-A, Liu G, Berisha A, Walsh R, Kelleher R. Lycium barbarum for Health and Longevity: A Review of Its Biological Significance. Obesities. 2025; 5(2):35. https://doi.org/10.3390/obesities5020035
Chicago/Turabian StyleZhang, Tao, Elena-Alexandra Alexa, Gavin Liu, Alois Berisha, Rhys Walsh, and Robbie Kelleher. 2025. "Lycium barbarum for Health and Longevity: A Review of Its Biological Significance" Obesities 5, no. 2: 35. https://doi.org/10.3390/obesities5020035
APA StyleZhang, T., Alexa, E.-A., Liu, G., Berisha, A., Walsh, R., & Kelleher, R. (2025). Lycium barbarum for Health and Longevity: A Review of Its Biological Significance. Obesities, 5(2), 35. https://doi.org/10.3390/obesities5020035






