The Possible Impact of COVID-19 on Glycated Hemoglobin and Systolic Blood Pressure in Type 2 Diabetes and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population/Participants
2.2. Inclusion Criteria
- -
- Adults aged between 18 and 80 years, with the aim of expanding the sample size and recruiting as many participants as possible.
- -
- Both male and female participants, with the goal of increasing the sample size and enrolling as many patients as feasible.
- -
- Diagnosis of T2D, confirmed by fasting plasma glucose levels of ≥126 mg/dL and HbA1c levels of ≥6.5%. The diagnosis of T2D was confirmed before the start of the study. It was previously performed by a doctor during consultation based on changes in blood glucose and glycated hemoglobin tests. All participants underwent the necessary diagnostic tests before enrollment in the study.
- -
- Willingness to participate in quarterly meetings over a 36-month period.
- -
- BMI ≥ 30 kg/m2.
- -
- Sedentary lifestyle.
2.3. Exclusion Criteria
- -
- Individuals who were unable to complete the requested assessments.
- -
- Participants who could not maintain regular attendance for data collection.
- -
- Those without a confirmed diagnosis of T2D and obesity.
- -
- Individuals undergoing insulin therapy.
- -
- Participants taking sodium-glucose cotransporter-2 (SGLT-2) inhibitors and/or glucagon-like peptide-1 (GLP-1) analogues. These medications were excluded to prevent confounding variables that could have skewed the study results. Their significant impact on glycemic control and other metabolic parameters could interfere with the measured outcomes.
- -
- Individuals with chronic kidney disease.
- -
- Eutrophic or malnourished participants.
- -
- Individuals engaging in more than 150 min of exercise per week.
2.4. Study Design and General Information
2.5. Evaluation of Physical Exercises and Medications
2.6. Nutritional Interventions and Protocols
2.7. COVID-19 Pandemic
2.8. Ethical Aspects
2.9. Research Construction, Management, and Databases—REDCap FAMERP/FUNFARME
2.10. Statistical Analysis
3. Results
3.1. Demographics, Anthropometric, Biochemical, and Cardiovascular Data
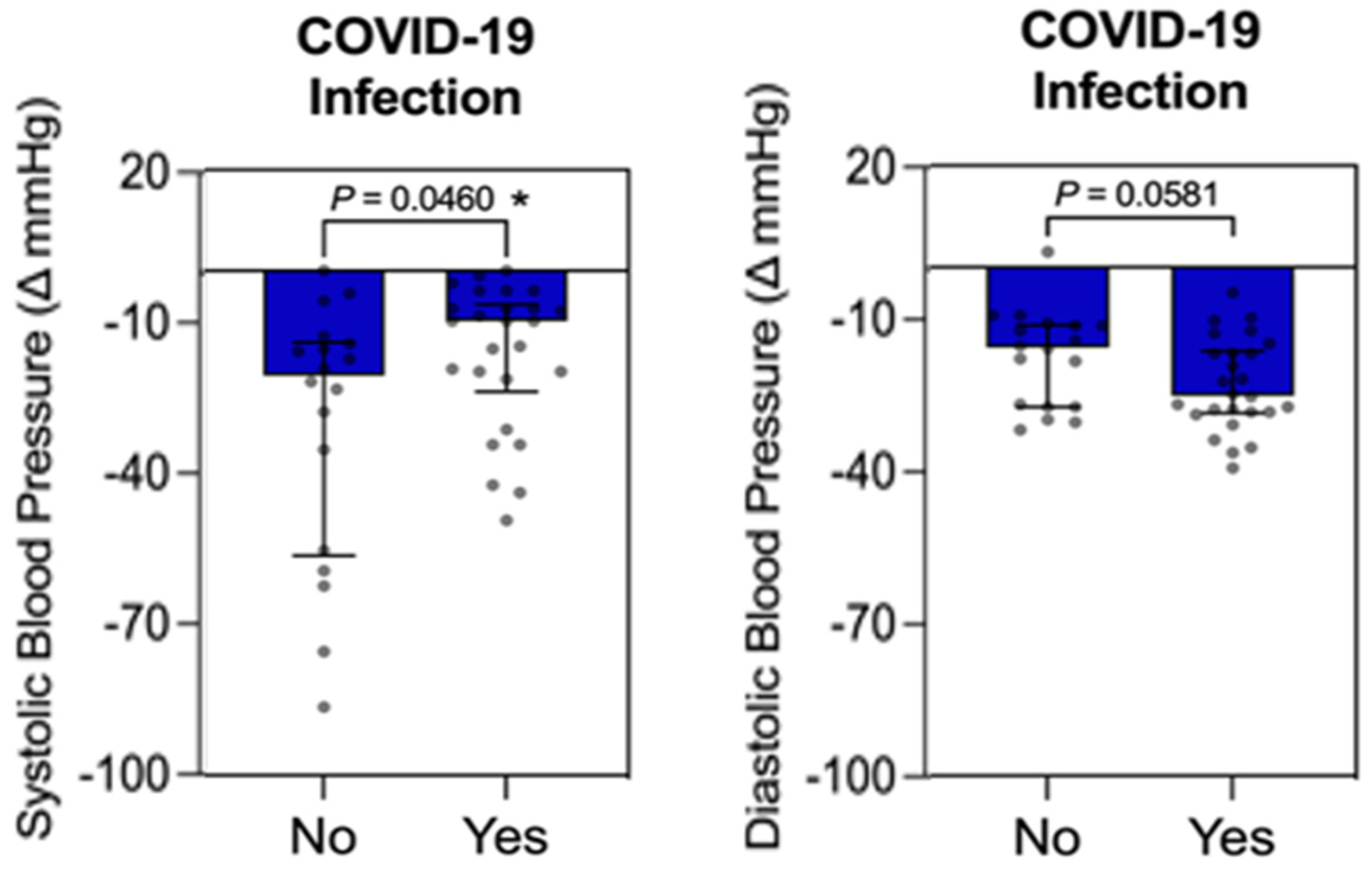
3.2. COVID-19 Infection
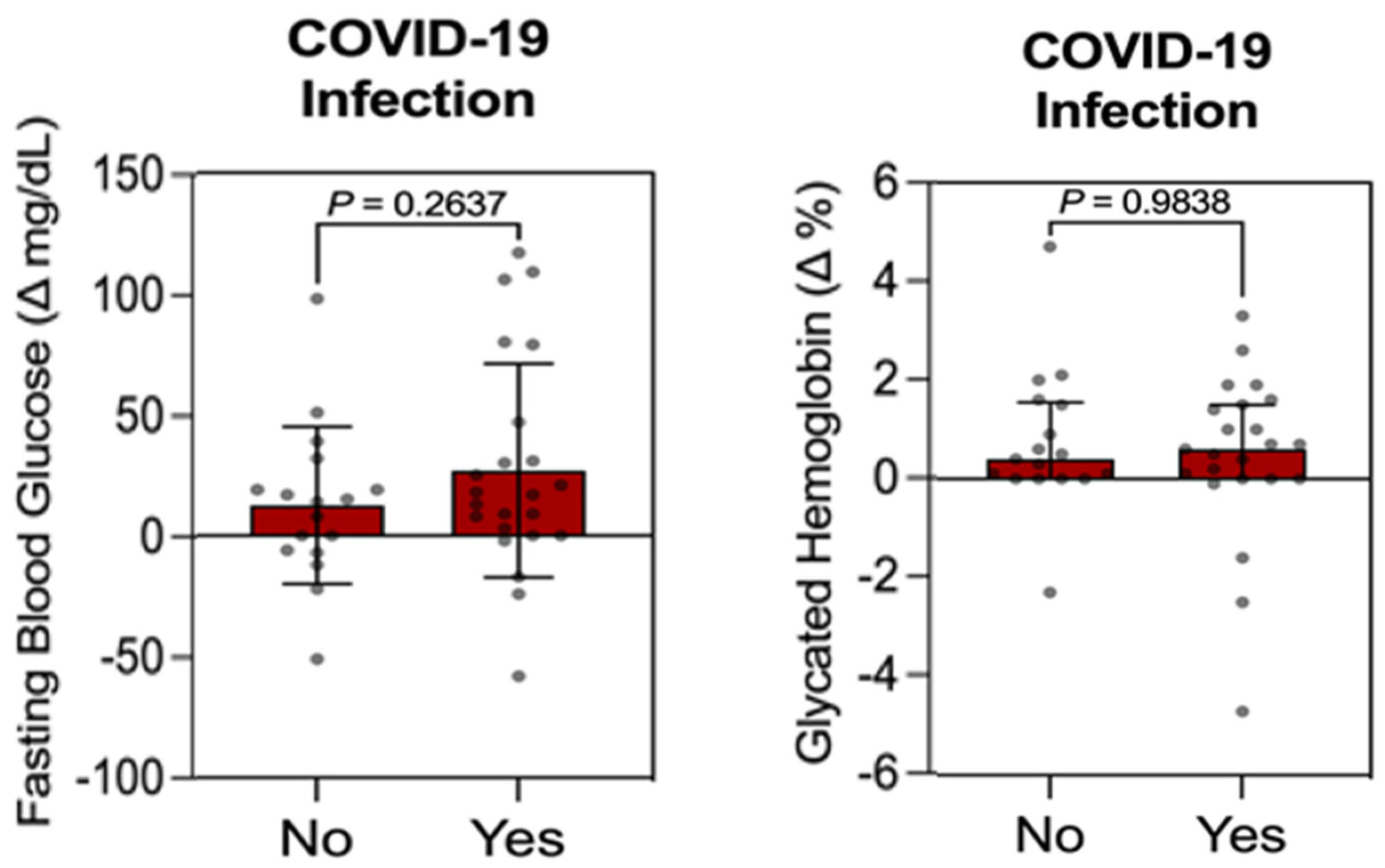
3.3. Individual Influence Analysis Between Qualitative Variable (COVID-19 Infection) vs. Quantitative Variables (FBG, HbA1c, Weight, and BMI) in the Control Group
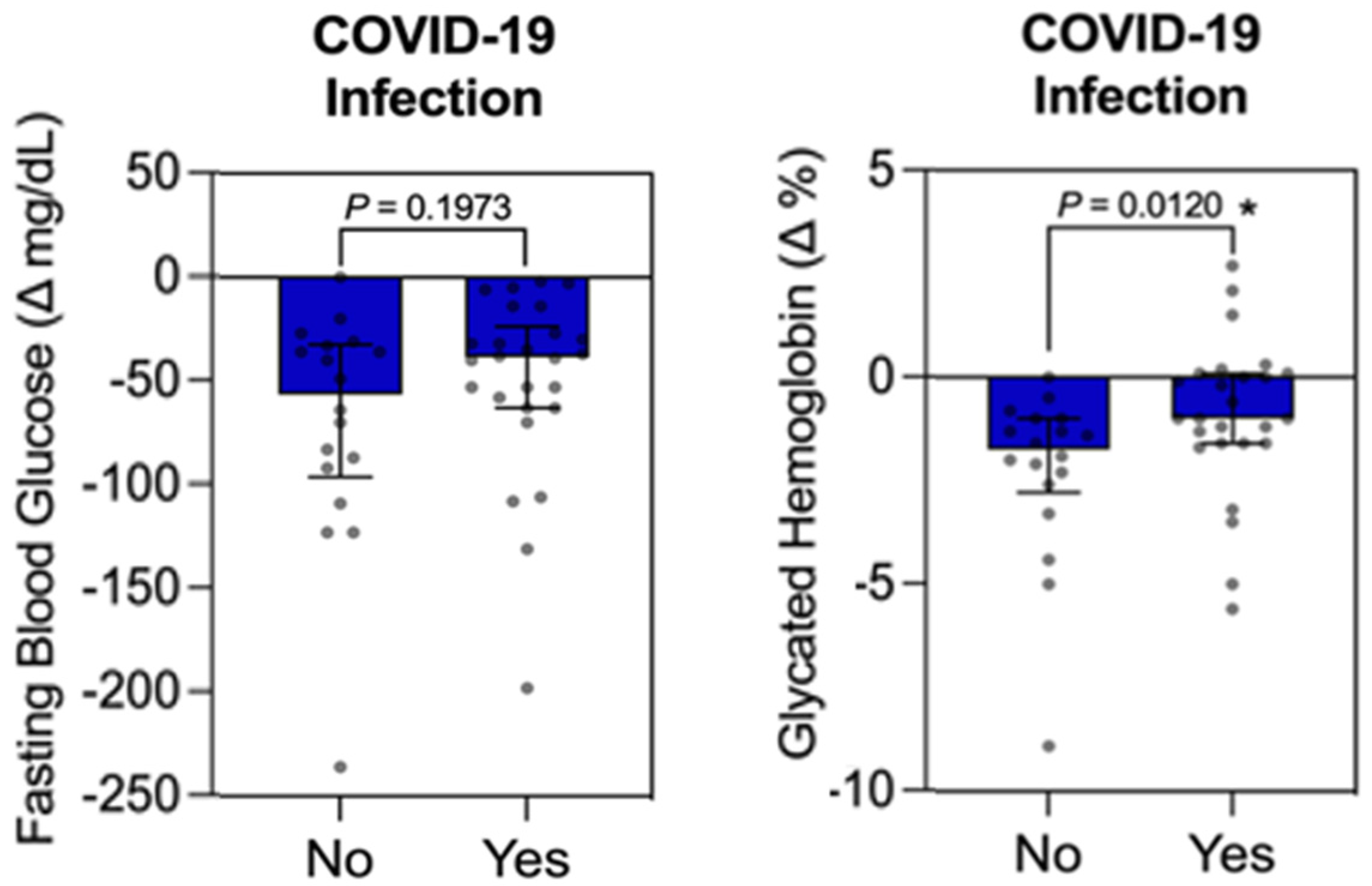
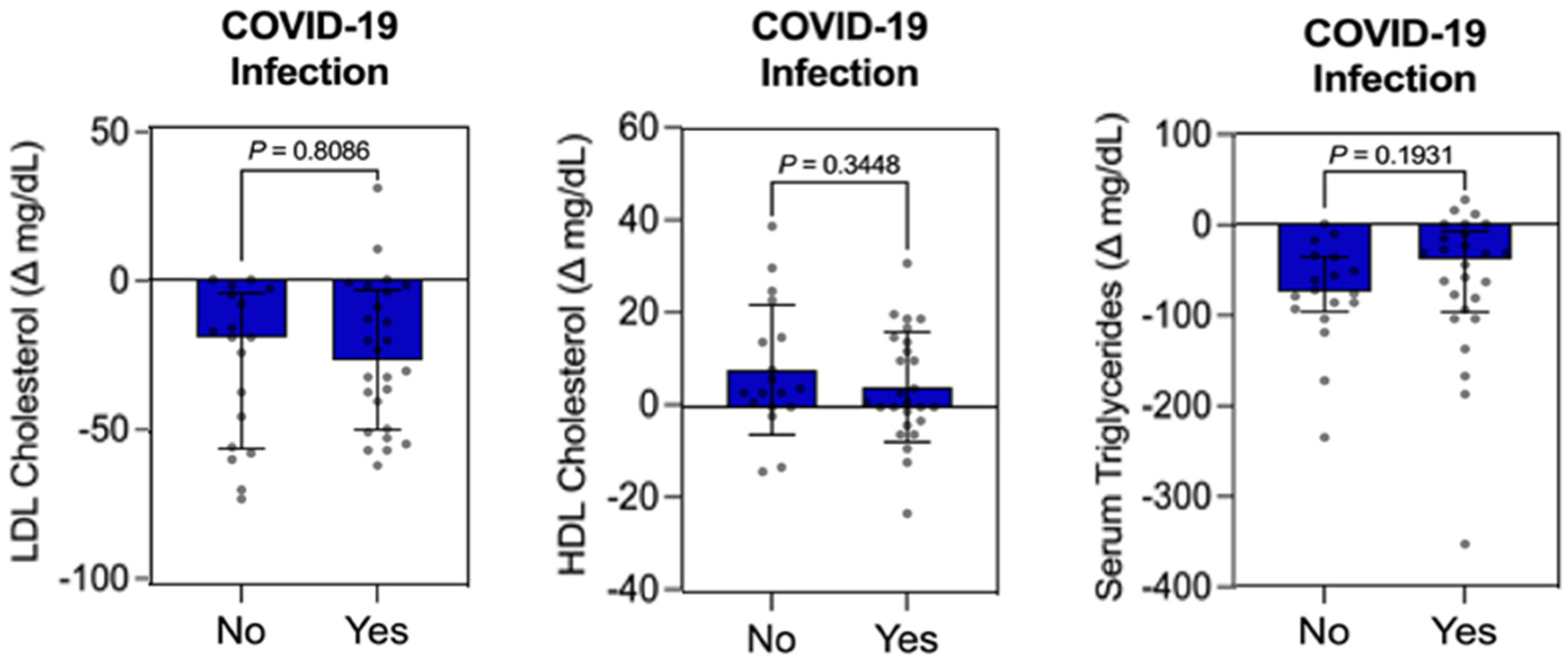
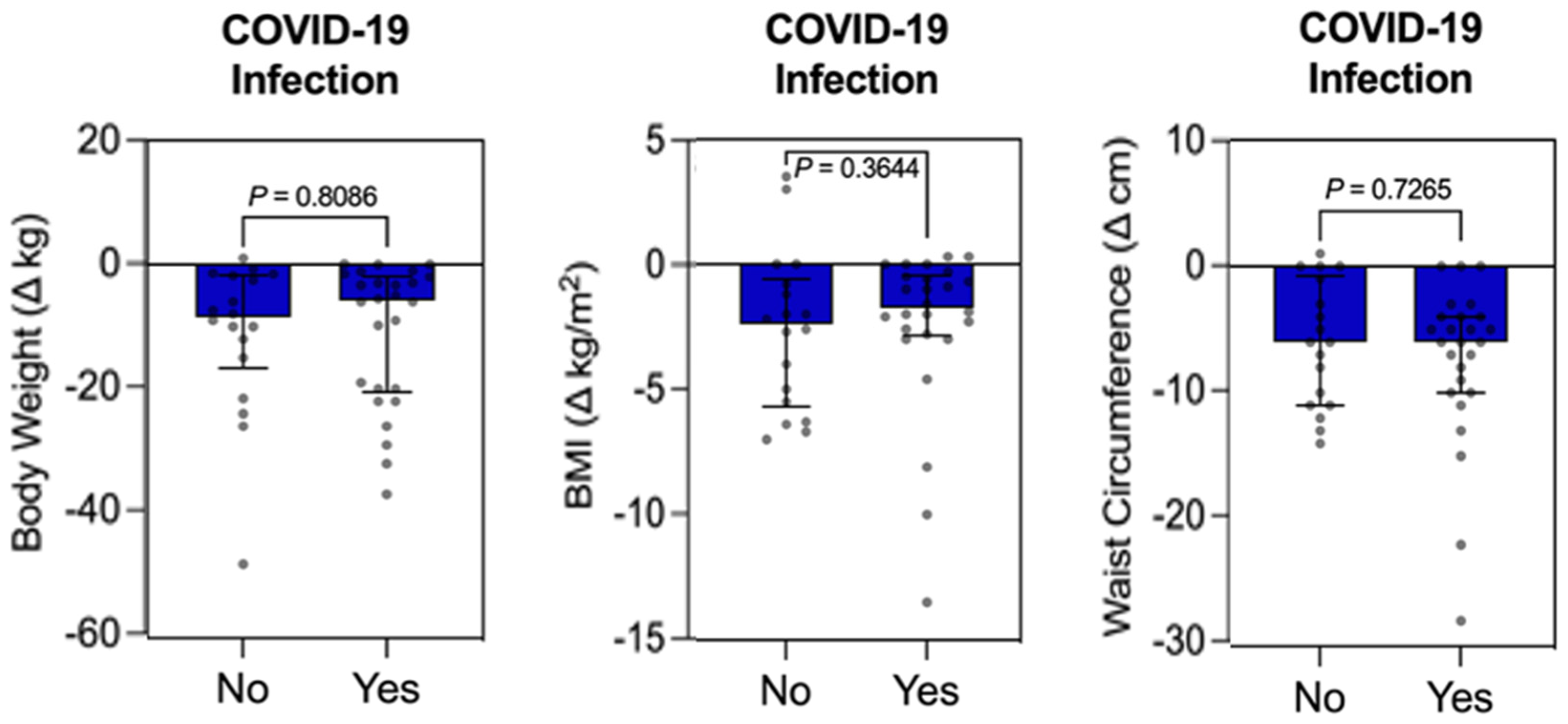
3.4. Individual Influence Analysis Between Qualitative Variable (COVID-19 Infection) vs. Quantitative Variables (FBG, HbA1c, LDL-C, HDL-C, TG, Weight, BMI, WC, SBP, and DBP) in the Intervention Group
3.5. Qualitative Results
- (a)
- Physical Exercise and Medications
- (b)
- COVID-19 Infection
4. Discussion
Limitations
5. Conclusions
6. Take-Home Message
- COVID-19 may significantly interfere with glycemic and blood pressure control in patients with T2D and obesity. This highlights the importance of managing these health parameters during and after the pandemic, particularly in a public health context. However, the results should be generalized with caution due to study limitations that might introduce bias.
- Nutritional strategies are crucial for enhancing quality of life by managing blood glucose, reducing weight, and lowering cardiovascular risk in patients with T2D and obesity. Despite these interventions, medication use, and constant monitoring, the effects of COVID-19 on SBP and HbA1c were not fully mitigated. Encouraging adherence to the MedDiet, alongside lifestyle modifications for weight loss, could reduce the COVID-19 risk in older adults with high cardiovascular risks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: http://www.diabetesatlas.org (accessed on 20 May 2024).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S128–S139. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140, Erratum in Eur. Heart J. 2023, 44, 5060; Erratum in Eur. Heart J. 2024, 45, 518. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Fact Sheet: Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 May 2024).
- World Health Organization (WHO). Proposed Policy Priorities for Preventing Obesity and Diabetes in the Eastern Mediterranean Region; Technical Report Series; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef]
- Minari, T.P.; Tácito, L.H.B.; Yugar, L.B.T.; Ferreira-Melo, S.E.; Manzano, C.F.; Pires, A.C.; Moreno, H.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Yugar-Toledo, J.C. Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review. Nutrients 2023, 15, 5096. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S158–S190. [Google Scholar] [CrossRef]
- Kökten, T.; Hansmannel, F.; Ndiaye, N.C.; Heba, A.C.; Quilliot, D.; Dreumont, N.; Arnone, D.; Peyrin-Biroulet, L. Calorie Restriction as a New Treatment of Inflammatory Diseases. Adv. Nutr. 2021, 12, 1558–1570. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Minari, T.P.; Araújo-Filho, G.M.d.; Tácito, L.H.B.; Yugar, L.B.T.; Rubio, T.d.A.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Fattori, A.; Yugar-Toledo, J.C.; et al. Effects of Mindful Eating in Patients with Obesity and Binge Eating Disorder. Nutrients 2024, 16, 884. [Google Scholar] [CrossRef]
- Khunti, K.; Valabhji, J.; Misra, S. Diabetes and the COVID-19 pandemic. Diabetologia 2023, 66, 255–266. [Google Scholar] [CrossRef]
- Farman, M.; Akgül, A.; Sultan, M.; Riaz, S.; Asif, H.; Agarwal, P.; Hassani, M.K. Numerical study and dynamics analysis of diabetes mellitus with co-infection of COVID-19 virus by using fractal fractional operator. Sci. Rep. 2024, 14, 16489. [Google Scholar] [CrossRef]
- Naidu, A.S.; Wang, C.K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.F.; Yen, C.H.; Porretta, S.; Mathai, I.; et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. NPJ Sci. Food 2024, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B.; RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86002. [Google Scholar] [CrossRef] [PubMed]
- Minari, T.P.; Manzano, C.F.; Tácito, L.H.B.; Yugar, L.B.T.; Sedenho-Prado, L.G.; Rubio, T.d.A.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Moreno, H.; et al. The Impact of a Nutritional Intervention on Glycemic Control and Cardiovascular Risk Markers in Type 2 Diabetes. Nutrients 2024, 16, 1378. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.S.; Araujo, M.A.; Ornelas, G.C.; Logrado, M.H. Validação de Instrumento de Triagem Nutricional [Validation of a nutritional screening tool]. Acta Med. Port. 2012, 25, 10–14. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metada-ta-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Graphpad Prism for Windows, Version 9.0. GraphPad Software: La Jolla, CA, USA, 2015. Available online: www.graphpad.com (accessed on 16 January 2024).
- Calcaterra, V.; Zanelli, S.; Foppiani, A.; Verduci, E.; Benatti, B.; Bollina, R.; Bombaci, F.; Brucato, A.; Cammarata, S.; Calabrò, E.; et al. Long COVID in Children, Adults, and Vulnerable Populations: A Comprehensive Overview for an Integrated Approach. Diseases 2024, 12, 95. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symp-toms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- LaVergne, S.M.; Stromberg, S.; Baxter, B.A.; Webb, T.L.; Dutt, T.S.; Berry, K.; Tipton, M.; Haberman, J.; Massey, B.R.; McFann, K.; et al. A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae. BMC Infect. Dis. 2021, 21, 677. [Google Scholar] [CrossRef]
- Sneller, M.C.; Liang, C.J.; Marques, A.R.; Chung, J.Y.; Shanbhag, S.M.; Fontana, J.R.; Raza, H.; Okeke, O.; Dewar, R.L.; Higgins, B.P.; et al. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann. Intern. Med. 2022, 175, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946, Erratum in JAMA 2024, 331, 1505. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Post-COVID dysautonomias: What we know and (mainly) what we don’t know. Nat. Rev. Neurol. 2024, 20, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Skrypnik, D. New-Onset Diabetes Mellitus, Hypertension, Dyslipidaemia as Sequelae of COVID-19 Infection-Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 13280. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 and diabetes: A bidirectional relationship. Clin. Investig. Arterioscler. 2021, 33, 151–157. [Google Scholar] [CrossRef]
- Kim, S.H.; Arora, I.; Hsia, D.S.; Knowler, W.C.; LeBlanc, E.; Mylonakis, E.; Pratley, R.; Pittas, A.G. New-Onset Diabetes After COVID-19. J. Clin. Endocrinol. Metab. 2023, 108, e1164–e1174. [Google Scholar] [CrossRef]
- Shyam, S.; García-Gavilán, J.F.; Paz-Graniel, I.; Gaforio, J.J.; Martínez-González, M.Á.; Corella, D.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; Vioque, J.; et al. Intensive Weight-Loss Lifestyle Intervention Using Mediterranean Diet and COVID-19 Risk in Older Adults: Secondary Analysis of PREDIMED-Plus Trial. J. Nutr. Health Aging 2023, 27, 1162–1167. [Google Scholar] [CrossRef]
- Chui, T.K.; Cedillo, Y.E.; El Zein, A.; Pavela, G.; Caldwell, A.E.; Peters, J.C.; Friedman, J.E.; DebRoy, S.; Oslund, J.L.; Das, S.K.; et al. Evaluation of socioecological factors on health behaviors and weight change during major life event: A cross-sectional study using data collected during the COVID-19 pandemic. Obes. Sci. Pract. 2024, 10, e785. [Google Scholar] [CrossRef]
- Varghese, J.S.; Ali, M.K.; Guo, Y.; Donahoo, W.T.; Chakkalakal, R.J. Risk of New-Onset Diabetes Before and During the COVID-19 Pandemic: A Real-World Cohort Study. J Gen Intern Med. 2024. [Google Scholar] [CrossRef]
- Minari, T.P.; Manzano, C.F.; Yugar, L.B.T.; Sedenho-Prado, L.G.; de Azevedo Rubio, T.; Tácito, L.H.B.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Ludovico, N.D.; et al. Demystifying Obesity: Understanding, Prevention, Treatment, and Stigmas. Nutr. Rev. 2024, nuae144. [Google Scholar] [CrossRef]
- Gruneck, L.; Marriott, L.K.; Gentekaki, E.; Kespechara, K.; Sharpton, T.J.; Denny, J.; Shannon, J.; Popluechai, S. A Non-Randomized Trial Investigating the Impact of Brown Rice Consumption on Gut Microbiota, Attention, and Short-Term Working Memory in Thai School-Aged Children. Nutrients 2022, 14, 5176. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.K.; Taillie, L.; Blanchard, L.M.; Wixom, N.; Harrington, D.K.; Peterson, D.R.; Wittlin, S.D.; Campbell, T.M. Post hoc analysis of food costs associated with Dietary Approaches to Stop Hypertension diet, whole food, plant-based diet, and typical baseline diet of individuals with insulin-treated type 2 diabetes mellitus in a nonrandomized crossover trial with meals provided. Am. J. Clin. Nutr. 2024, 119, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Jooste, B.R.; Kolivas, D.; Brukner, P.; Moschonis, G. Effectiveness of Technology-Enabled, Low Carbohydrate Dietary Interventions, in the Prevention or Treatment of Type 2 Diabetes Mellitus in Adults: A Systematic Literature Review of Randomised Controlled and Non-Randomised Trials. Nutrients 2023, 15, 4362. [Google Scholar] [CrossRef] [PubMed]
- Levintow, S.N.; Nielson, C.M.; Hernandez, R.K.; Breskin, A.; Pritchard, D.; Lash, T.L.; Rothman, K.J.; Gilbertson, D.; Muntner, P.; Critchlow, C.; et al. Pragmatic considerations for negative control outcome studies to guide non-randomized comparative analyses: A narrative review. Pharmacoepidemiol. Drug Saf. 2023, 32, 599–606. [Google Scholar] [CrossRef]

| Parameter | Control Group | Intervention Group | p-Value |
|---|---|---|---|
| Gender | Male: 50%, Female: 50% | Male: 27.3%, Female: 72.7% | n/a |
| Age (years) | 62.2 ± 8.0 | 64.2 ± 8.6 | >0.05 |
| Race | White: 25%, Brown: 35%, Black: 40% | White: 25%, Brown: 35%, Black: 40% | n/a |
| Socioeconomic Status | Class C: 65%, Class D: 35% | Class C: 63.6%, Class D: 36.4% | n/a |
| Exercise Habits | None: 100% | None: 100% | n/a |
| FBG (mg/dL) | 159.0 (196.5–132.0) | 148.0 (195.3–130.0) | >0.05 |
| HbA1c (%) | 8.7 (9.3–7.3) | 7.5 (10.0–6.8) | >0.05 |
| TC (mg/dL) | 171.5 (199.8–142.0) | 159.0 (211.5–129.0) | >0.05 |
| LDL-C (mg/dL) | 102.4 ± 15.1 | 102.3 ± 19.7 | >0.05 |
| HDL-C (mg/dL) | 43.0 (49.5–35.5) | 46.0 (53.0–40.0) | >0.05 |
| TG (mg/dL) | 165.5 (201.5–154.0) | 168.0 (195.0–151.8) | >0.05 |
| Body Weight (kg) | 87.7 (98.0–71.5) | 87.0 (92.9–79.9) | >0.05 |
| BMI (kg/m2) | 30.0 (35.5–30.0) | 31.5 (35.7–30.0) | >0.05 |
| WC (cm) | 106.8 ± 12.0 | 106.7 ± 7.5 | >0.05 |
| SBP (mmHg) | 145.3 ± 11.0 | 146.8 ± 17.1 | >0.05 |
| DBP (mmHg) | 88.5 (98.0–87.0) | 95.7 (99.0–91.6) | 0.0464 * |
| Parameter | Intervention Group (p-Value) | Control Group (p-Value) |
|---|---|---|
| Weight | <0.0001 * | <0.0001 * |
| BMI (kg/m2) | <0.0001 * | <0.0001 * |
| WC (cm) | <0.0001 * | >0.05 |
| FBG (mg/dL) | <0.0001 * | 0.0028 * |
| HbA1c (%) | <0.0001 * | <0.0001 * |
| TC (mg/dL) | >0.05 | >0.05 |
| LDL-C (mg/dL) | <0.0001 * | >0.05 |
| HDL-C (mg/dL) | 0.0105 * | >0.05 |
| TG (mg/dL) | <0.0001 * | >0.05 |
| SBP (mmHg) | <0.0001 * | >0.05 |
| DBP (mmHg) | <0.0001 * | >0.05 |
| Control | Intervention | ||||
|---|---|---|---|---|---|
| COVID-19 infection Over 12 months | No 12 (30.0%) | Yes 28 (70.0%) | No 12 (27.3%) | Yes 32 (72.7%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minari, T.P.; Manzano, C.F.; Tácito Yugar, L.B.; Sedenho-Prado, L.G.; Rubio, T.d.A.; Tácito, L.H.B.; Pires, A.C.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Ludovico, N.D.; et al. The Possible Impact of COVID-19 on Glycated Hemoglobin and Systolic Blood Pressure in Type 2 Diabetes and Obesity. Obesities 2024, 4, 412-426. https://doi.org/10.3390/obesities4040033
Minari TP, Manzano CF, Tácito Yugar LB, Sedenho-Prado LG, Rubio TdA, Tácito LHB, Pires AC, Vilela-Martin JF, Cosenso-Martin LN, Ludovico ND, et al. The Possible Impact of COVID-19 on Glycated Hemoglobin and Systolic Blood Pressure in Type 2 Diabetes and Obesity. Obesities. 2024; 4(4):412-426. https://doi.org/10.3390/obesities4040033
Chicago/Turabian StyleMinari, Tatiana Palotta, Carolina Freitas Manzano, Louise Buonalumi Tácito Yugar, Luis Gustavo Sedenho-Prado, Tatiane de Azevedo Rubio, Lúcia Helena Bonalumi Tácito, Antônio Carlos Pires, José Fernando Vilela-Martin, Luciana Neves Cosenso-Martin, Nelson Dinamarco Ludovico, and et al. 2024. "The Possible Impact of COVID-19 on Glycated Hemoglobin and Systolic Blood Pressure in Type 2 Diabetes and Obesity" Obesities 4, no. 4: 412-426. https://doi.org/10.3390/obesities4040033
APA StyleMinari, T. P., Manzano, C. F., Tácito Yugar, L. B., Sedenho-Prado, L. G., Rubio, T. d. A., Tácito, L. H. B., Pires, A. C., Vilela-Martin, J. F., Cosenso-Martin, L. N., Ludovico, N. D., Fattori, A., Yugar-Toledo, J. C., Moreno, H., & Pisani, L. P. (2024). The Possible Impact of COVID-19 on Glycated Hemoglobin and Systolic Blood Pressure in Type 2 Diabetes and Obesity. Obesities, 4(4), 412-426. https://doi.org/10.3390/obesities4040033







