Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays
Abstract
:1. Introduction
2. Inhibition Assays for Fat Metabolizing Enzymes
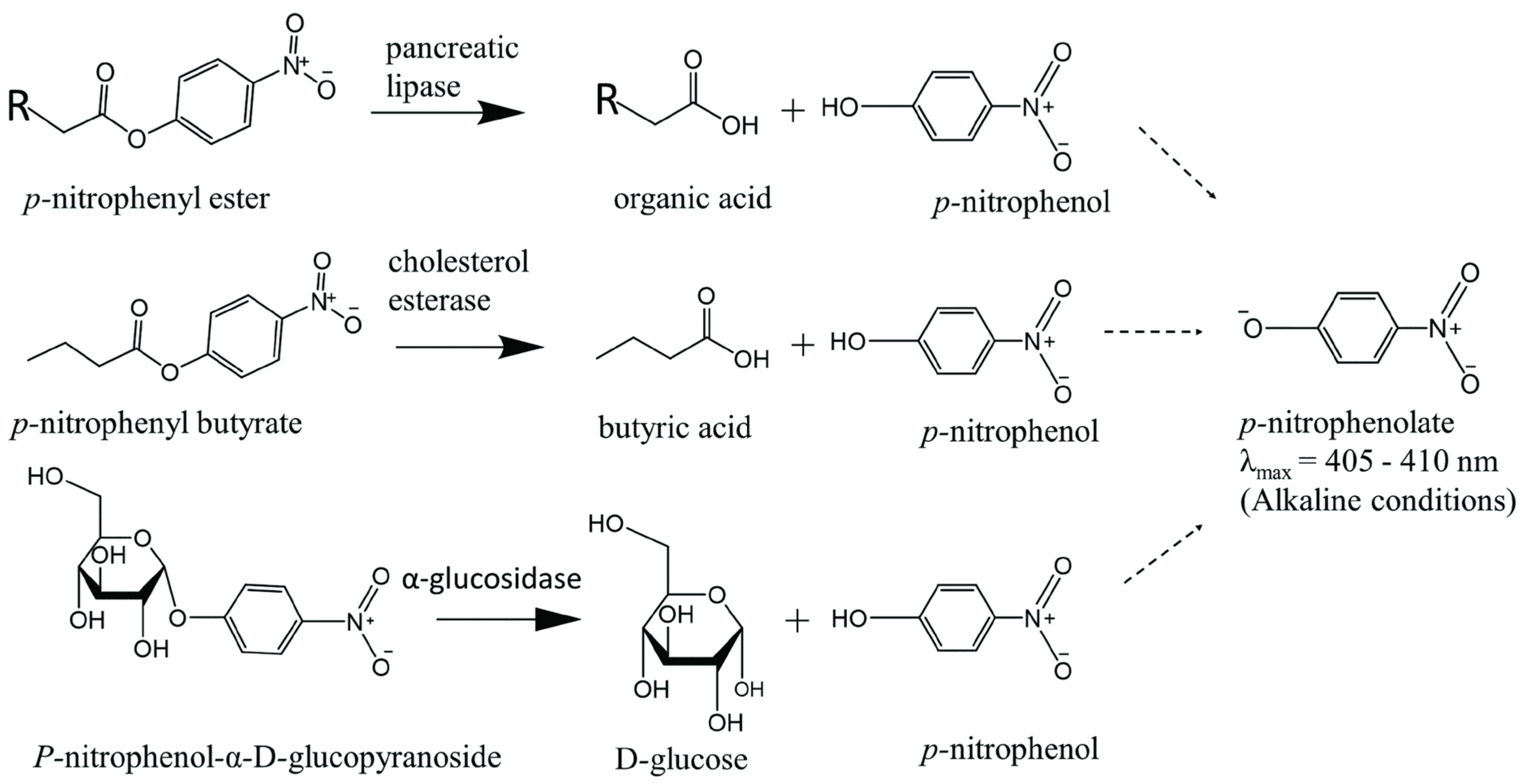
2.1. Assay Methods to Screen for Pancreatic Lipase Inhibitors
2.2. Assay Methods to Screen for Pancreatic Cholesterol Esterase Inhibitors
3. Inhibition Assays for Carbohydrate Metabolizing Enzymes
3.1. Assay Methods to Screen for α-Glucosidase Inhibitors
3.2. Assay Methods to Screen for α-Amylase Inhibitors
| Enzyme Concentration | Substrate Used | Buffer Used | Extract Concentration | Preincubation (Before Substrate) | Incubation | Solution/Method Used for Termination | Wavelength (nm) | Ref |
|---|---|---|---|---|---|---|---|---|
| 0.5 units/mL | 5 mM p-nitrophenyl- α-D-glucopyranoside | 10 mM potassium phosphate buffer (pH 6.8) | Various concentrations in buffer | 37 °C for 15 min | 37 °C for 15 min | 200 mM sodium carbonate | 405 | [15] |
| 1 unit/mL | p-nitrophenyl- α-D-glucopyranoside | Phosphate buffer | 100 to 500 µg/mL in buffer | 37 °C for 15 min | 37 °C for 20 min | 0.1 M sodium carbonate | 405 | [17] |
| Not given | 20 mM p-nitrophenyl-α-D-glucopyranoside | 0.5 M phosphate buffer, pH 6.5 | Not given | 37 °C for 15 min | 37 °C for 35 min | Not given | 405 | [23] |
| 1 unit/mL in buffer | 5 mM p-nitrophenyl-α-D-glucopyranoside | 50 mM phosphate buffer, pH 6.8 | 100 µg in 70 µL buffer | 37 °C for 10 min | 37 °C for 30 min | Not given | 405 | [50] |
| 50 µg/mL | p-nitro-phenyl-a-D-glucopyranoside | Not given | Not given | 37 °C for 5 min | 37 °C for 30 min | sodium carbonate solution | 405 | [29] |
| Not given | 5 mM p-nitrophenyl-α-D-glucopyranoside | 0.1 M Citrate-phosphate buffer, pH 7.0 | Not given | 37 °C for four periods of time | Not given | 0.05 M sodium hydroxide | 410 | [30] |
| 0.2 units/mL | 0.5 mM p-nitrophenyl-α-D-glucopyranoside | 0.1 M phosphate buffer, pH 7.0 | 25 to 85 µg/mL | N/A | 37 °C for 30 min | 0.2 M sodium carbonate | 410 | [31] |
| 0.26 units/mL in buffer | 0.3125 mM p-nitrophenyl-α-D-glucopyranoside | 0.1 M phosphate buffer, pH 6.8 | Various concentrations of diluted sample in buffer | 37 °C for 15 min | 37 °C for 15 min | 0.2 M sodium carbonate | 405 | [32] |
| Enzyme dissolved in buffer (concentration not given) | 20 mM p-nitrophenyl-α-D-glucopyranoside | 0.5 M phosphate buffer, pH 6.5 | Samples dissolved in 80% methanol | 37 °C for 15 min | 37 °C for 35 min | Not given | 405 | [33] |
| 1 unit/mL | 5 mM p-nitrophenyl-α-D-glucopyranoside | 100 mM phosphate buffer, pH 6.8 | 100 to 500 mg/mL | 37 °C for 15 min | 37 °C for 20 min | 0.1 M sodium carbonate | 405 | [35] |
| 1 unit/mL | p-nitrophenyl-α-D-glucopyranoside | Not given | 3.12 to 200 µg/mL | 37 °C for 10 min | 37 °C for 30 min | 0.2 M sodium carbonate | 405 | [36] |
| Enzyme Concentration | Substrate Used | Buffer Used | Extract Concentration | Preincubation (Before Substrate) | Incubation | Reagent Added to Generate Color | Solution/Method Used for Termination | Wavelength (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 unit/mL in buffer | 0.5% starch in buffer | 20 mM sodium phosphate buffer with 6 mM NaCl (pH 6.9) | 15.6 to 250 mg/L in buffer | 37 °C for 15 min | 37 °C for 15 min | 3,5-dinitrosalicylic acid | boiling for 5 min | 540 | [15] |
| 2 units/mL in 10% DMSO and buffer | 1 g/100 mL corn starch in water | Not given | 5 to 500 µg/mL in 10% DMSO and buffer | 30 °C for 10 min | 30 °C for 3 min | 3,5-dinitrosalicylic acid | Boiling 85–90 °C for 10 min | 540 | [17] |
| 1.25 µg/mL in buffer | 200 µg/mL DQ™ starch from corn, BODIPY® FL conjugate | Same buffer used in Lipase activity assay * | 2.5 mg/mL in DMSO (final concentration) | none | 37 °C for 30 min | none | N/A | Em/Ex = 535/485 | [18] |
| Not given | 0.5% potato starch in buffer | Phosphate buffer, pH 6.9 | Not given | 37 °C for 5 min | 37 °C for 3 min | 96 mM 3,5-dinitrosalicylic acid in 5.31 M sodium potassium tartrate, 2 M NaOH | 85 °C heater for 15 min | 540 | [23] |
| 2 units/mL in 10% DMSO | 1% starch solution | 0.02 M sodium phosphate, 0.006 M NaCl, at pH 6.9 | 10 to 500 µg/mL in 10% DMSO and buffer | 30 °C for 10 min | 30 °C for 3 min | 3,5-dinitrosalicylic acid | Boiling at 90 °C for 10 min | 540 | [24] |
| 2 units/mL in buffer | 1% starch mixed with buffer | 0.02 M sodium phosphate buffer, pH 6.9 | 10 to 500 µg/mL | 25 °C for 10 min | 25 °C for 10 min | 3,5-dinitrosalicylic acid | Boiling for 5 min | 540 | [27] |
| 2 units/mL stock prepared by dissolving 25 mg in 10% DMSO and diluting in buffer up to 100 mL | 1% corn starch in water | Not given | 1 mg/mL stock in 10% DMSO diluted to 10 to 500 µg/mL | 30 °C for 10 min | 30 °C for 3 min | 3,5-dinitrosalicylic acid | Boiling 85–90 °C for 10 min | 540 | [28] |
| 0.1 mg/mL | Starch solution | Phosphate buffer | Not given | 37 °C for 10 min | 37 °C for 30 min | Iodine reagent | Added 1 M HCl | 580 | [29] |
| Not given | 1% starch in buffer | 0.05 M Tris, pH 7.0 buffer with 38 mM NaCl and 0.1 mM CaCl2 | Not given | 37 °C for 20 min | 37 °C for four periods of time | 3,5-dinitrosalicylic acid | Not given | 540 | [30] |
| 2.0 units/mL in buffer | Starch azure solution in buffer | 50 mM Tris-HCl buffer, pH 6.9, containing 10 mM CaCl2 | 20 to 100 µg/mL in 25% DMSO | 37 °C for 10 min | 37 °C for 10 min | N/A | Added 50% acetic acid | 595 | [31] |
| 0.25 units/mL in buffer | 2.5% w/v potato starch in buffer | 20 mM phosphate buffer, pH 6.9 | Samples in 80% methanol (concentration not given) | Room temperature for 5 min | 15 min (temperature not specified) | 3,5-dinitrosalicylic acid | 80 °C for 40 min | 540 | [33] |
| 50 mg enzyme in 100 mL buffer | 2-chloro-4-nitrophenol-α-D-maltotrioside | 40 mM phosphate buffer, pH 6.9 | 25 to 100 µg/mL in DMSO | N/A | 37 °C for 5 min | N/A | N/A | 405 | [34] |
| 2 units/mL in 10% DMSO | 1% starch solution | 0.02 M Na2HPO4/NaH2PO4, 0.006 M NaCl, pH 6.9 | 10 to 500 µg/mL | 30 °C for 10 min | 30 °C for 3 min | 3,5-dinitrosalicylic acid | 90 °C for 10 min | 540 | [35] |
| 500 µg/mL | 0.25% starch solution | Not given | 3.12 to 200 µg/mL | 37 °C for 10 min | 37 °C for 30 min | Lugol’s solution | 1 M HCl | 620 | [36] |
4. Additional Considerations and Limitations for the Enzyme Inhibitory Assays
4.1. Pancreatic Lipase Inhibitory Assays
4.2. Cholesterol Esterase and α-Glucosidase Inhibitory Assays
4.3. α-Amylase Inhibitory Assays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.L.; Wu, Q.X.; Wei, X.; Qin, X.M. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Kass, D.A. COVID-19 and Severe Obesity: A Big Problem? Ann. Intern. Med. 2020, 173, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Karale, P.; Dhawale, S.C.; Karale, M.A. Quantitative Phytochemical Profile, Antioxidant and Lipase Inhibitory Potential of Leaves of Momordica charantia L. and Psoralea corylifolia L. Indian J. Pharm. Sci. 2022, 84, 189–196. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef] [Green Version]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2021, 12, 807548. [Google Scholar] [CrossRef]
- Tucci, S.A.; Boyland, E.J.; Halford, J.C. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: A review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes. 2010, 3, 125–143. [Google Scholar] [CrossRef] [Green Version]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Olivares-Vicente, M.; Encinar, J.A.; Barrajón-Catalán, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients 2017, 9, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr. J. 2011, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and Epigenetics Action Mechanisms of Antiobesity Medicinal Plants and Phytochemicals. Evid.-Based Complement. Altern. Med. 2021, 2021, 9995903. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Ali-Shtayeh, M.S. Antiobesity and Antioxidant Potentials of Selected Palestinian Medicinal Plants. Evid.-Based Complement. Altern. Med. 2018, 2018, 8426752. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Jaradat, N.; Qneibi, M.; Hawash, M.; Emwas, N. Free radicals and enzymes inhibitory potentials of the traditional medicinal plant Echium angustifolium. Eur. J. Integr. Med. 2020, 38, 101196. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus—Screening for Pancreatic Lipase and alpha-Amylase Inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Jiao, P.; Tseng-Crank, J.; Corneliusen, B.; Yimam, M.; Hodges, M.; Hong, M.; Maurseth, C.; Oh, M.; Kim, H.; Chu, M.; et al. Lipase Inhibition and Antiobesity Effect of Atractylodes lancea. Planta Med. 2014, 80, 577–582. [Google Scholar] [CrossRef]
- Ekanem, A.P.; Wang, M.; Simon, J.E.; Moreno, D.A. Antiobesity properties of two African plants (Afromomum meleguetta and Spilanthes acmella) by pancreatic lipase inhibition. Phytother. Res. 2007, 21, 1253–1255. [Google Scholar] [CrossRef]
- Velusami, C.C.; Agarwal, A.; Mookambeswaran, V. Effect of Nelumbo nucifera Petal Extracts on Lipase, Adipogenesis, Adipolysis, and Central Receptors of Obesity. Evid.-Based Complement. Altern. Med. 2013, 2013, 145925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Karmase, A.; Jagtap, S.; Shekhar, R.; Bhutani, K.K. Pancreatic Lipase Inhibitory Activity of Cassiamin A, a Bianthraquinone from Cassia siamea. Nat. Prod. Commun. 2013, 8, 195–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gok, H.N.; Orhan, N.; Ozupek, B.; Pekacar, S.; Selvi, S.N.; Orhan, D.D. Standardization of Juniperus macrocarpa Sibt. & Sm. and Juniperus excelsa M. Bieb. Extracts with Carbohydrate Digestive Enzyme Inhibitory and Antioxidant Activities. Iran. J. Pharm. Res. 2021, 20, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Qadi, M.; Jaradat, N.; Al-lahham, S.; Ali, I.; Abualhasan, M.N.; Shraim, N.; Hussein, F.; Issa, L.; Mousa, A.; Zarour, A.; et al. Antibacterial, Anticandidal, Phytochemical, and Biological Evaluations of Pellitory Plant. BioMed Res. Int. 2020, 2020, 6965306. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Raza, M.A.; Mukhtar, H.; Irfan, A.; Raza, S.A.; Touqeer, T.; Nadeem, M.; Saari, N. Cassia fistula Leaves; UHPLC-QTOF-MS/MS Based Metabolite Profiling and Molecular Docking Insights to Explore Bioactives Role towards Inhibition of Pancreatic Lipase. Plants 2021, 10, 1334. [Google Scholar] [CrossRef]
- Suh, D.H.; Jung, E.S.; Park, H.M.; Kim, S.H.; Lee, S.; Jo, Y.H.; Lee, M.K.; Jung, G.; Do, S.G.; Lee, C.H. Comparison of Metabolites Variation and Antiobesity Effects of Fermented versus Nonfermented Mixtures of Cudrania tricuspidata, Lonicera caerulea, and Soybean According to Fermentation In Vitro and In Vivo. PLoS ONE 2016, 11, e0149022. [Google Scholar] [CrossRef]
- Jaradat, N.; Qneibi, M.; Hawash, M.; Sawalha, A.; Qtaishat, S.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antiobesity, and Antidiabetic Effects ofHelichrysum sanguineum (L.) Kostel. from Palestine. Arab. J. Sci. Eng. 2021, 46, 41–51. [Google Scholar] [CrossRef]
- Jaradat, N.; Qadi, M.; Ali, I.; Hussein, F.; Issa, L.; Rashdan, D.; Jamoos, M.; Najem, R.; Zarour, A.; Arar, M. Phytochemical screening, antiobesity, antidiabetic and antimicrobial assessments of Orobanche aegyptiaca from Palestine. BMC Complement. Med. Ther. 2021, 21, 256. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. In vitro alpha-amylase, alpha-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon 2018, 4, e00810. [Google Scholar] [CrossRef] [Green Version]
- Simao, A.A.; Marques, T.R.; Marcussi, S.; Correa, A.D. Aqueous extract of Psidium guajava leaves: Phenolic compounds and inhibitory potential on digestive enzymes. Anais Acad. Bras. Cienc. 2017, 89, 2155–2165. [Google Scholar] [CrossRef]
- Dos Santos, U.P.; Tolentino, G.S.; Morais, J.S.; Souza, K.D.; Estevinho, L.M.; dos Santos, E.L. Physicochemical Characterization, Microbiological Quality and Safety, and Pharmacological Potential of Hancornia speciosa Gomes. Oxidative Med. Cell. Longev. 2018, 2018, 2976985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, G.L.; Zhang, Y.L.; Yang, M.; Chen, J.M.; Guo, M.Q. Potential hypoglycemic, hypolipidemic, and anti-inflammatory bioactive components in Nelumbo nucifera leaves explored by bioaffinity ultrafiltration with multiple targets. Food Chem. 2022, 375, 131856. [Google Scholar] [CrossRef] [PubMed]
- Pekacar, S.; Orhan, D.D. Investigation of Antidiabetic Effect of Pistacia atlantica Leaves by Activity-Guided Fractionation and Phytochemical Content Analysis by LC-QTOF-MS. Front. Pharmacol. 2022, 13, 826261. [Google Scholar] [CrossRef]
- Jerbi, A.; Derbali, A.; Elfeki, A.; Kammoun, M. Essential Oil Composition and Biological Activities of Eucalyptus globulus Leaves Extracts from Tunisia. J. Essent. Oil Bear. Plants 2017, 20, 438–448. [Google Scholar] [CrossRef]
- Jaradat, N.; Dacca, H.; Hawash, M.; Abualhasan, M.N. Ephedra alata fruit extracts: Phytochemical screening, anti-proliferative activity and inhibition of DPPH, alpha-amylase, alpha-glucosidase, and lipase enzymes. BMC Chem. 2021, 15, 41. [Google Scholar] [CrossRef]
- Mba, J.R.; Zouheira, D.; Dairou, H.; Yadang, F.S.A.; Gael, N.N.; Ayong, L.; Kuiate, J.R.; Agbor, G.A. In Vitro Antioxidant, Anti-Inflammatory, and Digestive Enzymes Inhibition Activities of Hydro-Ethanolic Leaf and Bark Extracts of Psychotria densinervia (K. Krause) Verdc. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8459943. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Amate, L.; Gil, A. Absorption and distribution of dietary fatty acids from different sources. Early Hum. Dev. 2001, 65, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Carlier, H.; Bernard, A.; Caselli, C. Digestion and absorption of polyunsaturated fatty acids. Reprod. Nutr. Dev. 1991, 31, 475–500. [Google Scholar] [CrossRef]
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-Lowering Activity of the Major Polyphenols in Grape Seed. Molecules 2011, 16, 5054. [Google Scholar] [CrossRef]
- Pietsch, M.; Gütschow, M. Synthesis of Tricyclic 1,3-Oxazin-4-ones and Kinetic Analysis of Cholesterol Esterase and Acetylcholinesterase Inhibition. J. Med. Chem. 2005, 48, 8270–8288. [Google Scholar] [CrossRef]
- Vo, C.-V.T.; Luu, N.V.H.; Nguyen, T.T.H.; Nguyen, T.T.; Ho, B.Q.; Nguyen, T.H.; Tran, T.-D.; Nguyen, Q.-T. Screening for pancreatic lipase inhibitors: Evaluating assay conditions using p-nitrophenyl palmitate as substrate. All Life 2022, 15, 13–22. [Google Scholar] [CrossRef]
- Pohanka, M. Biosensors and Bioassays Based on Lipases, Principles and Applications, a Review. Molecules 2019, 24, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Xenoulis, P.; Stavroulaki, E.; Lidbury, J.A.; Suchodolski, J.S.; Carrière, F.; Steiner, J.M. The 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6’-methylresorufin) ester (DGGR) lipase assay in cats and dogs is not specific for pancreatic lipase. Vet. Clin. Pathol. 2020, 49, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.; Roy, S.K.; Singh, A.; Bhutani, K.K. Pancreatic lipase inhibitory alkaloids of Murraya koenigii leaves. Nat. Prod. Commun. 2009, 4, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Vahouny, G.V.; Weersing, S.; Treadwell, C.R. Function of specific bile acids in cholesterol esterase activity in vitro. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1965, 98, 607–616. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Keharom, S.; Mahachai, R.; Chanthai, S. The optimization study of α-amylase activity based on central composite design-response surface methodology by dinitrosalicylic acid method. Int. Food Res. J. 2016, 23, 10–17. [Google Scholar]
- Lehoczki, G.; Kandra, L.; Gyémánt, G. The use of starch azure for measurement of alpha-amylase activity. Carbohydr. Polym. 2018, 183, 263–266. [Google Scholar] [CrossRef]
- Niaz, A.; Adnan, A.; Bashir, R.; Mumtaz, M.W.; Raza, S.A.; Rashid, U.; Tan, C.P.; Tan, T.B. The In Vitro alpha-Glucosidase Inhibition Activity of Various Solvent Fractions of Tamarix dioica and H-1-NMR Based Metabolite Identification and Molecular Docking Analysis. Plants 2021, 10, 1128. [Google Scholar] [CrossRef]
- Gupta, R.; Rathi, P.; Gupta, N.; Bradoo, S. Lipase assays for conventional and molecular screening: An overview. Biotechnol. Appl. Biochem. 2003, 37, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hriscu, M.; Chris, L.; Tosa, M.; Irimie, F.D. pH-Profiling of thermoactive lipases and esterases: Caveats and further notes. Eur. J. Lipid Sci. Technol. 2013, 115, 571–575. [Google Scholar] [CrossRef]
- Hotta, Y.; Ezaki, S.; Atomi, H.; Imanaka, T. Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Appl. Environ. Microbiol. 2002, 68, 3925–3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kademi, A.; Aït-Abdelkader, N.; Fakhreddine, L.; Baratti, J. Purification and characterization of a thermostable esterase from the moderate thermophile Bacillus circulans. Appl. Microbiol. Biotechnol. 2000, 54, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nyambe-Silavwe, H.; Villa-Rodriguez, J.A.; Ifie, I.; Holmes, M.; Aydin, E.; Jensen, J.M.; Williamson, G. Inhibition of human α-amylase by dietary polyphenols. J. Funct. Foods 2015, 19, 723–732. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]

| Plant Name | Parts Used | Extraction Solvent | Enzyme Inhibited | Ref | |||
|---|---|---|---|---|---|---|---|
| Pancreatic Lipase | Pancreatic α-Amylase | α-Glucosidase | Cholesterol Esterase | ||||
| Sweet gale (Myrica gale L.) | Stems & leaves | Hot water extract and ethanol extract | – | √ | √ | – | [15] |
| Roseroot (Rhodiola rosea L.) | |||||||
| Sheep sorrel (Rumex acetosa L.) | |||||||
| Stinging nettles (Utrica dioica L.) | |||||||
| Dandelion (Taraxacum officinale L.) | |||||||
| 39 plant species from several different families | Different parts | 70% ethanol | √ | – | – | – | [16] |
| Bugloss (Echium angustifolium Mill.) | Leaves | Methanol, water, acetone and hexane as separate extracts | √ | √ | √ | – | [17] |
| 23 plant species from few different families | Different parts | Water extract and methanol extract | √ | √ | – | – | [18] |
| Cang zhu (Atractylodes lancea (Thunb.) DC.) | rhizome | Methylene chloride/methanol mix (1:1) | √ | – | – | – | [19] |
| Guinea pepper (Aframomum melegueta K.Schum.) and Toothache plant (Spilanthes acmella (L.) L.) | Seeds or flower buds | 70% ethanol | √ | – | – | – | [20] |
| Lotus lily (Nelumbo nucifera Gaertn.) | Flower petals | Methanol extraction followed by water extraction | √ | – | – | – | [21] |
| Siamese cassia (Cassia siamea Lam.) | roots | Ethyl acetate | √ | – | – | – | [22] |
| Large-fruited juniper (Juniperus macrocarpa Sm.) and Greek Juniper (Juniperus excelsa M.Bieb.) | Branches, fruits and leaves | Water, ethyl acetate and methanol (2.5% w/v) as separate extracts | √ | √ | √ | – | [23] |
| Spreading pellitory (Parietaria judaica L.) | Leaves | Methanol, water, acetone and hexane as separate extracts | √ | √ | – | – | [24] |
| Golden shower tree (Cassia fistula L.) | Leaves | 20% to 80% hydroethanol | √ | – | – | – | [25] |
| Bitter melon (Momordica charantia L.) and Babchi (Psoralea corylifolia L.) | Leaves | Successive extraction with chloroform, acetone and 70% ethanol | √ | – | – | – | [4] |
| Mixture of Chinese mulberry (Cudrania tricuspidata (Carrière) Bureau ex Lavallée), Blue Honeysuckle (Lonicera caerulea L.), and Soybean (Glycine hispida (Moench) Maxim.) | Fruits/seeds | 80% methanol | √ | – | – | – | [26] |
| Red everlasting (Helichrysum sanguineum (L.) Kostel.) | Aerial parts | Hexane, acetone, methanol and water as separate extracts | √ | √ | – | – | [27] |
| Egyptian broomrape (Orobanche aegyptiaca Pers.) | Aerial parts | Successive extraction with petroleum ether, methylene chloride, chloroform, methanol and collected separately | √ | √ | – | – | [28] |
| Yellow camellia (Camellia nitidissima C.W.Chi) | Flower | 90% ethanol | √ | – | – | √ | [1] |
| Saltcedar (Tamarix dioica Roxb. ex Roth) | Aerial parts | Methanol (containing 10% water) | – | – | √ | – | [25] |
| Baboon’s cucumber (Kedrostis africana (L.) Cogn.) | Tuber | Ethanol extract and water extract | √ | √ | √ | – | [29] |
| Common guava (Psidium guajava L.) | Leaves | Water | √ | √ | √ | – | [30] |
| Mangaba fruit (Hancornia speciosa Gomes) | Leaves | 96% ethanol | √ | √ | √ | – | [31] |
| East Indian lotus (Nelumbo nucifera Gaertn.) | Leaves | 75% ethanol | √ | – | √ | – | [32] |
| Atlas mastic tree (Pistacia atlantica Desf.) | Leaves | 100% methanol | √ | √ | √ | √ | [33] |
| Southern blue gum (Eucalyptus globulus Labill.) | Leaves | Sequential extraction with hexane, ethyl acetate and methanol | √ | √ | – | – | [34] |
| White shrubby horsetail (Ephedra alata Decne.) | Fruits | Sequential extraction with hexane, acetone, methanol and water | √ | √ | √ | – | [35] |
| Wild coffee (Psychotria densinervia (K.Krause) Verdc.) | Leaves and Bark | 70% ethanol and 30% water mix | √ | √ | √ | √ | [36] |
| Enzyme Concentration | Substrate Used | Product Formed | Buffer Used | Extract Concentration | Preincubation (Before Substrate Addition) | Incubation | Solution/Method Used for Termination | Wavelength (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 10 mg/mL (crude) in buffer | 100 mM p-nitrophenyl butyrate in acetonitrile | p-nitrophenol | 20 mM Tris-HCl buffer, pH 8 | 5 mg/mL in ethanol | 37 °C for 5 min | Not given | N/A | 410 | [16] |
| 1 mg/mL in 10% DMSO | 20.9 mg p-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris-HCl buffer | 20 to 400 µg/mL in 10% DMSO and buffer | 37 °C for 15 min | 37 °C for 30 min | N/A | 405 | [17] |
| 0.5 mg/mL in buffer | 0.5 mM 4-methylumbelliferyl oleate | 4-methylumbelliferone | 13 mM Tris-HCl buffer, pH 8.0 | 2.5 mg/mL final concentration in DMSO | 10 min (temperature not specified) | 37 °C for 30 min | N/A | Em/Ex = 465/360 | [18] |
| Recombinant human pancreatic lipase (concentration Not given) | 1,2-O-dilaury-rac-glycero-3-glutaric acid-(6′-methylresorufin)-ester (concentration not given) | methylresorufin | Assay buffer, pH 8.4 | 120 µg/mL | 10 min (temperature not specified) | 37 °C for 30 min | N/A | 580 nm | [19] |
| Human pancreatic lipase 248 units/L | Reconstituted substrate solution | Quinone diamine dye | Not given | Not given | N/A | 37 °C for 5 min followed by 37 °C for 3 min after adding activator reagent | N/A | 550 nm | [20] |
| Conc. Not given | 4-methyl umbelliferyl oleate | 4-methyl umbelliferone | Tris buffer | 12.5 to 200 µg/mL | Not given | 25 °C for 30 min | N/A | Em/Ex = 460/360 | [21] |
| 1 mg/mL in buffer (0.1 mg/mL final conc.) | 4-nitrophenyl palmitate stock solution (10 mM) in acetonitrile and diluted in ethanol (1:2 v/v) to 3.33 mM (0.167 mM final conc.) | p-nitrophenol | 0.1 mM Tris-HCl buffer, pH 8.5 | 250 µg/mL final conc. in buffer and DMSO | Not given | 37 °C for 30 min | N/A | 405 nm | [22,45] |
| Dissolved In 4-morpholinepropanesulfonic acid (10 mM) and ethylenediaminetetraacetic acid (EDTA, 1 mM) buffer solution pH 6.8 (concentration Not given) | 4-nitrophenyl butyrate | p-nitrophenol | Tris–HCl, 100 mM, and CaCl2, 5 mM, pH 7.0 | 80% w/v in ethanol at logarithmic concentrations | 37 °C for 15 min | 37 °C for 30 min | N/A | 405 | [23] |
| 1 mg/mL stock solution in buffer | 20.9 mg 4-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris–HCl buffer | 50 to 400 µg/mL in 10% DMSO | 37 °C for 15 min | 37 °C for 30 min | N/A | 410 | [24] |
| 1 mg/mL (25 units/mL) | Substrate solution containing Arabic gum (10 g) and olive oil (10% w/v in buffer) | Free fatty acids | 0.01 Tris-HC1 buffer | In buffer (Conc. Not given) | 4 °C for 30 min | 37 °C for 30 min | Added acetone: ethanol (1:1) mixture | N/A | [25] |
| 1 mg/mL in 0.1 mM in potassium phosphate buffer, pH 6.0 | 20.9 mg para-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris-HC1 buffer, pH 7.4 | 50 to 400 µg/mL in 10% DMSO | 25 °C for 15 min | 37 °C for 30 min | N/A | 405 | [4] |
| Prepared in buffer (conc. Not given) | 10 mM 4-nitrophenyl butyrate | p-nitrophenol | 0.1 M Tris-HC1 buffer, pH 8 | Not given | 37 °C for 15 min | 37 °C for 15 min | N/A | 405 | [26] |
| 1 mg/mL stock in buffer | 20.9 mg para-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris-HCl | 1 mg/mL stock in 10% DMSO diluted to 50 to 400 µg/mL | 37 °C for 15 min | 37 °C for 30 min | N/A | 405 | [27] |
| 1 mg/mL stock | 20.9 mg para-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris-HC1 buffer, pH 7.4 | 1 mg/mL stock in 10% DMSO diluted to 50 to 400 µg/mL | 37 °C for 15 min | 37 °C for 30 min | N/A | 405 | [28] |
| 1 mg/mL in buffer | 2 mg/mL para-nitrophenyl butyrate in buffer | p-nitrophenol | 13 mM Tris-HC1 buffer, pH 8, 1.3 mM CaCl2 and 150 mM NaCl | Not given | 37 °C for 10 min | 37 °C for 20 min | N/A | 405 | [1] |
| Added at four times the volume of test samples (conc. Not given) | para-nitrophenyl butyrate in buffer | p-nitrophenol | Not given | 50 to 200 µg/mL in DMSO | 37 °C for 15 min | 37 °C for 25 min | N/A | 405 | [29] |
| Not given | 4 mM p-nitrophenyl laurate in buffer | p-nitrophenol | 0.05 mM Tris-HCl, pH 8.0 buffer containing 0.5% Triton-X100 | Not given | 37 °C for four periods of time | Not specified | Transferred to an ice bath and added 0.05 mM Tris-HCl, pH 8.0 | 410 | [30] |
| 1 mg/mL | para-nitrophenyl butyrate | p-nitrophenol | 0.1 mM potassium phosphate buffer, pH 7.2 with 0.1% Tween 80 | 2.5 to 35 µg/mL | 30 °C for 1 h | 30 °C for 5 min | N/A | 405 | [31] |
| Not given | 10 mM para-nitrophenyl phosphate | p-nitrophenol | 0.1 M phosphate buffer, pH 7.4 | Various concentrations of sample in buffer | 37 °C for 5 min | 37 °C for 20 min | N/A | 405 | [32] |
| 1 mg/mL in 10 mM MOPS and 1 mM EDTA buffer | 10 mM para-nitrophenyl butyrate in acetonitrile | p-nitrophenol | 100 mM Tris-HCl and 5 mM CaCl2, pH 7.4 | Not given | 37 °C for 15 min | 37 °C for 30 min | N/A | 405 | [33] |
| 2 units/mL in buffer | 0.5 mM 4-methylumbelliferyl oleate in buffer | 4-methylumbelliferone | 50 mM Tris-HCl, pH 8.0 | Not given | Not given | 37 °C for 30 min | N/A | Em/Ex = 455/360 | [34] |
| 1 mg/mL in buffer | 20.9 mg para-nitrophenyl butyrate in 2 mL acetonitrile | p-nitrophenol | Tris-HCl buffer | 50 to 400 µg/mL | 37 °C for 15 min | 37 °C for 30 min | N/A | 410 | [35] |
| 4 mg/mL | 10 mM para-nitrophenyl butyrate in dimethylformamide | p-nitrophenol | Phosphate buffer | 3.125 to 200 µg/mL | 37 °C for 37 min | 37 °C for 30 min | N/A | 405 | [36] |
| Enzyme Concentration | Substrate Used | Buffer Used | Extract Concentration | Preincubation (Before Enzyme) | Incubation | Wavelength (nm) | Ref |
|---|---|---|---|---|---|---|---|
| 0.163 units/mL in 0.1 M sodium phosphate, pH 7.0 | 1 mg/mL para-Nitrophenyl Butyrate in buffer | 100 mM NaCl, 5.16 mM sodium taurocholate, 100 mM sodium phosphate, pH 7.0 | Not given | 37 °C for 10 min | 37 °C for 20 min | 405 | [1] |
| Enzyme in buffer (concentration not given) | 5 mM para-Nitrophenyl Butyrate in dimethylformamide | 100 mM phosphate buffer, pH 7.0 | Samples dissolved in 80% methanol (concentration not given) | Room temperature for 5 min | 15 min (temperature not specified) | 405 | [33] |
| Not given | 0.2 M para-Nitrophenyl Butyrate | Not given | 3.12 to 200 µg/mL | 25 °C for 10 min | 25 °C for 5 min | 405 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandara, S.; Devereaux, S.; Weerasooriya, A. Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays. Obesities 2023, 3, 13-35. https://doi.org/10.3390/obesities3010003
Bandara S, Devereaux S, Weerasooriya A. Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays. Obesities. 2023; 3(1):13-35. https://doi.org/10.3390/obesities3010003
Chicago/Turabian StyleBandara, Subhani, Shelby Devereaux, and Aruna Weerasooriya. 2023. "Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays" Obesities 3, no. 1: 13-35. https://doi.org/10.3390/obesities3010003
APA StyleBandara, S., Devereaux, S., & Weerasooriya, A. (2023). Methods to Evaluate the Antiobesity Effects of Medicinal Plants Using Enzyme Assays. Obesities, 3(1), 13-35. https://doi.org/10.3390/obesities3010003





