Facile Engineering of Pt-Rh Nanoparticles over Carbon for Composition-Dependent Activity and Durability Toward Glycerol Electrooxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of PtxRhy/C NPs
2.3. Electrochemical Measurements
2.4. Density Functional Tight-Binding (DFTB) Methodology
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soffiati, G.; Bott-Neto, J.L.; Yukuhiro, V.Y.; Pires, C.T.G.V.M.T.; Lima, C.C.; Zanata, C.R.; Birdja, Y.Y.; Koper, M.T.M.; San-Miguel, M.A.; Fernández, P.S. Electrooxidation of C4 Polyols on Platinum Single-Crystals: A Computational and Electrochemical Study. J. Phys. Chem. C 2020, 124, 14745–14751. [Google Scholar] [CrossRef]
- Yukuhiro, V.Y.; Gibson, A.J.; Sitta, E.; Cuesta, A.; Fernández, P.S. Effect of Cations on the Electro-Oxidation of Alcohols and Polyols on Pt: Activity, Selectivity, and Mechanistic Insights. Curr. Opin. Electrochem. 2025, 52, 101705. [Google Scholar] [CrossRef]
- Teixeira, L.T.; de Lima, S.L.S.; Rosado, T.F.; Liu, L.; Vitorino, H.A.; dos Santos, C.C.; Mendonça, J.P.; Garcia, M.A.S.; Siqueira, R.N.C.; da Silva, A.G.M. Sustainable Cellulose Nanofibers-Mediated Synthesis of Uniform Spinel Zn-Ferrites Nanocorals for High Performances in Supercapacitors. Int. J. Mol. Sci. 2023, 24, 9169. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.L.S.; Pereira, F.S.; Lima, R.B.; Freitas, I.C.; Spadotto, J.; Connolly, B.J.; Barreto, J.; Stavale, F.; Vitorino, H.A.; Fajardo, H.V.; et al. MnO2-Ir Nanowires: Combining Ultrasmall Nanoparticle Sizes, O-Vacancies, and Low Noble-Metal Loading with Improved Activities towards the Oxygen Reduction Reaction. Nanomaterials 2022, 12, 3039. [Google Scholar] [CrossRef]
- Ribeiro, G.A.C.; Lima, S.L.S.; Santos, K.E.R.; Mendonça, J.P.; Macena, P.; Pessanha, E.C.; Cordeiro, T.C.; Gardener, J.; Solórzano, G.; Fonsaca, J.E.S.; et al. Zn-doped MnOx Nanowires Displaying Plentiful Crystalline Defects and Tunable Small Cross-Sections for an Optimized Volcano-Type Performance towards Supercapacitors. Discov. Nano 2023, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.R.M.; Ferreira, R.M.; Pereira, F.S.; Silva, F.A.; Silva, A.C.A.; Vitorino, H.A.; Júnior, J.J.G.V.; Tanaka, A.A.; Garcia, M.A.S.; Rodrigues, T.S. Application of AgPt Nanoshells in Direct Methanol Fuel Cells: Experimental and Theoretical Insights of Design Electrocatalysts over Methanol Crossover Effect. ChemCatChem 2022, 14, e202200605. [Google Scholar] [CrossRef]
- Li, T.; Harrington, D.A. An Overview of Glycerol Electrooxidation Mechanisms on Pt, Pd and Au. ChemSusChem 2021, 14, 1472–1495. [Google Scholar] [CrossRef] [PubMed]
- Terekhina, I.; White, J.; Cornell, A.; Johnsson, M. Electrocatalytic Oxidation of Glycerol to Value-Added Compounds on Pd Nanocrystals. ACS Appl. Nano Mater. 2023, 6, 11211–11220. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, J.; Zhao, J.; Meng, Y.; Li, Z.; Meng, X.; Wang, J. Glycerol Electrooxidation to Value-Added C 1–C 3 Chemicals: Mechanism Analyses, Influencing Factors, Catalytic Regulation, and Paired Valorization. Renewables 2024, 2, 89–110. [Google Scholar] [CrossRef]
- Fernández, P.S.; Tereshchuk, P.; Angelucci, C.A.; Gomes, J.F.; Garcia, A.C.; Martins, C.A.; Camara, G.A.; Martins, M.E.; Da Silva, J.L.F.; Tremiliosi-Filho, G. How Do Random Superficial Defects Influence the Electro-Oxidation of Glycerol on Pt (111) Surfaces? Phys. Chem. Chem. Phys. 2016, 18, 25582–25591. [Google Scholar] [CrossRef]
- Ye, H.; Favero, S.; Tyrrell, H.; Plub-in, K.; Hankin, A.; Rao, R.R.; Stephens, I.E.L.; Titirici, M.; Luo, H. Progress and Challenges in Electrochemical Glycerol Oxidation: The Importance of Benchmark Methods and Protocols. ChemCatChem 2025, 17, e00152. [Google Scholar] [CrossRef]
- Zhao, Y.; Björk, E.M.; Yan, Y.; Schaaf, P.; Wang, D. Recent Progress in Transition Metal Based Catalysts and Mechanism Analysis for Alcohol Electrooxidation Reactions. Green Chem. 2024, 26, 4987–5003. [Google Scholar] [CrossRef]
- Coutinho, J.W.D.; Figueiredo, P.B.S.; Colmati, F.; Tofanello, A.; Pereira, L.N.S.; Silva, A.C.A.; Garcia, M.A.S.; Lima, R.B. Correlating Composition and Electronic Effects of Self-Assembled PtMo Electrocatalysts for Ethylene Glycol Oxidation: An Experimental and Theoretical Approach. Int. J. Hydrogen Energy 2023, 48, 33875–33885. [Google Scholar] [CrossRef]
- Pereira, F.S.; Santana, V.F.; Silva, A.C.A.; Tofanello, A.; Romano, P.N.; Almeida, J.M.A.R.; Rodrigues, T.S.; Rodrigues, I.A.; Lima, R.B.; Garcia, M.A.S. Electrooxidation of Ethylene Glycol Using Ag-Pt Nanotubes Supported on Silica: Correlating the Unexpected O-Vacancies Creation with Catalytic Performance. Catal. Today 2024, 441, 114914. [Google Scholar] [CrossRef]
- Souza, M.B.C.; Soffiati, G.; Lemos, V.S.; Yukuhiro, V.Y.; San-Miguel, M.A.; Fernández, P.S. P-Block Elements Activate Pt Surfaces for the Electrooxidation of Alcohols and Polyols When Promoting the −OH Formation. ACS Catal. 2024, 14, 13105–13111. [Google Scholar] [CrossRef]
- Garcia, M.A.S.; Ibrahim, M.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; Santos, E.N.; Philippot, K.; Rossi, L.M. Study of the Influence of PPh3 Used as Capping Ligand or as Reaction Modifier for Hydroformylation Reaction Involving Rh NPs as Precatalyst. Appl. Catal. A Gen. 2017, 548, 136–142. [Google Scholar] [CrossRef]
- Bai, J.; Mei, J.; Liao, T.; Sun, Z. Engineering Structure-Activity Relationships in Rhodium-Based Catalysts for Electrocatalysis. Coord. Chem. Rev. 2025, 528, 216418. [Google Scholar] [CrossRef]
- Shilpa, T.; Dhanya, R.; Saranya, S.; Anilkumar, G. An Overview of Rhodium-Catalysed Multi-Component Reactions. ChemistrySelect 2020, 5, 898–915. [Google Scholar] [CrossRef]
- Garcia, M.A.S.; Heyder, R.S.; Oliveira, K.C.B.; Costa, J.C.S.; Corio, P.; Gusevskaya, E.V.; dos Santos, E.N.; Bazito, R.C.; Rossi, L.M. Support Functionalization with a Phosphine-Containing Hyperbranched Polymer: A Strategy to Enhance Phosphine Grafting and Metal Loading in a Hydroformylation Catalyst. ChemCatChem 2016, 8, 1951–1960. [Google Scholar] [CrossRef]
- Gupta, S.; Datta, J. A Comparative Study on Ethanol Oxidation Behavior at Pt and PtRh Electrodeposits. J. Electroanal. Chem. 2006, 594, 65–72. [Google Scholar] [CrossRef]
- Valerio Neto, E.; Almeida, C.; Eguiluz, K.; Salazar-Banda, G. The Effect of SnO2 and Rh on Pt Nanowire Catalysts for Ethanol Oxidation. Catal. Res. 2024, 04, 1–16. [Google Scholar] [CrossRef]
- Lam, B.T.X.; Chiku, M.; Higuchi, E.; Inoue, H. Rhodium Nanoparticle-Loaded Carbon Black Electrocatalyst for the Glycerol Oxidation Reaction in Alkaline Medium. Adv. Nanopart 2016, 5, 60–66. [Google Scholar] [CrossRef]
- Wang, B.; Tao, L.; Cheng, Y.; Yang, F.; Jin, Y.; Zhou, C.; Yu, H.; Yang, Y. Electrocatalytic Oxidation of Small Molecule Alcohols over Pt, Pd, and Au Catalysts: The Effect of Alcohol’s Hydrogen Bond Donation Ability and Molecular Structure Properties. Catalysts 2019, 9, 387. [Google Scholar] [CrossRef]
- Piwowar, J.; Lewera, A. On the Absence of a Beneficial Role of Rh towards C C Bond Cleavage during Low Temperature Ethanol Electrooxidation on PtRh Nanoalloys. J. Electroanal. Chem. 2020, 875, 114229. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective Electrooxidation of Glycerol into Value-Added Chemicals: A Short Overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.M.; Green, S.; Tompsett, G.A.; Lee, S.H.; Huber, G.W.; Kim, W.B. Highly Active and Stable PtRuSn/C Catalyst for Electrooxidations of Ethylene Glycol and Glycerol. Appl. Catal. B 2011, 101, 366–375. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All Spd-Block Elements (Z = 1−86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Cavalcante Lima, C.; Silva Fonseca, W.; Colmati, F.; Ribeiro, L.K.; Carvalho França, M.; Longo, E.; Suller Garcia, M.A.; Atsushi Tanaka, A. Enhancing the Methanol Tolerance of Ultrasmall Platinum Nanoparticles and Manganese Oxide onto Carbon for Direct Methanol Fuel Cell: The Importance of the Synthesis Procedure. Electrochim. Acta 2020, 363, 137256. [Google Scholar] [CrossRef]
- Qin, B.; Yu, H.; Chi, J.; Jia, J.; Gao, X.; Yao, D.; Yi, B.; Shao, Z. A Novel Ir/CeO2–C Nanoparticle Electrocatalyst for the Hydrogen Oxidation Reaction of Alkaline Anion Exchange Membrane Fuel Cells. RSC Adv. 2017, 7, 31574–31581. [Google Scholar] [CrossRef]
- Fang, L.; Vidal-Iglesias, F.J.; Huxter, S.E.; Attard, G.A.; Wells, P.B. RhPt/Graphite Catalysts for CO Electrooxidation: Performance of Mixed Metal and Alloyed Surfaces. Surf. Sci. 2015, 631, 258–266. [Google Scholar] [CrossRef]
- Carmargo, V.F.; Fontes, E.H.; Nandenha, J.; Souza, R.F.B.; Neto, A.O. High Activity of Pt–Rh Supported on C–ITO for Ethanol Oxidation in Alkaline Medium. Res. Chem. Intermed. 2020, 46, 1555–1570. [Google Scholar] [CrossRef]
- Fontes, E.H.; da Silva, S.G.; Spinacé, E.V.; Neto, A.O.; de Souza, R.F.B. In Situ ATR-FTIR Studies of Ethanol Electro-Oxidation in Alkaline Medium on PtRh/C Electrocatalyst Prepared by an Alcohol Reduction Process. Electrocatalysis 2016, 7, 297–304. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.W.; Lee, S.; Han, J.; Lee, D.; Kim, J.; Kim, T.; Kim, C.; Jeong, S.; Chae, H.; et al. The Role of Ruthenium on Carbon-Supported PtRu Catalysts for Electrocatalytic Glycerol Oxidation under Acidic Conditions. ChemCatChem 2017, 9, 1683–1690. [Google Scholar] [CrossRef]
- Jin, C.; Sun, C.; Dong, R.; Chen, Z. Electrocatalytic Activity of PtAu/C Catalysts for Glycerol Oxidation. J. Nanosci. Nanotechnol. 2012, 12, 324–329. [Google Scholar] [CrossRef]
- Anil, A.; White, J.; Campos Santos, E.; Terekhina, I.; Johnsson, M.; Pettersson, L.G.M.; Cornell, A.; Salazar-Alvarez, G. Effect of Pore Mesostructure on the Electrooxidation of Glycerol on Pt Mesoporous Catalysts. J. Mater. Chem. A Mater. 2023, 11, 16570–16577. [Google Scholar] [CrossRef]
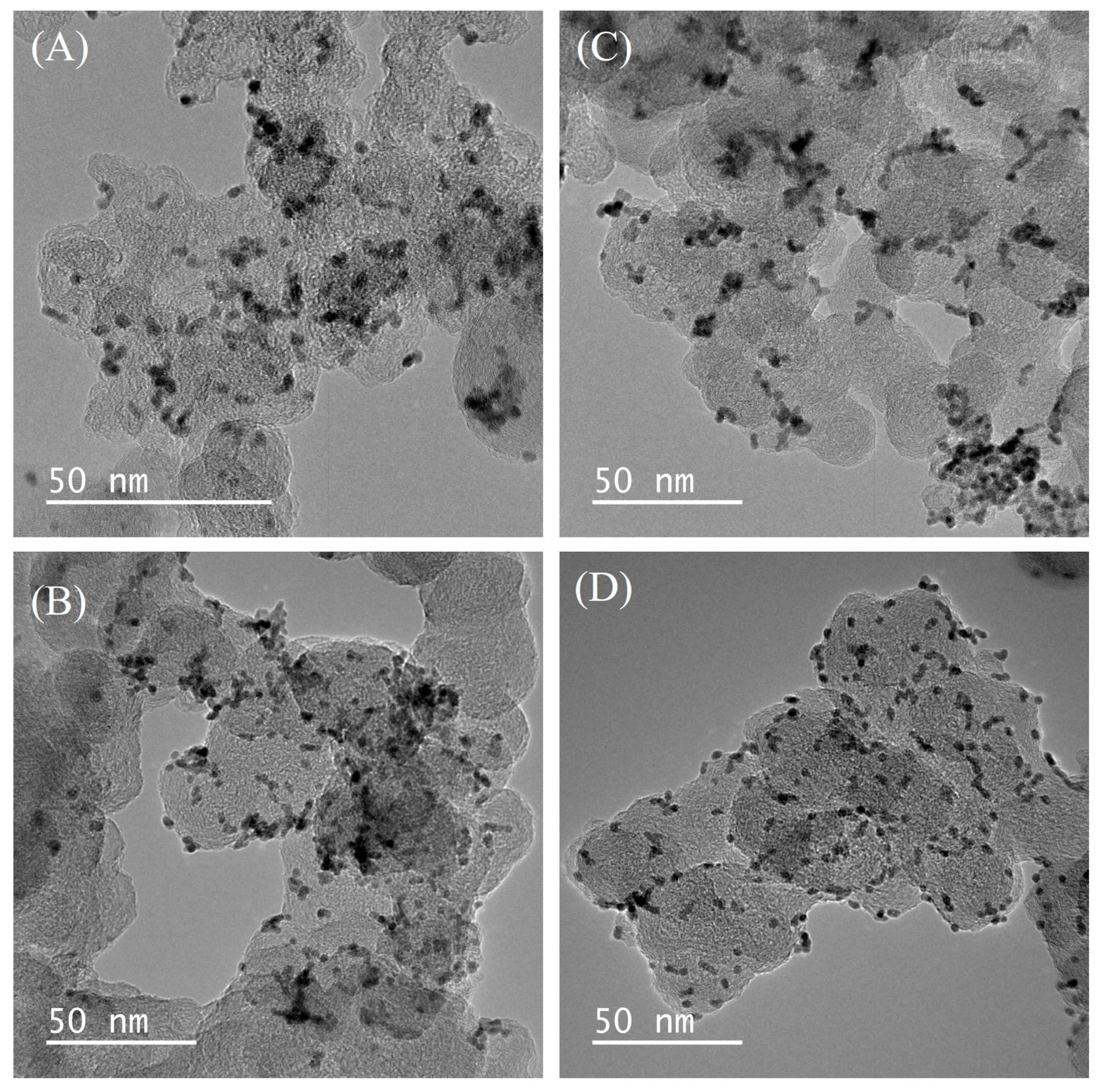


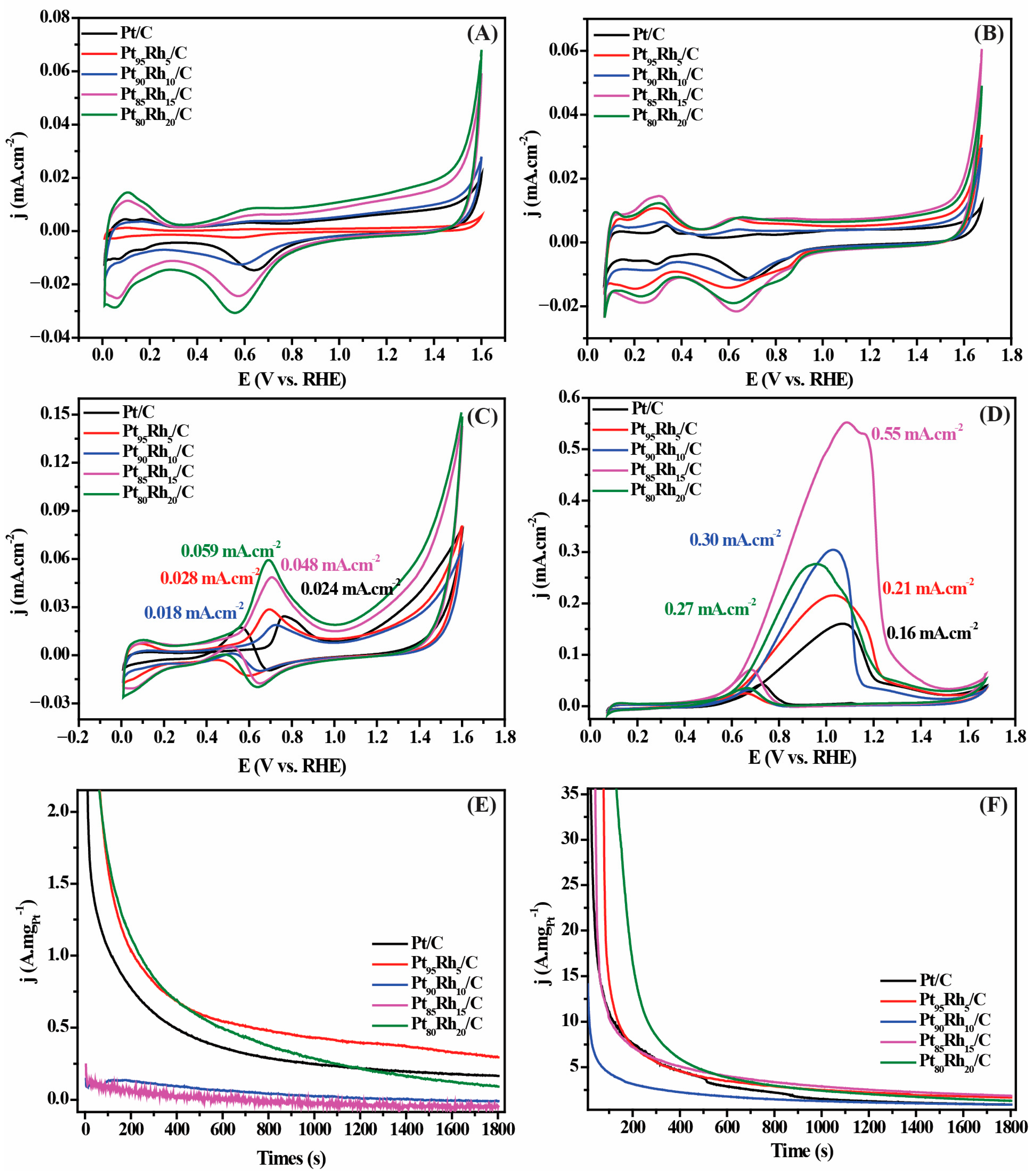
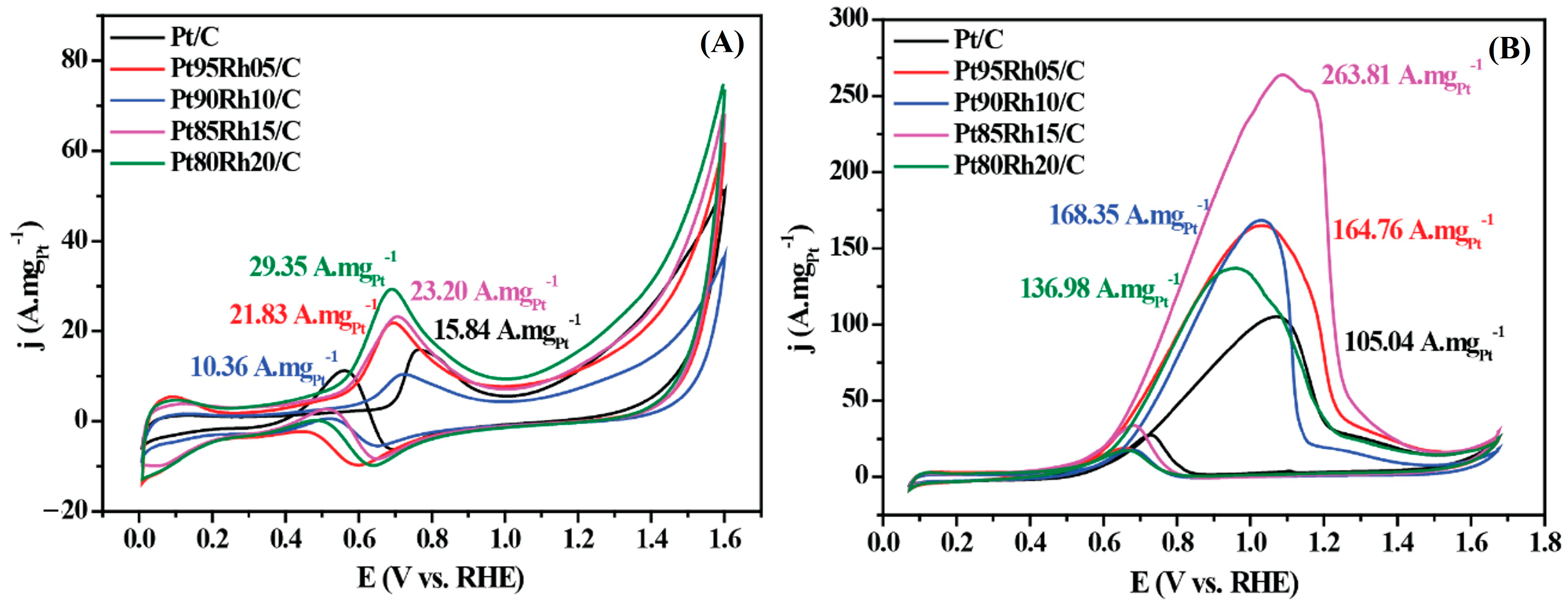

| Catalyst | Medium | Retention over the Chronoamperometric Test Period | References |
|---|---|---|---|
| Pt95Rh5/C | Acidic and Alkaline | ≈100–105% retention after 1800 s (acid and alkaline); no significant decay, indicating excellent stability | This work |
| PtRu/C | Acidic (H2SO4 + glycerol) | ~70% retention after 1800 s; better than Pt/C (~50%) due to higher CO tolerance | [34] |
| PtAu/C | Alkaline (NaOH + glycerol) | ~60% retention after 1800 s; better than Pt/C but limited by Au segregation | [35] |
| Mesoporous Pt | Alkaline (1 M NaOH + glycerol) | ~80% retention after 2000 s; hierarchical porosity delays deactivation compared to Pt/C (~45%) | [36] |
| No Glycerol | 0.1 M C3H8O3 | |||
|---|---|---|---|---|
| Electrocatalysts | KOH | HClO4 | KOH | HClO4 |
| Rct (Ω.cm−2) | ||||
| Pt/C | 1.90 × 105 | 1.54 × 105 | 3.54 × 103 | 2.19 × 105 |
| Pt95Rh5/C | 2.82 × 106 | 2.61 × 105 | 2.64 × 103 | 3.54 × 105 |
| Pt90Rh10/C | 1.66 × 106 | 4.89 × 106 | 9.91 × 105 | 2.38 × 106 |
| Pt85Rh15/C | 7.27 × 106 | 2.82 × 105 | 1.44 × 103 | 2.34 × 105 |
| Pt80Rh20/C | 3.93 × 106 | 3.48 × 105 | 2.41 × 103 | 1.76 × 105 |
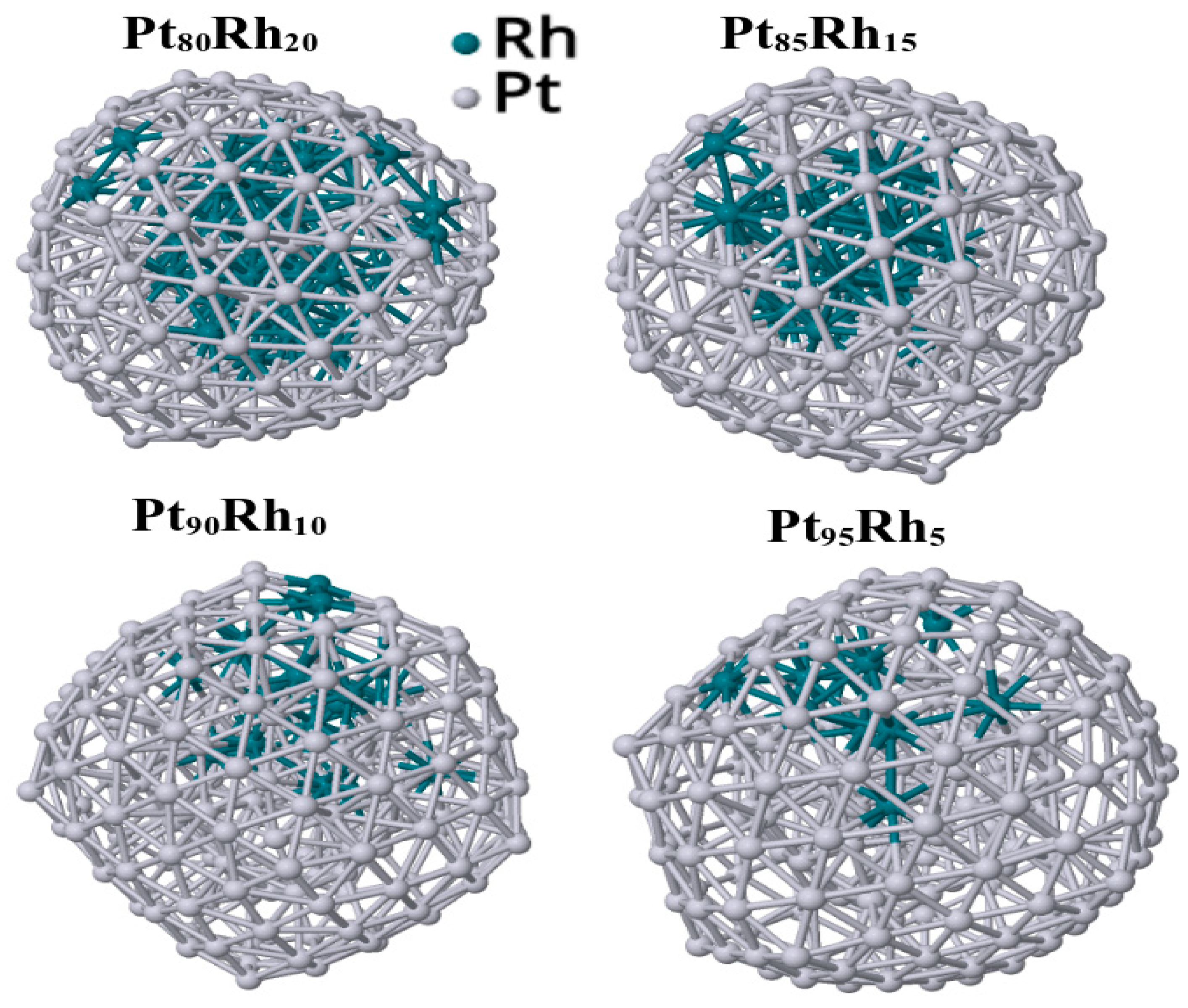
| Metal Composition | Energy (Binding) | Energy (Excess) |
|---|---|---|
| Pt95Rh5 | −0.313 | −6.584 |
| Pt90Rh10 | −0.308 | −5.710 |
| Pt85Rh15 | −0.304 | −4.806 |
| Pt80Rh20 | −0.300 | −4.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.V.F.; Santos, W.D.C.d.; Pereira, F.d.S.; Silva, A.C.A.; Liu, L.; Sant’Anna, M.C.; D’Elia, E.; Lima, R.B.d.; Garcia, M.A.S. Facile Engineering of Pt-Rh Nanoparticles over Carbon for Composition-Dependent Activity and Durability Toward Glycerol Electrooxidation. Hydrogen 2025, 6, 78. https://doi.org/10.3390/hydrogen6040078
Rodrigues MVF, Santos WDCd, Pereira FdS, Silva ACA, Liu L, Sant’Anna MC, D’Elia E, Lima RBd, Garcia MAS. Facile Engineering of Pt-Rh Nanoparticles over Carbon for Composition-Dependent Activity and Durability Toward Glycerol Electrooxidation. Hydrogen. 2025; 6(4):78. https://doi.org/10.3390/hydrogen6040078
Chicago/Turabian StyleRodrigues, Marta Venancia França, Wemerson Daniel Correia dos Santos, Fellipe dos Santos Pereira, Augusto César Azevedo Silva, Liying Liu, Mikele Candida Sant’Anna, Eliane D’Elia, Roberto Batista de Lima, and Marco Aurélio Suller Garcia. 2025. "Facile Engineering of Pt-Rh Nanoparticles over Carbon for Composition-Dependent Activity and Durability Toward Glycerol Electrooxidation" Hydrogen 6, no. 4: 78. https://doi.org/10.3390/hydrogen6040078
APA StyleRodrigues, M. V. F., Santos, W. D. C. d., Pereira, F. d. S., Silva, A. C. A., Liu, L., Sant’Anna, M. C., D’Elia, E., Lima, R. B. d., & Garcia, M. A. S. (2025). Facile Engineering of Pt-Rh Nanoparticles over Carbon for Composition-Dependent Activity and Durability Toward Glycerol Electrooxidation. Hydrogen, 6(4), 78. https://doi.org/10.3390/hydrogen6040078








