1. Introduction
Green hydrogen is a promising renewable energy vector for the decarbonization of various industrial and mobility sectors. The technology for making green hydrogen is known as electrolysis and is a mature technology; however, the electrolyzer needed for this purpose has faced integration problems associated with various power sectors such as PV arrays (photovoltaic arrays) and grid-based electricity due to the intermittent nature of both. The integration of renewable energy into existing grid systems also faced inherent challenges in terms of wind and solar variability in different geographic locations. The grid stability is further affected in terms of power imbalances by the changes in wind/sun/weather patterns or fluctuations in daily/seasonal patterns. In the case of solar insolation and wind speed, it causes inconsistencies in voltage and frequency parameters, which in the future will need AI-powered central systems to supply stable grid power and thus enhance the efficiency of electrolyzer technologies to produce green hydrogen and to fulfill the required demand. Following this, further optimization is needed to integrate the electrolyzer directly with the PV array with definite sizing. In the case of wind-based sources, BESS systems (battery energy storage systems) could be the best option.
Several types of electrolyzer technologies are currently running, which vary regarding efficiency and cost analysis. For instance, the PEM (proton exchange membrane) electrolyzer BOSCH 1.25 MW system shows the highest efficiency of 80% with a ramping rate of < 1 s, but its cost is affected by requiring scarce materials such as IrO
2/Pt, and the upfront cost comes at 700–1400 USD/kg [
1]. While alkaline electrolyzers are more mature technology and cheaper (500–1000 USD/kg), their slow ramping (min) and lower efficiency (65–70%) make them less suitable for intermittent renewables. The highest efficiency obtained is for an SOEC (solid oxide electrolyzer), but it is not suitable under dynamic grid conditions. The current cost of green H
2 comes at 3.75–6 USD/kg when compared to fossil fuel alternatives (1.5–2 USD/kg). The 2040 vision for green H
2 cost would be around USD 2 with the scale-up in production and cheaper renewable power availability [
1]. This can be cut short with the help of recent advances in automotive manufacturing and new materials innovation to achieve the DOE (Department of Energy USA) target of 1 USD/kg of hydrogen production by 2031.
More studies have recently been conducted with respect to the integration of renewable energy with electrolyzers. For instance, Su et al. [
2] studied the optimization and analysis of a PV—coupled electrolyzer with a direct coupling system. Here, focus was put on effectively designing and sizing the system to enhance hydrogen production from solar energy. The main goal of the work was to improve PV modules and electrolyzers to achieve high efficiency and reduce costs. The PV source operating point matching that of an electrolyzer is found to be an important parameter to achieve the best performance. It is found that a well-designed direct coupling system can significantly increase energy transfer efficiency, thereby offering deep insights into the efficiency challenges associated with power electronics. Next, Khalil Nejad and Riahy [
3] studied the effect of a hybrid wind–PV based system embedded with an alkaline electrolyzer. The goal of studying this was to obtain stable hydrogen output throughout the year by the integration of wind turbines, PV arrays, and energy storage solutions. The study also emphasizes how to reduce the effect of intermittent renewable energy supply by utilizing a BESS (battery energy storage system)-based system to coordinate hydrogen production with energy supply requirements. When renewable energy generation is insufficient, the operational mode involves the combined input powers from both batteries and the grid. This hybrid approach not only enhances hydrogen production but also decreases carbon emission reductions. Another team coupled PV with an alkaline electrolyzer system and discussed the performance of a commercial alkaline electrolyzer of 1 Nm
3/h capacity using actual weather data for irradiance, temperature, and wind speed [
4]. The capacity of both PV and wind turbines is 6.8 kW and 6 kW, respectively. The main finding of the work is related to the application of two strategies for proper integration: operations between the smallest limit for a brief period and the addition of an extra battery bank. The overall service life and performance of the electrolyzer increased the number of operational steps by 63.1% and increased in energy efficiency by 7.6%. Following this, another study [
5] assessed the technical and economic viability of using a photovoltaic–fuel cell battery hybrid system in the Amazon region of Brazil. HOMER software was used to make a comparative cost analysis, and it was found that the first cost was around USD87,138 and electricity cost USD 351/kWh. In addition to onshore renewable energy sources, offshore wind firms are coupled to electrolyzers in Germany [
6]. The idea is to check the economic viability in ancillary service markets. The best bidding approach for ancillary services and best sizing power to hydrogen facilities are developed as strategies, and it was found that the offshore wind-based H
2 production is more sustainable. It is suggested that providing subsidies like those for conventional offshore wind farms could reduce further costs.
The volatile power sources significantly hamper hydrogen production. Various advanced control systems, such as the battery–hysteresis cycle, model-based scheduling, and frequency response, were developed by Alsagher and Wilcken et al. [
7] to mitigate this effect. A model predictive control (MPC) algorithm was developed by researchers, which dynamically adjusts electrolyzer load to support a breakdown energy balance on the DC bus bar linking generation and demand. The system is assessed for a 5 kW PEM electrolyzer, and it achieves automated energy balancing for both grid-connected and standalone systems. Key outcomes of this study are 6.3–7.6% improved energy efficiency and enhanced H
2 yield under variable renewable inputs. In addition, few studies associated with the successful coupling of electrolyzers with renewable energy sources have been conducted in the recent past [
8,
9,
10,
11,
12,
13,
14,
15,
16]. Next, a bilevel optimization model is proposed by Li et al. [
17] for the wind–photovoltaic electrolyzer system sizing embedded with hydrogen energy storage (HESS) and economic performance. The volatility is addressed via source load interaction at the interface between wind and solar as well as demand response, thus reducing cost and improving output matching. Following this, a total annual cost (TAC) model is developed by integrating the levelized cost of storage (LCOS) for HESS, which shows a 7.3% reduction in TAC and an LCOS value drop by 10.3%. Sonya et al. [
18] recently developed a scale-up project using an area over 10 m
2 by exploring various PV–electrolysis combinations, which includes a thermally integrated PV electrolyzer and the direct coupling of PV modules to a PEM electrolyzer. The main outcome of the so-called PECSYS (photoelectrochemical system) project is to increase the solar-to-H
2 efficiencies ranging from 4% to 13% by using bifacial PV modules, which exceed 10% over extended periods. Similarly, a kilowatt-scale solar H
2 production system using an integrated photoelectrochemical device was recently developed by Holmes-Gentle et al. [
19]. The thermally integrated PEC device can achieve solar-to-hydrogen efficiency at 20% and system efficiency at 5%. This makes it further scale up from lab scale to a kW-scale pilot facility. The research applies novel strategies such as managing solar irradiation, thermal integration, and electrolysis processes to find major energy losses as well as increase the system- level efficiency.
The operational experience, performance testing, and system integration of alkaline and PEM electrolyzers coupled with PV and wind-based renewable sources were recently discussed in an NREL case study report [
20]. The wind-2-H
2 project (wind to hydrogen), which is a collaboration between Xcel Energy (industry partner) and NREL, is a pioneering demonstration facility at NREL that is specifically focused on the production of hydrogen from renewable energy sources. The project shows the production and storage of H
2 and converting it into electricity by the integration of wind turbines and photovoltaic arrays with electrolyzers. The key aim of the project was to reduce H
2 costs to 2–3 USD/kg to match the DOE target and to improve the system efficiency with the inclusion of power electronics. The project further employs H
2 storage as an effective solution to meet the challenges of variable renewable energy sources, aiming to further enhance system performance and reduce the cost of renewable H
2 production. The grid integration advancement has been incorporated in another case study [
21], as it is needed for balancing renewable energy variability and enhancing grid stability. Under this study, which involves a 1.25 MW PEM electrolyzer project, predictive modeling tools are developed that focus on performance characterization under diverse grid scenarios. The future direction in this case is to mitigate the associated challenges in communication protocols, safety compliance, and grid dynamics management and to scale up both centralized and decentralized systems and the integration of technology synergies like offshore H
2 islands and digital twins for the hardware-in-the-loop testing. A further study has been conducted for the optimization of an integrated renewable energy–electrolysis system [
22]. In this case, the main priority was to refine the constructive interaction between renewable energy and that of the grid with a balanced approach to absorb the excess energy for H
2 production with an electrolyzer. However, in this case, the cost is still highest at 5–6 USD/kg, which is mostly driven by electrolyzer expenses and renewable energy variability. The U.S. DOE H
2 shot program was launched recently to obtain the desired 1 USD/kg of H
2 by 2031 to address the cost issue.
With reference to cost analysis, a recent report published by IRENA (The International Renewable Energy Agency) [
1] stressed the scale-up of electrolyzers to produce green hydrogen with a final goal of obtaining net zero carbon energy to not surpass the global rise in the temperature of 1.5 °C as agreed within the Paris Agreement on climate change. The study mentioned that the cost of H
2 production is controlled by key components such as electricity supply, electrolyzer CAPEX (capital expenditure), and use rates. In this case, electricity cost ranges from 20 to 65 USD/MWh depending on location, and the next electrolyzer costs accounted for 25–40% of the upfront cost with the current cost of 700–1400 USD/kWh, and it is projected that the cost significantly reduced by 2030 through scaled manufacturing. Strategically optimized approaches include electrolyzer innovation, policy support (carbon pricing and mandates), and the inclusion of leverageable low-cost renewables in ideal locations. Islanded H
2 production with huge renewable energy potential serves as H
2 export hubs. Mazzotti et al. [
23] recently studied the cost and environmental impact of such systems. The researcher proposed that using hybrid systems combining different electricity sources offers the best economic and environmental performance. The main challenges met here are the associated catalysts for electrolyzer scale-up due to materials scarcity (IrO
2 and Pt used for PEM electrolyzer) and the required land for the installation of renewable energy infrastructure. With reference to the export hub, Mio et al. [
24] recently proposed a case study by considering the Trieste port with the application of different hydrogen production pathways to make gray, blue, green, and grid-based H
2. The levelized cost of hydrogen (LCOH) for gray hydrogen is comparable to that of grid-based and green hydrogen, the study found. Green hydrogen is at a competitive disadvantage because the total cost of ownership (TCO) for hydrogen-powered vehicles is still higher than that of diesel equivalents.
So far, we have discussed various integrated systems and related techno-economics associated with the production of green H2. Now, we will focus on recent progress in electrolyzer research and gaps behind their performance integration with both renewable and grid-based electrical power, which is followed by establishing the objectives of the current experimental and performance study of a 25 kW commercial alkaline electrolyzer integrated with a hybrid power supply from PV and the grid.
Electrolyzers are the primary support mechanism for grid-based electricity power management combined with renewable energy. The idea here is that the electrolyzer can consume the surplus power by converting it into hydrogen and electricity in the form of hydrogen storage. A recent review [
25] discussed the electrolyzer-based system for providing ancillary services. Electrolyzers support functions like voltage and frequency control and grid balancing by using excess electricity and converting it into H
2. One of the recent challenges faced by alkaline electrolyzers is their system efficiency when running under low loads (<30% capacity) due to the electrical limitations imposed by fluctuating RES (renewable energy sources). To address this, Xia et al. [
26] developed a multi-mode self-optimization electrolysis strategy that adjusted dynamically voltage/current based on real-time RESs (renewable energy systems) inputs and AWE (alkaline water electrolyzer) operating states, enhancing adaptability to variable power conditions. The approach improves efficiency from 30% to 53.21% under 15% load and expands the operational range from 30% to 100% of rated capacity, which was confirmed for both lab-scale and commercial systems. The method also stabilizes H
2 output by improving thermal management and electrical response without requiring hardware modifications. Such approaches are important to reduce energy consumption to 51–57 kWh/kg H
2 and to achieve a stack lifetime of 7–10 years. In addition to dynamical adjustment, alkaline electrolyzers also suffer from H
2 crossovers, which are a major challenge for renewable energy integration.
The current zero-gap designs show high H
2 crossover (supersaturation level: 8–80 at the diaphragm electrolyte interface) due to imperfect electrode—diaphragm contact, which causes unstable performance under dynamic loads. A finite-gap configuration [500 μm] has been designed by Lua Garcia Boras et al. [
27] to mitigate this. Innovation reduces crossover dramatically to a supersaturation level (2–4). Here, cathodes gaps are proving particularly effective at lowering gas impurities compared to anode gaps. It enables AWE to run safely across a broad range without compromising efficiency. Next, the effect of operating temperature on the electrolyzer at low current density limitations has been reviewed by Lohman-Richters et al. [
28]. Higher operating temperature [70–200 °C] reduces cell voltage requirements, thus improving energy efficiency and allowing thermal integration with industrial processes. It is found that material stability is still a challenge for components like catalysts, diaphragms, and electrodes. They must be stable under corrosive molten hydroxides and elevated temperatures. Recent advances show stable operation up to 150 °C with experimental systems achieving 200 °C as the operating temperature when using robust materials like porous metal oxide matrices and PTFE (polytetrafluoroethylene)-based components. However, the technology is not yet conducted for higher TRL (technology readiness level), and to be scale up, it requires addressing the capital costs, durability (which targets 7–10 years for stacks), and infrastructure gaps.
From the above critical analysis of the current state of the art, the electrolyzer parameter study is still lacking in terms of a pilot-level system integrated with grid-based electricity and PV for green hydrogen production. The main goal of the current work is to analyze various aspects of electrolyzer parameters such as stack efficiency, stack current, and voltage behavior with respect to electrolyte temperature and the balance of plant discussion in a real-case scenario. Here, we have adapted protocols from the NREL case study wind 2H2 project. But the main novelty here is to understand how the hydrogen flow rate behaves with the stack current at various times of day with the integration of PV and grid-based hybrid power sources. We also made a correlation between stack power and hydrogen output in kg following system efficiency, which had not been undertaken for a commercial pilot system of kW capacity to the best of our knowledge. Following this, our next goal is to calculate the LCOH by applying the AGORA tools to obtain a rough estimate of the cost of green hydrogen production and build a strategy to further improve the condition in a reverse engineering scale from pilot scale to lab level.
2. Methods
Here, we will describe the commissioning and testing of an alkaline electrolyzer. The 25 KW alkaline electrolyzer (Piel M, McPhy Italia) was installed and commissioned in 2023. It is an integrated electrolyzer with a built-in power electronics controller by Unitronics systems (PLC = programmable logic controller). The electrolyzer operation is performed by supporting the ATEX (Atmosphères Explosibles, Equipment for potentially explosive atmospheres) safety standard. The electrolyte used was 20 wt % of NaOH.H2O solution (26 L) with allowed impurities of dry NaOH.
The goal of the work is to understand the effect of grid-based power on its operational characteristics. The electrolyzer input power is provided by both PV and grid-based electricity. The PV array is connected to a DC–AC inverter, which supplies electricity to it in parallel to the grid load. The ramp-up time of the electrolyzer until the hydrogen production or generation step is around 5–10 min. If the pressure built up is not adequate, the electrolyzer is not in operation mode.
After the generation step, the H2 generated is passed via a deoxo unit and finally via a gas-drying system fitted with a heat exchanger to remove the moisture content from hydrogen gas as condensates, which are collected later, and the output gas is 99.5 ± 0.3% purity. The condensates mixed with generated H2 and O2 gases from untreated water molecules from the electrolyte during the electrocatalytic water splitting process. Note that condensate removal is necessary to support the purity of product H2 as its application, for instance in fuel cells, needs a 99.999% pure form. After passing through the drying process, the hydrogen produced is not stored and passed via the water reservoir to air. The aim of this step is to mitigate any explosion-related incidents in the safety scenario.
After the above demonstration step was conducted and it was found that the electrolyzer continuously is able to generate H
2 at the desired rate, we set up the operational protocol. Here, the electrolyzer is coupled with a grid-based power source provided electricity by a PV array of 200 kW capacity. It is found that during the peak solar insolation, a capacity of 200 kW has been obtained. Note that the electrolyzer energy input is 25 kW, and a power load of 10 kW more is needed to run different components of the electrolyzer, such as the lye fan for cooling purposes, the heat exchanger to support the output temperature, and a dryer with vacuum pump installation. The electrolyzer comprised two alkaline stacks of 10 kW capacity and is connected to the gas collection chamber along with more cylinders to collect the unreacted electrolyte. The collected gas is further transferred to the dryer to remove the condensate as described above. The oxygen gas is collected and directly vented out to the environment. The power electronics and associated reaction engineering balance of plants (BOP) is controlled by a PLC (programmable logic controller, made by Unitronics) system, as shown in
Figure 1. The electrolyzer shows a maximum hydrogen production rate of 4.1 Nm
3/h, which has an equivalent output pressure of 0.80 bar under full load operation. The electrolyzer operation mode is pressurization–generation. First, the electrolyzer oxygen valve is open to build up the necessary pressure with the closure of the hydrogen vent valve. Once the hydrogen generation is initiated, the hydrogen valve is open and gas flows toward the drying chamber.
The drying chamber was installed with more valves, which are opened slowly until the gas passes via the various stages of drying and finally passes via distilled water and then into the air. The gas is not stored, as the desired pressure of 3 bar is not achievable in this case. However, the gas generated will be used in further power-to-X applications such as in PEM fuel cell-based power generation. After each operation, O2 gases are vented out and condensate is drained from the drying chamber. The condensate has been drained, or it can be used as a recycled electrolyte. The electrolyte needed to produce 1 kg of H2 is around nine liters of 6M KOH. Following this calculation, the water consumption for one ton of H2 plant would be nine thousand liters.
3. Results and Discussion
3.1. Balance of Plant of the Electrolyzer and Its Reaction Engineering Discussion
The BOP consists of electrolyzer stacks, where liquid electrolyte is fed with the help of a centrifugal pump premixed with water with another pump, which is called the electrolyte circulation pump. The electrolyte temperature is kept with the help of a heat exchanger and another fan, as shown in
Figure 2. The minimum voltage requirement for an electrolyzer is 200 V and 17 A current from one stack. As the electrolyzer investigated here has two stacks, the total voltage and current requirements will be 400 V and 34 A. As the hydrogen generation steps progress, the gas will be collected in respective electrolyte–gas separation tanks. From here, it is collected into catch pots for both H
2 and O
2. After this step, it will be passed to the deoxo step, where condensates associated with both oxygen and hydrogen gases will be collected. The deoxo consists of packed bed Pt/Pd catalysts, which work by heating to around 200 °C. The oxygen generated is vented out into the air, and condensates associated with it will be directly collected from the output valve from the rear of the electrolyzer. The H
2 generated is passed via the same step and finally collected in the drying chamber for removing the residual water molecule as well as condensate collection. After the drying step, the output H
2 pressure is around 4.2 Nm
3/h, giving a total of 369 g/h, which will result in 8.85 kg/day of electrolyzer operation. After the shutdown of the electrolyzer and the production target is reached, the dryer is working by condensation using heat exchanger plates with recovery and condensate drain devices. The temperature is controlled by a refrigerant chiller. The hydrogen constant dew point is obtained by these types of dryers.
After an operational and construction overview of the electrolyzer, we will now discuss the various results obtained regarding the electrolyzer reaction engineering perspective, such as the stack current resistance, stack efficiency, and system efficiency. Also, we will study the effect of electrolyte temperature on the performance of the electrolyzer. We have seen and compared this result with well-established cases studied and performed at the NREL. The NREL studies focus more on total system installation and various outcomes of associated H2 production in both wind and solar energy scenarios, which are followed by the development of advanced power electronics. The novelty of this paper lies in the direct correlation of system efficiency with that of stack efficiency and maximum gas output volume or production rates; furthermore, we used an innovative methodology to understand the correlation, while the NREL studies did not directly compare it with an alkaline electrolyzer.
Following the understanding of various parameters, we applied the AGORA LCOH calculation tool to understand the cost of H2 production under the current operational scenario. Then, we discussed various electrolyzer parameters along with the outlook for further improvement in electrolyzer performance.
3.2. Coupling of PV Array and Grid-Based Electricity with Electrolyzer and the Effect of Proximity of Electrolyzer to the Power Sources
Next, to see the effect of input power supplied from both PV array and grid-based powers, we studied the electrolyzer characteristics related to its proximity to the power sources. We have seen that the electrolyzer functions normally in terms of its stack current and the associated H2 production. We believe that there are no transmission losses, as the electrolyzer shows stable voltage operation and stack current. However, during cloudy conditions, when the PV array output power is limited, it has been observed that the stack current decreases, but the desired input power is automatically adjusted by the in-built power electronics of the electrolyzer with added load provided by the grid. The electrolyzer’s stable operation is made possible by the combined power input of both grid- and PV-powered electricity.
3.3. Effect of Shutdown Action on the Operational Performance of the Electrolyzer
We have performed experiments related to the effect of shutdown operation on the performance of the electrolyzer. It is worthwhile to mention that the electrolyzer shutdown action causes the stack degradation, resulting in the decrease in stack current, and thus it finally affects the associated power needed to run the electrolyzer to achieve the desired H
2 flow rate output. The shutdown action leaves the electrolyzer’s anodes and cathodes undergoing a spontaneous self-discharge process due to bipolar plates acting as a galvanic cell and thereby generating an electromotive force. This in turn generates a reverse current, which flows in the opposite direction of the electrolysis current between the anode and cathode until an equilibrium is reached. This condition creates a reverse redox current, and thereby the anode is reduced, and the cathode becomes oxidized [
29,
30,
31]. In this case, the alkaline electrolyzer cathode is made of nickel, which upon oxidation produces β–Ni(OH)
2 according to a recent in situ study with X-ray spectroscopy [
32]. In this case, we have not noticed any observational change in current density during the next day of operation within one month of running the electrolyzer by following the on–off strategy, so the degradation mechanism is momentarily ruled out. The shutdown action of the electrolyzer usually brings it back to the equilibrium state, and on the next day also, the ramp-up time to the hydrogen generation step remained the same as that of the very first day of operation and before each consecutive shutdown, and the magnitude of the stack current did as well. There is no loss of either potential or current in this case, and hence the effect of shutdown on the operational performance is confirmed.
3.4. Effect of Intermittency on the PV-Coupled Electrolyzer
An intermittency of PV supplied electricity is not seen here, as the electrolyzer is indirectly coupled to the PV array. However, at the end of the day, when PV panels are not producing electricity anymore, voltage stepdown has been observed during the operation of the electrolyzer. Due to the reduction in stack voltage, the stack current also decreases along with the output pressure.
3.5. Stack Efficiency
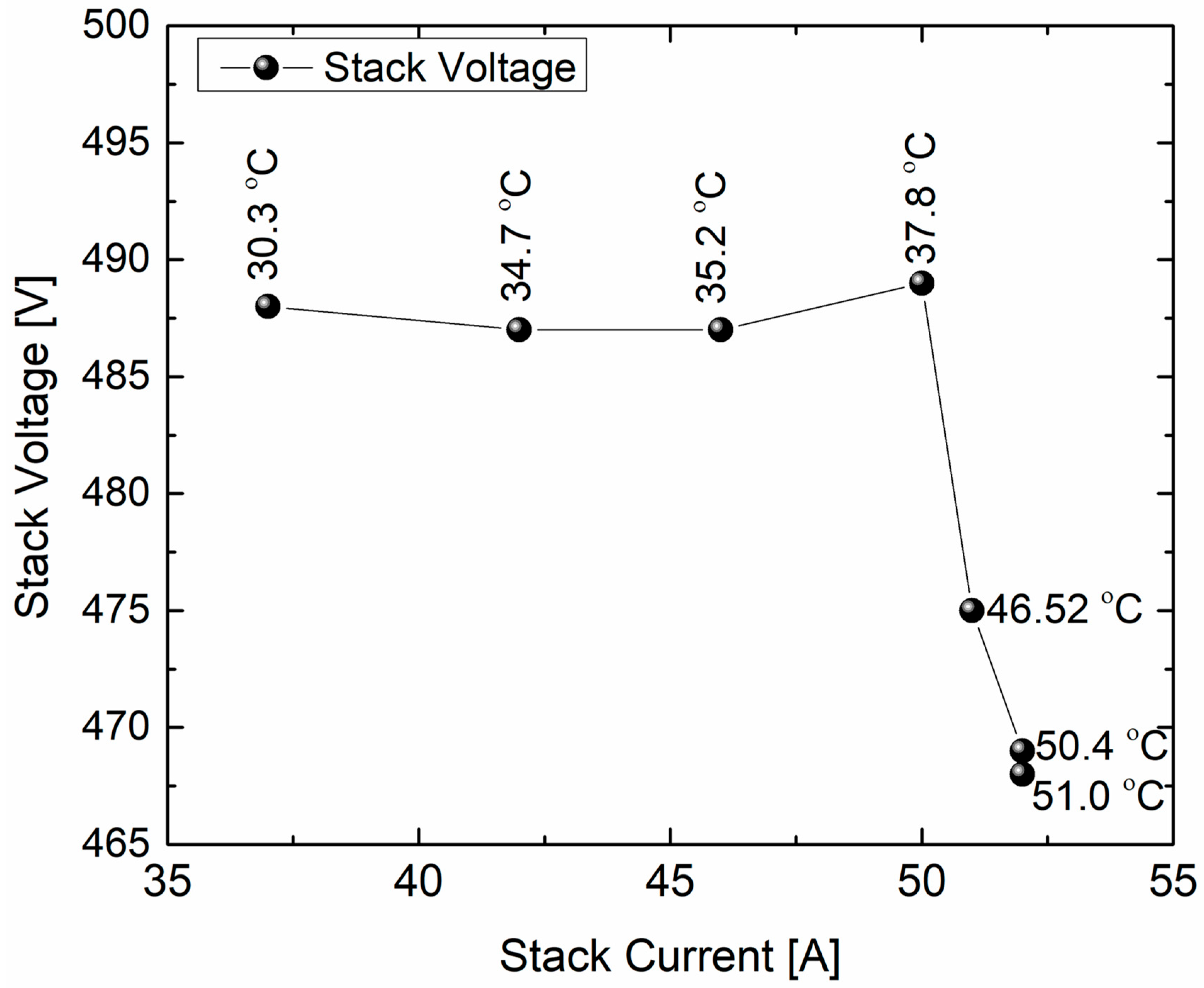
Next, to understand the correlation of stack voltage [V] against stack current [I], we have plotted them in
Figure 3. From the results obtained, the trend is a linear pattern in which the stack voltage stays the same as the stack current increases in the range of 35–55 A. This phenomenon is due to the Ohmic losses related to the ionic resistance of the electrolyte. A similar type of observation had been made by an NREL study involving an alkaline electrolyzer equipped with power electronics [
20]. The electrolyte temperature does play a role in the performance of stack current, as the electrolyte temperature influences it, and that is why it is cooled down with the help of the lye fan and heat exchanger as described in the balance of the plant. In addition to electrolyte temperature, overall, the electrolyzer system temperature increases from 37.8 °C to 51 °C. Above 37 °C, the stack voltage decreases further, and ultimately, the reaction engineering parameters such as output hydrogen pressure and gas flow become affected.
Here, temperature and pressure remain constant. The cathode and anode pressures are 0.86 bar and 0.80 bar, respectively. This value is used further to calculate the ideal stack voltage needed to calculate the stack efficiency according to the following equations [
20]:
where
Vn stands for Nernst potential, including the total potential of 1.48 V at HHV (higher heating value). R is the universal gas constant (8.341 J/mol), T is the operating temperature in kelvin, and
Z stands for the number of electrons taking part in the overall reaction.
F is the Faraday constant (96,485 C/mol).
PH2 is the pressure of the cathode,
PO2 is the pressure of the anode, and
PH2O is the pressure of the anode feed water. The Nernst potential (
Vn) is added here as an extra term to account for the electrochemical compression energy, which is used to generate the cathode pressure (hydrogen) of the cell within stacks.
Stack efficiency is defined as the voltage efficiency figured out by comparing the ideal stack potential with the actual stack potential. The measured operating voltage is always compared to this ideal voltage to calculate stack efficiency, as shown in
Figure 4. Stack efficiency is plotted against the stack current (A), as shown in the figure. Hereby, it is seen that stack efficiency is still at maximum ability when the stack is working at low current. Next, it decreases with the increase in stack current. It is also clear from the figure that a stack current of 50 A results in a stable stack voltage. The stack current also increases because of the rise in electrolyte temperature. A stack current operating in the range of 36–50 A results in good stack efficiency, so it is recommended that the best stack current is 52 A with the largest stack efficiency of 99%. Also, it is clear from the figure that 52 A gives maximum hydrogen output [
Figure 5 as discussed below], and with a voltage of 490 V, the output power is 25 kW, which is equivalent to the maximum electrolyzer capacity in terms of power output. Also, stack resistance decreases with the rise in stack current [
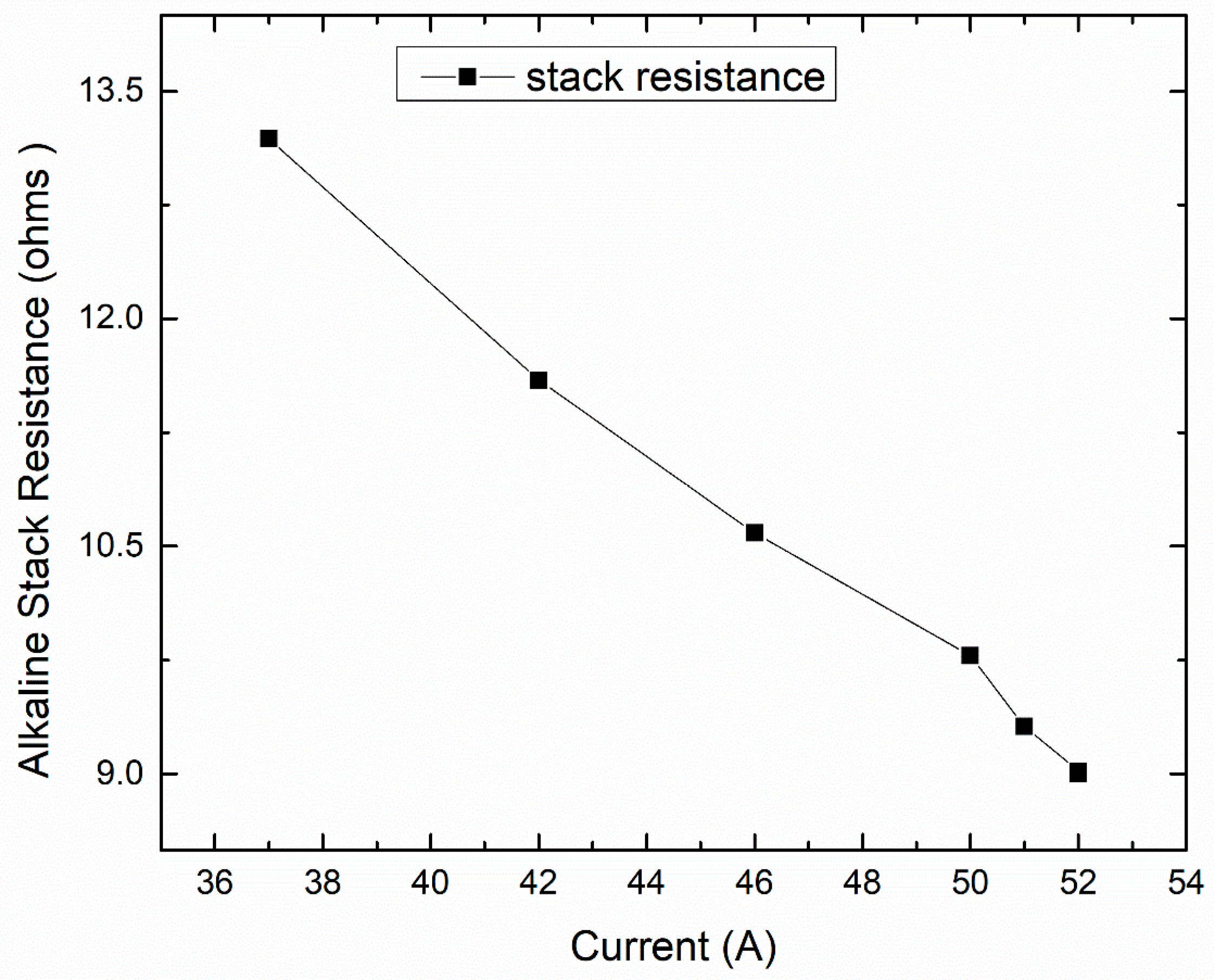
Figure 6]. It points to the fact that the electrolyzer’s best operable current is 52 A.
3.6. Correlation of Hydrogen Flow Rate [Nm3/h] with Stack Current [A]
The hydrogen flow rate follows an exponential asymptotic growth pattern with respect to the stack current and time stamp for a day of operation, as shown in
Figure 6. Here, generation starts at 10.00 a.m., and from the generation step until the hydrogen flow rate saturation, it takes around 20 min from the startup of the electrolyzer for hydrogen production. As can be seen from the results obtained, after 20 min to 1 h of operation, the electrolyzer hydrogen flow rate stays stable. The graph obtained upon fitting with an exponential asymptotic function clearly shows an exponential asymptotic growth for hydrogen flow rate with respect to the stack current. This finding is novel in terms of the hydrogen flow rate with respect to the stack current in the case of an industrial pilot alkaline electrolyzer.
We also came across other research dealing with the MATLAB simulation of a parameter-adjustable dynamic mass and energy balance model of a large-scale industrial alkaline electrolyzer plant of 3 MW capacity [
33]. The study also simulates the experimental data of a working electrolyzer in terms of hydrogen production vs. DC current, but the ramp-up time in this case is 7 h, and hydrogen production rates are stable with respect to DC current. In this case, both measured and modeled H
2 production shows a linear pattern, while in our case, it shows an exponential asymptotic growth in H
2 flow rate during the electrolyzer ramp-up time.
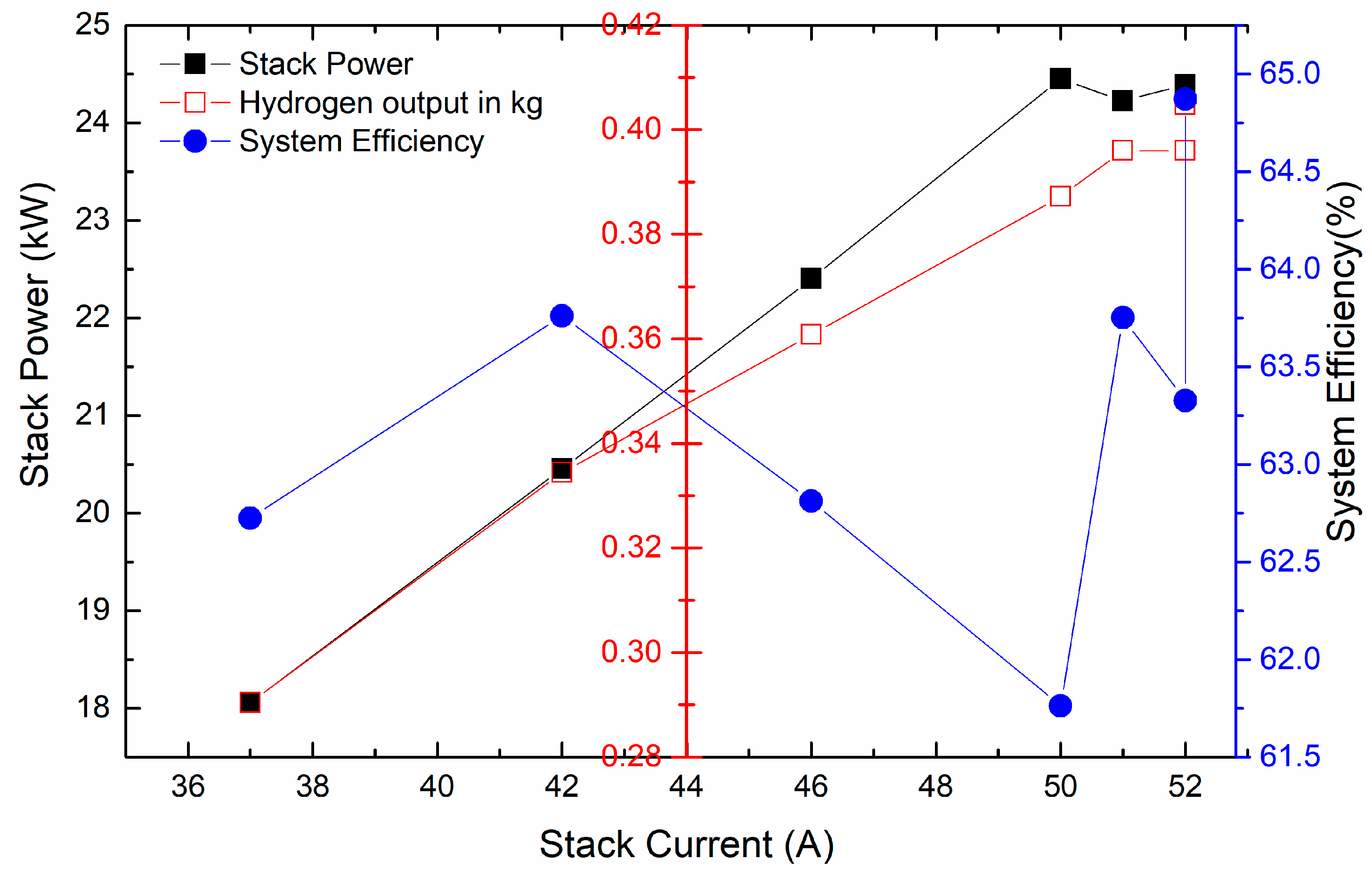
3.7. Correlation of Stack Power (kW) with That of System Efficiency (%)
System efficiency is defined as the amount of energy needed to produce 1 kg of H
2. For a system to be 100% efficient, an output power of 39.4 kWh/kg is needed [
20]. Based on this, we have calculated the system efficiency, and it is plotted against the stack power, stack current, and amount of H
2 generated in kg. The results obtained are shown in
Figure 7. From here, it is found that the system efficiency is maximum when the stack current is highest, and here, we have seen the highest hydrogen production capacity of 0.42 kg. The system efficiency is calculated and is found to be 65%. The system efficiency, stack power (kW), and hydrogen output (kg) all match the same stack current, which is 52 A, where stack resistance is still at minimum. So, we finally conclude here that 52 A is the ideal current to have maximum hydrogen output as well as support the capacity of the electrolyzer, which is 25 kW.
3.8. Techno-Economic Investigation
Three key components decide the cost of green hydrogen production. They are electricity supply, electrolyzer capex, and use rates. Here, 60–70% of the total production cost accounts for electricity costs, and the price is declining due to falling renewable energy prices related to regions with abundant solar and wind resources. Electrolyzer costs, which are currently at 700–1400 USD/kW, are expected to decrease by 40–80% in 2030 if scaled manufacturing and technological improvements are made currently. A further cost reduction in electrolyzers is possible by increasing the system efficiency, hybrid systems integration, and induction of carbon pricing and supportive policies. The production cost of green hydrogen is two to three times more than that of blue mostly due to renewable electricity price fluctuations and the CAPEX of the electrolyzer. The research gap in cost minimization balancing is mostly driven by location-specific factors. Production cost optimization and environmental sustainability need favorable locations with high renewable energy ability. The current production cost of islanded green H2 production via electrolysis from solar and wind energy is at 3.7–5 USD/kg and is competitive with reference to gray H2 when natural gas prices are high. The current cost can be reduced further in 2040 by the adoption of renewable deployment scaled-up and hybrid systems. This can be conducted by the use of cost drivers such as infrastructure, where an “offshore island” can reduce grid cost and intermittency can be managed well with the integration of both wind and solar renewables. But the desalination cost can affect the price due to the limited availability of fresh water. The final scale- up of such islanded green hydrogen production can be conducted by the development of strategic infrastructure, hybrid renewable systems, and policy support.
3.9. AGORA Data Analysis
The levelized cost of H
2 production (LCOH) in the current study is calculated using LCOH tools, Equation (1) developed by Agora Energiewende and the parameters shown in
Figure 8. Note that the currency used for this calculation is set as euro (EUR) rather than US dollar (USD). As the electrolyzer was procured from McPhy inc. Italy(SanMiniato basso), we used the associated cost of the electrolyzer (CAPEX) in EUR as needed by the LCOH calculation model, as shown in
Figure 8. The LCOH is found to be at the high end because the system under investigation is a pilot plant with only a 25 kW capacity alkaline electrolyzer. As described above and discussed in various scenarios, the scaling up of the plant will further reduce the cost. Hence, electrolyzer scale-up by enhance sizing capacity will provide higher hydrogen output along with a reduced cost by consideration of the same parameters as those listed in
Table 1.
LCOH = levelized cost of hydrogen [EUR/kg H2];
LHV = lower heating value [kWh/kg H2];
I = discount rate [%];
n = lifetime [year];
E = electricity costs [EUR/kWh];
ηsys, LHV = system efficiency relates to LHV;
τ = full load hours [h];
OPEX = operational expenditures [% CAPEX/a];
CAPEX = capital expenditures [EUR/kW].
Table 1.
Parameters used for the calculation of the levelized cost of hydrogen (LCOH).
Table 1.
Parameters used for the calculation of the levelized cost of hydrogen (LCOH).
| Parameters | User Input | Unit |
|---|
System
specification | Discount rate | 7 | % |
| Lifetime electrolyzer system | 3 | a |
| Lifetime stack (manufacturer’s data) | 17,520 | h |
| Annuity factor | 0.553 | - |
| Specific energy consumption | 25.0 | kWh/kgH2 |
| Energy consumption (pressure < 30 bar) | 68.1 | kWh/kgH2 |
| Full load hours | 2333 | h |
| Capacity factor | 0.266 | - |
| System pressure | 0.8 | bar |
| Compressor efficiency | 5 | % |
| | Electricity costs | 107 | €/MWh |
| | O2 selling price | 0.00 | €/kgO2 |
| | Heat selling price | 0 | €/MWh |
| OPEX | OPEX | 3.00 | % of CAPEX per year |
| Input CAPEX | CAPEX electrolyzer system | 200.00 | €/kW |
| EPC | 30 | % of CAPEX electrolyzer system |
| Lifetime stack (calculated) | 7.5 | a |
| Stack replacement costs | 30 | % of CAPEX electrolyzer system |
| CAPEX system without stack | 140.00 | €/kW |
| Compressor costs | 1000.00 | €/kW compressor |
| Building | 0.00 | €/kW |
| CAPEX free user input | 0.00 | €/kW |
| Total CAPEX | 848.67 | €/kW |
| LCOH | LCOH | 17.56 | €/kgH2 |
| LCOH (incl. sale of heat and O2) | 17.56 | €/kgH2 |
| LCOH (LHV specific) | 0.65 | €/kWhH2, LHV |
| Overarching cost drivers | CAPEX depreciation | 12.38 | €/kgH2 |
| OPEX | 0.74 | €/kgH2 |
| Electricity costs | 7.28 | €/kgH2 |
| Cost of capital | 1.33 | €/kgH2 |
Figure 8.
LCOH (levelized cost of hydrogen) calculation using the AGORA tools from real-time 500 hydrogen production with a 25 kW alkaline electrolyzer.
Figure 8.
LCOH (levelized cost of hydrogen) calculation using the AGORA tools from real-time 500 hydrogen production with a 25 kW alkaline electrolyzer.