Abstract
This review presents a critical analysis of Japan’s hydrogen strategy, focusing on the broader context of its decarbonization efforts. Japan aims to achieve carbon neutrality by 2050, with intermediate targets including 3 million tons of hydrogen use by 2030 and 20 million tons by 2050. Unlike countries with abundant domestic renewables, Japan’s approach emphasizes hydrogen imports and advanced storage technologies, driven by limited local renewable capacity. This review not only synthesizes policy and project-level developments but also critically evaluates Japan’s hydrogen roadmap by examining its alignment with global trends, technology maturity, and infrastructure scalability. The review integrates recent policy updates, infrastructure developments, and pilot project results, providing insights into value chain modeling, cost reduction strategies, and demand forecasting. Three policy conclusions emerge. First, Japan’s geography justifies an import-reliant pathway, but it heightens exposure to price, standards, and supply-chain risk; diversification across LH2 and ammonia with robust certification and offtake mechanisms is essential. Second, near-term deployment is most credible in industrial feedstocks (steel, ammonia, methanol) and the maritime sector, while refueling rollout lags materially behind plan and should be recalibrated. Third, cost competitiveness hinges less on electrolyzer CAPEX than on electricity price, liquefaction, transport; policy should prioritize bankable offtake, grid-connected renewables and transmission, and targeted CAPEX support for import terminals, bunkering, and cracking. Japan’s experience offers a pathway in the global hydrogen transition, particularly for countries facing similar geographic and energy limitations. By analyzing both the progress and the limitations of Japan’s hydrogen roadmap, this study contributes to understanding diverse national strategies in the rapidly changing state of implementation of clean energy.
1. Introduction
In recent years, the global economy and energy sector have been increasingly pressured to address vital issues of environmental pollution, energy security, and the need for alternative energy sources [1,2]. This has accelerated the transition away from traditional fossil fuels towards renewable energy options [3,4]. Hydrogen power has emerged as a promising solution for sustainable energy production due to its potential for low emissions and diverse applications across sectors [5,6,7,8].
Hydrogen supports decarbonization by lowering greenhouse gas emissions and enabling flexible energy storage. It can also blend with natural gas for transitional use [9,10]. However, challenges remain, including high production costs, energy losses, complex storage due to low volumetric density (e.g., ~5300 MJ/m3 at 700 bar; ~8500 MJ/m3 as liquid at 20 K) [11]. Additionally, green hydrogen production depends on access to ample renewable energy, which varies by region [12].
Clean hydrogen is projected to supply around 10% of global energy by 2050, driven by demand in heavy industry (e.g., steel, ammonia, methanol) and transport sectors like freight, shipping, and aviation [13,14]. Today, most hydrogen is produced using steam methane reforming (SMR), a fossil-based method that generates significant CO2 emissions; the resulting product is commonly referred to as grey hydrogen [15,16]. The transition toward “green hydrogen” by electrolysis using renewables is accelerating, with “blue hydrogen” (with carbon capture) as a bridge. Other methods include “pink” and “purple” hydrogen from nuclear energy and naturally occurring “white hydrogen” [17,18,19].
In response to growing energy security concerns and global decarbonization goals, Japan has adopted a long-term strategy to develop a clean energy system centered in part on hydrogen. The Basic Hydrogen Strategy, first released in 2017 and updated in 2023, outlines Japan’s roadmap toward carbon neutrality by 2050, including specific targets for hydrogen production, infrastructure development, and sectoral integration [20,21,22,23]. The strategy emphasizes the deployment of green hydrogen (produced via renewable electricity) and blue hydrogen (produced from natural gas with carbon capture and storage), with Japan aiming to use 3 Mt of hydrogen annually by 2030 and 20 Mt by 2050. While Japan was an early adopter of hydrogen planning, it now faces increasing competition from countries such as China and Germany, which have rapidly scaled investment, renewable integration, and domestic manufacturing capabilities. Japan’s approach remains distinct, however, due to its focus on liquefied hydrogen (LH2) logistics and its reliance on international hydrogen partnerships to address domestic renewable limitations.
Japan’s hydrogen roadmap focuses on scaling up infrastructure, including hydrogen production facilities and refueling stations, and predicting a future where hydrogen powers not only the transportation sector but also industry, residential energy, and heavy-duty applications [22,23,24]. Hydrogen power in Japan can lead the way in transitioning towards a low-carbon society, setting a model for hydrogen utilization that balances energy security, environmental responsibility, and economic growth [25,26].
This review provides an in-depth overview of Japan’s hydrogen society, considering logistical strategies and potential for optimization, and a roadmap for Japan’s ambitious hydrogen economy and its implications for the global energy transition.
2. Hydrogen Value Chain
In Japan, efficient liquefaction and mid-term storage are essential for long-distance hydrogen transport. However, large-scale hydrogen liquefaction facilities have not yet been implemented at the scale required to meet expected demand. This is attributed to a variety of technical, technological, and economic challenges inherent in managing hydrogen’s unique characteristics under cryogenic temperatures and high-pressure conditions [27,28].
Optimizing Japan’s hydrogen value chain is vital for making hydrogen a cost-effective energy carrier. The hydrogen supply chain includes several stages [29,30]: production, transformation, transportation, storage, distribution, and end-use applications. Production often occurs through methods such as electrolysis or natural gas reforming. Subsequently, hydrogen is transformed, typically through liquefaction, a process in which it is cooled to extremely low temperatures to facilitate compact storage and long-distance transportation. Specialized cryogenic tanks and vessels transport liquefied hydrogen across regions, while high-pressure or cryogenic tanks are used for storage [31,32]. Finally, Japan’s distribution network delivers hydrogen to key sectors, including transportation (via fuel cells), industrial applications (such as steel and ammonia production), and energy generation [4,33,34].
2.1. Hydrogen Production
In Japan, hydrogen production is evolving with various methods, each with unique costs, resources, and environmental impacts [35,36]. Green hydrogen, produced from renewable sources such as wind, hydro, and solar power, is the cleanest option with zero emissions and is currently prioritized [37]. Conventional methods, such as grey and brown hydrogen from natural gas and coal, are highly polluting, whereas blue hydrogen mitigates emissions through carbon capture. Emerging lower-emission alternatives include pink hydrogen, produced via nuclear power, and white hydrogen, either naturally occurring or biotechnologically generated.
As Japan advances toward its hydrogen economy, the focus is increasingly on clean production methods to support sectors where direct electrification is challenging. Although most hydrogen today is produced through fossil-fuel-based natural gas reforming, a significant shift toward green hydrogen is underway, largely due to the increased use of water electrolysis. This process, which splits water into hydrogen and oxygen without CO2 emissions, is projected to play a central role in future hydrogen production [34,38].
Japan’s hydrogen production goals align with broader global ambitions, as global demand for hydrogen is projected to surge from under 1 million metric tons (Mt) per year to 10 Mt by 2030, potentially reaching 600 Mt worldwide by 2050 [39,40,41]. Achieving these levels may necessitate substantial renewable energy resources, estimated at approximately 200 GW, particularly if electrolysis emerges as the predominant method of hydrogen production. Projected hydrogen production costs vary, with proton exchange membrane (PEM) electrolysis estimated at 1.8 USD/kgH2, alkaline electrolysis at 1.6 USD/kgH2, and SMR with carbon capture and storage at 1.2 USD/kgH2 [42,43]. While these estimates provide useful cost benchmarks, the practical competitiveness of CCS remains subject to regional policy stimulus, infrastructure readiness, and long-term viability. Norway, for instance, has led early CCS initiatives but still faces challenges in achieving widespread commercial scalability [1,15]. This economic and technological landscape demonstrates Japan’s ongoing investments and innovations in achieving a sustainable, hydrogen-based energy future.
2.1.1. Natural Gas Steam Methane Reforming
Natural gas steam methane reforming (SMR) is one of the most widely used methods for producing hydrogen today [44,45]. It involves the extraction of hydrogen from natural gas by using steam at high temperatures. This process is the dominant technology for industrial hydrogen production, but it has significant carbon emissions unless paired with carbon capture and storage (CCS).
In SMR, natural gas (methane) reacts with steam (H2O) at high temperatures (700–1000 °C) in the presence of a catalyst, typically made from nickel. This reaction produces hydrogen and carbon monoxide (CO).
The primary reaction is:
CH4 + H2O → CO + 3H2
The byproduct of this reaction, carbon monoxide, is then further reacted with steam in the water-gas shift reaction to produce additional hydrogen:
CO + H2O → CO2 + H2
The overall process produces hydrogen and CO2, which is the main environmental concern with this method.
The hydrogen is separated from the other gases, typically using techniques like pressure swing adsorption (PSA), leaving behind carbon dioxide (CO2) and any remaining methane. The SMR process is highly efficient at producing hydrogen, with typical yields of about 3 moles of hydrogen per mole of methane.
The reaction is endothermic, meaning significant heat is required to proceed. This heat is typically supplied by burning some of the methane itself, which leads to carbon dioxide emissions during the process. For every ton of hydrogen produced through SMR, about 9–12 tons of CO2 are emitted if no CCS is applied [16,46]. Without CCS, this process results in a high carbon footprint, making it less environmentally friendly compared to methods such as water electrolysis powered by renewable energy (which produces green hydrogen).
CCS is a technology that captures the CO2 produced during the SMR process and stores it underground to prevent it from entering the atmosphere. With CCS, SMR can produce low-carbon hydrogen, which is sometimes referred to as blue hydrogen. The degree of CO2 capture depends on the efficiency of the CCS system but typically ranges from 70% to 90% of the CO2 produced during the SMR process [47]. However, the economic feasibility of CCS remains a challenge due to high capital and operational costs, and large-scale deployment has been limited despite technological maturity. Blue hydrogen is considered a bridge technology that can reduce emissions in the hydrogen sector, although it still depends on fossil fuels (natural gas) for hydrogen production [48].
SMR is the most mature and widely used method for industrial hydrogen production, accounting for approximately 75% of global hydrogen production. The process is well-established, efficient, and relatively cost-effective compared to newer methods like water electrolysis or biological hydrogen production. The natural gas infrastructure (e.g., pipelines and storage) is already in place in many regions, making it easier to use existing infrastructure for hydrogen production via SMR [10]. The natural gas supply chain is already well-developed and global, providing a stable and easily accessible feedstock for hydrogen production [49]. Additionally, hydrogen produced by SMR can support energy storage applications for grid balancing and seasonal storage in integrated energy systems.
SMR will likely remain an important method of hydrogen production in the near to medium term, particularly for blue hydrogen, where CCS can be applied to mitigate carbon emissions. The future of blue hydrogen could involve integrating SMR with renewable energy sources. For example, renewable electricity could be used to power the CO2 capture and compression processes or to offset some of the energy required for SMR, reducing the overall carbon footprint [50]. Although blue hydrogen may contribute to emission reductions in the short term, the long-term objective is likely to focus on the widespread adoption of green hydrogen derived from renewable energy sources, such as water electrolysis powered by solar, wind, or hydropower, which represents the most environmentally sustainable solution.
In Japan, SMR has a significant meaning due to its industrial efficiency and reliance on natural gas as a primary feedstock. Currently, SMR is employed by major hydrogen producers in Japan, including large-scale industrial facilities that support industries like refining, petrochemicals, and ammonia production. Companies such as Linde and Air Liquide, which operate globally, also have a presence in Japan, providing SMR-based hydrogen solutions. The hydrogen produced through SMR in Japan is largely used for industrial applications, but it is also being integrated into the country’s energy transition plans, particularly when paired with CCS to mitigate CO2 emissions [51,52,53].
Japan’s efforts to decarbonize its hydrogen production are also implementing innovation in reforming technologies. For instance, SMR is being supplemented or replaced in some cases by emerging methods like chemical looping or autothermal reforming, both of which aim to improve energy efficiency and reduce emissions. However, SMR remains the dominant technology due to its established infrastructure and cost-effectiveness. Additionally, high-pressure storage technologies, such as Toyota’s Type 4 composite cylinders capable of 700 bar, offer a viable alternative to liquid hydrogen storage, minimizing the need for energy-intensive cryogenic systems.
In Japan, several companies are currently using SMR for hydrogen production as part of efforts to advance hydrogen-based energy systems. INPEX is involved in the Kashiwazaki Blue Hydrogen/Ammonia Project in Niigata Prefecture, which utilizes SMR combined with carbon capture and storage (CCS) to produce blue hydrogen. This project is part of a broader effort to commercialize hydrogen production in Japan by leveraging existing natural gas infrastructure [54,55]. Known as a leader in hydrogen-related technologies, Iwatani uses SMR to produce hydrogen, which is then distributed for industrial uses and refueling stations in Japan [56,57]. ENEOS has projects using SMR to produce hydrogen for mobility and other applications. The company also integrates CCS into its processes to reduce the carbon footprint of hydrogen production [58].
These companies reflect Japan’s strategy to develop blue hydrogen as a transition fuel while scaling up green hydrogen production in the future. Japan’s adoption of SMR aligns with its goal to establish a reliable hydrogen supply chain and contribute to achieving carbon neutrality by 2050 [20,22].
2.1.2. Natural Gas Autothermal Reforming
Natural Gas Autothermal Reforming (ATR) is another method for producing hydrogen from natural gas. It shares some similarities with SMR but has a main difference in how heat is supplied to the process. ATR is a more efficient alternative to SMR in certain applications, particularly in large-scale hydrogen production [44,59].
In ATR, methane (CH4) reacts with oxygen (O2) and steam (H2O) in the presence of a nickel-based catalyst or rhodium/platinum group metal catalysts at high temperatures (typically around 800–1000 °C) [60]. The reaction occurs in a two-step process [60,61]:
- Partial oxidation, where oxygen is introduced into the system to partially oxidize methane. This reaction generates heat:
CH4 + O2 → CO + 2H2
- 2.
- Steam reforming, where the carbon monoxide (CO) produced in the partial oxidation reaction then reacts with steam (H2O) in the water–gas shift reaction to produce additional hydrogen and carbon dioxide:
CO + H2O → CO2 + H2
The combination of these two reactions provides the heat required for the reaction to proceed. The autothermal part of the process refers to the fact that the heat needed for the reaction is generated internally through the partial oxidation of methane.
Unlike SMR, which is endothermic (requiring external heat, usually from burning some of the methane), ATR is exothermic in the partial oxidation step, meaning it generates heat. This leads to a more efficient process with a better energy balance. Hydrogen is typically separated from the other gases using PSA, while CO2 can be captured using CCS technologies if desired, making the process more environmentally friendly [62].
ATR can handle a wider range of feedstock compositions and can tolerate some impurities in the natural gas, which makes it more flexible compared to SMR. It also typically has better performance when processing high-sulfur natural gas [63].
Like SMR, the CO2 generated in ATR can be captured using CCS technologies. This makes the process potentially low-carbon when paired with CCS, producing blue hydrogen.
Without the use of CCS, NG ATR remains a high-carbon process, like SMR. It still produces significant amounts of CO2 (around 9–12 tons per ton of hydrogen produced) unless the CO2 is captured and stored [64]. The integration of CCS with ATR adds costs and complexity to the process. The infrastructure required to capture and store CO2 can be expensive and may limit the widespread adoption of blue hydrogen. The blue hydrogen from ATR with CCS can serve as a transitional technology for industries that are difficult to decarbonize using other methods [65].
In Japan, autothermal reforming (ATR) is being explored as a promising technology for hydrogen production, particularly in conjunction with carbon capture to create low-carbon hydrogen and ammonia. A key example is the Kashiwazaki Clean Hydrogen and Ammonia Project in Niigata Prefecture, where the Japanese energy company INPEX Corporation has partnered with Air Liquide to implement ATR technology. This demonstration project is Japan’s first to integrate ATR with carbon capture, aiming to produce low-carbon hydrogen and ammonia efficiently and cost-effectively [66,67]. The project highlights ATR’s role in Japan’s strategy to decarbonize its energy and industrial sectors while supporting clean energy initiatives.
This effort reflects Japan’s extensive interest in advancing hydrogen technologies. ATR is considered a viable solution for large-scale hydrogen production due to its efficiency and compatibility with existing infrastructure. While still in the demonstration phase, this project could lead to the wider adoption of ATR in Japan’s hydrogen economy.
2.1.3. Coal Gasification
Coal Gasification is a process that converts coal into synthesis gas (syngas), primarily composed of hydrogen (H2), carbon monoxide (CO), and carbon dioxide (CO2). The gasification process involves reacting coal with oxygen and steam at high temperatures (typically 900–1200 °C). Coal gasification can be used to produce hydrogen, but it is a carbon-intensive process unless coupled with CCS [68,69].
Coal is heated in a controlled environment with a combination of oxygen and steam. This produces syngas, which contains mainly H2, CO, and CO2 [70]. The syngas is then processed to separate hydrogen from the other gases. After gasification, hydrogen is typically extracted through a water-gas shift reaction (to convert CO into CO2 and more H2). Hydrogen is then separated and purified, often using PSA technologies.
Coal is widely available and abundant, making it a potential hydrogen source in regions with significant coal reserves. Coal gasification is a mature and well-established technology used to produce syngas for power generation, chemicals, and hydrogen production. CCS can significantly reduce the carbon emissions associated with coal gasification, making it a potential option for low-carbon hydrogen production [71].
Besides CO2, coal gasification can produce other pollutants such as nitrogen oxides (NOx), sulfur compounds, and particulates, depending on the coal quality and gasification technology. Additionally, the cost of implementing CCS is high, making coal gasification with CCS more expensive than other low-carbon hydrogen production methods, such as water electrolysis. Coal gasification with CCS could be implemented in regions with abundant coal reserves as a transition technology for producing blue hydrogen [72,73].
While coal gasification with CCS offers technological maturity and short-term reliability for hydrogen production, its high emissions (1.2–2.0 kg CO2/kg H2), costs (2.92–5.00 USD/kgH2), and energy security concerns make it an unsustainable option in Japan’s long-term strategy. Coal-based hydrogen risks being phased out as Japan prioritizes cleaner alternatives like electrolysis powered by renewables and explores transitional technologies such as ATR with CCS. Japan’s focus should remain on accelerating green hydrogen solutions while limiting coal gasification to a temporary, localized role in industrial sectors where alternatives are not yet viable [74,75].
2.1.4. Coal/Torrefied Woody Biomass Gasification
Coal/Torrefied Woody Biomass Gasification is a hybrid process that combines coal and torrefied woody biomass as feedstocks for gasification to produce hydrogen and other valuable gases such as carbon monoxide (CO) and carbon dioxide (CO2) [76,77]. The integration of torrefied woody biomass—a processed form of biomass characterized by higher energy density and improved handling properties compared to raw biomass—has the potential to enhance the sustainability of the process relative to conventional coal gasification. However, the overall environmental impact is contingent upon the extent to which carbon capture and storage (CCS) is implemented.
The torrefied woody biomass serves as a partial renewable feedstock, potentially reducing the overall carbon intensity of the process compared to pure coal gasification. Coal and torrefied woody biomass are gasified together in a reactor with a mixture of oxygen and steam at high temperatures (900–1200 °C) [78]. The resulting syngas (synthesis gas) contains mainly hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and trace amounts of other gases such as methane (CH4) and nitrogen (N2). After gasification, hydrogen is separated from the syngas using methods such as pressure swing adsorption, and the remaining gases are often processed further for other uses. Torrefied biomass has a higher energy content than raw biomass, which makes it a more efficient and attractive feedstock when mixed with coal for hydrogen production.
Coal gasification is carbon-intensive, as it involves fossil fuel (coal) as a feedstock. However, using torrefied woody biomass can reduce the overall carbon intensity because biomass is considered carbon-neutral (the CO2 released during combustion or gasification is offset by the CO2 absorbed by the plants during their growth). CCS can be integrated with this process to capture the CO2 generated from both coal and biomass components, which helps reduce the overall carbon footprint. If the torrefied woody biomass proportion is significant in the feedstock, the overall carbon emissions may be lower than pure coal gasification. The biomass portion offsets some of the CO2 produced, but this depends on how much of the feedstock is biomass vs. coal [79]. However, unless CCS is applied, this process still emits significant amounts of CO2, and without the capture of these emissions, the process could be categorized as grey hydrogen.
Biomass sourcing can have land-use implications, particularly if large-scale cultivation of energy crops is involved. The availability of sustainable biomass is a potential limitation, especially if biomass demand competes with food production or biodiversity concerns. The cost of torrefied biomass is typically higher than raw coal, and the economic viability of the process will depend on the relative prices of coal and biomass, as well as the costs of CCS. If biomass prices are high or if the market does not sufficiently reward the low-carbon benefits of this process, it could limit widespread adoption [80,81].
2.1.5. Alkaline Electrolysis
Alkaline Electrolysis (ALK) is a well-established method for producing hydrogen through the electrolysis of water using an alkaline electrolyte (typically potassium hydroxide (KOH) or sodium hydroxide (NaOH) solution) [44,82]. The electrolyte helps improve the conductivity of the solution, allowing for more efficient electron transfer and reducing the voltage required for the process. The electrolyte is a key component because it affects the efficiency, durability, and overall performance of the electrolysis system [83]. It is a mature and reliable technology used to generate hydrogen using a clean and sustainable method, provided that the electricity used is from renewable sources.
In ALK, water (H2O) is split into hydrogen gas (H2) and oxygen gas (O2) by passing an electric current through an alkaline solution [45,84]. The process occurs in an electrolyzer consisting of two electrodes, known as the anode and the cathode.
Anode: the positive electrode where oxygen (O2) is produced:
2H2O → O2 + 4H+ + 4e−
Cathode: the negative electrode where hydrogen (H2) is produced:
4H+ + 4e− → 2H2
The hydrogen produced at the cathode is then separated and collected, while the oxygen is released at the anode (Figure 1).
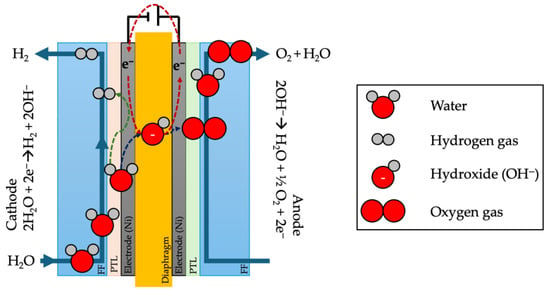
Figure 1.
A schematic representation of alkaline electrolysis, in which water is introduced at the cathode, is provided. Upon the application of an electrical potential, hydrogen is evolved at the cathode, while oxygen is evolved at the anode. FF: flow field; PTL: porous transport layer (inspired by [82]).
ALK is one of the most established forms of electrolysis, with decades of proven industrial use, particularly for producing hydrogen in industries such as chlor-alkali production. Alkaline electrolyzers can be scaled to a wide range of sizes, from small-scale installations for localized hydrogen production to large industrial plants capable of producing significant quantities of hydrogen [85].
ALK electrolyzers are generally less expensive than other types of electrolyzers, such as PEM electrolyzers. The materials used in ALK (such as stainless steel and potassium hydroxide) are relatively low-cost compared to the more advanced materials used in other electrolyzers. ALK typically has an electrical efficiency of around 60–70%, although newer advancements in technology are improving its efficiency further [86]. The efficiency depends on factors such as the current density, operating temperature, and electrolyte composition.
In comparison to PEM electrolyzers, alkaline (ALK) systems typically exhibit lower current densities, necessitating larger electrolyzer volumes to achieve equivalent hydrogen production outputs. Consequently, ALK systems may be less compact and exhibit reduced efficiency in certain high-demand applications. Furthermore, ALK electrolyzers are generally larger than other types, such as PEM, and may demonstrate lower efficiency at smaller scales. As a result, they are predominantly deployed in large-scale, centralized installations, rather than in small-scale, decentralized applications.
Alkaline electrolysis is a key near-term solution for Japan’s hydrogen economy, offering a mature, reliable, and zero-emission method of producing green hydrogen. Examples such as the Fukushima Hydrogen Energy Research Field [87] and Hokkaido wind-to-hydrogen projects [88] demonstrate ALK’s project ENEOS [89,90] viability when coupled with renewable energy. However, high electricity costs and infrastructure development remain barriers to scaling. Japan should continue investing in renewable energy, optimizing ALK efficiency, and expanding hydrogen infrastructure to drive down costs and solidify ALK’s role in achieving its carbon-neutrality goals by 2050.
2.1.6. Proton Exchange Membrane Electrolysis
Proton Exchange Membrane Electrolysis is an advanced and highly efficient method for producing hydrogen through the electrolysis of water. It uses a solid polymer membrane as the electrolyte to separate the hydrogen and oxygen produced during the electrolysis process. PEM electrolysis is increasingly recognized as a promising technology for green hydrogen production, particularly when powered by renewable electricity such as from solar or wind sources [91,92].
In PEM electrolysis, water (H2O) is split into hydrogen gas (H2) and oxygen gas (O2) by passing an electric current through catalytic electrodes coated onto a solid proton-conducting membrane [93,94]. Reactions are like (5) and (6).
The protons (H+) evolved at the anode travel through the proton-conducting membrane to the cathode, where they combine with electrons (e−) from the external electrical circuit to produce hydrogen. The oxygen produced at the anode is released as gas.
The solid polymer membrane is a proton-conductive material that separates the hydrogen and oxygen gases. The materials used in PEM electrolysis rely on precious metals such as platinum and iridium as catalysts, which enhance efficiency but contribute to high system costs. Platinum is primarily used at the cathode for hydrogen evolution reactions, while iridium is utilized at the anode for oxygen evolution reactions due to its stability under acidic conditions [95,96]. The process occurs in high-pressure environments (often around 30–80 bar), which makes it suitable for applications where pressurized hydrogen is needed, such as in fuel cells [97,98].
PEM electrolysis is one of the most efficient electrolysis technologies, with efficiencies typically ranging from 65% to 80% depending on the system design and operating conditions. The high efficiency makes it ideal for green hydrogen production when powered by renewable energy. PEM electrolyzers are compact and modular, making them easier to scale and integrate into different applications, from small-scale installations to large industrial plants [99]. Their modular design allows for scalability based on demand. They can also operate at high current densities, which leads to smaller system sizes compared to other electrolysis technologies, making them suitable for distributed generation systems.
PEM electrolysis produces high-purity hydrogen because the proton-exchange membrane prevents any impurities from mixing with the hydrogen, making it particularly useful for applications that require high-purity hydrogen, such as in fuel cells or semiconductor manufacturing [100].
The materials cost can be a barrier to large-scale adoption, although costs are expected to decrease with advancements in technology and economies of scale [101]. Research is ongoing to find more cost-effective, durable catalysts that can reduce reliance on precious metals.
It should be noted that PEM electrolyzers are being deployed in the Tohoku region to produce green hydrogen from local solar and wind power, supporting industrial clusters and hydrogen refueling stations [102].
Japan is actively expanding its hydrogen infrastructure to support fuel cell vehicles (FCVs) and promote a hydrogen-based society. As of April 2024, the Kanto region, covering Tokyo, leads with 47 hydrogen refueling stations, contributing to a nationwide total of 161 stations. The Japanese government aims to increase this number to 1000 by 2030 to facilitate the adoption of FCVs [103,104].
Kawasaki Heavy Industries is at the forefront of Japan’s hydrogen initiatives through its “Kawasaki Hydrogen Road” project [105]. This comprehensive plan includes the development of technologies for hydrogen production, liquefaction, transportation, storage, and utilization. A notable achievement is the construction of the world’s first liquefied hydrogen carrier, the Suiso Frontier, designed to transport large volumes of hydrogen, thereby establishing a global hydrogen supply chain.
2.1.7. Anion Exchange Membrane Electrolysis
Anion Exchange Membrane Electrolysis (AEM) is the newest and most promising electrolysis technology for producing hydrogen from water. It uses an AEM instead of the PEM to separate hydrogen and oxygen during the electrolysis process. AEM electrolysis is considered to offer several advantages over traditional methods, such as ALK and PEM, but also comes with its own set of challenges [106].
Like other types of water electrolysis, AEM electrolysis splits water (H2O) into hydrogen gas (H2) and oxygen gas (O2) (Figure 2). The primary difference is that AEM electrolysis uses an anion-conducting membrane instead of a proton-conducting membrane like in PEM [107]. The process consists of two half-reactions:
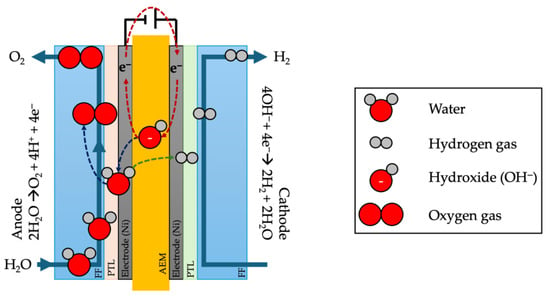
Figure 2.
A schematic illustrating anion exchange membrane electrolysis, in which water is supplied to the anode, is presented. Upon the application of an electrical potential, oxygen is evolved at the anode, while hydrogen is evolved at the cathode. FF: flow field; PTL: porous transport layer (inspired by [108]).
Anode (positive electrode):
2H2O → O2 + 4H+ + 4e−
Cathode (negative electrode):
4OH− + 4e− → 2H2 + 2H2O
The main component of AEM electrolysis is the anion exchange membrane, which allows for the transfer of hydroxide ions (OH−) between the cathode and anode while preventing the mixing of hydrogen and oxygen. It is typically made from a polymer-based material that can conduct anions. AEMs are generally cheaper and more abundant than the proton exchange membranes used in PEM electrolysis, making them a potential solution for reducing the cost of hydrogen production.
One of the important advantages of AEM electrolysis over PEM electrolysis is the lower cost of the ion-exchange membrane and the electrode materials. AEMs do not require precious metal catalysts (platinum or iridium), which are needed in PEM systems. Instead, AEM electrolyzers can use more abundant and cheaper materials, such as nickel or iron, for the electrodes [109].
AEM electrolysis typically offers higher efficiency than ALK, as it can operate at higher current densities and has a more compact design compared to alkaline systems [110]. The current density of AEM electrolysis is comparable to PEM, making it suitable for higher efficiency and faster response times than ALK, especially when integrating with intermittent renewable energy sources (wind, solar). Since AEM electrolysis does not require precious metal catalysts and operates with alkaline electrolytes, the system design is simpler and more cost-effective compared to PEM electrolysis. The lower costs make it a more attractive option for large-scale hydrogen production.
AEM electrolysis can operate at higher pressures, which is beneficial for directly producing pressurized hydrogen for storage or use in fuel cells, thereby excluding the need for additional compression equipment. AEM electrolysis uses alkaline electrolytes (such as potassium hydroxide (KOH) or sodium hydroxide (NaOH)) [111]. This can contribute to longer operational lifetimes and reduced maintenance costs.
Japan is actively advancing AEM electrolysis technology to produce green hydrogen efficiently and cost-effectively. In July 2023, Tokyo Gas introduced Japan’s first AEM water electrolyzer at its Senju Hydrogen Station. This facility utilizes renewable electricity to produce hydrogen, supporting Tokyo Gas’s goal of achieving carbon neutrality by 2050 [112]. Researchers from Waseda University and the University of Yamanashi have developed a new polyphenylene-based AEM with robust hydrophobic components, enhancing durability and ion conductivity. This advancement is crucial for improving the performance and longevity of AEM water electrolyzers [113,114]. A comprehensive project led by the Japan Science and Technology Agency (JST) Project focuses on developing innovative water electrolysis systems, including AEM types, aiming for low-cost, high-efficiency, and durable solutions [115].
2.1.8. Solid Oxide Electrolysis
Solid Oxide Electrolysis (SOEC) is an advanced and highly efficient method for producing hydrogen through the electrolysis of water. Unlike traditional electrolysis methods such as alkaline electrolysis or proton exchange membrane electrolysis, SOEC operates at high temperatures (typically 700 °C to 1000 °C) and uses a solid oxide electrolyte to split water into hydrogen and oxygen [116].
In SOEC, water (H2O) is split into hydrogen gas (H2) and oxygen gas (O2) by passing electricity through a solid oxide electrolyte at high temperatures. This method is based on high-temperature electrolysis (HTE), where the heat plays a significant role in driving the electrochemical reactions and enhancing the efficiency of the process [117,118]. The main components and reactions of the SOEC system are [119]:
Anode:
2O2− → O2 + 4e−
Cathode:
H2O + 2e− → H2 + O2−
The oxygen is released at the anode, and hydrogen is produced at the cathode. The solid oxide electrolyte conducts oxide ions (O2−) between the electrodes, allowing the reactions to take place.
The electrolyte used in SOEC is typically a ceramic material, such as yttria-stabilized zirconia (YSZ), that conducts oxide ions (O2−) at high temperatures. The electrolyte is a key component that differentiates SOEC from other electrolysis technologies, as it operates at higher temperatures, unlike PEM and alkaline electrolysis, which are based on liquid or solid polymer electrolytes. The high operating temperatures of SOEC reduce the amount of electrical energy required to split water, as thermal energy from the heat source can contribute to the electrolysis process, improving overall efficiency [120].
SOEC is one of the most efficient electrolysis methods, with efficiencies of up to 85% or higher, depending on the system design and operating conditions [119,121]. This is because the high operating temperatures reduce the electrical energy required to split water by using thermal energy, which enhances the overall energy efficiency. The efficiency of SOEC can be significantly improved by integrating it with high-temperature processes such as industrial waste heat or nuclear power, making it a promising option for large-scale hydrogen production. The ability to operate at high temperatures and pressures makes it suitable for integration with existing industrial infrastructures, such as steelmaking or refining industries.
SOEC systems tend to have higher capital costs compared to other electrolysis technologies. The high temperature and the materials required to withstand those temperatures (such as ceramic electrolytes and high-temperature resistant components) add to the complexity and expense of SOEC systems. The high operating temperatures of SOEC systems present challenges related to material degradation [122,123]. The materials used for the electrolyte and electrodes (typically ceramics and nickel-based alloys) must withstand both thermal stresses and the corrosive environment created during electrolysis. The lifetime of these materials can be a limiting factor [124].
Japan is actively advancing SOEC technology to produce green hydrogen efficiently. In July 2023, DENSO launched a pilot program at its Hirose Plant utilizing an SOEC device developed in-house. This system produces green hydrogen through high-temperature steam electrolysis, aiming to enhance the sustainability of DENSO’s manufacturing operations and contribute to its goal of carbon neutrality by 2035 [125,126]. In April 2024, MHI commenced the operation of a 400 kW SOEC test module at its Takasago Hydrogen Park in Hyogo Prefecture. Based on solid oxide fuel cell technology, this system seeks to achieve an overall efficiency of 90% (Higher Heating Value) and is a significant step toward high-efficiency hydrogen production [127]. Toshiba is developing an SOEC system as part of its efforts to establish a hydrogen society. Their research focuses on enhancing the efficiency and durability of SOEC technology to facilitate large-scale hydrogen production [128].
2.1.9. Other Methods
Japan is actively exploring diverse hydrogen production technologies to meet its carbon neutrality goals. Methane pyrolysis offers an innovative route to turquoise hydrogen production, decomposing methane into hydrogen and solid carbon at high temperatures (1000–1300 °C). This process eliminates direct CO2 emissions, producing a usable solid carbon byproduct and aligning with Japan’s focus on low-emission solutions. However, the technology’s maturity (technology readiness level (TRL) 3–8) requires further R&D to achieve scalable and economic competitiveness [129].
Japan is also prioritizing biomass-based hydrogen production, using abundant local resources such as agricultural residues and forestry byproducts [130,131]. Biomass steam reforming and biomass pyrolysis produce hydrogen alongside byproducts: bio-oil, syngas, and char. These methods can deliver green hydrogen with a net-zero carbon impact when coupled with carbon capture and sustainable sourcing. Emerging methods such as biomass hydrothermal liquefaction and photo-fermentation provide additional flexibility in utilizing wet biomass and sunlight-driven processes, supporting Japan’s renewable energy strategy [132]. However, it should be noted that scaling up hydrogen production from biomass in Japan faces challenges related to limited domestic biomass availability, high logistics costs, and competition with other bioenergy applications.
2.1.10. Analysis of Hydrogen Production Methods in the Context of Japan’s Hydrogen Strategy
While SMR without CCS has low production costs, its high emissions (9–12 kg CO2/kg H2) conflict with Japan’s low-carbon goals. SMR with CCS offers a balance between cost and emissions reduction, with emissions as low as 0.4–0.7 kg CO2/kg H2 (Figure 3). This option is more favorable but is not fully aligned with carbon neutrality.
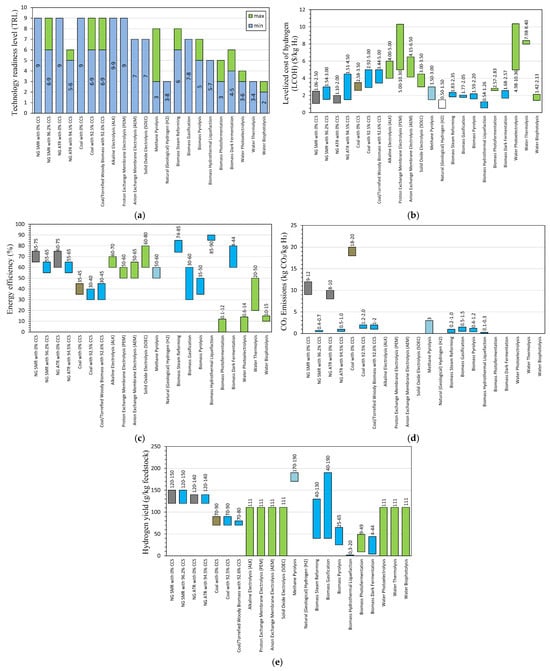
Figure 3.
Overview of hydrogen production methods, including technological readiness level (a), cost of hydrogen (b), energy efficiency (c), CO2 emissions (d), hydrogen yield (e). Values are based on the literature listed in Section 2.1. Colors for (b–e) correspond to the type of hydrogen.
ATR has similar costs to SMR and slightly lower CO2 emissions. With CCS, it becomes a more viable low-carbon option. Japan’s hydrogen strategy may leverage ATR with CCS as a bridge technology, but further advancements in CCS technology will be needed to reduce costs and improve feasibility.
Even with CCS, coal-based hydrogen production remains high in emissions and is costly. Due to Japan’s reliance on imported coal, this method poses energy security risks and is less favorable in the long term.
Electrolysis aligns well with Japan’s strategy due to zero CO2 emissions when powered by renewables. Alkaline and PEM electrolysis are more mature technologies (TRL 9), while SOEC and AEM are emerging (TRL 7) with higher efficiency potential. Despite high production costs, Japan is focusing on scaling electrolysis, coupled with its growing renewable energy sector.
Biomass pathways align with Japan’s strategy as they leverage biogenic CO2 emissions, which are considered carbon neutral. These methods support Japan’s goal to diversify hydrogen production sources, especially in rural areas with biomass availability. Biomass steam reforming and gasification have relatively mature TRLs and offer lower Levelized cost of hydrogen (LCOH), making them attractive for local, decentralized hydrogen production.
Analyzing the goals of Japan’s strategy, it can be highlighted that Japan is relying on blue hydrogen (e.g., NG SMR or ATR with CCS) as a bridge technology due to its relatively low cost and reduced emissions compared to grey hydrogen (Table 1). This approach enables the scaling of hydrogen use in industries and transportation without the significant investments of fully transitioning to green hydrogen. Table 1 presents an analysis and evaluation alignment with Japan’s strategy:

Table 1.
Prospects of the hydrogen production methods and Japan’s Hydrogen Strategy.
- Low level—minimal relevance to goals, lacks scalability or compatibility with strategy;
- Medium level—partial relevance, potential for niche or regional applications with further development; and,
- High level—fully aligned with goals, supports scalability, decarbonization, and infrastructure.
At the same time, Japan’s long-term goal emphasizes green hydrogen production through various electrolysis methods powered by renewables. However, high LCOH (4–10 USD/kgH2) is a significant barrier. Government subsidies and investments in renewable infrastructure are part of the strategy to bring these costs down over time.
On the other side, technologies such as SOEC are promising due to higher efficiencies, although they are currently less mature. Japan’s strategy includes funding R&D for such innovations to make green hydrogen economically viable.
Biomass-based hydrogen production methods provide a renewable option that utilizes domestic resources. While these processes emit biogenic CO2, they may serve as a viable part of the mix due to their carbon-neutral status when using sustainable biomass. In addition, approaches such as natural geological hydrogen and methane pyrolysis also show promise and minimize emissions, but they still have low TRL.
Due to this, we can say that Japan’s hydrogen strategy is based on a balanced, phased approach:
- Short-term: Focus on blue hydrogen production (e.g., NG SMR/ATR with CCS) to scale hydrogen usage with lower emissions.
- Medium-term: Invest in lowering the costs of green hydrogen via electrolysis and advancing technologies such as SOEC and AEM.
- Long-term: Shift towards renewable-powered green hydrogen to achieve a fully sustainable hydrogen economy.
At the same time, it should be noted that, based on estimates for 2023–2025, hydrogen production costs in Japan remain relatively high compared to global benchmarks. Green hydrogen produced via PEM or alkaline electrolysis ranges between 3.0 and 10.3 USD/kgH2, largely influenced by electricity prices and project scale. In contrast, blue hydrogen produced by SMR with CCS is estimated at 1.2–2.3 USD/kgH2. These costs are higher than those in countries such as Australia, where green hydrogen costs can be within 1.5–3.5 USD/kgH2.
The success of Japan’s strategy depends on reducing the cost of green hydrogen production, improving efficiency, and creating a resilient supply chain that integrates domestic and international resources.
2.1.11. Environmental Lifecycle Assessment of Hydrogen Production
To comprehensively evaluate the sustainability of Japan’s hydrogen strategy, it is essential to consider the environmental lifecycle impacts of various hydrogen production routes. Lifecycle assessment (LCA) provides insights into total greenhouse gas emissions, resource use, and environmental impacts from production to end-use. For instance, gray hydrogen produced by SMR without carbon capture results in lifecycle emissions of approximately 9–12 kgCO2/kgH2, while blue hydrogen can reduce this to 1–3 kgCO2/kgH2 and lower, depending on capture efficiency. Green hydrogen, derived from electrolysis using renewable electricity, has the lowest emissions footprint, but it depends on the electricity mix and the efficiency of electrolyzers. Incorporating LCA into hydrogen policy development is crucial to ensure climate goals.
2.2. Hydrogen Transportation
Transporting hydrogen efficiently and safely is essential to Japan’s strategy for establishing a hydrogen economy. Given hydrogen’s low energy density and challenges in long-distance transport, Japan is investing in multiple methods for hydrogen transportation.
2.2.1. Compressed Hydrogen Gas
One of the main methods for transporting and distributing hydrogen is transporting it as a compressed gas in high-pressure cylinders (typically 350–700 bar). It is mainly suited for short- and medium-distance use. Today, the Japanese industry uses compressed hydrogen for local distribution, especially for fueling hydrogen stations in urban areas. For example, today, there are about 160 stations in operation and development [103,104]. However, compressed gas is not ideal for long-distance transport due to the limited capacity of cylinders and the cost of high-pressure compression.
2.2.2. Liquid Hydrogen (LH2)
Hydrogen can be liquefied by cooling it to −253 °C, which increases energy density and makes it suitable for long-distance shipping, though the process is energy-intensive.
It should be noted that Japan has pioneered the use of liquid hydrogen tankers, with the Suiso Frontier, the world’s first liquefied hydrogen carrier, transporting hydrogen from Australia to Japan [133]. This method is central to Japan’s strategy for importing hydrogen from overseas. As Japan lacks domestic hydrogen production resources, liquid hydrogen transport is vital to its plan to import hydrogen from countries such as Australia, where hydrogen can be produced using local resources and renewable energy.
2.2.3. Ammonia as a Hydrogen Carrier
Ammonia (NH3) can store hydrogen in a stable, easily liquefiable form (liquid at −33 °C) and can be transported in conventional chemical tankers. Ammonia can then be “cracked” to release hydrogen or used directly in fuel cells and combustion.
Japan considers ammonia to be a promising hydrogen carrier for international shipping due to its existing infrastructure for ammonia transport and storage, with lower storage costs and fewer safety challenges compared to liquefied hydrogen.
2.2.4. Liquid Organic Hydrogen Carriers (LOHCs)
LOHCs are organic compounds (such as methylcyclohexane) that can chemically bind hydrogen, allowing it to be transported at ambient temperatures and pressures. Hydrogen is extracted through a catalytic process at the destination.
Nowadays, Japan is testing LOHC technology, especially because of the flexibility it offers in hydrogen storage and transportation. Japanese company Chiyoda Corporation is involved in LOHC projects for secure, long-distance transport. In this case, LOHCs could simplify hydrogen logistics by utilizing existing fuel distribution infrastructure and avoiding the extreme temperatures or pressures required by other methods.
2.2.5. Hydrogen Pipelines
Pipelines are a widely used method of transporting hydrogen and offer a continuous and direct way to transport hydrogen over short to medium distances. However, modifying natural gas pipelines or building hydrogen pipelines requires significant investment and technical adjustments. Hydrogen pipelines are being considered for local distribution around industrial hubs and ports, but are not yet widespread. Japan will consider pipeline networks in high-demand regions as its domestic hydrogen market grows.
2.2.6. Alignment with Japan’s Hydrogen Goals
In analyzing hydrogen transportation methods in the context of Japan’s hydrogen strategy, cost considerations, and specific infrastructure options, in Table 2 and Table 3 and Figure 4, we can see that Japan’s hydrogen strategy is heavily focused on cost-effective, large-scale hydrogen import due to limited domestic production capabilities [134,135]. Given geographic constraints, Japan’s system will be anchored by large- and mid-tonnage maritime carriers for long-haul supply (primarily from Australia and the Middle East), which offer the lowest unit costs at scale but require portside cryogenic capability and boil-off management for liquid hydrogen [136]. Domestically, pipelines are the most efficient backbone where feasible on the main islands, while truck/rail combinations provide flexible delivery to hydrogen refueling stations and industrial hubs despite higher per-kg costs—truck-only routes should be limited to short hauls or pilots. For maritime carriers, ammonia and LOHCs avoid extreme cryogenic conditions but introduce cracking/dehydrogenation penalties. At the same time, ammonia is especially attractive given the established shipping infrastructure and energy density. Taken together, this two-level chain (ocean carriers—pipelines/trucks) aligns with Japan’s geography and minimizes delivered cost.

Table 2.
The main performance of the hydrogen transportation methods [28,30,137,138].

Table 3.
Relevance of the transportation hydrogen ways to Japan’s Hydrogen Strategy.
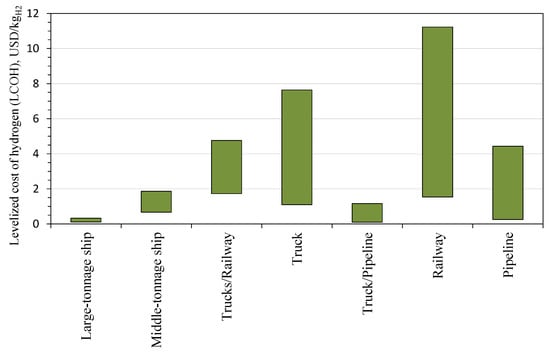
Figure 4.
The levelized cost of hydrogen via different modes of transportation.
2.3. Hydrogen Storage
There are a lot of efficient methods for storing hydrogen, which can be divided into two main parts: physical storage and material-based or chemical storage. In the hydrogen strategy, methods can be chosen depending on the direction of the use of hydrogen and the scale of the distribution [27,28,30].
2.3.1. Compressed Gaseous Hydrogen (CGH2)
Compressed Gaseous Hydrogen (CGH2) is one of the most widely used and mature hydrogen storage methods. It involves compressing hydrogen gas to high pressures (typically 350 to 700 bar) for storage in specially designed tanks [139,140]. This form of storage is common in hydrogen refueling stations, industrial sites, and mobile hydrogen applications, such as fuel cell vehicles (FCVs). In such a method, hydrogen gas is compressed using industrial compressors to pressures of 350 bar (for industrial use) or 700 bar (for mobility and FCVs). The CGH2 is stored in cylindrical or spherical high-pressure tanks made from advanced materials such as carbon fiber composites or metal alloys to withstand extreme pressures [141,142]. However, high-pressure tanks can cost between 500 and 1000 USD/kgH2 of storage capacity, influenced by material selection (steel vs. composite materials) [143,144].
Hydrogen refueling stations across Japan, such as those in Tokyo and Osaka, primarily use CGH2 technology. Compared to LH2 or advanced storage methods, CGH2 infrastructure requires less capital investment and is ideal for short to medium distances and urban refueling networks due to the fast-refueling times (3–5 min for FCVs) [145].
However, this approach has challenges, such as hydrogen in gaseous form having low volumetric energy density and requiring large, high-pressure tanks to store meaningful quantities of hydrogen. Also, tube trailers used to transport CGH2 can carry limited amounts of hydrogen, making long-distance transport inefficient. Distributed storage systems in urban areas or industrial clusters could use CGH2 to supply local refueling stations or small-scale industrial applications in medium-scale decentralized storage. Osaka Hydrogen Industrial Cluster is part of the Kansai Hydrogen Consortium, which uses CGH2 storage to supply hydrogen to local industries, fuel cell buses, and forklifts in industrial zones. Medium-scale CGH2 tanks installed at industrial facilities and refueling stations sourced from regional production sites provide a decentralized hydrogen supply tailored to industrial and urban energy needs [146,147].
In addition, hydrogen’s flammability and diffusion rate require reliable safety protocols, including sensors, venting systems, and double-walled tanks.
Today’s Japan’s hydrogen stations primarily utilize CGH2 to fuel FCVs such as the Toyota Mirai [148,149,150] and in industrial hubs. Multiple hydrogen stations across Tokyo provide CGH2 refueling at 700 bar, supporting Japan’s fuel cell vehicle fleet expansion [103]. Fukushima’s facility produces and stores CGH2, which is distributed to local refueling stations and industrial applications [151,152]. Kawasaki City utilizes CGH2 storage for local hydrogen applications, including fueling hydrogen buses and commercial vehicles [153].
2.3.2. Liquid Hydrogen Storage
LH2 is produced by cooling gaseous hydrogen to −253 °C at atmospheric pressure, significantly increasing its energy density and allowing for more efficient transport and storage compared to compressed gaseous hydrogen (CGH2) [68,154].
LH2 is stored in double-walled, vacuum-insulated cryogenic tanks that minimize heat transfer and reduce boil-off losses (evaporation of hydrogen during storage). It can be transported via specialized liquid hydrogen tankers or cryogenic containers to distribution points, where it is either used directly or converted back to gas for industrial and mobility applications [155].
It should be noted that efficiency is 60–70% due to the liquefaction being energy-intensive, consuming 30–40% of the hydrogen’s energy content during the process. In addition, continuous evaporation results in 0.3–1.0% daily losses during storage and up to 5–10% during transport for long voyages [156]. Cryogenic tanks cost 1000–1500 USD/kgH2 of hydrogen capacity, higher than CGH2 tanks [157]. At the same time, LH2 technology has been in use for decades, particularly in the space industry (rocket fuel), and is now being adapted for large-scale energy applications [158].
However, such a way of hydrogen storage needs LH2 infrastructure (liquefaction plants, cryogenic tankers, and specialized terminals) and requires significant capital investment. On the other hand, handling cryogenic hydrogen at −253 °C poses technical and safety challenges, as leaks or tank failures can lead to embrittlement or frostbite hazards [159,160].
Japan’s hydrogen import projects, such as the partnership with Australia’s Hydrogen Energy Supply Chain (HESC), heavily rely on LH2 for transporting hydrogen produced from brown coal and renewables [161]. The development of the Suiso Frontier, the world’s first liquefied hydrogen tanker, is a significant milestone in Japan’s hydrogen import infrastructure, demonstrating the feasibility of large-scale LH2 transport [29,162].
There is an example of the LH2 infrastructure and large-scale storage is Kobe LH2 Terminal. Japan’s first liquefied hydrogen receiving terminal is in Kobe, where LH2 from Australia is offloaded and distributed to local hydrogen refueling stations and industrial users [136]. This facility serves as a blueprint for future hydrogen import terminals. An industry company such as Iwatani operates several LH2-based hydrogen refueling stations across Japan, supporting the country’s fuel cell vehicle rollout and operating small-scale and short-term LH2 storage [163].
2.3.3. Cryo-Compressed Hydrogen
Cryo-compressed hydrogen (CcH2) is an advanced hydrogen storage method that combines cryogenic cooling (to temperatures near −253 °C) with compression (typically 200–350 bar) [164,165]. This hybrid approach increases hydrogen density beyond what is achievable by CGH2 or LH2 alone, providing greater storage capacity with fewer boil-off losses. Japan is actively exploring this technology to address its hydrogen import and domestic distribution needs, but widespread adoption remains limited due to technological complexity, high costs, and infrastructure challenges (Table 4).

Table 4.
General methods of hydrogen storage.
CcH2 requires double-walled, vacuum-insulated pressure vessels designed to withstand both high pressures and low temperatures [166]. These tanks reduce boil-off rates, allowing for longer storage times [167,168]. In this case, approximately 25–35% of the hydrogen’s energy is consumed during the liquefaction and compression stages. Production and storage costs are 4–7 USD/kgH2 of hydrogen, while cryo-compressed tanks are more expensive than standard CGH2 or LH2 tanks, with costs ranging from 1200 to 2000 USD/kgH2 of hydrogen capacity [169,170]. CcH2 achieves up to 80 kg/m3, which makes it ideal for long-range hydrogen vehicles and space-constrained applications [171,172].
Japan’s automotive leaders, such as Toyota and Honda, are investigating CcH2 as a next-generation storage method for hydrogen fuel cell trucks and trains. Toyota is exploring CcH2 for heavy-duty hydrogen trucks and buses, aiming to extend driving ranges and reduce refueling frequency through higher hydrogen storage densities [173,174,175]. The Japan Aerospace Exploration Agency (JAXA) is researching CcH2 technologies for potential use in space applications and fueling hydrogen-powered rockets [176,177].
2.3.4. Material-Based (Chemical) Storage: Adsorption
Adsorption-based hydrogen storage utilizes porous materials, such as activated carbon, metal–organic frameworks (MOFs), zeolites, and carbon nanotubes, to store hydrogen on the surface of the material. This method allows for low-pressure hydrogen storage at relatively low temperatures (typically 77 K or −196 °C, near liquid nitrogen temperatures) [178,179]. Adsorption is considered a safe, compact, and potentially scalable hydrogen storage method, though it remains experimental mainly and is limited in capacity and efficiency (Table 4).
According to the process and technology of adsorption, hydrogen gas is introduced into high-surface-area materials, which adhere to the surface of the porous material at low pressures and temperatures. This process is reversible, allowing hydrogen to be released when needed by increasing the temperature or reducing the pressure. Activated carbon, MOFs, zeolites, and carbon nanotubes are the most common materials explored for adsorption due to their high porosity and large internal surface area [179].
Adsorption-based storage systems use insulated vessels to maintain low temperatures, with hydrogen distributed and stored in compact storage units. It should be noted that desorption or hydrogen release requires energy input to raise temperatures or reduce pressure, further contributing to energy losses [180,181].
On the one hand, adsorption allows for hydrogen storage at low pressures (10–100 bar), enhancing safety compared to high-pressure CGH2 storage (350–700 bar). Adsorption materials offer higher volumetric storage efficiency at low pressures, allowing for compact hydrogen tanks in vehicles and industrial applications [182,183].
Also, it should be highlighted that the low-pressure nature of adsorption storage reduces the risk of leaks, explosions, or embrittlement, making it safer for use in urban environments or sensitive areas. Adsorption technology is being explored for hydrogen-powered drones, portable energy systems, and small hydrogen vehicles, where safety and size constraints are paramount [184,185]. On the other hand, despite advancements, adsorption materials store less hydrogen by weight (typically 1–3% of their mass) compared to LH2 or CGH2, limiting the feasibility of large-scale storage or transport. Also, repeated adsorption–desorption cycles can lead to material degradation over time, reducing the storage system’s lifespan and efficiency.
At present, Japan is investing in the development of adsorption technology through initiatives at academic institutions and private enterprises, though it remains in the experimental phase. Adsorption is being explored for specialized applications, including portable hydrogen storage for drones, backup generators, and hydrogen-powered forklifts. Considering Japan’s emphasis on safety and disaster resilience, the low-pressure, compact characteristics of adsorption align with hydrogen storage strategies designed for urban areas or small-scale hydrogen hubs.
Kyushu University is leading research into MOFs and activated carbon for hydrogen adsorption [186,187,188]. The university is exploring materials capable of achieving higher hydrogen densities at lower pressures. Toyota is evaluating adsorption-based storage as part of its effort to develop next-generation hydrogen fuel cell vehicles with safer and more compact hydrogen storage systems [189,190].
2.3.5. Material-Based (Chemical) Storage: Absorption
Absorption-based hydrogen storage utilizes metal hydrides and chemical materials that chemically bond with hydrogen, forming stable compounds capable of dense hydrogen storage. This method offers safe, compact, and reversible storage, making it attractive for stationary industrial applications or mobile systems requiring stable hydrogen containment [191,192]. Hydrogen stored in this form can be released by applying heat, which breaks the chemical bonds, freeing the hydrogen gas, although slow kinetics present challenges for large-scale applications [193].
In the absorption process, hydrogen gas reacts with metal or chemical compounds (metal hydrides), forming a solid or semi-solid hydride. This is a reversible reaction that allows hydrogen to be released when heat is applied. Hydrogen is released by heating the hydride material, typically requiring 200–400 °C. This process can be slower than other storage methods, limiting real-time hydrogen availability [191,194]. Storage materials include elemental hydrides (magnesium hydride), complex metal hydrides (sodium alanate, borohydrides), and interstitial hydrides (palladium alloys) [194,195].
The need for high-temperature heating during desorption reduces overall system efficiency, and the energy efficiency of the absorption method is 60–80%. Energy losses occur during both hydrogen absorption and release, requiring external heat sources [196,197]. High costs (8–30 USD/kgH2) are driven by the need for rare metals, heating systems, and material degradation over time [192].
It should be noted that hydrides provide stable hydrogen storage, reducing the risk of leaks or explosions compared to compressed or cryogenic storage. An important advantage of absorption technology is its ability to store hydrogen at low pressures (<10 bar), enhancing safety for applications in urban areas or environmentally sensitive settings [198].
On the other hand, slow hydrogen uptake and release in metal hydrides can take hours, limiting the practicality of absorption systems for applications requiring rapid refueling or high energy demand. Over time, hydride materials degrade, reducing hydrogen capacity and requiring regeneration or replacement [199]. There are different types of hydride storage [187,200,201,202]:
- Elemental hydrides (e.g., MgH2, TiH2)—stable, dense storage but heavy, with slow kinetics.
- Complex metal hydrides (e.g., sodium alanate, borohydrides) have higher capacity and are lighter than elemental hydrides, but are expensive to produce and difficult to scale.
- Interstitial hydrides (e.g., palladium alloys)—hydrogen is stored within metal lattices, allowing low-pressure hydrogen uptake.
Today, Japan continues to research and develop absorption technologies, primarily for industrial uses rather than mass hydrogen transport or storage. Kyoto University leads metal hydride development projects, focusing on magnesium and complex hydrides for stationary hydrogen storage and fuel cell backup systems [203]. Tohoku University is researching sodium alanate and borohydride materials to improve hydrogen capacity and reduce absorption/desorption temperatures [204,205]. Hitachi Zosen is developing metal hydride storage systems for use in industrial decarbonization projects, providing compact, dense hydrogen storage solutions [206].
2.3.6. Scale Hydrogen Storage
The analysis of hydrogen storage technologies across scales reveals a need for diversified strategies to meet different storage requirements (Table 5). Table 5 shows hydrogen storage methods by scale and provides insights into each technology’s suitability, challenges, and real-world applications. Small-scale solutions require advancements in materials and efficiency, medium- and large-scale storage faces cost and safety challenges, and very large-scale storage depends on geological options that may not be available in every region [207,208,209,210].

Table 5.
Main trends and scales of hydrogen storage methods [211,212].
2.3.7. Alternative Solutions and Trends
Based on the information provided, it was analyzed that Japan’s hydrogen storage strategy focuses on large-scale storage solutions and their alignment with the country’s overall hydrogen strategy:
Salt caverns. With capacities around, for example, 300,000 m3, salt caverns provide an estimated 122 GWh of energy (based on the lower heating value) [213,214,215]. Although onshore storage facilities incur higher construction costs, estimated at 8.75 USD/kgH2, and offshore facilities are even more expensive due to infrastructure challenges, they provide significant storage capacity, with hydrogen storage costs ranging from 0.66 to 1.75 EUR/kgH2 [216,217]. However, the efficiency of round-trip hydrogen storage is only about 40%, meaning Japan would need to consider ways to boost this efficiency.
Depleted gas fields. These fields offer an economical storage option with a much lower cost of ~1.07 USD/kgH2, with a 45–55% damper gas requirement to maintain pressure [218,219]. Delivery pressures of 50–100 bar make these fields suitable for large-scale, long-term storage. Given the relatively lower infrastructure costs, depleted gas fields may offer a feasible option for Japan, particularly if paired with renewable sources to support large-scale energy storage. However, both large storage options face a low round-trip efficiency (~40%), which could result in significant energy losses.
Compressed gas vessels are suitable for buffering hydrogen, with a usable density of about 1.2 kg H2/m3 [137,140]. Though compact, they might serve as short-term or emergency storage rather than a primary option for Japan’s large-scale needs.
Metal hydrides. AB2-type metal hydrides, which have a hydrogen density of up to 13.9 kg H2/m3, exhibit fast absorption/desorption kinetics. This makes them a viable buffer storage option for industrial applications where hydrogen demand is variable [220].
Underwater storage. Underwater storage offers a relatively low-cost (1.50 USD/kgH2) option at depths of 500 m, where pressures reach 50 bar [12,221]. Fixed-walled storage solutions using materials like HDPE provide a secure, isobaric environment for hydrogen storage. This type of storage might suit Japan’s island geography, allowing hydrogen to be stored offshore at reduced land usage.
2.4. Hydrogen Distribution and Infrastructure, Key Ports for Hydrogen Importation
Japan is rapidly developing its hydrogen economy, and several of its seaports are being positioned as future hubs for hydrogen importation. These ports are either upgrading their infrastructure or are already involved in various hydrogen-related projects to support the country’s push toward a clean energy transition. Table 6 is an overview of the main ports in Japan that could be important in hydrogen imports [133,136,222,223,224,225].

Table 6.
Japanese seaports and potential for hydrogen imports.
These ports (Figure 5), both large and smaller, are crucial for developing a comprehensive hydrogen import infrastructure across Japan. Larger ports like Kawasaki, Chiba, and Osaka will likely handle significant hydrogen import volumes due to their strategic locations and proximity to major industrial zones. Meanwhile, regional ports such as Niigata, Onahama, Tokuyama, and Kudamatsu will complement the larger ports by providing localized distribution networks for hydrogen to various industrial sectors. The Fukushima Hydrogen Supply Chain project, with Onahama as a main port, highlights the importance of several of these ports in receiving hydrogen from international sources, particularly from Australia, and ensuring that Japan can scale its hydrogen economy. Ports like Tomakomai and Muroran are also poised to become integral players, particularly for hydrogen production and transport from nearby hydrogen-rich regions.
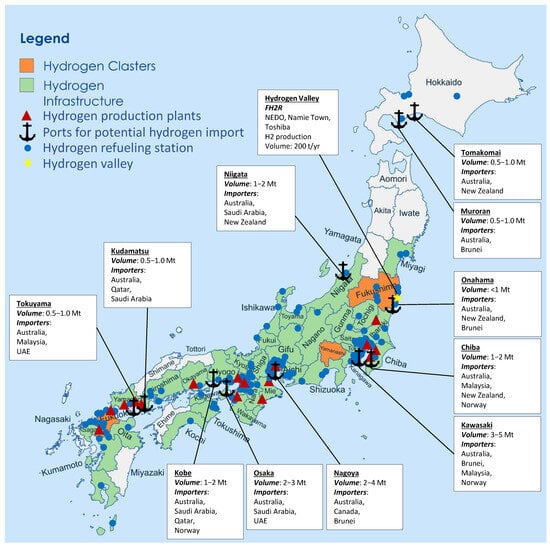
Figure 5.
Japan’s domestic hydrogen geographic landscape, including hydrogen clusters, infrastructure, production plants, potential import ports, and refilling stations.
Ports Kawasaki, Nagoya, and Osaka have the potential to import 2–5 Mt/year by 2030, making them central to Japan’s hydrogen economy. Smaller regional ports such as Muroran, Tomakomai, and Kudamatsu are expected to handle 0.5–1.0 Mt/year, with volumes increasing as demand for hydrogen grows. Japan’s hydrogen import network is expected to involve a diverse mix of ports handling various forms of hydrogen, with LH2 and ammonia.
Kawasaki, Osaka, and Nagoya are pivotal for both green and blue hydrogen, with blue hydrogen expected to dominate in industrial centers such as Nagoya and Tokuyama, while green hydrogen derived from renewable sources is more likely to be found in Muroran, Tomakomai, and Onahama. The predominant forms of hydrogen anticipated at these ports are liquid hydrogen (LH2) and ammonia. LH2 is a key focus for Kawasaki, Kobe, and Nagoya, while ammonia is widely anticipated at ports such as Chiba, Niigata, and Tokuyama, serving as a hydrogen carrier. By 2030, the total volume of hydrogen imports at these ports could range from 0.5 Mt/year at smaller ports like Muroran and Kudamatsu to 3–5 Mt/year at larger, more central ports such as Kawasaki, Nagoya, and Osaka.
Figure 5 shows the location of major hydrogen production plants in Japan, which are primarily located in the major urban and industrial areas of Kanto and Kansai. Industry, academia, and government are collaborating to create hydrogen clusters (also known as hydrogen valleys, hubs, or ecosystems). These clusters are hydrogen value chain demonstrations and pilot projects that cut across sector applications [151,226]. Each cluster leverages its unique comparative advantages and has set out local hydrogen strategies. Fukuoka is the “hydrogen hub of Japan” designated by METI, Fukushima is focused on becoming a renewable energy leader, considering the Fukushima disaster, and Yamanashi is focused on energy storage and becoming a ‘fuel cell valley’, considering its solar resources. Each cluster comprises universities, local government, industry stakeholders, and public research institutions. Further, Japan’s 2021 Strategic Energy Plan outlines a policy plan to transition service stations to “comprehensive energy hubs” to supply energy to electric and fuel cell electric vehicles and “local community infrastructure” to provide services to meet local needs.
Fukuoka:
- As the METI-designated “hydrogen hub of Japan”, Fukuoka has been supporting the development of a sustainable society based on hydrogen energy since 2004 and holds the largest hydrogen conference in Japan, the Fukuoka Strategy Conference for Hydrogen Energy.
- The stakeholders active in the Fukuoka cluster projects: Kyushu University, Kyushi Electric Power Co., Taiyo Nippon Sanso Co., Kyuky Co., Iwatani Co., Nippon Steel Co., JXTG Nippon Oil & Energy, Saibu Gas Energy Co., Sumimoto Metal, Yawate Steel Works, Mitsubishi Kakoki Kaisha Ltd., and Toyota, Tsusho Corp.
- Initiatives include the Hy-Life Project, Fukuoka FCV Club, Q-PIT: Kyushu University Platform of Inter/Transdisciplinary Energy Research, and the Fukuoka Strategy Conference for Hydrogen Energy (~800 members across industry, university, administrative, and research organizations).
- Key demonstration projects include the “Hydrogen Highway”, Kyushu Station, “Fukuoka Hydrogen Town” (Itoshima City), “Hydrogen Town” Kitakyushu City, and Hydrogen production from sewage sludge.
- R&D infrastructure capabilities include Kyushu University (several laboratories on campus, including the world’s largest hydrogen research facility), the Hydrogen Energy Test and Research Centre (HyTReC), and the Centre for Research Activities and Development of Large-scale Pressure Vessel Equipment (CRADLE).
Fukushima:
- As an emerging global center for renewable energy research, Fukushima has been hosting AIST activities in hydrogen energy carriers, as well as hydrogen and heat utilization systems.
- Fukushima hosts a major hydrogen project called the Fukushima Hydrogen Energy Research Field (FH2R) [227], comprising a hydrogen production facility using solar power and electrolysis, transport and storage of compressed hydrogen, and utilization in gas networks and for electricity generation and grid balancing.
- Stakeholders in this cluster include AIST’s Fukushima Renewable Energy Institute (FREA), Toshiba ESS, Tohoku Electric Power Co., and Iwatani Corporation.
Yamanashi:
- Positioning itself as a “Fuel Cell Valley” and national center for storage and fuel cell development, Yamanashi hosts several demonstration projects within its Komekurayama Facilities. This facility includes the Electric Power Storage Technology Research Site (solar power station) and a power-to-gas demonstration.
- Stakeholders contributing to projects in the Yamanashi area include Iwate University, Shinshu University, Tohoku University, AIST, Yamanashi University, TEPCO, Kobe Steel Ltd., Panasonic, Toray Research Centre Inc, Takaoka Toko, HySUT, Miura, Hitz Hitachi Zosen, Japan Steel Works Ltd., Tanaka Kinkinzoku Kogyo KK, Kaneka Corp., and Nissan Arc Ltd.
- R&D infrastructure in the area includes the Clean Energy Research Center, the Hydrogen and Fuel Cell Technical Support Center, the Fuel Cell Nanomaterials Center, and the Hydrogen Technical Center (HySUT).
The Japanese automakers Toyota and Honda launched their fuel cell vehicles (FCVs), MIRAI and Clarity, in 2014 and 2016, respectively, with initial sales prices of around 7 million JPY (approximately 52,000 USD as of 2023) [228,229,230]. A typical HRS has a supply capacity of 300 Nm3/h. The roadmap targets the establishment of 200 HRSs by 2025 and 900 HRSs by 2030.
In addition to passenger vehicles, the use of fuel-cell technology is expanding into other mobility sectors, including fuel-cell buses and fuel-cell loaders. The deployment of fuel cell buses is expected to reach around 100 units by 2025, with a target of 1200 units by 2030. Similarly, the number of fuel cell loaders is projected to grow, with an estimated 500 units expected by 2025 and 10,000 units by 2030 [226].
At the same time, the widespread deployment of hydrogen technologies in Japan faces notable social acceptance and safety challenges. Public concerns often relate to the perceived risks associated with hydrogen’s flammability and high-pressure storage. To address these issues, it has implemented strict safety regulations for hydrogen refueling stations and transport systems.
3. National Hydrogen Strategy
3.1. Companies and Projects
While in European countries, governments determine hydrogen strategies based on energy security and geopolitical challenges, in Japan, private companies take the lead, shaping strategy around forecasts of economic growth, particularly in heavy industries such as steel manufacturing. The top hydrogen suppliers in Japan:
- Iwatani, a pioneer since 1941, is the largest hydrogen producer, operating multiple large-scale liquid hydrogen plants and supplying approximately 70% of the domestic market, including for FCVs and industrial use.
- Kawasaki Heavy Industries plays a key role in both production and logistics, having developed Japan’s first liquefied hydrogen carrier (“Suiso Frontier”) and supplying compressed and liquid hydrogen trailers.
- JXTG Nippon Oil & Energy (now Eneos) and Air Liquide Japan also contribute significantly, particularly in industrial gas supply and fueling stations.
- Toyota, NEDO, and several utility companies support hydrogen station networks through the Japan H2 Mobility consortium (JHyM).
It should be highlighted that newer projects starting from 2021 onwards focus on large-scale hydrogen imports, with Japan targeting Australia and the Middle East for green hydrogen. Domestic infrastructure, such as the Green Innovation Fund, supports transitioning to a carbon-neutral society. Importantly, Japan’s national hydrogen strategy includes both green and blue hydrogen pathways, with early-phase targets relying significantly on blue hydrogen produced from natural gas with carbon capture.
Across the last decades of pilots, Japan’s programs (Table 7) demonstrate the operational readiness of electrolysis (ALK, PEM), power generation and mobility integration at the city and regional scale. Electrolysis pilots (Tohoku Pilot Plant, THEUS H2 storage, Shoro Dam hydropower-to-H2, Sendai, Musashi-Mizonokuchi, and other urban sites) validated stack durability under real-world duty cycles, grid-following operation, and end-use synergy (mobility, heating) while exposing limitations in water treatment, capacity, and purification. The hydrogen refueling demonstrations (urban stations and venue deployments) allowed for the identification of technology readiness of stations and safety risks, and identified components of cost relevant to FCV uptake. The FH2R 10 MW plant provided the first Japan-scale evidence of variable renewable integration into electrolysis, highlighting that electricity price and utilization dominate delivered H2 cost, not just stack CAPEX. NEDO’s thematic programs shifted emphasis from isolated pilots to system integration: the HRS expansion program lead to increasing the pace of development of networks; “Technologies for Realizing a Hydrogen Society” progressed balance-of-plant standardization; and the Green Innovation Fund reoriented R&D toward large-scale supply chains (import terminals, ammonia/LH2 logistics), where trials have surfaced energy penalties (liquefaction), boil-off gas (BOG) management, and standards gaps as principal bottlenecks. In parallel, hydrogen-fired gas turbine combustion work has delivered stable hydrogen flames with NOx control strategies, mapping out materials and burner designs required for higher H2 shares in power generation.

Table 7.
Leading projects in the Japanese industry.
Japan’s project portfolio shows that electrolyzer integration works technically, refueling can be operated reliably, and industry co-use is feasible, but cost and scale depend on terminal efficiency, utilization, and standardized logistics. These outcomes justify a policy pivot toward terminal CAPEX support and the development of standards, with near-term deployment strongest in ammonia co-firing, industrial feedstocks, and the maritime sector.
3.2. Transport Sector
Hydrogen mobility was once a leadership part of Japan’s energy transition, but recent developments show that the country has fallen behind other major players such as China and South Korea. While Japan was an early adopter of the national hydrogen roadmap in 2017 and introduced FCVs like Toyota’s Mirai, the pace of adoption has slowed due to policy, economic, and infrastructural challenges.
Hydrogen in transportation covers a range of applications, including passenger cars, buses, trucks, forklifts, and trains [231,232,233,234,235]. In 2020, Japan had deployed around 5170 fuel cell vehicles (FCVs). However, actual deployment has significantly lagged these projections. As of 2025, the number of FCVs in operation is estimated to be around 10,000, highlighting a considerable gap between policy ambition and market reality. This gap reflects technological, infrastructural, and economic barriers, including high vehicle costs, limited hydrogen refueling stations, and competition from battery electric vehicles [236].
Despite constructing over 160 hydrogen refueling stations (HRS) by 2023, these stations remain significantly underutilized. A typical HRS is designed to support 200–300 vehicles per day, but actual throughput averages less than 10% of capacity. In contrast, China has a rapidly growing FCV sector with over 15,000 vehicles as of 2022, with an expanding HRS network. Stronger public investment, deployment strategies, and vehicle manufacturing support have enabled China and South Korea to exceed Japan’s early lead in this domain [237].
Japan’s underperformance in hydrogen mobility is attributed to several factors:
- High vehicle cost and limited model diversity.
- Policy fragmentation, especially in subsidies and regional coordination.
Nevertheless, public–private initiatives such as Japan H2 Mobility (JHyM) continue to promote HRS deployment through regulatory coordination and co-investment. New initiatives in 2024 under METI’s revised hydrogen roadmap include increased FCV purchase subsidies, infrastructure integration with logistics hubs. Emerging opportunities for hydrogen use in transport include:
- Expansion of hydrogen engines and hybrid fuel systems in heavy vehicles (e.g., Mitsubishi Fuso).
- Decentralized hydrogen storage solutions for forklifts and material-handling systems (e.g., Toyota, Mitsubishi).
- International market entry by Japanese hydrogen technology companies, including partnerships in Europe and Southeast Asia.
Japan’s hydrogen transport strategy remains part of a long-term decarbonization plan, but recent developments underscore the need for recalibrated targets, stronger industrial coordination, and clearer alignment with global trends.
3.3. Maritime Sector
The marine sector is ready to adopt hydrogen-based technologies to transition away from fossil fuels and reduce greenhouse gas emissions. Recent studies highlight the potential of hydrogen-based energy systems in maritime applications, reinforcing the role of liquid hydrogen in future transportation networks [238,239]. While there are currently no commercial hydrogen-powered marine deployments, significant R&D efforts are underway in Japan, leveraging the country’s reliable shipbuilding industry [240]. A consortium of major engine manufacturers—Kawasaki Heavy Industries, Yanmar Power Technology, and Japan Engine—is collaborating to develop hydrogen-fueled marine engines for various applications, including ocean-going vessels and coastal ferries [133,241]. These engines aim to address diverse operational requirements, such as main engines, auxiliary engines, and power generators.
Japan is advancing innovative projects like small hydrogen-powered ferries and hydrogen fuel cell systems, planning pilot operations in Yokohama by 2024 [22,23]. Yanmar has already completed high-pressure hydrogen refueling tests for demonstration vessels and plans to commercialize hydrogen fuel cell systems by 2023. Additionally, international collaborations, such as the partnership between Belgian company Compagnie Maritime Belge (CMB) and Japan’s Tsuneishi Group, are contributing to the development of hydrogen-powered passenger ferries [242].
Japan’s Shipping Zero Emission Project, launched in 2018, sets ambitious goals for zero-emission ships by 2030 through a combination of hydrogen, ammonia, and carbon recycling technologies [243,244]. Ammonia-based propulsion, better suited for large ocean-going vessels due to its higher energy density and existing handling infrastructure, is also under active research in Japan. Companies such as IHI, Japan Marine United, and Mitsui E&S are leading efforts to integrate ammonia engines and bunkering infrastructure into new builds and retrofits.
Key opportunities for hydrogen in maritime applications:
- Fuel cells for auxiliary power: replacing diesel generators on ferries and cargo ships during port stays.
- Propulsion systems: hydrogen for small- to mid-sized vessels; ammonia for long-haul maritime transport.
- Port infrastructure: hydrogen-ready refueling terminals, such as those being built at Kobe and Yokohama ports
- International collaboration: Corvus Energy, ABB, and other global leaders in maritime fuel systems integration.
3.4. Main Exporters of Hydrogen
Nowadays, Japan’s Hydrogen industry considers around 10 top countries for importing hydrogen in such forms as LH2 and ammonia (Table 8) [20,245]. High-readiness countries (Australia, Saudi Arabia, Norway, and the United States) have robust infrastructure, projects under development, and strong government backing for scaling hydrogen production and export [134,246,247,248]. Medium-readiness countries (UAE, Chile, New Zealand, Canada) have good potential due to renewable energy resources or initial hydrogen projects but require further investments in infrastructure and policy support [249,250,251,252]. Low-readiness countries (Brunei, South Korea) are in earlier stages of hydrogen export development, often relying on specific pilot projects or partnerships to progress [252,253].

Table 8.
The potential hydrogen imports partners.
3.5. Analysis of the Hydrogen Strategy and Main Goals Until 2050
Below are presented data about the strategic importance and readiness of measures in hydrogen strategy timelines (2024–2050) in readiness levels: “high,” “medium,” and “low” (Table 9, Table 10 and Table 11).

Table 9.
The strategic importance and readiness for the implementation of hydrogen technology and energy.

Table 10.
Main Goals/Drivers of Japan’s Hydrogen Strategy.

Table 11.
The implementation in the sectors of the economy.
It should be noted that by 2050, global scale-up, efficient infrastructure, and cheap renewable energy could reduce green hydrogen costs to below 1.5 USD/kgH2, especially in resource-rich nations. The values are inferred from trends in hydrogen strategies, global priorities, and readiness levels for policies, technologies, and investments [19,20,43,252].
4. Comparable Analysis of the Renewable Energy Production and Forecast of the Hydrogen Demand
4.1. Expansion Prospects of the Green Electricity
To reach the main target by 2050 regarding decarbonization and the full shift to green renewable energy resources, one needs to understand how the growth of renewable energy corresponds to the hydrogen demand. An analysis of the projection of Japan’s primary energy demand and the shift in energy mix over time, with a focus on renewable energy versus conventional and nuclear energy, is presented below (Figure 6).
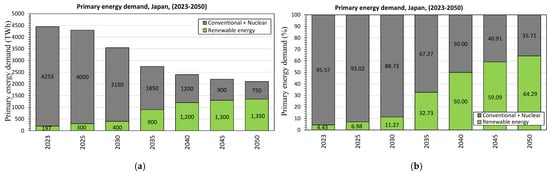
Figure 6.
Primary energy demand in Japan for the upcoming decades in TWh (a) and % (b).
Japan’s total energy demand is projected to decline significantly, from 4450 TWh in 2023 to 2100 TWh in 2050 [1,254,255,256]. This reflects efforts to enhance energy efficiency and potentially a decrease in energy-intensive activities. In this case, the total energy demand decreases by an average of approximately 2.15% per year from 2023 to 2050.
Renewable energy will grow from 197 TWh in 2023 to 1350 TWh in 2050, representing a significant expansion in clean energy infrastructure and technology deployment. Renewable energy will increase by a factor of approximately 6.85 over the period. The share of renewable energy will rise from 4.43% in 2023 to 64.29% in 2050. This illustrates Japan’s strong shift toward decarbonizing its energy system. It should be noted that, by 2030, renewable energy will contribute 11.27%, nearly tripling its 2023 share. At the same time, by 2040, renewables achieve 50% of the energy mix, reaching parity with conventional and nuclear sources, and, finally, by 2050, renewables dominate nearly two-thirds of the energy mix.
On the other hand, conventional and nuclear energy drops sharply, from 4253 TWh in 2023 to 750 TWh in 2050. This represents an 82.37% reduction over the period. The share of conventional and nuclear energy falls from 95.57% in 2023 to 35.71% in 2050, reflecting Japan’s transition away from fossil fuels and reliance on nuclear energy. By 2030, conventional and nuclear sources will still dominate, but will be reduced to 88.73%. In 2040, their share equals renewables at 50%, and by 2050, their contribution will shrink to just over one-third of the energy mix.
It should be stressed that this transition aligns with global trends toward achieving net-zero emissions and Japan’s goals under international agreements such as the Paris Agreement. The steep increase in renewables between 2030 and 2040 suggests significant investments in technology, infrastructure, and policies to boost clean energy deployment. A shift from imported fossil fuels to domestic renewable sources enhances energy security by reducing dependence on volatile global energy markets.
However, expanding renewable capacity (e.g., wind, solar) at this scale will require reliable investments, although it can create jobs and stimulate green technologies. In addition, balancing a high proportion of renewables in the energy mix will demand advanced grid management systems and energy storage solutions.
It is extremely important to determine trends in the share of renewables in Japan’s total energy consumption and renewable electricity generation from 2000 to 2050 because exactly renewable electricity is planned for use in the hydrogen production process (Figure 7). Starting at 3.7% in 2000, the share of renewables in total energy consumption grew very slowly, reaching only 8.84% by 2021. Growth was minimal for over a decade (2000–2011), averaging less than 0.1 percentage points per year. After 2011 (post-Fukushima disaster), the growth rate slightly improved, reflecting a policy shift toward renewables, but remained gradual. Renewables are expected to rise sharply from 8.84% in 2021 to 11.27% by 2030 and then surge to 32.73% in 2035, 50% in 2040, and 64.29% by 2050. This represents a 7.27-fold increase from 2000 levels and suggests an extremely aggressive shift in energy policies and investments post-2021. The transition appears slow historically but accelerates dramatically after 2030, likely reflecting planned policy stages in Japan, and by 2050, nearly two-thirds of Japan’s total energy consumption will be renewable.
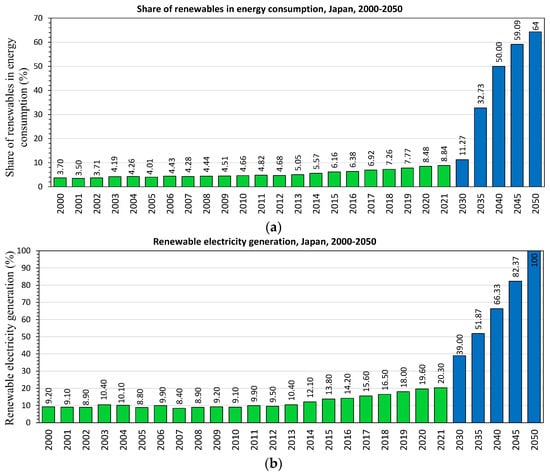
Figure 7.
Share renewable energy consumption (a) and electricity generation (b) in Japan in the coming years.
On the other hand, renewable electricity generation showed moderate growth from 9.2% in 2000 to 20.3% in 2021. Progress was uneven, with declines observed in years like 2005 and 2007, but more consistent growth from 2013 onwards. The average growth rate was approximately 0.5 percentage points per year during this period.
Renewable electricity generation is expected to rise rapidly, reaching 39% by 2030 (a doubling from 2021), 51.87% by 2035, 66.33% by 2040, and ultimately 100% by 2050. Renewable electricity grows faster than the broader share of renewables in total energy consumption, reflecting electricity’s central role in decarbonization.
It should be noted that the renewable electricity share consistently outpaces the broader renewable energy share, indicating that electricity decarbonization is prioritized over other sectors such as transport and heating. By 2050, renewable electricity will reach 100%, while total energy consumption will reach 64.29%, leaving a portion of energy demand still met by other sources. Heavy focus will likely be placed on electrifying other sectors (e.g., transport and industrial processes) to further increase the overall share of renewables in total energy.
4.2. Forecast Hydrogen Demand and Import
The data analysis outlines hydrogen demand in Japan across sectors from 2023 to 2050, showing its anticipated rise and the changing sectoral contributions (Figure 8).
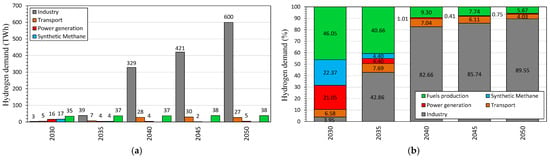
Figure 8.
Hydrogen demand in Japan between 2023 and 2050 in TWh (a) and % (b), depending on the sector of use.
Hydrogen demand grows to 76 TWh by 2030 and surges to 670 TWh by 2050 [256,257]. This represents a dramatic expansion in hydrogen deployment as Japan transitions to a hydrogen-based economy to achieve decarbonization goals. The largest increases occur between 2035 and 2040 (307 TWh growth) and 2040 to 2050 (272 TWh growth), reflecting an acceleration in hydrogen adoption.
From 3 TWh in 2030 to 600 TWh in 2050, industrial use becomes the dominant driver of hydrogen demand. By 2050, the industrial sector will represent 89.55% of total hydrogen demand. The steep increase reflects a shift to hydrogen as a replacement for fossil fuels in high-energy, hard-to-electrify industries like steelmaking, chemical production, and cement manufacturing.
While hydrogen can be used in decarbonizing heavy transport (e.g., trucks, ships, and possibly aviation), its relatively small share indicates competition with other technologies like battery-electric solutions. Transport’s hydrogen demand grows modestly, from 3 TWh in 2025 to a peak of 30 TWh in 2045, before slightly reduced to 27 TWh in 2050. Its share of total hydrogen demand falls from 100% in 2025 to 4.03% in 2050.
Hydrogen demand for power generation peaks at 16 TWh in 2030, then declines significantly to just 5 TWh by 2050. Its share falls from 21.05% in 2030 to 0.75% in 2050. Hydrogen’s diminishing role in power generation suggests a transition toward other renewable sources (solar, wind) or improved storage technologies, with hydrogen possibly reserved for backup power or peak demand periods.
Hydrogen demand for fuel production remains stable, fluctuating between 35 and 38 TWh from 2030 to 2050. Its share, however, declines from 46.05% in 2030 to 5.67% in 2050, indicating its relative importance decreases as industrial hydrogen use grows. This category likely includes hydrogen use for refining or as feedstock for synthetic fuels, which may become less critical over time due to direct electrification and renewable energy expansion.
Hydrogen demand for synthetic methane appears only in 2030 and 2035, at 17 TWh and 4 TWh, respectively. The declining role suggests synthetic methane might serve as a transitional fuel in Japan’s decarbonization strategy, but is ultimately replaced by direct hydrogen or other cleaner solutions.
It should be highlighted that, by 2030, fuel production will dominate demand (46.05%), followed by synthetic methane (22.37%) and power generation (21.05%). Industry becomes the primary hydrogen consumer, reaching 82.66% share by 2040. Hydrogen used for transport and power generation declines in relative importance. Industry solidifies its position as the primary consumer of hydrogen, with nearly 90% of demand. The overall hydrogen economy reaches its full potential, with 670 TWh of demand in 2050.
In Figure 9, it was presented the data that provides insights into Japan’s hydrogen supply development, including domestic production from green hydrogen, domestic hydrogen production using imported natural gas (SMR), and imported hydrogen.
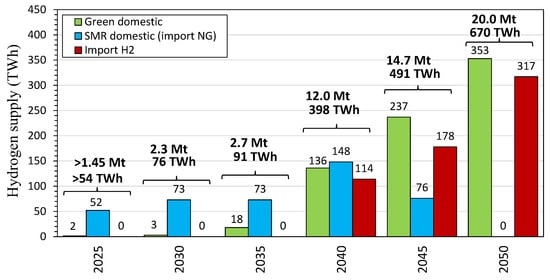
Figure 9.
Hydrogen supply in Japan (National hydrogen strategy) up until 2050.
The total hydrogen supply is projected to increase significantly, rising from 2 TWh (0.06 Mt) in 2023 to 670 TWh (20.102 Mt) by 2050, highlighting a substantial expansion in hydrogen consumption. This growth emphasizes the role of hydrogen in Japan’s decarbonization and energy transition strategy. Green hydrogen production, which commences in 2023, is expected to reach 353 TWh by 2050, accounting for 52.7% of the total hydrogen supply. A notable acceleration in production will occur between 2035 (18 TWh) and 2040 (136 TWh), with further rapid expansion through 2050. This increase reflects significant investments in renewable energy infrastructure and electrolyzers to produce green hydrogen from water and renewable electricity. Achieving this scale will necessitate substantial reductions in the costs associated with green hydrogen production and storage.
In 2023, hydrogen from SMR is the sole contributor (2 TWh, 0.06 Mt). It peaks at 148 TWh (4.44 Mt) in 2040, then phases out entirely by 2050. The decline from 2040 to 2050 reflects Japan’s move away from fossil-based hydrogen (even with carbon capture) toward fully renewable solutions.
At the same time, imported hydrogen begins in 2040 at 114 TWh, growing to 317 TWh by 2050. By 2050, imported hydrogen will contribute 47.3% of total hydrogen demand, which is slightly less than domestic green hydrogen. The large share of imports highlights potential challenges in scaling domestic production rapidly enough to meet demand. From 2023 to 2040, total imports dominate the hydrogen supply chain, with 262 TWh of imports by 2040. The reliance on imported natural gas will end by 2050, while direct hydrogen imports grow significantly.
Based on analysis of the future hydrogen demand and trends in the development and improvement of renewable electricity and energy, it can be formulated the following challenges and advantages:
Challenges:
- Domestic green hydrogen production must grow exponentially after 2035, requiring rapid expansion of renewable energy and electrolyzer capacity.
- Dependence on imports for nearly half of the supply by 2050 presents geopolitical and logistical risks.
- During the transition phase, SMR-based hydrogen (from imported natural gas) must utilize carbon capture and storage to align with emission goals.
Advantages:
- Hydrogen demand will drive renewable energy expansion, benefiting Japan’s overall energy security.
- Japan can establish itself as a leader in hydrogen technology, exporting know-how and equipment (e.g., electrolyzers, fuel cells).
- Strong ties with hydrogen-exporting countries will strengthen Japan’s geopolitical position and provide stable energy sources.
It should be noted that it is essential to consider the risks associated with potential technological or policy failures to ensure the robustness of Japan’s hydrogen strategy. Delays in scaling electrolysis capacity, cost overruns in CCS deployment, or changes in government energy policy could significantly alter projected timelines and cost pathways. At the same time, it should be highlighted that Artificial Intelligence (AI) and digital twin technologies are increasingly being explored in Japan to optimize the hydrogen value chain. This approach enables real-time monitoring, predictive maintenance, and dynamic optimization of hydrogen production, storage, and distribution systems. Japan’s integration of such technologies remains in the early stages but represents a promising direction for improving efficiency and system flexibility.
5. Conclusions
Japan’s hydrogen strategy exemplifies a comprehensive and forward-looking approach to achieving carbon neutrality by 2050. The hydrogen strategy focuses on both short-term and long-term goals, balancing economic viability, technological readiness, and environmental impact.
In the short term, Japan is prioritizing the production and utilization of blue hydrogen, primarily through natural gas reforming with 96.2% CCS. This serves as a transitional solution to scale up hydrogen infrastructure while minimizing emissions from 9 to 12 kg CO2/kg H2 to as low as 0.4–0.7 kg CO2/kg H2. On the other side, investments in green hydrogen production—driven by water electrolysis powered by renewable energy—highlight Japan’s support for achieving zero-emission energy systems despite current production costs ranging from 3 to 10.3 USD/kgH2.
Hydrogen storage and transportation present critical challenges, and Japan is exploring diverse technologies:
- Liquid hydrogen: Central to Japan’s hydrogen import projects, enabling large-scale transportation from Australia. LH2 offers high energy density but has energy efficiency rates of 60–70% and storage costs of 3–6 USD/kgH2.
- Ammonia: Japan targets 30 million tons of ammonia per year by 2050, positioning it as a viable hydrogen carrier with lower transport costs.
Japan’s national hydrogen strategy includes installing 1000 hydrogen refueling stations by 2030 and expanding hydrogen use in 30% of the power sector by 2050. Efforts to scale electrolysis capacity to 15 GW by 2030 further demonstrate Japan’s drive to lead in hydrogen production and utilization.
Despite notable progress, significant barriers remain, including production costs, infrastructure gaps, and the need for technological breakthroughs. Japan’s ongoing investment in pilot projects, such as the 10 MW Fukushima Hydrogen Energy Research Field, reflects trends to find efficiency solutions. Collaboration between government agencies, industry leaders, and academic institutions is fostering innovation and scalability. Future implementation of hydrogen strategies must also account for potential technological and policy risks and emphasize the importance of flexible scenario planning.
Japan is actively exploring hydrogen imports from Norway as part of its strategy to secure stable, long-term supplies of clean hydrogen to meet its decarbonization goals. Norway, with its abundant renewable energy resources and advanced CCS capabilities, is positioned to export blue hydrogen produced from natural gas with over 95% CO2 capture rates. The partnership leverages Norway’s offshore wind potential and hydropower infrastructure, enabling the production of green hydrogen for future exports. Japan sees Norway as one of the key partners in diversifying hydrogen supply chains, reducing reliance on fossil fuels, and accelerating the transition to a hydrogen-based economy. This collaboration aligns with Japan’s goal to import 300,000 tons of hydrogen annually by 2030, reinforcing its leadership in the global hydrogen market.
A key risk in Japan’s hydrogen strategy is the heavy reliance on imported hydrogen, which increases concerns about energy security, geopolitical stability, and the sustainability of the supply chain.
While liquid hydrogen creates known challenges from the point of view of liquefaction energy and usage, inclusion in Japan’s hydrogen strategy is justified by ongoing national projects and infrastructure development. Japan is also diversifying its strategy with green ammonia, which benefits from existing infrastructure, transport, and storage systems. By developing LH2 and ammonia pathways, Japan looks for secure, flexible, long-term supply options for industrial use, energy generation, and decarbonization goals.
In summary, Japan’s hydrogen roadmap reflects a balanced, phased strategy that aligns with global decarbonization efforts. By fostering international partnerships and pioneering new hydrogen technologies, Japan is positioning itself as a leader in the hydrogen economy, contributing to global CO2 reductions and paving the way for a sustainable energy future.
It should be emphasized that the development of hydrogen value chain models and simulations of the different scenarios must be directed to optimize production, storage, transportation, and utilization, and, as a result, to identify optimal solutions from the point of view of using hydrogen as a future energy carrier. Advanced simulations can provide an efficient forecast of the hydrogen demand growth, infrastructure needs, and carbon reduction impacts, supporting the realization of a commercial hydrogen strategy by 2050.
Author Contributions
Conceptualization, Y.I., D.K. and I.T.; methodology, Y.I., D.K. and I.T.; formal analysis, D.K., I.T. and Y.I.; investigation, D.K., I.T. and Y.I.; resources, I.T.; writing—original draft preparation, D.K., I.T., Y.I. and J.J.L.; writing—review and editing, D.K., I.T., J.J.L. and Y.I.; project administration, I.T.; funding acquisition, I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This publication has been produced with support from: The program International Partnerships for Excellent Education, Research and Innovation, IntER-Cold project (Project number: 309841, funded by the Research Council of Norway); The HYDROGENi Research Centre (hydrogeni.no), performed under the Norwegian research program FMETEKN. The authors acknowledge the industry partners in HYDROGENi for their contributions and the Research Council of Norway (333118).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AEM | Anion exchange membrane electrolysis |
| ALK | Alkaline electrolysis |
| ATR | Autothermal reforming |
| CcH2 | Cryo-compressed hydrogen |
| CCS | Carbon capture and storage |
| CGH2 | Compressed gaseous hydrogen |
| CMB | Compagnie Maritime Belge |
| FCVs | Fuel cell vehicles |
| FF | Flow field |
| FH2R | Fukushima hydrogen energy research field |
| HESC | Hydrogen energy supply chain |
| HRS | Hydrogen refueling station |
| HTE | High-temperature electrolysis |
| JAXA | Japan Aerospace Exploration Agency |
| JHyM | Japan H2 Mobility |
| JST | Japan science and technology agency |
| LCOH | Levelized cost of hydrogen |
| LH2 | Liquid hydrogen |
| LOHCs | Liquid organic hydrogen carriers |
| MEGC | Multiple-element gas containers |
| MOFs | Metal–organic frameworks |
| Mt | Million metric tons |
| NG | Natural gas |
| PEM | Proton exchange membrane |
| PSA | Pressure swing adsorption |
| PTL | Porous transport layer |
| SMR | Steam methane reforming |
| SOEC | Solid oxide electrolysis |
| TRL | Technology readiness level |
| YSZ | Yttria-stabilized zirconia |
References
- DNV. Energy Transition Outlook 2024: A Global and Regional Forecast to 2050; DNV: Bærum, Norway, 2024; p. 261. [Google Scholar]
- Dejonghe, M.; Van de Graaf, T.; Belmans, R. From natural gas to hydrogen: Navigating import risks and dependencies in Northwest Europe. Energy Res. Soc. Sci. 2023, 106, 103301. [Google Scholar] [CrossRef]
- Otaki, T.; Shaw, R. Cost Estimates and Policy Challenges of Transporting Renewable Energy Derived Ammonia from Gujarat, India to Japan. Hydrogen 2023, 4, 961–974. [Google Scholar] [CrossRef]
- Iida, S.; Sakata, K. Hydrogen technologies and developments in Japan. Clean Energy 2019, 3, 105–113. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Joshi, M.; Chernyakhovskiy, I.; Chung, M. Hydrogen 101: Frequently Asked Questions About Hydrogen for Decarbonization; NREL: Golden, CO, USA, 2022. [Google Scholar]
- Li, F.; Liu, D.; Sun, K.; Yang, S.; Peng, F.; Zhang, K.; Guo, G.; Si, Y. Towards a Future Hydrogen Supply Chain: A Review of Technologies and Challenges. Sustainability 2024, 16, 1890. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.H.; Cho, S.M.; Na, Y.; Kim, S.; Kim, D.K. Review of hydrogen infrastructure: The current status and roll-out strategy. Int. J. Hydrogen Energy 2023, 48, 1701–1716. [Google Scholar] [CrossRef]
- Habibi, M.; Hosseini, M.G.; Wang, K. Toward sustainable energy: A comprehensive review of hydrogen production, storage, and utilization. Renew. Sustain. Energy Rev. 2026, 226, 116193. [Google Scholar] [CrossRef]
- Adams, M.J.; Wadge, M.D.; Sheppard, D.; Stuart, A.; Grant, D.M. Review on onshore and offshore large-scale seasonal hydrogen storage for electricity generation: Focusing on improving compression, storage, and roundtrip efficiency. Int. J. Hydrogen Energy 2024, 73, 95–111. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050. In A Roadmap for the Global Energy Sector; IEA: Paris, France, 2021. [Google Scholar]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Nyangon, J.; Darekar, A. Advancements in hydrogen energy systems: A review of levelized costs, financial incentives and technological innovations. Innov. Green Dev. 2024, 3, 100149. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Absi Halabi, M.; Rana, M.S.; Vinoba, M. Blue hydrogen: Current status and future technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Makepeace, R.W.; Tabandeh, A.; Hossain, M.J.; Asaduz-Zaman, M. Techno-economic analysis of green hydrogen export. Int. J. Hydrogen Energy 2024, 56, 1183–1192. [Google Scholar] [CrossRef]
- Wendt, D.S.; Knighton, L.T. High Temperature Steam Electrolysis Process Performance and Cost Estimates—DOE Hydrogen Program AMR Presentation; Idaho National Laboratory: Idaho Falls, ID, USA, 2022. [Google Scholar]
- Zhu, L.; Hu, L.; Yüksel, S.; Dinçer, H.; Karakuş, H.; Ubay, G.G. Analysis of Strategic Directions in Sustainable Hydrogen Investment Decisions. Sustainability 2020, 12, 4581. [Google Scholar] [CrossRef]
- Japan: Hydrogen Strategy—November 2023. Available online: https://www.mfat.govt.nz/en/trade/mfat-market-reports/japan-hydrogen-strategy-november-2023 (accessed on 18 November 2024).
- Akimoto, D. A Look at Japan’s Latest Hydrogen Strategy. Available online: https://thediplomat.com/2023/07/a-look-at-japans-latest-hydrogen-strategy/ (accessed on 18 November 2024).
- Li, H.-W.; Nishimiya, N. Insight from Japan’s Hydrogen Strategy and Activities. Engineering 2021, 7, 722–725. [Google Scholar] [CrossRef]
- Weijun, S. Japan fine tunes its hydrogen strategy. Pet. Econ. 2023, 90, 83. [Google Scholar]
- IRENA and AEA. Innovation Outlook: Renewable Ammonia; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates; Ammonia Energy Association: Brooklyn, NY, USA, 2022; p. 144. [Google Scholar]
- Japan Hydrogen Basic Strategy. Available online: https://www.whitecase.com/insight-alert/japan-hydrogen-basic-strategy (accessed on 18 November 2024).
- Sakaba, N.; Sato, H.; Ohashi, H.; Nishihara, T.; Kunitomi, K.; Shiozawa, S. Development strategy for non-nuclear grade hydrogen production system coupled with the Japan’s HTTR. In Proceedings of the American Nuclear Society Embedded Topical Meeting—2007 International Topical Meeting on Safety and Technology of Nuclear Hydrogen Production, Control, and Management, Boston, MA, USA, 24–28 June 2007; pp. 355–362. [Google Scholar]
- Aliaksei, P.; Rahmatallah, P. Hydrogen Storage for a Net-Zero Carbon Future; Oxford Institute for Energy Studies: Oxford, UK, 2023; p. 43. [Google Scholar]
- Burke, A.; Ogden, J.; Fulton, L.; Cerniauskas, S. Hydrogen Storage and Transport: Technologies and Costs; Working Paper Series; Institute of Transportation Studies: Davis, CA, USA, 2024; p. 33. [Google Scholar]
- Cao, T.; Sugiyama, M.; Ju, Y. Prospects of regional supply chain relocation for iron & steel industry decarbonization: A case study of Japan and Australia. Resour. Conserv. Recycl. 2024, 209, 107804. [Google Scholar] [CrossRef]
- European Industrial Gases Association Aisbl. Hydrogen Overview Distribution, Storage, Applications; European Industrial Gases Association Aisbl: Brussels, Belgium, 2022; p. 37. [Google Scholar]
- European Hydrogen Observatory. Hydrogen Basics Factsheets. In Hydrogen Value Chains; European Hydrogen Observatory: Brussels, Belgium, 2023; p. 7. Available online: https://observatory.clean-hydrogen.europa.eu/learn-about-hydrogen/hydrogen-basics/hydrogen-value-chains (accessed on 18 November 2024).
- Parolin, F.; Colbertaldo, P.; Campanari, S. Optimal design of hydrogen delivery infrastructure for multi-sector end uses at regional scale. Int. J. Hydrogen Energy 2024, 79, 839–849. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Fulton, L.; Shams, M.; Sperling, D. Creating a global hydrogen economy: Review of international strategies, targets, and policies with a focuse on japan, germany, South Korea and California. In Proceedings of the WHEC 2022—23rd World Hydrogen Energy Conference: Bridging Continents by H2, Istanbul, Turkey, 26–30 June 2022; pp. 1235–1238. [Google Scholar]
- Ishimoto, Y.; Voldsund, M.; Nekså, P.; Roussanaly, S.; Berstad, D.; Gardarsdottir, S.O. Large-scale production and transport of hydrogen from Norway to Europe and Japan: Value chain analysis and comparison of liquid hydrogen and ammonia as energy carriers. Int. J. Hydrogen Energy 2020, 45, 32865–32883. [Google Scholar] [CrossRef]
- Turning Hydrogen Demand Into Reality: Which Sectors Come First? International Chamber of Shipping Publications: London, UK, 2024; p. 44.
- Global Hydrogen Review 2021; International Energy Agency: Paris, France, 2021; p. 223.
- IRENA. Global Hydrogen Trade to Meet the 1.5 °C Climate Goal: Part II—Technology Review of Hydrogen Carriers; IRENA: Abu Dhabi, United Arab Emirates, 2022; p. 156. [Google Scholar]
- Berstad, D.; Gardarsdottir, S.; Roussanaly, S.; Voldsund, M.; Ishimoto, Y.; Nekså, P. Liquid hydrogen as prospective energy carrier: A brief review and discussion of underlying assumptions applied in value chain analysis. Renew. Sustain. Energy Rev. 2022, 154, 111772. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; p. 106. [Google Scholar]
- Ghassan, W.; Magnolia, T. Hydrogen for Decarbonization: A Realistic Assessment; CAFT: Boston, MA, USA, 2023; p. 13. Available online: https://www.catf.us/resource/hydrogen-decarbonization-realistic-assessment/ (accessed on 21 November 2023).
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the green hydrogen market—Current state and outlook on green hydrogen demand and electrolyzer manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- Aorere, M. Japan Hydrogen Strategy; Foreign Affairs and Trade: Wellington, New Zealand, 2023; p. 7. [Google Scholar]
- Salimi, M.; Hosseinpour, M.; N. Borhani, T. The Role of Clean Hydrogen Value Chain in a Successful Energy Transition of Japan. Energies 2022, 15, 6064. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- European Industrial Gases Association. Overview of Hydrogen Production Methods—Doc 251/24; AISBL: Brussels, Belgium, 2024; p. 26. [Google Scholar]
- Stiller, C.; Svensson, A.M.; Møller-Holst, S.; Bünger, U.; Espegren, K.A.; Holm, Ø.B.; Tomasgård, A. Options for CO2-lean hydrogen export from Norway to Germany. Energy 2008, 33, 1623–1633. [Google Scholar] [CrossRef]
- Wu, W.; Zhai, H.; Holubnyak, E. Technological evolution of large-scale blue hydrogen production toward the U.S. Hydrogen Energy Earthshot. Nat. Commun. 2024, 15, 5684. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Kim, K.-J.; Kim, B.-J.; Hong, G.-R.; Jang, W.-J.; Bae, J.W.; Park, Y.-K.; Jeon, B.-H.; Roh, H.-S. From gray to blue hydrogen: Trends and forecasts of catalysts and sorbents for unit process. Renew. Sustain. Energy Rev. 2023, 186, 113635. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue hydrogen production from natural gas reservoirs: A review of application and feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.-V.N.; et al. Grey, blue, and green hydrogen: A comprehensive review of production methods and prospects for zero-emission energy. Int. J. Green Energy 2024, 21, 1383–1397. [Google Scholar] [CrossRef]
- Ganguli, A.; Bhatt, V. Hydrogen production using advanced reactors by steam methane reforming: A review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Jumah, A.b. A comprehensive review of production, applications, and the path to a sustainable energy future with hydrogen. RSC Adv. 2024, 14, 26400–26423. [Google Scholar] [CrossRef]
- Mohd Yunus, S.; Yusup, S.; Johari, S.S.; Mohd Afandi, N.; Manap, A.; Mohamed, H. Comparative Hydrogen Production Routes via Steam Methane Reforming and Chemical Looping Reforming of Natural Gas as Feedstock. Hydrogen 2024, 5, 761–775. [Google Scholar] [CrossRef]
- INPEX Visio @ 2022. Available online: https://www.inpex.co.jp/english/company/pdf/inpex_vision_2022.pdf (accessed on 22 November 2024).
- JOGMEC and INPEX Commence a Demonstration Project for the Production of Clean Hydrogen and Ammonia Combined with CO2 Storage and Utilization in Kashiwazaki-City, Niigata Prefecture, Japan. Available online: https://www.jogmec.go.jp/english/news/release/news_10_00018.html (accessed on 22 November 2024).
- Iwatani’s Hydrogen Activities. Available online: https://cdnw8.eu-japan.eu/sites/default/files/imce/seminars/ying_yu_ban_yan_gu_chan_ye_.pdf (accessed on 22 November 2024).
- Iwatani Advanced Hydrogen Technology Center. Available online: https://www.iwatani.co.jp/eng/business/rd/hydrogen/ (accessed on 22 November 2024).
- Hydrogen Business Strategy. Available online: https://www.eneos.co.jp/english/business/hydrogen/strategy.html (accessed on 22 November 2024).
- Lewis, E.; McNaul, S.; Jamieson, M.; Henriksen, M.S.; Matthews, H.S.; Walsh, L.; Grove, J.; Shultz, T.; Skone, T.J.; Stevens, R. Comparison of Commercial, State-of-the-Art, Fossil-Based Hydrogen Production Technologies; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2022. [Google Scholar]
- Amin, A. Review of autothermal reactors: Catalysis, reactor design, and processes. Int. J. Hydrogen Energy 2024, 65, 271–291. [Google Scholar] [CrossRef]
- Anwar, S.; Li, X. Production of hydrogen from fossil fuel: A review. Front. Energy 2023, 17, 585–610. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Wang, H.; Huang, Z.; Liu, H.; Tian, X.; Yan, F.; Yin, R. Catalyst, reactor, reaction mechanism and CO remove technology in methanol steam reforming for hydrogen production: A review. Fuel Process. Technol. 2023, 252, 108000. [Google Scholar] [CrossRef]
- Prato-Garcia, D.; Robayo-Avendaño, A.; Vasquez-Medrano, R. Hydrogen from natural gas and biogas: Building bridges for a sustainable transition to a green economy. Gas Sci. Eng. 2023, 111, 204918. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Wassie, S.A.; Medrano, J.A.; Zaabout, A.; Cloete, S.; Melendez, J.; Tanaka, D.A.P.; Amini, S.; van Sint Annaland, M.; Gallucci, F. Hydrogen production with integrated CO2 capture in a membrane assisted gas switching reforming reactor: Proof-of-Concept. Int. J. Hydrogen Energy 2018, 43, 6177–6190. [Google Scholar] [CrossRef]
- Air Liquide Autothermal Reforming Technology Selected For First Low-carbon Hydrogen And Ammonia Production In Japan. Available online: https://hydrogen-central.com/air-liquide-autothermal-reforming-technology-selected-first-low-carbon-hydrogen-and-ammonia-production-in-japan/ (accessed on 22 November 2024).
- Wright, A. Air Liquide’s Technology Chosen for Low-Carbon Hydrogen Test in Japan. Available online: https://www.gasworld.com/story/air-liquides-technology-chosen-for-low-carbon-hydrogen-test-in-japan/ (accessed on 22 November 2024).
- El-Adawy, M.; Dalha, I.B.; Ismael, M.A.; Al-Absi, Z.A.; Nemitallah, M.A. Review of Sustainable Hydrogen Energy Processes: Production, Storage, Transportation, and Color-Coded Classifications. Energy Fuels 2024, 38, 22686–22718. [Google Scholar] [CrossRef]
- Su, S.; Tahir, M.H.; Cheng, X.; Zhang, J. Modification and resource utilization of coal gasification slag-based material: A review. J. Environ. Chem. Eng. 2024, 12, 112112. [Google Scholar] [CrossRef]
- Yan, S.; Xuan, W.; Cao, C.; Zhang, J. A review of sustainable utilization and prospect of coal gasification slag. Environ. Res. 2023, 238, 117186. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, D.; Wei, Z.; Chen, Z.; Mirzayev, M.; Chen, Y.; Chen, S. Coal decarbonization: A state-of-the-art review of enhanced hydrogen production in underground coal gasification. Energy Rev. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Hydrogen Roadmap: A Commitment to Renewable Hydrogen; Ministry of Ecological Transition and Demography: Madrid, Spain, 2020; p. 70. Available online: https://www.miteco.gob.es/en/ministerio/planes-estrategias/hidrogeno.html/ (accessed on 22 November 2024).
- Gray, D.; Tomlinson, G. Hydrogen from Coal. Mitretek Technical Paper; DE-AM26-99FT40465; Centre for Science and Technology: Washington, DC, USA, 2002; p. 30. [Google Scholar]
- Kaneko, S. Integrated Coal Gasification Combined Cycle: A Reality, Not a Dream. J. Energy Eng. 2016, 142, E4015018. [Google Scholar] [CrossRef]
- Nakata, T.; Sato, T.; Wang, H.; Kusunoki, T.; Furubayashi, T. Modeling technological learning and its application for clean coal technologies in Japan. Appl. Energy 2011, 88, 330–336. [Google Scholar] [CrossRef]
- Yazdanpanah Jahromi, M.-A.; Atashkari, K.; Kalteh, M. Comparison of different woody biomass gasification behavior in an entrained flow gasifier. Biomass Convers. Biorefin. 2023, 13, 3165–3177. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, P.; Liu, R. Synergistic combination of biomass torrefaction and co-gasification: Reactivity studies. Bioresour. Technol. 2017, 245, 225–233. [Google Scholar] [CrossRef]
- Yazdanpanah Jahromi, M.-A.; Atashkari, K.; Kalteh, M. Development of a high-temperature two-stage entrained flow gasifier model for the process of biomass gasification and syngas formation. Int. J. Energy Res. 2019, 43, 5864–5878. [Google Scholar] [CrossRef]
- Clausen, L.R.; Elmegaard, B.; Houbak, N. Technoeconomic analysis of a low CO2 emission dimethyl ether (DME) plant based on gasification of torrefied biomass. Energy 2010, 35, 4831–4842. [Google Scholar] [CrossRef]
- Trehan, K.; Molintas, H.; Gupta, A.K. Gasification of Torrefied and Soft Wood Pellets in Air and CO2. In Proceedings of the ASME 2014 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. Volume 4: 19th Design for Manufacturing and the Life Cycle Conference; 8th International Conference on Micro- and Nanosystems, Buffalo, NY, USA, 17–20 August 2014; V004T06A028. ASME: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Chien, K.-L.; Lu, C.-H. Characterization and comparison of biomass produced from various sources: Suggestions for selection of pretreatment technologies in biomass-to-energy. Appl. Energy 2012, 100, 164–171. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, G.; Zhu, G.; Sun, B.; Sun, Z.; Li, S.; Lan, Y.-Q. Recent advancements in noble-metal electrocatalysts for alkaline hydrogen evolution reaction. Chin. Chem. Lett. 2025, 36, 109557. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Chen, Z.; Yang, Z.; Yun, J.; Zhang, J. NiCoCe/NF nanosheet clusters for alkaline electrolyzed water oxygen evolution reaction. Mater. Today Commun. 2025, 42, 111173. [Google Scholar] [CrossRef]
- Liu, S.; Wei, Y.; Wang, M.; Shen, Y. The future of alkaline water splitting from the perspective of electrocatalysts-seizing today’s opportunities. Coord. Chem. Rev. 2025, 522, 216190. [Google Scholar] [CrossRef]
- Aguirre, O.A.; Ocampo-Martinez, C.; Camacho, O. Control strategies for alkaline water electrolyzers: A survey. Int. J. Hydrogen Energy 2024, 86, 1195–1213. [Google Scholar] [CrossRef]
- The World’s Largest Hydrogen-Production Facility on the Path to Zero Emissions. Available online: https://www.japan.go.jp/kizuna/2021/03/hydrogen-production_facility.html (accessed on 18 December 2024).
- Martin, P. Japanese Energy Companies Launch the Country’s Largest Green Hydrogen Project. Available online: https://www.hydrogeninsight.com/production/japanese-energy-companies-launch-the-countrys-largest-green-hydrogen-project/2-1-1602552 (accessed on 18 December 2024).
- An Initiative Aimed at Decarbonization of Tokyo International Airport (Haneda Airport). Japan Airport Terminal Co., Ltd., ENEOS Corporation: Japan. 2024. Available online: https://www.tokyo-airport-bldg.co.jp/files/news_release/000014679.pdf (accessed on 18 December 2024).
- Establishment of a Hydrogen Supply Chain for the Introduction of Hydrogen-Powered Train. Central Lapan Railway Company, ENEOS Corporation, Hitachi Ltd.: Japan 2024. Available online: https://global.jr-central.co.jp/en/news/_pdf/2024/0008.pdf (accessed on 18 December 2024).
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef]
- Badgett, A.; Brauch, J.; Thatte, A.; Rubin, R.; Skangos, C.; Wang, X.; Ahluwalia, R.; Pivovar, B.; Ruth, M. Updated Manufactured Cost Analysis for Proton Exchange Membrane Water Electrolyzers; National Renewable Energy Laboratory: Applewood, CO, USA, 2024. [Google Scholar]
- Ahmed, K.; Salam, M.A.; Ali Shaikh, M.A.; Murugaiah, D.K.; Shahgaldi, S.; Sweety, M.N. A Review on Most Recent Development of Electrode Structures for Proton Exchange Membrane Fuel Cell Application with Upcoming Prospects. ACS Appl. Energy Mater. 2024, 7, 9052–9083. [Google Scholar] [CrossRef]
- Ajeeb, W.; Costa Neto, R.; Baptista, P. Life cycle assessment of green hydrogen production through electrolysis: A literature review. Sustain. Energy Technol. Assess. 2024, 69, 103923. [Google Scholar] [CrossRef]
- Abdol Rahim, A.H.; Tijani, A.S.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Grandi, M.; Rohde, S.; Liu, D.J.; Gollas, B.; Hacker, V. Recent advancements in high performance polymer electrolyte fuel cell electrode fabrication—Novel materials and manufacturing processes. J. Power Sources 2023, 562, 232734. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, L.; Li, Q.; Yang, Y.; Zhang, C.; Dou, Z. Review of electric hydrogen production technology combined with technology maturity analysis. Int. J. Hydrogen Energy 2025, 98, 429–440. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, S.; Cheng, X.; Tu, F.; Zhou, J.; Shen, S.; Yin, J.; Yan, X.; Zhang, J. Applications of model electrode for investigations of reaction and transport issues in proton exchange membrane water electrolyzer. Curr. Opin. Electrochem. 2025, 49, 101601. [Google Scholar] [CrossRef]
- Sezer, N.; Bayhan, S.; Fesli, U.; Sanfilippo, A. A comprehensive review of the state-of-the-art of proton exchange membrane water electrolysis. Mater. Sci. Energy Technol. 2025, 8, 44–65. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, F.; Yang, Z. Strategies for the enhancements in catalytic performance and stability of anodic electrocatalyst in PEM water splitting. Energy Rev. 2024, 3, 100103. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Wang, J.; Yang, B.; Jiang, L. Water electrolyzer operation scheduling for green hydrogen production: A review. Renew. Sustain. Energy Rev. 2024, 203, 114779. [Google Scholar] [CrossRef]
- Oki, N. Japan’s Tohoku to Test H2 Co-Firing at CCGT Unit. Available online: https://www.argusmedia.com/en/news-and-insights/latest-market-news/2493260-japan-s-tohoku-to-test-h2-co-firing-at-ccgt-unit (accessed on 18 December 2024).
- Japan Targets 1000 Hydrogen Stations by End of Decade. Available online: https://hydrogen-central.com/japan-1000-hydrogen-stations/?utm_source (accessed on 18 December 2024).
- Number of Hydrogen Fueling Stations for Road Vehicles in Japan as of April 2024, by Region. Available online: https://www.statista.com/statistics/1236606/japan-hydrogen-fuel-station-number-by-region/?utm_source (accessed on 18 December 2024).
- Kawasaki Hydrogen Road. Available online: https://global.kawasaki.com/en/hydrogen/?utm_source (accessed on 18 December 2024).
- Mulk, W.U.; Aziz, A.R.A.; Ismael, M.A.; Ghoto, A.A.; Ali, S.A.; Younas, M.; Gallucci, F. Electrochemical hydrogen production through anion exchange membrane water electrolysis (AEMWE): Recent progress and associated challenges in hydrogen production. Int. J. Hydrogen Energy 2024, 94, 1174–1211. [Google Scholar] [CrossRef]
- Qayoom, A.; Ahmad, M.S.; Fayaz, H.; Qazi, A.; Selvaraj, J.; Zainul, R.; Krismadinata; Rahim, N.A.; Atamurotov, F.; Tran, T.K.; et al. Recent advances in anion exchange membrane technology for water electrolysis: A review of progress and challenges. Energy Sci. Eng. 2024, 12, 5328–5352. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Comprehensive impedance investigation of low-cost anion exchange membrane electrolysis for large-scale hydrogen production. Sci. Rep. 2021, 11, 293. [Google Scholar] [CrossRef]
- Shaik, S.; Kundu, J.; Yuan, Y.; Chung, W.; Han, D.; Lee, U.; Huang, H.; Choi, S.-I. Recent Progress and Perspective in Pure Water-Fed Anion Exchange Membrane Water Electrolyzers. Adv. Energy Mater. 2024, 14, 2401956. [Google Scholar] [CrossRef]
- Akyüz, E.S.; Telli, E.; Farsak, M. Hydrogen generation electrolyzers: Paving the way for sustainable energy. Int. J. Hydrogen Energy 2024, 81, 1338–1362. [Google Scholar] [CrossRef]
- Sriram, G.; Dhanabalan, K.; Ajeya, K.V.; Aruchamy, K.; Ching, Y.C.; Oh, T.H.; Jung, H.-Y.; Kurkuri, M. Recent progress in anion exchange membranes (AEMs) in water electrolysis: Synthesis, physio-chemical analysis, properties, and applications. J. Mater. Chem. A 2023, 11, 20886–21008. [Google Scholar] [CrossRef]
- Dokso, A. Tokyo Gas Unveils Japan’s First AEM Water Electrolyzer Hydrogen Station. Available online: https://energynews.biz/tokyo-gas-unveils-japans-first-aem-water-electrolyzer-hydrogen-station/?utm_source (accessed on 18 December 2024).
- Transforming Anion Exchange Membranes in Water Electrolysis for Green Hydrogen Production. Available online: https://www.waseda.jp/top/en/news/82506?utm_source (accessed on 18 December 2024).
- Liu, F.; Miyatake, K.; Mahmoud, A.M.A.; Yadav, V.; Xian, F.; Guo, L.; Wong, C.Y.; Iwataki, T.; Shirase, Y.; Kakinuma, K.; et al. Polyphenylene-Based Anion Exchange Membranes with Robust Hydrophobic Components Designed for High-Performance and Durable Anion Exchange Membrane Water Electrolyzers Using Non-PGM Anode Catalysts. Adv. Energy Mater. 2024, 15, 2404089. [Google Scholar] [CrossRef]
- Development of Innovative Water Electrolysis Systems for Green Hydrogen Production. Available online: https://www.catec.t.u-tokyo.ac.jp/wing/en (accessed on 18 December 2024).
- Flis, G.; Wakim, G. Solid Oxide Electrolysis: A Technology Status Assessment; Clean Air Task Force: Boston, MA, USA, 2023; p. 47. [Google Scholar]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Eslick, J.; Noring, A.; Susarla, N.; Okoli, C.; Allan, D.; Wang, M.; Ma, J.; Zamarripa-Perez, M.A.; Iyengar, A.; Burgard, A. Technoeconomic Evaluation of Solid Oxide Fuel Cell Hydrogen-Electricity Co-Generation Concepts; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2023. [Google Scholar]
- Du, Y.; Ling, H.; Zhao, L.; Jiang, H.; Kong, J.; Liu, P.; Zhou, T. The development of solid oxide electrolysis cells: Critical materials, technologies and prospects. J. Power Sources 2024, 607, 234608. [Google Scholar] [CrossRef]
- Jang, I.; Carneiro, J.S.A.; Crawford, J.O.; Cho, Y.J.; Parvin, S.; Gonzalez-Casamachin, D.A.; Baltrusaitis, J.; Lively, R.P.; Nikolla, E. Electrocatalysis in Solid Oxide Fuel Cells and Electrolyzers. Chem. Rev. 2024, 124, 8233–8306. [Google Scholar] [CrossRef]
- Lahrichi, A.; El Issmaeli, Y.; Kalanur, S.S.; Pollet, B.G. Advancements, strategies, and prospects of solid oxide electrolysis cells (SOECs): Towards enhanced performance and large-scale sustainable hydrogen production. J. Energy Chem. 2024, 94, 688–715. [Google Scholar] [CrossRef]
- Han, X.; Ostrikov, K.K.; Chen, J.; Zheng, Y.; Xu, X. Electrochemical Reduction of Carbon Dioxide to Solid Carbon: Development, Challenges, and Perspectives. Energy Fuels 2023, 37, 12665–12684. [Google Scholar] [CrossRef]
- Qiu, P.; Li, C.; Liu, B.; Yan, D.; Li, J.; Jia, L. Materials of solid oxide electrolysis cells for H2O and CO2 electrolysis: A review. J. Adv. Ceram. 2023, 12, 1463–1510. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.-A.; Menzler, N.H. Solid oxide electrolysis cells—Current material development and industrial application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- DENSO to Begin SOEC Demonstration at Hirose Plant to Produce and Use Green Hydrogen for Manufacturing. Available online: https://www.denso.com/global/en/news/newsroom/2023/20230627-g01/?utm_source%20%28 (accessed on 18 December 2024).
- DENSO Is Challenging Itself to Develop a Solid Oxide Electrolysis Cell System Looking Ahead to a Future of Hydrogen Society. Available online: https://www.denso.com/global/en/driven-base/tech-design/soec/ (accessed on 18 December 2024).
- MHI Begins Operation of SOEC Test Module the Next-Generation High-Efficiency Hydrogen Production Technology at Takasago Hydrogen Park. Available online: https://www.mhi.com/news/240425.html?utm_source (accessed on 20 December 2024).
- Osada, N. Development of Solid Oxide Electrolysis Cell System at TOSHIBA. Available online: https://www.nedo.go.jp/content/100939729.pdf (accessed on 20 December 2024).
- Takasago Hydrogen Park, the World’s First Integrated Validation Facility for Technologies from Hydrogen Production to Power Generation, Enters Full-Scale Operation—Electrolysis Hydrogen Production Begins. Available online: https://www.mhi.com/news/23092003.html?utm_source (accessed on 20 December 2024).
- Qiu, Y.; Zeng, D.; Xiao, R. Hydrogen Production from Biomass-Based Chemical Looping: A Critical Review and Perspectives. Energy Fuels 2024, 38, 13819–13836. [Google Scholar] [CrossRef]
- Alqarzaee, F.; Al Bari, M.A.; Razzak, S.A.; Uddin, S. Biomass-based hydrogen production towards renewable energy sources: An advance study. Emergent Mater. 2024, 8, 1287–1309. [Google Scholar] [CrossRef]
- Bio-Hydrogen Plant Opens in Japan. Available online: https://www.worldbiogasassociation.org/bio-hydrogen-plant-opens-in-japan/?utm_source (accessed on 20 December 2024).
- Takaoka, Y.; Mizumukai, K.; Kameno, Y. Suiso Frontier, the First LH2 Carrier—Demonstration of Technologies and Operational Phase. Int. J. Offshore Polar Eng. 2023, 33, 225–233. [Google Scholar] [CrossRef]
- Kar, S.K.; Sinha, A.S.K.; Bansal, R.; Shabani, B.; Harichandan, S. Overview of hydrogen economy in Australia. WIREs Energy Environ. 2023, 12, e457. [Google Scholar] [CrossRef]
- Van’t Noordende, H.; Tubben, G.; Rouwenhorst, K.H.; Fruytier, M. Clean Ammonia Roadmap Report; Institute for Sustainable Process Technology (ISPT): Jisp, The Netherlands, 2024; p. 72. [Google Scholar]
- Chen, P.S.; Fan, H.; Enshaei, H.; Zhang, W.; Shi, W.; Abdussamie, N.; Miwa, T.; Qu, Z.; Yang, Z. Opportunities and Challenges of Hydrogen Ports: An Empirical Study in Australia and Japan. Hydrogen 2024, 5, 436–458. [Google Scholar] [CrossRef]
- Zhaka, V.; Samuelsson, B. Hydrogen as fuel in the maritime sector: From production to propulsion. Energy Rep. 2024, 12, 5249–5267. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Q.; Su, G.; Lu, W. A Review of Hydrogen Storage and Transportation: Progresses and Challenges. Energies 2024, 17, 4070. [Google Scholar] [CrossRef]
- Ali Lashari, Z.; Haq, B.; Al-Shehri, D.; Zaman, E.; Al-Ahmed, A.; Lashari, N.; Zakir Hossain, S.M. Recent Development of Physical Hydrogen Storage: Insights into Global Outlook and Future Applications. Chem. Asian J. 2024, 19, e202300926. [Google Scholar] [CrossRef]
- Rampai, M.M.; Mtshali, C.B.; Seroka, N.S.; Khotseng, L. Hydrogen production, storage, and transportation: Recent advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, L.; Han, Y.; Chen, X.; Sheng, P.; Wang, S.; Huang, X.; Wang, X.; Lu, C.; Luo, H.; et al. Development of a gaseous and solid-state hybrid system for stationary hydrogen energy storage. Green Energy Environ. 2021, 6, 528–537. [Google Scholar] [CrossRef]
- Chen, L.; Lv, H.; Shang, Y.; Zhang, Z.; Chen, S.; Hou, Y. Strategies for improving the thermal insulation performance of liquid hydrogen tanks: A review of multilayer insulation structure. Int. J. Hydrogen Energy 2024, 94, 1419–1434. [Google Scholar] [CrossRef]
- Shu, K.; Guan, B.; Zhuang, Z.; Chen, J.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Dang, H.; et al. Reshaping the energy landscape: Explorations and strategic perspectives on hydrogen energy preparation, efficient storage, safe transportation and wide applications. Int. J. Hydrogen Energy 2025, 97, 160–213. [Google Scholar] [CrossRef]
- Wu, J.K.; Chen, S.; Yu, M.; Zhang, X.J.; Jiang, L. Techno-economic analysis on low-temperature and high-pressure cryo-adsorption hydrogen storage. Fuel 2025, 381, 133532. [Google Scholar] [CrossRef]
- Gómez, J.A.; Santos, D.M.F. The Status of On-Board Hydrogen Storage in Fuel Cell Electric Vehicles. Designs 2023, 7, 97. [Google Scholar] [CrossRef]
- The Union of Kansai Governments. Roadmap for Establishing a Hydrogen Supply Chain in the Kansai Area (Japan); The Union of Kansai Governments: Osaka, Japan, 2021; p. 3. Available online: https://www.kouiki-kansai.jp/material/files/group/15/HydrogenSupplyChain.pdf (accessed on 20 December 2024).
- Hydrogen-Related Businesses in the Kansai Region. METI: Osaka, Japan, 2024; 41p. Available online: https://www.kansai.meti.go.jp/5-1shiene/smart_energy_initiative/hydrogen_data/index_en.html (accessed on 15 August 2025).
- Soleimani, A.; Hosseini Dolatabadi, S.H.; Heidari, M.; Pinnarelli, A.; Mehdizadeh Khorrami, B.; Luo, Y.; Vizza, P.; Brusco, G. Progress in hydrogen fuel cell vehicles and up-and-coming technologies for eco-friendly transportation: An international assessment. Multiscale Multidiscip. Model. Exp. Des. 2024, 7, 3153–3172. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Martinez, A.; Hong, P.; Xu, H.; Bockmiller, F.R. Polymer electrolyte membrane fuel cell and hydrogen station networks for automobiles: Status, technology, and perspectives. Adv. Appl. Energy 2021, 2, 100011. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Fukushima Hydrogen Energy Research Field in Japan ready for green hydrogen production. Fuel Cells Bull. 2020, 2020, 1. [CrossRef]
- The World’s Largest-Class Hydrogen Production, Fukushima Hydrogen Energy Research Field (FH2R) Now Is Completed at Namie Town in Fukushima. Available online: https://www.nedo.go.jp/english/news/AA5en_100422.html (accessed on 2 January 2025).
- Kawasaki Heavy Industries, Ltd. Japan’s Movement for Hydrogen Society and Global Hydrogen Supply Chain; 191210 Hyper Open Seminar; SINTEF: Trondheim, Norway, 2019; p. 27. Available online: https://www.sintef.no/globalassets/project/hyper/presentations-day-1/day1_1510_taira_kawasakihi.pdf (accessed on 2 January 2025)191210 Hyper Open Seminar.
- Naseem, K.; Qin, F.; Khalid, F.; Suo, G.; Zahra, T.; Chen, Z.; Javed, Z. Essential parts of hydrogen economy: Hydrogen production, storage, transportation and application. Renew. Sustain. Energy Rev. 2025, 210, 115196. [Google Scholar] [CrossRef]
- Kang, D.; Yun, S.; Kim, B.-k. Review of the Liquid Hydrogen Storage Tank and Insulation System for the High-Power Locomotive. Energies 2022, 15, 4357. [Google Scholar] [CrossRef]
- Fang, W.; Ding, C.; Chen, L.; Zhou, W.; Wang, J.; Huang, K.; Zhu, R.; Wu, J.; Liu, B.; Fang, Q.; et al. Review of Hydrogen Storage Technologies and the Crucial Role of Environmentally Friendly Carriers. Energy Fuels 2024, 38, 13539–13564. [Google Scholar] [CrossRef]
- Naquash, A.; Agarwal, N.; Lee, M. A Review on Liquid Hydrogen Storage: Current Status, Challenges and Future Directions. Sustainability 2024, 16, 8270. [Google Scholar] [CrossRef]
- Hartwig, J.; Johnson, W.; Bamberger, H.; Meyer, M.; Wendell, J.; Mullins, J.; Craig Robinson, R.; Arnett, L. NASA Glenn Research Center Creek Road Cryogenic Complex: Testing between 2005–2019. Cryogenics 2020, 106, 103038. [Google Scholar] [CrossRef]
- Davies, E.; Ehrmann, A.; Schwenzfeier-Hellkamp, E. Safety of Hydrogen Storage Technologies. Processes 2024, 12, 2182. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of hydrogen storage and transportation: An overview on mechanisms, techniques, and challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Rezaei, M.; Akimov, A.; Gray, E.M. Techno-economics of renewable hydrogen export: A case study for Australia-Japan. Appl. Energy 2024, 374, 124015. [Google Scholar] [CrossRef]
- Rhee, Y.; O’Neill, K.; Al Ghafri, S.Z.S.; May, E.F.; Johns, M.L. Effect of location on green steel production using Australian resources. Int. J. Hydrogen Energy 2024, 90, 827–841. [Google Scholar] [CrossRef]
- Transition to a Hydrogen Energy-Based Society. Available online: https://www.iwatani.co.jp/eng/sustainability/environment/hydrogen/ (accessed on 2 January 2025).
- Cao, Q.; Chen, Y.; Wang, Z.; Wang, M.; Wang, P.; Ge, L.; Li, P.; Zhao, Q.; Wang, B.; Gan, Z. Improving the cooling efficiency of cryo-compressed hydrogen based on the temperature-distributed method in regenerative refrigerators. Energy 2025, 314, 134234. [Google Scholar] [CrossRef]
- Baetcke, L. Hydrogen Transport and Storage Options. In Powerfuels: Status and Prospects; Bullerdiek, N., Neuling, U., Kaltschmitt, M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 281–297. [Google Scholar]
- Jaramillo, D.E.; Moreno-Blanco, J.; Aceves, S.M. Evaluation of cryo-compressed hydrogen for heavy-duty trucks. Int. J. Hydrogen Energy 2024, 87, 928–938. [Google Scholar] [CrossRef]
- Stops, L.; Siebe, D.; Stary, A.; Hamacher, J.; Sidarava, V.; Rehfeldt, S.; Klein, H. Generalized thermodynamic modeling of hydrogen storage tanks for truck application. Cryogenics 2024, 139, 103826. [Google Scholar] [CrossRef]
- Li, S.; Han, F.; Liu, Y.; Xu, Z.; Yan, Y.; Ni, Z. Evaluation criterion for filling process of cryo-compressed hydrogen storage vessel. Int. J. Hydrogen Energy 2024, 59, 1459–1470. [Google Scholar] [CrossRef]
- Boretti, A. Technology readiness level of hydrogen storage technologies for transport. Energy Storage 2024, 6, e546. [Google Scholar] [CrossRef]
- Schmitt, J.; Roychowdhury, B.; Swanger, A.; Otto, M.; Kapat, J. Techno-economic analysis of green hydrogen energy storage in a cryogenic flux capacitor. In Proceedings of the ASME Turbo Expo, London, UK, 24–28 June 2024. [Google Scholar]
- Clarke, J.; Dettmer, W.; Wen, J.; Ren, Z. Cryogenic Hydrogen Jet and Flame for Clean Energy Applications: Progress and Challenges. Energies 2023, 16, 4411. [Google Scholar] [CrossRef]
- Boretti, A. Progress in cold/cryo-pressurized composite tanks for hydrogen. MRS Commun. 2023, 13, 400–405. [Google Scholar] [CrossRef]
- Aceves, S.M.; Espinosa-Loza, F.; Ledesma-Orozco, E.; Ross, T.O.; Weisberg, A.H.; Brunner, T.C.; Kircher, O. High-density automotive hydrogen storage with cryogenic capable pressure vessels. Int. J. Hydrogen Energy 2010, 35, 1219–1226. [Google Scholar] [CrossRef]
- De Boever, J.; Fisher, C. Development of a prototype fuel cell powered Toyota Hilux. In Proceedings of the Powertrain Systems for a Sustainable Future—Proceedings of the International Conference on Powertrain Systems for a Sustainable Future, Lille, France, 19–20 June 2024; pp. 421–431. [Google Scholar]
- Moura, L.; González, M.; Silva, J.; Silva, L.; Braga, I.; Ferreira, P.; Sampaio, P. Evaluation of technological development of hydrogen fuel cells based on patent analysis. AIMS Energy 2024, 12, 190–213. [Google Scholar] [CrossRef]
- Mochizuki, K.; Habaguchi, Y.; Tsujioka, M.; Nonaka, S.; Ogawa, H.; Ito, T.; Noboru, S. A road map toward Japan’s future reusable space transportation system. In Proceedings of the International Astronautical Congress, IAC, Guadalajara, Mexico, 26–30 September 2016. [Google Scholar]
- Nagasaki, A.; Watanabe, D.; Tamura, T.; Onga, T.; Ogawara, A.; Higuchi, N.; Sakai, H.; Nunome, Y.; Kawashima, H. Additive manufacturing development for rocket engine in Japan. In Proceedings of the International Astronautical Congress, IAC, Adelaide, Australia, 25–29 September 2017; pp. 8713–8719. [Google Scholar]
- Qureshi, F.; Yusuf, M.; Ahmed, S.; Haq, M.; Alraih, A.M.; Hidouri, T.; Kamyab, H.; Vo, D.-V.N.; Ibrahim, H. Advancements in sorption-based materials for hydrogen storage and utilization: A comprehensive review. Energy 2024, 309, 132855. [Google Scholar] [CrossRef]
- Letwaba, J.; Uyor, U.O.; Mavhungu, M.L.; Achuka, N.O.; Popoola, P.A. A review on MOFs synthesis and effect of their structural characteristics for hydrogen adsorption. RSC Adv. 2024, 14, 14233–14253. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Dhilip Kumar, T.J.; Ahuja, R. Furtherance of the material-based hydrogen storage based on theory and experiments. Int. J. Hydrogen Energy 2023, 48, 12767–12795. [Google Scholar] [CrossRef]
- Harisankar, U.S.; Menon, S.K.; Babu, J.S.; Shankar, B. Exploring Carbon Nanotubes for Enhanced Hydrogen Storage: A Review on Synthesis, Mechanisms, and Evaluation. Korean J. Chem. Eng. 2025, 42, 13–42. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H.; Kolhe, M.L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Mahmoud, L.A.M.; Rowlandson, J.L.; Fermin, D.J.; Ting, V.P.; Nayak, S. Porous carbons: A class of nanomaterials for efficient adsorption-based hydrogen storage. RSC Appl. Interfaces 2025, 2, 25–55. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Singh, S. Chapter 4—Hydrogen surface storage. In Subsurface Hydrogen Energy Storage; Bera, A., Kumar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 91–122. [Google Scholar]
- Shao, H.; Lyth, S.M. Solid Hydrogen Storage Materials: High Surface Area Adsorbents. In Hydrogen Energy Engineering: A Japanese Perspective; Sasaki, K., Li, H.-W., Hayashi, A., Yamabe, J., Ogura, T., Lyth, S.M., Eds.; Springer: Tokyo, Japan, 2016; pp. 241–251. [Google Scholar]
- Akiba, E. Solid Hydrogen Storage Materials: Interstitial Hydrides. In Hydrogen Energy Engineering: A Japanese Perspective; Sasaki, K., Li, H.-W., Hayashi, A., Yamabe, J., Ogura, T., Lyth, S.M., Eds.; Springer: Tokyo, Japan, 2016; pp. 191–206. [Google Scholar]
- Chakraborty, A.; Saha, B.B.; Koyama, S.; Ng, K.C.; Yoon, S.-H. Thermodynamic trends in the uptake capacity of porous adsorbents on methane and hydrogen. Appl. Phys. Lett. 2008, 92, 201911. [Google Scholar] [CrossRef]
- Thanh, H.V.; Ebrahimnia Taremsari, S.; Ranjbar, B.; Mashhadimoslem, H.; Rahimi, E.; Rahimi, M.; Elkamel, A. Hydrogen Storage on Porous Carbon Adsorbents: Rediscovery by Nature-Derived Algorithms in Random Forest Machine Learning Model. Energies 2023, 16, 2348. [Google Scholar] [CrossRef]
- How Energy Observer Set Sail with a Toyota Fuel Cell. Available online: https://pressroom.toyota.com/how-energy-observer-set-sail-with-a-toyota-fuel-cell/ (accessed on 3 January 2025).
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kalibek, M.R.; Ospanova, A.D.; Suleimenova, B.; Soltan, R.; Orazbek, T.; Makhmet, A.M.; Rafikova, K.S.; Nuraje, N. Solid-state hydrogen storage materials. Discov. Nano 2024, 19, 195. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, M.P.; Park, C.H.; Tran, Q.N. Recent Developments in Materials for Physical Hydrogen Storage: A Review. Materials 2024, 17, 666. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Ye, J.; Wu, Y.; Guo, X.; Li, Z.; Li, H. Research progress of solid-state hydrogen storage technology. Taiyangneng Xuebao/Acta Energiae Solaris Sin. 2022, 43, 345–354. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Review of metal hydride hydrogen storage thermal management for use in the fuel cell systems. Int. J. Hydrogen Energy 2021, 46, 31699–31726. [Google Scholar] [CrossRef]
- Zohra, F.T.; Webb, C.J.; Lamb, K.E.; Gray, E.M. Degradation of metal hydrides in hydrogen-based thermodynamic machines: A review. Int. J. Hydrogen Energy 2024, 64, 417–438. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef] [PubMed]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Lim, K.L.; Kazemian, H.; Yaakob, Z.; Daud, W.R.W. Solid-state Materials and Methods for Hydrogen Storage: A Critical Review. Chem. Eng. Technol. 2010, 33, 213–226. [Google Scholar] [CrossRef]
- Ubukata, H.; Takeiri, F.; Shitara, K.; Tassel, C.; Saito, T.; Kamiyama, T.; Broux, T.; Kuwabara, A.; Kobayashi, G.; Kageyama, H. Anion ordering enables fast H<sup>−</sup> conduction at low temperatures. Sci. Adv. 2021, 7, eabf7883. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.-i.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Takagi, S.; Orimo, S.-I. Recent progress in hydrogen-rich materials from the perspective of bonding flexibility of hydrogen. Scr. Mater. 2015, 109, 1–5. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Kato, Z. The Use of Renewable Energy in the Form of Usable Fuel via Hydrogen. Corros. Eng. 2012, 61, 305–313. [Google Scholar] [CrossRef][Green Version]
- Khandoozi, S.; Li, P.; Ershadnia, R.; Dai, Z.; Zhang, Z.; Stauffer, P.H.; Mehana, M.; Cole, D.R.; Soltanian, M.R. An integrated approach for optimizing geological hydrogen storage. Appl. Energy 2025, 381, 125182. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Longe, P.O.; Mehrad, M.; Rukavishnikov, V.S. Underground hydrogen storage: A review of technological developments, challenges, and opportunities. Appl. Energy 2025, 381, 125172. [Google Scholar] [CrossRef]
- Viveros, F.E.; Medina, O.E.; Moncayo-Riascos, I.; Lysyy, M.; Benjumea, P.N.; Cortés, F.B.; Franco, C.A. Hydrogen storage in depleted gas reservoirs using methane cushion gas: An interfacial tension and pore scale study. J. Energy Storage 2024, 98, 113110. [Google Scholar] [CrossRef]
- Zeng, L.; Sander, R.; Chen, Y.; Xie, Q. Hydrogen Storage Performance During Underground Hydrogen Storage in Depleted Gas Reservoirs: A Review. Engineering 2024, 40, 211–225. [Google Scholar] [CrossRef]
- Mehr, A.S.; Phillips, A.D.; Brandon, M.P.; Pryce, M.T.; Carton, J.G. Recent challenges and development of technical and technoeconomic aspects for hydrogen storage, insights at different scales; A state of art review. Int. J. Hydrogen Energy 2024, 70, 786–815. [Google Scholar] [CrossRef]
- Muthukumar, P.; Kumar, A.; Afzal, M.; Bhogilla, S.; Sharma, P.; Parida, A.; Jana, S.; Kumar, E.A.; Pai, R.K.; Jain, I.P. Review on large-scale hydrogen storage systems for better sustainability. Int. J. Hydrogen Energy 2023, 48, 33223–33259. [Google Scholar] [CrossRef]
- Londe, L.; Réveillère, A. Different ways to store massive quantities of hydrogen. In Proceedings of the WHEC 2022—23rd World Hydrogen Energy Conference: Bridging Continents by H2, Istanbul, Turkey, 26–30 June 2022; pp. 494–496. [Google Scholar]
- Nassan, T.H.; Kirch, M.; Amro, M. Underground hydrogen storage in salt caverns: Laboratory experiments to determine integrity of rock salt and wellbore through effective permeability measurements. Int. J. Hydrogen Energy 2025, 99, 619–631. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Shi, X.; Bai, W.; Huang, Y.; Hong, Y.; Liu, X.; Ma, H.; Li, P.; Xu, M.; et al. The Role of Underground Salt Caverns in Renewable Energy Peaking: A Review. Energies 2024, 17, 6005. [Google Scholar] [CrossRef]
- Lange, J.; Schulthoff, M.; Puszkiel, J.; Sens, L.; Jepsen, J.; Klassen, T.; Kaltschmitt, M. Aboveground hydrogen storage—Assessment of the potential market relevance in a Carbon-Neutral European energy system. Energy Convers. Manag. 2024, 306, 118292. [Google Scholar] [CrossRef]
- Talukdar, M.; Blum, P.; Heinemann, N.; Miocic, J. Techno-economic analysis of underground hydrogen storage in Europe. iScience 2024, 27, 108771. [Google Scholar] [CrossRef]
- Saadat, Z.; Farazmand, M.; Sameti, M. Integration of underground green hydrogen storage in hybrid energy generation. Fuel 2024, 371, 131899. [Google Scholar] [CrossRef]
- Bade, S.O.; Taiwo, K.; Ndulue, U.F.; Tomomewo, O.S.; Aisosa Oni, B. A review of underground hydrogen storage systems: Current status, modeling approaches, challenges, and future prospective. Int. J. Hydrogen Energy 2024, 80, 449–474. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Sezgin, B.; Devrim, Y.; Ozturk, T.; Eroglu, I. Hydrogen energy systems for underwater applications. Int. J. Hydrogen Energy 2022, 47, 19780–19796. [Google Scholar] [CrossRef]
- Chapman, A.J.; Fraser, T.; Itaoka, K. Hydrogen import pathway comparison framework incorporating cost and social preference: Case studies from Australia to Japan. Int. J. Energy Res. 2017, 41, 2374–2391. [Google Scholar] [CrossRef]
- Toshiba H2One unit running in Yokohama, launches Truck Model. Fuel Cells Bull. 2016, 2016, 6. [CrossRef]
- Claussner, L.; Ustolin, F. System Resilience of a Liquid Hydrogen Terminal During Loading and Unloading Operations. IFAC-PapersOnLine 2024, 58, 359–364. [Google Scholar] [CrossRef]
- Suzuki, H.; Yoshiyama, T.; Shindo, K.; Nishimura, M. International liquefied hydrogen supply chain. In Proceedings of the ICOPE 2019—7th International Conference on Power Engineering, Proceedings, Kunming, China, 21–25 October 2019; pp. 833–838. [Google Scholar]
- Benedicte Delaval, T.R.; Sharma, R.; Hugh-Jones, W.; McClure, E.; Temminghoff, M.; Srinivasan, V. Hydrogen RD&D Collaboration Opportunities: Japan; CSIRO: Canberra, Australia, 2022; p. 51. Available online: http://hdl.handle.net/102.100.100/474026?index=1 (accessed on 15 August 2025).
- Japan takes a major step toward a H2-based economy. Chem. Eng. 2018, 125, 10.
- List of Hydrogen Stations in Japan. Available online: https://www.glpautogas.info/data/hydrogen-stations-map-japan.html (accessed on 3 January 2025).
- Position Paper on Hydrogen Economy; Academy of Sciences Malaysia: Kuala Lumpur, 2022; p. 139.
- List of hydrogen stations in the Tokyo metropolitan area. Available online: https://www.cev-pc.or.jp/suiso_station/area01.html (accessed on 6 January 2025).
- Li, Y.; Wang, Y.; Zhou, R.; Qian, H.; Gao, W.; Zhou, W. Energy transition roadmap towards net-zero communities: A case study in Japan. Sustain. Cities Soc. 2024, 100, 105045. [Google Scholar] [CrossRef]
- Meng, L.; Li, M.; Asuka, J. A scenario analysis of the energy transition in Japan’s road transportation sector based on the LEAP model. Environ. Res. Lett. 2024, 19, 044059. [Google Scholar] [CrossRef]
- Murayama, K.; Okamoto, S.; Iida, T. Development of Hydrogen Fuel Cell Train “HYBARI”. Jpn. Railw. Eng. 2023, 63, 24–26. [Google Scholar]
- Asabin, V.V.; Garanin, M.A.; Kurmanova, L.S.; Mishkin, A.A.; Svechnikov, A.A. Prospects for using hydrogen on railway transport. IOP Conf. Ser. Mater. Sci. Eng. 2020, 953, 012074. [Google Scholar] [CrossRef]
- Han, X.; Yan, H.-g.; Kang, J.-d.; Li, Y. Strategic Analysis of Hydrogen Energy Policies and Technology Layout in Major Countries. In Proceedings of the 10th Hydrogen Technology Convention, Foshan, China, 22–26 May 2023; Springer: Singapore, 2024; Volume 1, pp. 435–451. [Google Scholar]
- Toyota and Woven Planet Have Developed a New Portable Hydrogen Cartridge Prototype. Toyota: 2022. Available online: https://global.toyota/en/newsroom/corporate/37405994.html (accessed on 3 January 2025).
- Japan H2 Mobility, LLC, Established by Eleven Companies to Accelerate the Deployment of Hydrogen Stations in Japan. Available online: https://global.toyota/en/newsroom/corporate/21322195.html (accessed on 7 January 2025).
- Emblemsvåg, J. A Study on the Limitations of Green Alternative Fuels in Global Shipping in the Foreseeable Future. J. Mar. Sci. Eng. 2025, 13, 79. [Google Scholar] [CrossRef]
- Meca, V.L.; d’Amore-Domenech, R.; Crucelaegui, A.; Leo, T.J. Large-Scale Maritime Transport of Hydrogen: Economic Comparison of Liquid Hydrogen and Methanol. ACS Sustain. Chem. Eng. 2022, 10, 4300–4311. [Google Scholar] [CrossRef]
- LH2 ship carries hopes for a hydrogen society. Motor Ship 2018, 99, 42.
- Goldmeer, J.; Buck, C.; Suleiman, B.; Selim, H.; Tayebi, K.; Agostinelli, G.L.; Dawood, A. Techno-economic analysis of the hydrogen and ammonia as low carbon fuels for power generation. In Proceedings of the ASME Turbo Expo, Boston, MA, USA, 27–29 June 2023. [Google Scholar]
- Hydrogen-Powered Vessels|CMB X Tsuneishi Group. Available online: https://www.eu-japan.eu/publications/hydrogen-powered-vessels-cmb-x-tsuneishi-group (accessed on 7 January 2025).
- The Nippon Foundation Zero Emission Ships Project. Available online: https://www.nippon-foundation.or.jp/en/news/articles/2024/20240904-103973.html#:~:text=The%20Nippon%20Foundation%20launched%20the,of%20Fukuyama%20in%20Hiroshima%20Prefecture (accessed on 7 January 2025).
- Roadmap to Zero Emission from International Shipping; Ministry of Land, Infrastructure, Transport and Tourism, Maritime Bureau: Tokyo, Japan, 2020; p. 136. Available online: https://www.mlit.go.jp/en/maritime/GHG_roadmap_en.html (accessed on 7 January 2025).
- Working Paper. National Hydrogen Strategies; World Energy Council: London, UK, 2021; p. 20. Available online: https://www.worldenergy.org/publications/entry/working-paper-hydrogen-on-the-horizon-national-hydrogen-strategies (accessed on 7 August 2025).
- DNV—Hydrogen Forecast to 2050; DNV: Høvik, Norway, 2022; p. 114.
- Fam, A.; Fam, S. Review of the US 2050 long term strategy to reach net zero carbon emissions. Energy Rep. 2024, 12, 845–860. [Google Scholar] [CrossRef]
- Bade, S.O.; Tomomewo, O.S. A review of governance strategies, policy measures, and regulatory framework for hydrogen energy in the United States. Int. J. Hydrogen Energy 2024, 78, 1363–1381. [Google Scholar] [CrossRef]
- The Hydrogen Strategy for Canada. Seizing the Opportunities for Hydrogen; Natural Resources Canada: Ottawa, ON, Canada, 2020; p. 141. [Google Scholar]
- Canadian Hydrogen Sector Profile 2024; Canadian Hydrogen Association: Vancouver, BC, Canada, 2024; p. 55.
- National Green Hydrogen Strategy; Ministry of Energy, Government of Chile: Santiago, Chile, 2020; p. 33.
- Aditiya, H.B.; Aziz, M. Prospect of hydrogen energy in Asia-Pacific: A perspective review on techno-socio-economy nexus. Int. J. Hydrogen Energy 2021, 46, 35027–35056. [Google Scholar] [CrossRef]
- Nakano, J. South Korea’s Hydrogen Industrial Strategy. Available online: https://www.csis.org/analysis/south-koreas-hydrogen-industrial-strategy (accessed on 3 January 2025).
- Equinor. Energy Perspectives: Global Macroeconomic and Energy Market Outlook; Equinor: Stavanger, Norway, 2025; p. 42. Available online: https://www.equinor.com/sustainability/energy-perspectives#downloads (accessed on 3 August 2025).
- IEA. World Energy Transitions Outlook 2023; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 3 January 2025).
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 3 January 2025).
- IEA. Global Hydrogen Review; IEA: Paris, France, 2023; p. 176. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 3 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).