Hydrogen via Co-Electrolysis of Water and CO2: Challenge or Solution for Industrial Decarbonization?
Abstract
1. Introduction
1.1. Background on Hydrogen and Co-Electrolysis
1.2. Scientific Motivation and Objectives of the Work
2. Fundamentals and Energy Analysis of Co-Electrolysis of H2O and CO2
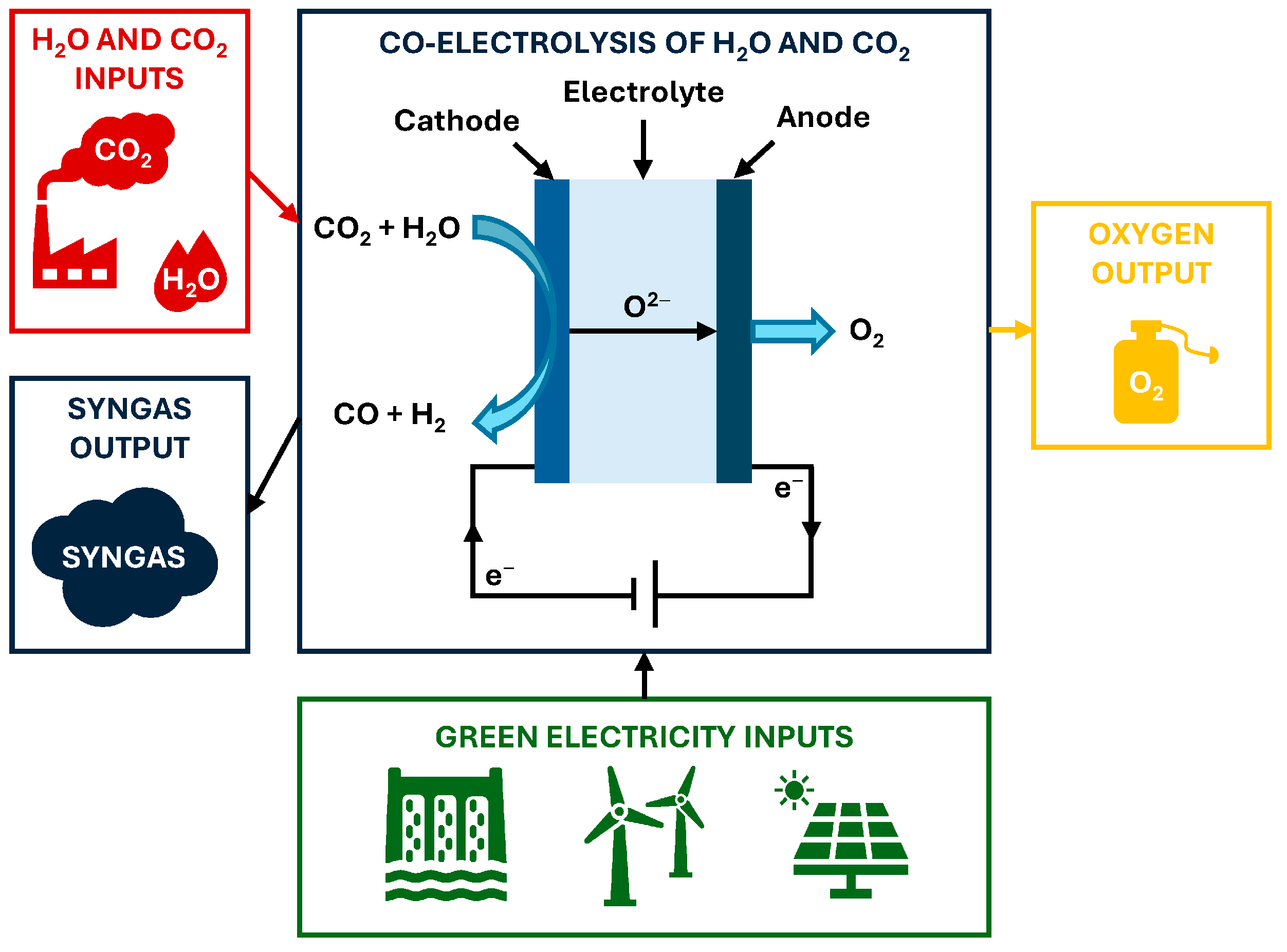
2.1. Operating Principles of Co-Electrolysis
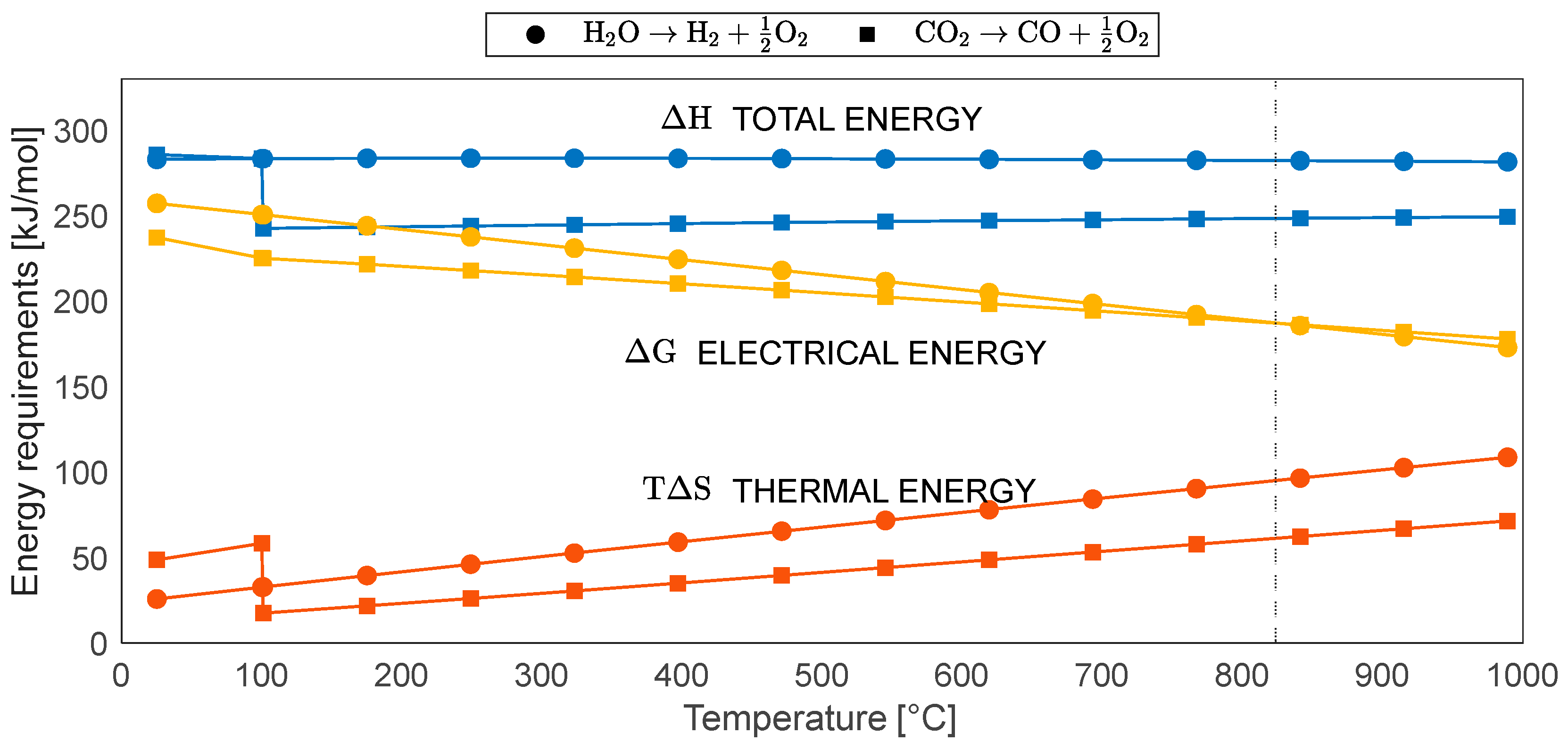
2.2. Energy Analysis of Co-Electrolysis
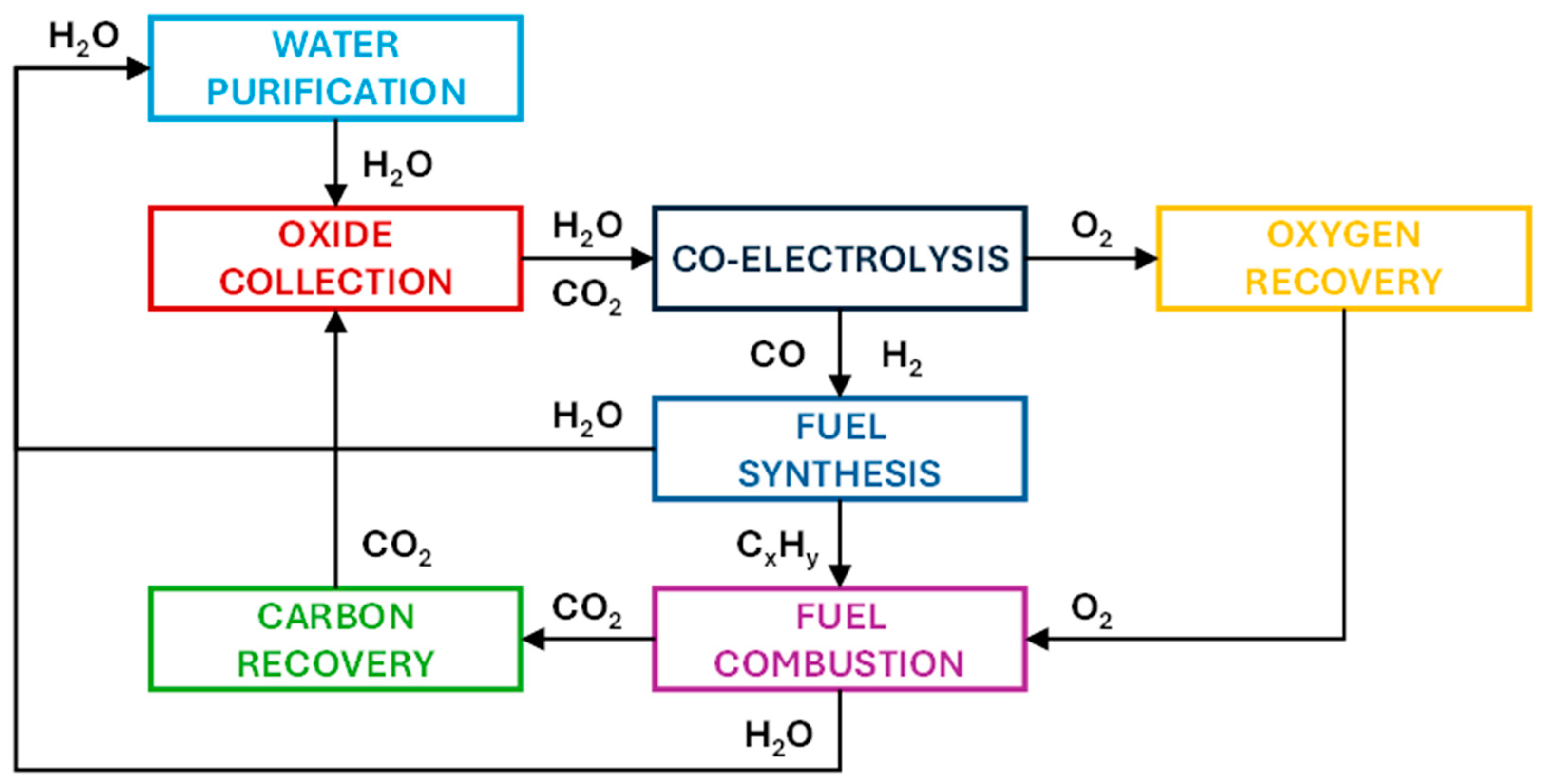
3. Outlook and Recent Developments of Co-Electrolysis of H2O and CO2
4. Potential Application of Co-Electrolysis in Specific Industrial Contexts
4.1. Cement Production
- -
- Calcination of Limestone: at high temperatures (approximately 900–1000 °C), limestone (CaCO3) undergoes a chemical reaction known as calcination. During this reaction, calcium carbonate decomposes into calcium oxide (CaO), also known as lime, and carbon dioxide (CO2):CaCO3 (s) → CaO (s) + CO2 (g)The CaO produced is solid and remains in the kiln, while the CO2 is released into the atmosphere as a gas.
- -
- Clinker Formation: the CaO produced in the calcination step then reacts with other materials in the kiln, such as silica (SiO2), alumina (Al2O3), and iron oxide (Fe2O3), at higher temperatures (around 1400–1450 °C). These reactions result in the formation of clinker, a solid material that is ground into cement.
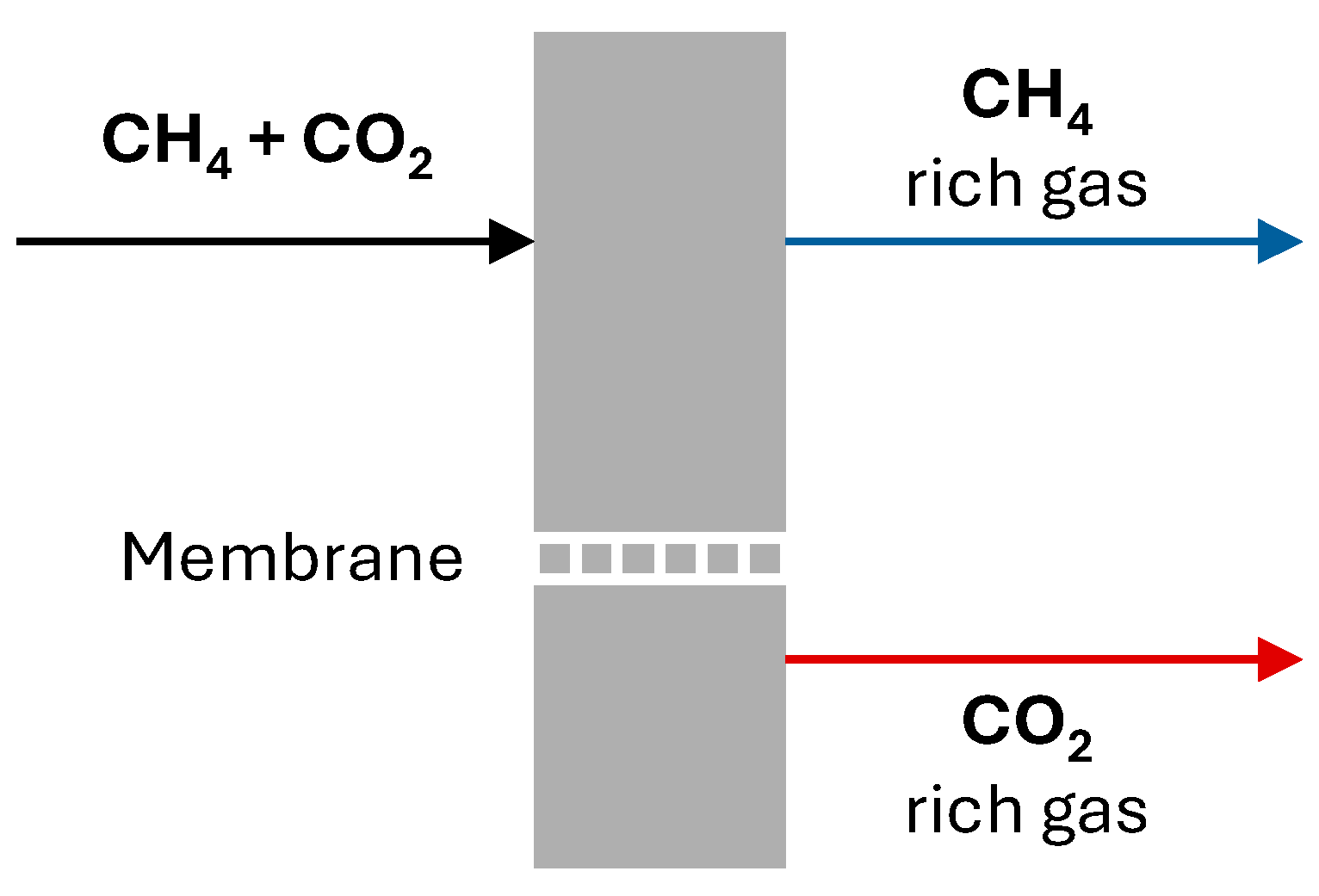
4.2. Natural Gas and Biogas Processing Facilities
4.3. Carbon Dioxide Generation and Capture in Corn Fermentation for Bioethanol Production
- -
- Milling and Starch Preparation in which the corn is ground to release starch, which is then broken down into fermentable sugars through enzymatic hydrolysis.
- -
- Liquefaction and Saccharification where enzymes (such as amylase) convert starch into simple sugars such as glucose and maltose.
- -
- Fermentation during which yeast and enzymes convert glucose into ethanol and CO2:C6H12O6 → 2 C2H5OH + 2 CO2
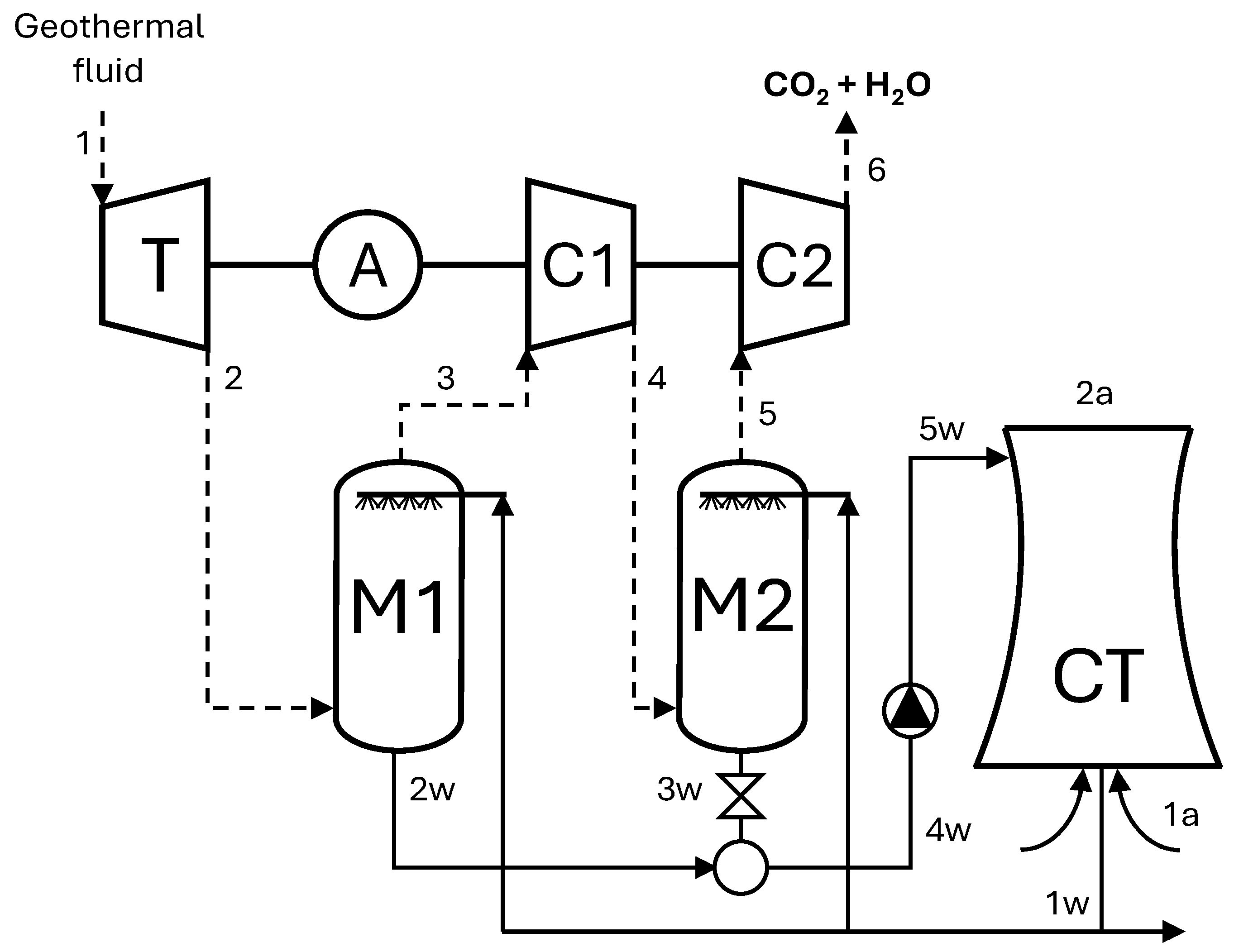
4.4. Co-Electrolysis of CO2 and H2O in the Power Sector: The Case of Geothermal Power Plants
4.5. Multi-Criteria Assessment of Industrial Co-Electrolysis Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary/Nomenclature/Abbreviations
| AC | Alternate current |
| act,a | Activation at anode |
| act,c | Activation at cathode |
| BoP | Balance of plant |
| conc,a | Concentration at anode |
| conc,c | Concentration at cathode |
| CO2RR | Electrochemical reduction of CO2 |
| CCUS | Carbon capture utilization and storage |
| DC | Direct current |
| E | Ideal potential [V] |
| ext | Relative to the external components |
| F | Faraday’s constant |
| I | Current [A] |
| LCOE | Levelized cost of energy |
| LCOH | Levelized cost of hydrogen |
| LHV | Lower heating value [MJ/kg] |
| ṁ | Mass flow rate [kg/s] |
| Ne | Number of “moles” of electrons |
| ohmic | Ohmic |
| P | Pressure [bar] |
| PEM | Proton exchange membrane |
| Q | Charge [Coulomb] |
| Thermal power [W] | |
| RES | Renewable energy systems |
| SEC | Specific energy consumption [kWh/kg] |
| SOEC | Solid oxide electrolytic cell |
| SMR | Steam methane reforming |
| stack | Relative to the stack |
| Syn | Syngas |
| SWOT | Strengths, weaknesses, opportunities, threats |
| T | Temperature |
| TRL | Technology readiness level |
| V | Potential [V] |
| Power [W] | |
| X | Steam quality |
| µ | Overpotential |
| η | Efficiency |
| ΔG | Gibbs free energy [kJ/kmol] |
| ΔH | Enthalpy of the reactions [kJ/kmol] |
References
- Hydrogen Council. Hydrogen Decarbonization Pathways Part 1: A Life-Cycle Assessment. 2021. Available online: https://hydrogencouncil.com/wp-content/uploads/2021/04/Hydrogen-Council-Report_Decarbonization-Pathways_Part-1-Lifecycle-Assessment.pdf (accessed on 22 August 2025).
- Blank, T.K.; Molly, P. Hydrogen’s Decarbonization Impact for Industry. Near-Term Challenges and Long-Term Potential. Report of the Rocky Mountain Institute. 2020. Available online: https://rmi.org/wp-content/uploads/2020/01/hydrogen_insight_brief.pdf (accessed on 22 August 2025).
- Franco, A.; Giovannini, C. Routes for Hydrogen Introduction in the Industrial Hard-to-Abate Sectors for Promoting Energy Transition. Energies 2023, 16, 6098. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the Climate Impacts of Blue Hydrogen Production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How Green Is Blue Hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Boston Metal. Zero CO2 Steel by Molten Oxide Electrolysis: A Path to 100% Global Steel Decarbonization. 2023. Available online: https://www.bostonmetal.com/news/zero-co2-steel-by-molten-oxide-electrolysis-a-path-to-100-global-steel-decarbonization/ (accessed on 22 August 2025).
- Crownhart, C. How Green Steel Made with Electricity Could Clean up a Dirty Industry. 2022. Available online: https://www.technologyreview.com/2022/06/28/1055027/green-steel-electricity-boston-metal/ (accessed on 22 August 2025).
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable Hydrocarbon Fuels by Recycling CO2 and H2O with Renewable or Nuclear Energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Zong, S.; Zhao, X.; Jewell, L.L.; Zhang, Y.; Liu, X. Advances and Challenges with SOEC High Temperature Co-Electrolysis of CO2/H2O: Materials Development and Technological Design. Carbon Capture Sci. Technol. 2024, 12, 100234. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and Synthetic Fuel Production from Renewable Energy Sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook (NIST Standard Reference Database Number 69); National Institute of Standards and Technology: Gaithersburg, MD, USA. [CrossRef]
- Belsa, B.; Xia, L.; de Arquer, F.P.G. CO2 Electrolysis Technologies: Bridging the Gap toward Scale-up and Commercialization. ACS Energy Lett. 2024, 9, 4293–4305. [Google Scholar] [CrossRef]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef]
- Gao, N.; Quiroz-Arita, C.; Diaz, L.A.; Lister, T.E. Intensified Co-Electrolysis Process for Syngas Production from Captured CO2. J. CO2 Util. 2021, 43, 101365. [Google Scholar] [CrossRef]
- Min, G.; Choi, S.; Hong, J. A Review of Solid Oxide Steam-Electrolysis Cell Systems: Thermodynamics and Thermal Integration. Appl. Energy 2022, 328, 120145. [Google Scholar] [CrossRef]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Thermodynamic and achievable efficiencies for solar-driven electrochemical reduction of carbon dioxide to transportation fuels. Proc. Natl. Acad. Sci. USA 2015, 112, E6111–E6118. [Google Scholar] [CrossRef]
- Küngas, R. electrochemical CO2 reduction for CO production: Comparison of low-and high-temperature electrolysis technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K. Recent advances in low-temperature electrochemical conversion of carbon dioxide. Rev. Chem. Eng. 2021, 37, 863–884. [Google Scholar] [CrossRef]
- Kupecki, J.; Motylinski, K.; Jagielski, S.; Wierzbicki, M.; Brouwer, J.; Naumovich, Y.; Skrzypkiewicz, M. Energy Analysis of a 10 kW-Class Power-to-Gas System Based on a Solid Oxide Electrolyzer (SOE). Energy Convers. Manag. 2019, 199, 111934. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Graves, C.; Mogensen, M. Production of Synthetic Fuels by Co-Electrolysis of Steam and Carbon Dioxide. Int. J. Green Energy 2009, 6, 646–660. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-Electrolysis of CO2 and H2O in High-Temperature Solid Oxide Electrolysis Cells: Recent Advance in Cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, H.; Yoon, K.J.; Lee, J.-H.; Kim, B.-K.; Choi, W.; Lee, J.-H.; Hong, J. Reactions and Mass Transport in High Temperature Co-Electrolysis of Steam/CO2 Mixtures for Syngas Production. J. Power Sources 2015, 280, 630–639. [Google Scholar] [CrossRef]
- Deka, D.J.; Gunduz, S.; Fitzgerald, T.; Miller, J.T.; Co, A.C.; Ozkan, U.S. Production of Syngas with Controllable H2/CO Ratio by High Temperature Co-Electrolysis of CO2 and H2O over Ni and Co- Doped Lanthanum Strontium Ferrite Perovskite Cathodes. Appl. Catal. B Environ. 2019, 248, 487–503. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-supported perovskite as an efficient bifunctional electrocatalyst for oxygen reduction and evolution: Substrate effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Chen, Z.; Hou, Y.; Peng, J.; Song, X.; An, S.; O’Hayre, R. An All-Oxide Electrolysis Cells for Syngas Production with Tunable H2/CO Yield via Co-Electrolysis of H2O and CO2. J. Power Sources 2021, 482, 228887. [Google Scholar] [CrossRef]
- Bimpiri, N.; Konstantinidou, A.; Tsiplakides, D.; Balomenou, S.; Papazisi, K.M. Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis. Nanomaterials 2023, 13, 299. [Google Scholar] [CrossRef]
- Cinti, G.; Discepoli, G.; Bidini, G.; Lanzini, A.; Santarelli, M. Co-Electrolysis of Water and CO2 in a Solid Oxide Electrolyzer (SOE) Stack: Study of High-Temperature Co-Electrolysis Reactions in SOEC. Int. J. Energy Res. 2016, 40, 207–215. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, J.; Han, M.; Hua, X.; Li, D.; Ni, M. The Development of Solid Oxide Co-Electrolysis of H2O and CO2 on Large-Size Cells and Stacks. iEnergy 2023, 2, 109–118. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, J.; Zhang, L.; Liu, Q.; Pan, Z.; Chan, S.H. High-Temperature Electrolysis of Simulated Flue Gas in Solid Oxide Electrolysis Cells. Electrochim. Acta 2018, 280, 206–215. [Google Scholar] [CrossRef]
- Sebastián, D.; Palella, A.; Siracusano, S.; Lo Vecchio, C.; Monforte, G.; Baglio, V.; Spadaro, L.; Aricò, A.S. Development of an Electrochemical Co-Electrolysis System for CO2 and H2O with a Polymer Electrolyte Operating at Low Temperature for CO2 Reduction (CO2CHEM)—R.E. 45/16. CNR-ITAE. 2016. Available online: https://publications.cnr.it/doc/359565 (accessed on 22 August 2025).
- Franco, A.; Diaz, A.R. Environmental Sustainability of CO2 Capture in Fossil Fuel Based Power Plants. In Ecosytems and Sustainable Development VI; WIT Press: Coimbra, Portugal, 2007; Volume I, pp. 251–261. [Google Scholar]
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef]
- Volaity, S.S.; Aylas-Paredes, B.K.; Han, T.; Huang, J.; Sridhar, S.; Sant, G.; Kumar, A.; Neithalath, N. Towards decarbonization of cement industry: A critical review of electrification technologies for sustainable cement production. Npj Mater. Sustain. 2025, 3, 23. [Google Scholar] [CrossRef]
- Bahadori, A. Natural Gas Processing: Technology and Engineering Design; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2014; ISBN 978-0-08-099971-5. [Google Scholar]
- Joyia, M.A.K.; Ahmad, M.; Chen, Y.-F.; Mustaqeem, M.; Ali, A.; Abbas, A.; Gondal, M.A. Trends and advances in sustainable bioethanol production technologies from first to fourth generation: A critical review. Energy Convers. Manag. 2024, 321, 119037. [Google Scholar] [CrossRef]
- Frondini, F.; Caliro, S.; Cardellini, C.; Chiodini, G.; Morgantini, N. Carbon Dioxide Degassing and Thermal Energy Release in the Monte Amiata Volcanic-Geothermal Area (Italy). Appl. Geochem. 2009, 24, 860–875. [Google Scholar] [CrossRef]
- Taussi, M.; Nisi, B.; Brogi, A.; Liotta, D.; Zucchi, M.; Venturi, S.; Cabassi, J.; Boschi, G.; Ciliberti, M.; Vaselli, O. Deep Regional Fluid Pathways in an Extensional Setting: The Role of Transfer Zones in the Hot and Cold Degassing Areas of the Larderello Geo-thermal System (Northern Apennines, Italy). Geochem. Geophys. Geosyst. 2023, 24, e2022GC010838. [Google Scholar] [CrossRef]
- Fridriksson, T.; Merino, A.M.; Yasemin Orucu, A.; Audinet, P. Greenhouse Gas Emissions from Geothermal Power Production. In Proceedings of the 42nd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 13–15 February 2017; Available online: https://cekh.ccreee.org/wp-content/uploads/2021/12/Greenhouse-Gas-Emissions-from-Geothermal-Power-Production-2.pdf (accessed on 25 August 2025).
- Steingrímsson, B.S.; Ármannsson, H. An overview of carbon dioxide emissions from Icelandic geothermal areas. Appl. Geochem. 2018, 97, 11–18. [Google Scholar] [CrossRef]
- Bloomberg, S.; Werner, C.; Rissmann, C.; Mazot, A.; Horton, T.; Gravley, D.; Kennedy, B.; Oze, C. Soil CO2 Emissions as a Proxy for Heat and Mass Flow Assessment, Taupō Volcanic Zone, New Zealand. Geochem. Geophys. Geosyst. 2014, 15, 4885–4904. [Google Scholar] [CrossRef]
- Lasco, R.D.; Lales, J.S.; Arnuevo, M.T.; Guillermo, I.Q.; De Jesus, A.C.; Medrano, R.; Bajar, O.F.; Mendoza, C.V. Carbon Dioxide (CO2) Storage and Sequestration of Land Cover in the Leyte Geothermal Reservation. Renew. Energy 2002, 25, 307–315. [Google Scholar] [CrossRef]
- Bettagli, N.; Bidini, G. Larderello-Farinello-Valle Secolo Geothermal Area: Exergy Analysis of the Transportation Network and of the Electric Power Plants. Geothermics 1996, 25, 3–16. [Google Scholar] [CrossRef]



| Chemical Species | Molar Mass (g/mol) | Moles [mol/s] | Mass Flow Rate [kg/s] | LHV [kJ/mol] | LHV [MJ/kg] |
|---|---|---|---|---|---|
| H2O | 18.02 | 22.73 | 0.41 | ||
| CO2 | 44.01 | 22.73 | 1 | ||
| CO | 28.01 | 22.73 | 0.637 | 283.0 | 10.1 |
| H2 | 2.02 | 22.73 | 0.046 | 241.8 | 120 |
| Number of Electrons [mol/s] | Total Charge [C/s] | Minimum Cell Potential, E [V] | P = Q × E [kW] |
|---|---|---|---|
| 90.92 | 8.76 × 106 | 1.46 | 12,680.9 |
| Parameter | Water Electrolysis (H2O → H2 + ½O2) | Co-Electrolysis (CO2 + H2O → CO + H2) |
|---|---|---|
| Reactants required | ~9 kg H2O | ~9 kg H2O + ~22 kg CO2 |
| Overall reaction | H2O → H2 + ½O2 | CO2 + H2O → CO + H2+ O2 |
| Technology used | PEM/Alkaline/SOEC | SOEC |
| Operating temperature | 60–80 °C (ALK, PEM); 700–850 °C (SOEC) | 700–850 °C |
| ΔG° | ~237 kJ/mol H2 | ~257 kJ/mol H2 |
| Minimum energy required | 39.6 kWh/kg H2 | 42.7 kWh/kg H2 |
| Electrical energy input | 55–60 kWh/kg H2 | 79–84 kWh/kg H2 |
| Thermal energy input (net) | ~0–1 kWh/kg H2 | ~5–10 kWh/kg H2 |
| Total energy required | ~55–61 kWh/kg H2 | ~84–94 kWh/kg H2 |
| Main product gas | H2 | Syngas (H2 + CO) |
| CO2 processed per kg of H2 | None | ~22 kg |
| Efficiency (on LHV basis) | ~60–70% | ~45–50% |
| Efficiency of Co-Electrolysis | Electricity Required [kWh] | SEC for Hydrogen Production [kWh/kg] |
|---|---|---|
| 70% | 9100 | 77.8 |
| 50% | 12,800 | 100.2 |
| 30% | 21,330 | 151.7 |
| Operating Temperature [°C] | Estimated Perspective Efficiency [%] |
|---|---|
| 50–80 | 20–30 |
| 200 | 30–40 |
| 400 | 40–50 |
| 600 | 50–60 |
| 800 | 60–70 |
| Factor | High-Temperature Co-Electrolysis | Low-Temperature Co-Electrolysis |
|---|---|---|
| Energy Requirement | ~3.0–4.5 MWh/tCO2 (electrical) + thermal input (~2 MWh/tCO2). | ~5.0–6.5 MWh/tCO2 (entirely electrical) |
| Reaction Kinetics | Fast kinetics at 700–850 °C (current densities up to 1.5–2.5 A/cm2). | Slow kinetics at <150 °C (current densities typically <0.5 A/cm2) |
| Electrolyte Conductivity | Solid oxide (e.g., YSZ) with oxygen ion conductivity ~0.1–0.2 S/cm at 800 °C. | Low proton or ion conductivity (~10−3–10−2 S/cm), leading to high ohmic losses |
| Water Phase | Requires steam (≥600 °C); utilizes heat from industrial integration | Requires additional energy to convert liquid water into steam (parasitic losses). |
| Overall Efficiency | 50–60% (electrical-to-syngas); 70–80% with heat recovery | <40% typical; high overpotentials |
| Cost Reduction | Stack cost: >1500 €/kW; high due to ceramics and balance of plant | Stack cost potentially <800 €/kW; lower materials cost but lower performance |
| Research Area | Key Challenges | Potential Solutions and Directions |
|---|---|---|
| Electrode Materials and Catalysts | Stability and durability: operation >1000 h under relevant conditions; resistance to anode/electrolyte interface degradation | Advanced perovskite oxides, transition metal doping, nanostructured catalysts |
| Electrochemical Performance | Efficiency and performance: high overall and Faradaic efficiency; current densities >1 A/cm2 with favorable reaction kinetics | Optimized doping, interface engineering, enhanced electrode architecture |
| Temperature Optimization | Operating conditions: elevated temperatures (700–900 °C) leading to high thermal energy demand, material stress, and complex thermal management | Exploring lower temperature SOECs, hybrid approaches integrating PEM technology |
| H2/CO Ratio Control | Process integration: precise tuning for downstream applications (e.g., Fischer–Tropsch synthesis) | Adjusting feed gas composition, operating voltage, and catalyst properties |
| System Scalability | Scalability: transition from laboratory (~10 kW) to industrial-scale systems (>100 MW) | Modular SOEC stacks, integration with renewable energy sources |
| Integration with Industrial Processes | System compatibility: integration with CO2 separation processes requiring high purity (>95%), with corresponding sizing and energy needs | Direct syngas utilization, coupling with carbon capture and utilization (CCUS) technologies |
| Parameter | Value |
|---|---|
| Annual Cement Production | 1,000,000 tons/year |
| Daily Production | 1,000,000 tons ÷ 365 days = 2740 tons/day |
| Hourly Production | 2740 tons ÷ 24 h = 114.17 tons/h |
| CO2 Emissions from Calcination | 0.68 × 114.17 tons = 77.76 tons CO2/hour |
| Annual CO2 Emissions | 77.76 tons/hour × 24 h × 365 days = 681,000 tons CO2/year |
| Parameter | Value |
|---|---|
| Natural gas treated (per day) | 2,000,000 m3 |
| Initial CO2 content (6% of total) | 120,000 m3 |
| CO2 separation efficiency | 85% |
| CO2 separated (per day) | 102,000 m3 |
| CO2 residual (in treated gas) | 18,000 m3 |
| CO2 separated (per year) | 37,230,000 m3 (approx. 37.23 million m3) |
| CO2 separated (mass per day) | 102,000 m3 × 1.977 kg/m3 = 201,654 kg (201.65 tons) |
| CO2 residual (mass per day) | 18,000 m3 × 1.977 kg/m3 = 35,346 kg (35.35 tons) |
| Geothermal Field | CO2 Concentration (Weight %) | CO2 Emissions (kg/MWh) | References |
|---|---|---|---|
| Monte Amiata (Italy) | ~5–8% | 250–520 | [37] |
| Larderello (Italy) | ~1–5% | Lower than Monte Amiata | [38] |
| The Geysers (USA) | ~0.5–2% | ~40–100 | [39] |
| Krafla (Iceland) | ~0.5–1.5% | ~10–50 | [40] |
| Taupo Volcanic Zone (NZ) | ~2–6% | ~100–300 | [41] |
| Philippines Fields | ~1–4% | ~50–200 | [42] |
| Point | ṁ [kg/s] | P [bar] | T [°C] | x CO2 |
|---|---|---|---|---|
| 1 | 111.11 | 5.00 | 195 | 5.0 |
| 2 | 111.11 | 0.08 | 41 | 5.0 |
| 3 | 7.65 | 0.07 | 26 | 72.6 |
| 4 | 7.65 | 0.272 | 177 | 72.6 |
| 5 | 6.10 | 0.260 | 33 | 91.1 |
| 6 | 6.10 | 1.013 | 176 | 91.1 |
| Scenario | Net Power | Energy Req. per Second | H2 Prod. | H2 Daily | CO2 Converted | CO2 Converted | CO Prod. |
|---|---|---|---|---|---|---|---|
| Full Power | 53 MW | 14.7 kWh | 0.147 kg/s | 12.7 t/day | 3.2 kg/s | 54% | 2.0 kg/s |
| 50% Power | 26.5 MW | 7.35 kWh | 0.073 kg/s | 6.3 t/day | 1.6 kg/s | 27% | 1.0 kg/s |
| 20% Power | 10.6 MW | 2.94 kWh | 0.029 kg/s | 2.5 t/day | 0.64 kg/s | 10.8% | 0.40 kg/s |
| Criterion | Quantitative Indicator | Unit | Normalization (0–3) | Weight [%] |
|---|---|---|---|---|
| CO2 availability | Capture potential × stream purity | tCO2/h, % | 0: <0.2 t/h and purity < 80% 1: 0.2–2 t/h 2: >2 t/h 3: >2 t/h and purity > 95% | 25 |
| Heat integration | Recoverable thermal power at T > 650 °C | MWth | 0: none 1: <5 MWth 2: 5–20 MWth 3: >20 MWth | 20 |
| Electricity cost/availability | Average cost or renewable LCOE | €/MWh | 0: >90 €/MWh 1: 70–90 €/MWh 2: 50–70 €/MWh 3: <50 €/MWh | 15 |
| Syngas demand | Substitutable demand vs. fossil feedstocks | MWh/year | 0: none; 1: <10%; 2: 10–30%; 3: >30% of internal demand | 15 |
| Operational flexibility | Load modulation range or storage capacity | % load range | 0: <20% 1: 20–40% 2: 40–60% 3: >60% plus storage capability | 10 |
| Utilities and space | Availability of water, oxygen handling, footprint | Qualitative | 0: critical 1: limited 2: adequate 3: abundant | 5 |
| Policy/incentives | Value of existing schemes | €/tCO2 | 0: <30 €/t 1: 30–50 €/t 2: 50–80 €/t 3: >80 €/t | 10 |
| Total score | — | — | Weighted sum | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Miserocchi, L. Hydrogen via Co-Electrolysis of Water and CO2: Challenge or Solution for Industrial Decarbonization? Hydrogen 2025, 6, 60. https://doi.org/10.3390/hydrogen6030060
Franco A, Miserocchi L. Hydrogen via Co-Electrolysis of Water and CO2: Challenge or Solution for Industrial Decarbonization? Hydrogen. 2025; 6(3):60. https://doi.org/10.3390/hydrogen6030060
Chicago/Turabian StyleFranco, Alessandro, and Lorenzo Miserocchi. 2025. "Hydrogen via Co-Electrolysis of Water and CO2: Challenge or Solution for Industrial Decarbonization?" Hydrogen 6, no. 3: 60. https://doi.org/10.3390/hydrogen6030060
APA StyleFranco, A., & Miserocchi, L. (2025). Hydrogen via Co-Electrolysis of Water and CO2: Challenge or Solution for Industrial Decarbonization? Hydrogen, 6(3), 60. https://doi.org/10.3390/hydrogen6030060







