1. Introduction
The amount of power originating from the sun and intercepted by the earth’s surface is immense, at the order of 1.73 × 10
8 GW [
1,
2]. Of this amount, 1.20 × 10
8 GW is absorbed by the earth, with the rest being radiated back into space for a net average power input of about 342 W per m
2 on the earth’s surface. By comparison, the amount of power currently used by mankind is about 13,000 GW, or 0.01% of the received sun’s power. This implies that the received sun power on the earth should be sufficient to supply 10,000 times the needs of humanity. This also implies that the sunlight that reaches the earth everyday dwarfs all the planet’s other energy sources employed by mankind today and is clearly sufficient in scale to meet all of mankind’s energy needs, essentially, in perpetuity.
The retention of the sun’s energy on the surface of the earth, including its atmosphere, manifests itself in numerous ways that are conducive to associated capture and utilization mechanisms. These ways comprise, among others, direct solar radiation, wind, rain fall, ocean tides, and biomass. However, the immense amount of solar power received by the earth has a relatively low density, as already indicted, and is also intermittent, requiring the development of specific harnessing methods along with suitable storage in a cost-effective manner. Thus, direct solar radiation can either be converted to electricity via photovoltaics or supply domestic hot water, while wind can be converted to electricity via wind turbines. Likewise, rainfall can be and has been collected for a very long time behind dams in rivers and lakes to be used to generate electricity. However, hydropower requires specific geological formations to be harnessed and consequently has a limited potential, most of which has already been exploited around the globe. Biomass constitutes a large component of the sun’s energy; it can be harvested out of a variety of crops; and it has been used historically to generate electricity and more recently a variety of renewable fuels such as bioethanol, biomethane, or renewable natural gas (RNG) and biodiesel. Because biomass can be stored after being harvested in a fashion similar to fossil fuels, it does not present any utilization issues other than the costs involved. Solar photovoltaic power and wind power also constitute a large component of the available sun energy, but their occurrence, more so than that of biomass, is highly dependent on geographic and climatic conditions. The intermittent occurrence of direct solar radiation (only daytime) and of winds clearly suggests that the generation of electricity out of these two sources cannot match the power demands. Consequently, the need for storage becomes critical for solar PV and wind power, as we will address shortly.
A traditional thermal power plant employing fossil fuels as the energy source, such as coal, oil, and natural gas, as well as a nuclear power plant typically have an operational capacity factor measured as the percent of the number of hours in a year (8760 h) that are generating power at its name plate capacity. Thus, a power plant with a name plate capacity of 1 GW and an annual operating capacity factor of 90% would generate 7884 GWh in that year. The actual operating capacity factor of a thermal power plant would be a function of its need to produce and dispatch power into the grid on a demand basis as well as its state of maintenance and fuel supply status, both of which are dependent on the operator of the plant. On the other hand, a renewable power plant instead has a natural capacity factor, which is entirely dependent on the availability of the natural source of energy and independent of the grid demand and the plant operator. As it turns out, natural capacity factors for solar PV plants can be in the 15–25% range, while for wind power plants, they can be in the 25–35% range, the range being the result of the prevailing climatic conditions at the power plant site. For obvious economic reasons, the implementation of solar PV and wind power plants attempts to utilize the most favorable climatically sites initially and proceeds to the less favorable ones over time. This is, for example, the major driving force for offshore wind vs. onshore wind despite the much higher capital cost of the former vs. that of the latter.
The most recent annual (2023) statistics of power generation characteristics, including average operational and natural capacity factors obtained, are summarized for the USA and Germany in
Table 1 and
Table 2, respectively [
3,
4]. These countries have been selected because both represent very large and highly industrialized economies following distinctively different pathways to the utilization of energy sources to supply electricity, including a large and ever-increasing utilization of renewable energy.
Several observations pertaining to the USA power sector can be derived from the data in
Table 1. Nuclear power shows a capacity factor of over 90%, signifying that nuclear power plants operate at a maximum practical capacity, essentially as baseload power plants, given their high cost per GW installed and slower response to output fluctuations to match the grid demand. Natural gas plants in a combined cycle configuration, which have a conversion efficiency of fuel to electricity of over 60%, have a capacity factor of just under 60%, and are also used as baseload power plants, have high flexibility to adjust the output to the demand by their nature. We may also note that in 2013, when coal was the predominant fossil fuel for baseload power generation in the USA, the coal capacity was 302 GW, and the respective capacity factor was 59.4% [
3]. Natural gas plants in a single mode, with a conversion efficiency of fuel to electricity of slightly over 40%, are only employed in a standby mode to meet daily and seasonal grid demands, such as in the summer months. Consequently, the capacity factor of these natural plants is quite low. Renewable energy plants composed of hydro, wind, and solar have natural capacity factors ranging from about 34% to 32% to 20%, respectively. Biomass plants, which employ burning mostly wood but also waste and landfill gas, have a capacity factor of over 60%, which is consistent with natural gas baseload plants, as already discussed. We may also note that as of 2023, there were 47 GW of small-scale solar PV in the US, i.e., non-utility, generating an estimated 74,000 GWh of electricity to reduce on-site demand through net-metering, which is the approach for site solar employed in the USA [
3]. Thus, small-scale solar PV has an average natural capacity factor of 18% and will offset 0.17% of annual electricity use in 2023. We may also note that in 2023, the production of energy in the USA consumed 31,290,105 GWh of primary energy, of which 11,386,482 GWh (37.75%) was used to generate the 4,178,000 GWh of delivered electricity for a conversion efficiency of 36.69% of the primary energy [
5]. However, 897,000 GWh of the generated electricity (21.5%) came from renewable resources, as the data in
Table 1 suggest.
Several observations pertaining to the power sector in Germany can be derived from the data in
Table 2. Nuclear power, even though it was totally phased out in 2023, still shows a capacity factor of almost 85%, and, in fact, it has had capacity factors of around 90% in earlier years for the reasons already discussed [
4]. Renewable electricity generation from solar PV, onshore and offshore wind, hydro, biomass, geothermal, and renewable wastes accounted for about 56% of the total generation in 2023, and thus, for the first time in the history of the country, it exceeded the 50% mark [
4]. The capacity factor for solar PV was about 9% in 2023 and was down from 11% in 2015, most likely due to (a) natural climate variability from year to year and (b) the progressive exploitation of less sunny sites [
4]. It should be noted that site-generated solar PV contributes to the overall installed power of the country due to the separate metering of it from the metering of site consumption and the differential pricing between the two in Germany. The capacity factor of onshore wind has apparently remained almost constant since 2015, albeit still being slightly lower [
4]. However, the capacity factor of offshore wind at 30.4 in 2023 was significantly lower than that in 2015, when it stood at well over 40%, the decrease most likely being attributable to the confluence of (a) annual climate variability and (b) the selection of less windy sites as the development of the offshore wind resource progresses. The capacity factors for biomass and renewable wastes are around 55%, which is consistent with what one would expect. Coal (lignite and hard coal) still accounts for the largest fossil fuel generation in the country. This makes economic sense given that coal is indigenous and abundant in Germany. However, a capacity factor of 42% suggests the utilization of fuel, mostly for baseload generation. On the other hand, natural gas, which is imported, accounts for slightly less than half of electricity generation compared to that of coal. With a capacity factor approaching 24%, natural gas is employed in some baseload generation and predominantly in peak-load generation. Lastly, we may note that the installed renewable power represents almost two-thirds (65.3%) of the total installed power, while the installed wind and solar PV power reflect 58.3% of the total power. It should be noted that electricity generation in Germany stood at 612,000 GWh prior to the severe disruption of imported natural gas from Russia, following the implementation of EU sanctions in 2022. Moreover, the ratio of about eight between the production of electricity in the USA and Germany can be explained as composed of higher energy efficiency by roughly a factor of two and a smaller population by a factor of four of the latter to the former.
The primary energy sources employed in the USA in 2023 are summarized in
Table 3. We note that natural gas accounts for about 45% of the total primary energy, including natural gas liquids (ethane, propane, and butane) that are extracted from natural gas production. Renewable primary energy accounts for 7.63% of the total production and is just almost as much as the production by nuclear power. Almost 70% of the renewable energy is due to biomass, and, in turn, almost 90% of the biomass energy represents the production of biofuels (ethanol, biodiesel, and renewable natural gas). However, less than 1/10th of the primary energy used comes from renewable sources. The primary energy sources utilized in Germany are also summarized in
Table 3. Crude oil employed essentially in road transport accounts for 40% of the primary energy use. Given the lack of indigenous oil and the relatively short travel distances encountered in Germany, a very high degree of electrification in personal transport, much more so than in the USA, makes good sense. Natural gas is the second largest primary energy source, accounting for almost 1/3 of the total use. Biomass use is roughly evenly divided between power and heat on the one hand and biofuels (mostly biodiesel) on the other [
5,
6]. Thus, slightly over 10% of the energy production/utilization in Germany comes from renewable sources.
The three major takeaways from
Table 3, which are applicable to both the USA and Germany, are as follows: (a) crude oil, which is essentially employed in transportation, accounts for a large component of primary energy and would require a very significant development of non-fossil energy sources to be replaced in the future, either by electricity or a combination of electricity and a fuel, such as green hydrogen or bio-methane (renewable natural gas); (b) biomass, essentially in the form of biofuels, represents an extremely promising large component in terms of its potential as a renewable energy source, notwithstanding that wind and solar are extensively being promoted in the public sphere; and (c) natural gas, which represents an extremely important but mostly underappreciated carrier of energy in a large economy with the attendant infrastructure, has therefore a huge potential in a renewable-energy-based economy as we will examine in the remainder of this article.
The intermittent nature of wind and solar PV generation would require the availability of storage to ensure the effective utilization of these renewable resources when fossil-fuel-powered peak power plants are no longer in operation. Moreover, a future efficient form of storage would also make the electrification of the transportation sector more sustainable. For the purposes of renewable electricity generation, one must differentiate among three types of storage as follows {7}: (a) short-term storage on the order of seconds to minutes as generated wind or solar electricity is injected into the grid to keep it balanced, i.e., stable; (b) intermediate storage to balance daily peak and low loads on the grid; and (c) long-term storage, also designated as long-duration energy storage (LDES), to account for seasonal as well as annual climatic variability [
7,
8,
9]. Thus, one study suggests that, based on computer simulations employing up to 30 years-worth of wind and solar radiation data, the required storage can be significantly reduced if about a 20% overcapacity in GWs is installed, whereby the required storage may be as short as one week for a climate with modest seasonal variability (e.g., Australia) to as long as four weeks in a climate with high seasonal variability (e.g., the United Kingdom) [
8]. In another study, the conclusion was that LDES storage equal to 10% of the likely electricity generated annually by the installed wind and solar power would suffice in a climate such as that of the USA [
9]. These two studies offer consistent results in terms of the required storage for wind and solar electricity. For simplicity, we will employ the latter one in this article. Thus, we have calculated the required amount of energy storage for two different scenarios of renewable energy penetration by wind and solar at 50% and 75% of primary energy utilization in the example of the two economies, the USA and Germany, that we have already discussed. The remainder of the supply of non-wind and solar supply in these two scenarios would come from either 25% biomass penetration plus 25% of another non-renewable source, e.g., nuclear or fossil natural gas or even crude oil. The required primary energy use that would result under these two scenarios has been calculated and is shown in
Table 4. Three key assumptions have been made for the calculation, as follows: (a) energy consumption remains at the current levels, suggesting that efficiency will make up for increased economic activity and population growth; (b) some 50% of the current energy used for transportation will be provided by electricity, with the other 50% to be provided by bio-fuels; and (c) an average reduction in the primary energy use of fossil fuel energy for power generation and for transportation at 50% of the current levels will occur [
7,
10]. The resulting storage numbers for wind and solar required in the two economies will be further discussed in a subsequent section.
2. Biomethane from Hydrogen as the Storage Medium of Choice
Consequently, the search for a sustainable and affordable LDES solution at the grid scale is at the forefront of energy research and remains important. Several forms of LDES storage have been identified, as follows [
9]: (a) chemical, such as hydrogen or another renewable compound; (b) electrochemical, such as lithium batteries or air–metal batteries; (c) thermal, such as a high heat capacity solid or liquid element or compound maintained at a high temperature; and (d) mechanical, such as a high kinetic energy (flywheel) or potential energy (pumped-hydro or compressed air) arrangement. In all cases, the storage process would consist of three steps, as follows:
Of these four possible options, two of them, i.e., (c) and (d), can be excluded as universal LDES because (c) has a short temperature lifetime of less than a week and (d) requires geological formations that are generally not available. Option (b) is problematic because of its low energy density and need for rare elements, plus it is competing with the electrification of transportation, i.e., vehicle batteries, relying on the same materials. Option (a) appears to be the best-suited technology as a universal, i.e., widely applicable, storage technology. Up to now, hydrogen has been considered the medium of choice. However, hydrogen suffers from certain, well-known for a long time, limitations, as we will address shortly. Ammonia has been mentioned as an alternative to hydrogen for some time, but it has its own limitations that we will also address briefly as well. Thus, other chemicals must be sought as the universal storage medium for renewable energy, perhaps being more suitable. Methane appears to be a suitable chemical for the storage and transport of hydrogen following its production from wind and solar power, as we will demonstrate in the remainder of this article.
Hydrogen can readily be produced via the electrolysis of water, which is a well-established commercially available technology that has been around for over one hundred years [
11]. The required electricity is supplied by wind and solar power. This hydrogen is classified as green to distinguish it from other production processes that utilize fossil fuels, such as coal and natural gas. The water electrolysis process of choice, particularly for large hydrogen production applications, is the alkaline one based on potassium hydroxide as the electrolyte. In addition, proton or polymer exchange membranes, phosphoric acid, and solid oxide electrolytes are also available in different stages of development. Alkaline electrolysis units with a nominal capacity of up to 4920 Nm
3/h of hydrogen production are available, and plants exceeding 30,000 Nm
3/h of hydrogen output and 60 MW of input power are already operational [
12]. The typical conversion efficiencies of alkaline units range from 3.8 kWh to 4.4 kWh per Nm
3 of the hydrogen produced. Assuming an average efficiency of 4.0 kWh/Nm
3, we conclude that 1 GWh of wind and solar electricity will produce 250,000 Nm
3 of hydrogen. Hydrogen fuel has the highest energy content on a mass basis of all fuels, but it suffers from an extremely low mass density, such that its volumetric energy density is extremely low, as the data in
Table 5 clearly show [
13,
14,
15]. And volumetric energy density is what matters most when it comes to the storage and the transport of energy in general and fuels more specifically. Ammonia in its liquid form is better than liquid hydrogen but not nearly as good as liquid methane. We also note that hydrogen liquefaction theoretically uses up 10% of its energy content and is practically close to twice as much. The issue of the storage of hydrogen has preoccupied researchers for a long time, but no viable solutions have been found [
16]. As a result, ammonia has been and is considered a potential solution [
16,
17,
18,
19]. But ammonia has its own issues, including the high energy required to produce it as well as the energy required to crack it to extract the hydrogen component of it. The least energy-intensive way to produce ammonia today is via the Haber–Bosch process, which requires 7.9 kWh per kg of ammonia if the source of hydrogen is methane (fossil natural gas) [
20]. Electrochemical production processes utilizing hydrogen from water electrolysis require 9 to 11 kWh/kg of ammonia produced [
20]. Since ammonia contains about 18% hydrogen by weight, we can estimate the energy required to produce this hydrogen via electrolysis at 0.18 × 4.00 kWh/kg H
2 = 0.72 kWh. We thus conclude that the energy required to produce 1 kg of ammonia, assuming that hydrogen is already available, would be 7.1 kWh. This is well above the energy content of ammonia at 5.2 kWh per kg We may also note that the hydrogen content and therefore the mass energy density of certain ammonia-derived compounds can be higher than that of ammonia, notably ammonia borane (H
3NBN
3) at 6.5 kWh per kg, but the improvement is not sufficient to overcome the already-discussed energy issues related to ammonia Several other chemical compounds that contain hydrogen have been and are being investigated as possible hydrogen storage media. Among these, formic acid and hydrazine hydrate are featured prominently. All these chemical compounds are considered terminal hydrogen sources, mainly for vehicular applications rather than as universal media for the large-scale storage and transportation of hydrogen. Notwithstanding a number of technological challenges confronting these compounds regarding the delivery of hydrogen, which could be resolved in due time, their main drawback is the low hydrogen-based energy content compared to methane. For example, the mass energy density of formic acid (HCOOH) is 1.5 kWh/kg and that of hydrazine hydrate (N
2H
4.H
2O) is 2.7 kWh/kg. As stated already, such compounds can have specialized hydrogen applications but cannot serve in the large-scale storage and transport of hydrogen addressed in this work Consequently, while it is fine to produce ammonia as a fertilizer even at a high energy input, it makes no sense energetically to use ammonia as a universal storage and transportation medium for green hydrogen, which in turn has to be produced from water electrolysis with renewable electricity.
It is apparent that methane is an ideal medium to store and transport hydrogen in an economy that relies on renewable energy. In fact, methane (fossil natural gas) is used for the current production of (gray) hydrogen in industrial applications, and it is already the dominant energy source in developed countries, transported via a vast network of pipelines on land as a gas and by ships across the oceans as a liquid (LNG) [
21]. Given the advantages that methane offers as a green hydrogen means of storage and transport, it is curious why up to now methane has not received any significant attention. The most likely explanation is that methane contains carbon and, as such, does not fit the “decarbonization” mantra that has been prevalent in recent decades as it pertains to climate change and global warming. It is only in recent years that it has been realized that carbon cannot be eliminated from the energy economy, and the emphasis has shifted to the appropriate term of a “net zero carbon” approach to dealing with climate change. It is then, in the spirit of a “net zero carbon” economy, that methane can achieve its place as the key element to facilitate the establishment and support the permanence of a hydrogen-based renewable energy economy. In fact, the advent of the so-called “Methane Age”, whereby methane fuel became the dominant energy source globally, was forecast over forty years ago [
22]. In the intervening period, while hydrogen has received a great deal of attention in the early 2000s and in the past four years, methane has gradually become the premier energy source in the industrialized world, as the data in
Table 3 clearly suggest, albeit in its fossil form as natural gas.
Besides hydrogen, carbon is also an excellent energy carrier, either by itself in the form of coal or in combination with hydrogen in the form of hydrocarbons. The chemical combination of hydrogen with carbon results in the closer packing of the hydrogen atoms, such that the volume energy density of the compound element increases. Thus, carbon combined with hydrogen produces very versatile and highly energetic fuels that occur naturally. It has been pointed out that “if we didn’t have carbon, we would have to invent it as the ideal tool for handling hydrogen” [
7,
23].
Methane is the lightest hydrocarbon and next to hydrogen, the fuel with the highest mass energy density. It is also a gas under typical ambient environmental conditions with a high enough volume energy density to be cost-effectively transportable via pipelines. Methane can also be readily liquefied for cost-effective transportation by suitable tanker ships across the oceans. The envisioned process to employ methane as the storage and transport medium for green hydrogen is summarized in the four steps presented in
Table 6.
There are several advantages to employing methane as the storage medium for wind and solar power generation and as the transportation medium for hydrogen on land and at sea. The first advantage is that it provides the necessary energy density for renewable-based electricity and hydrogen fuel. Second, the existing infrastructure of pipelines and ships can be used, thereby eliminating the need for new, costly storage and transport systems. Third, it provides by far the most energy-efficient process to introduce green hydrogen into the economy, given that conversion energy losses in renewable energy systems are critical but by-and-large rarely discussed [
7].
The superiority of methane as a renewable electricity and green hydrogen storage and transport medium has already been demonstrated and will not be discussed any further. Incidentally, the combination of up to 20% hydrogen by volume with at least 80% methane by volume ensures that no appreciable storage or transmission volumetric energy density occurs, while the combustion efficiency of the mixture improves because of the high velocity of the ignition of hydrogen [
24,
25]. The advantages of methane fitting well into the current energy infrastructure will be addressed in the remainder of this section. Lastly, the conversion efficiency advantage of the methane medium will be discussed in the next section, along with the technologies involved in the conversion of hydrogen to methane.
Natural gas, which typically consists of 97–99% methane, is the most diversified fuel in the industrialized world, and its diversification is expanding continually around the globe. This fuel is used to provide space heating, heat domestic hot water, cook food in homes, generate electricity either as baseload or as a peak-load demand, and is used as a raw material for products, such as fertilizers and plastics and even gray hydrogen in refineries and steel manufacturing. The diversified use of natural gas results in significant seasonal variations in which consumption is highest during winter months and lowest during mild-weather months, such as in the spring and fall. Natural gas production remains relatively constant throughout the year. Consequently, natural gas storage enables the supply to match the demand on any given day of the year by adjusting daily and seasonal fluctuations. Thus, when natural gas production is higher than natural gas consumption, extra gas is stored, while when consumption exceeds production, the deficit amount of natural gas is made up by withdrawing it from storage. For example, it is estimated that 20% of the use of natural gas in the USA in the winter months comes from storage [
26]. Storage then provides the flexibility and reliability of natural gas delivery. A five-year summary of the natural gas storage record is shown in
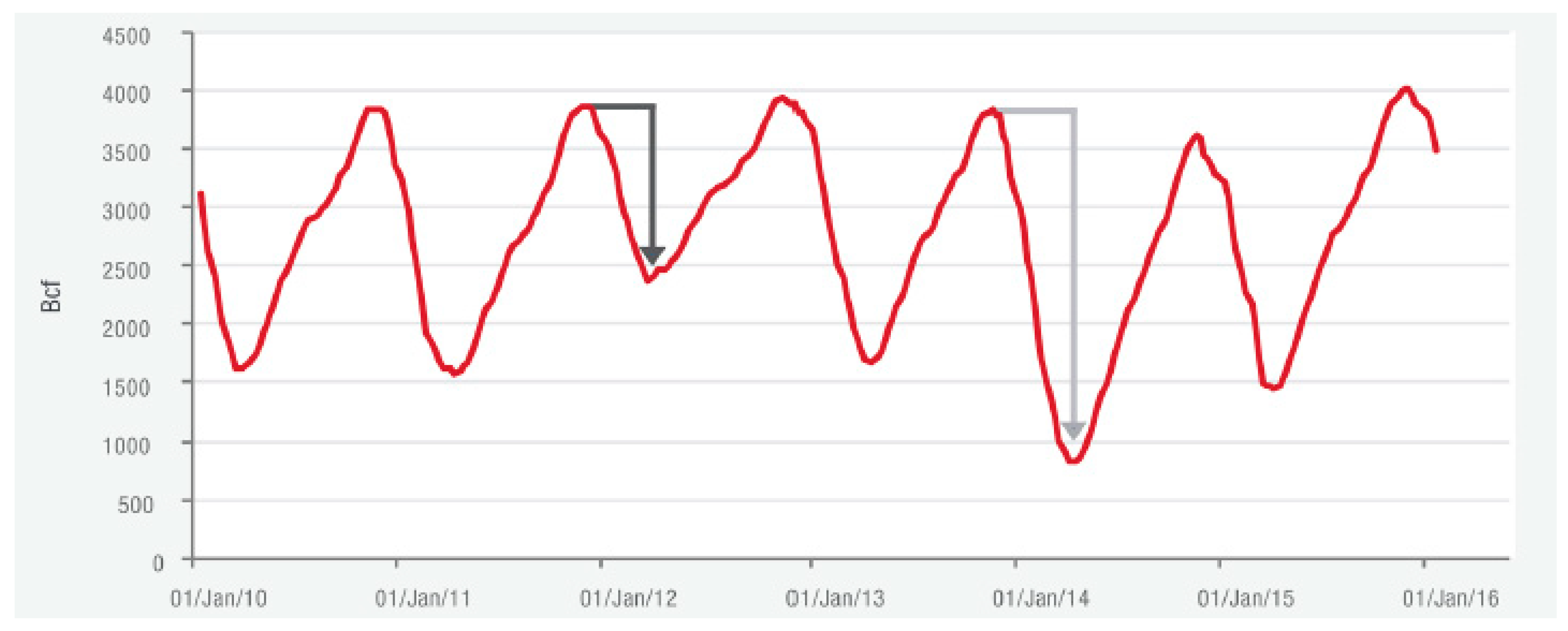
Figure 1.
Natural gas can be stored in
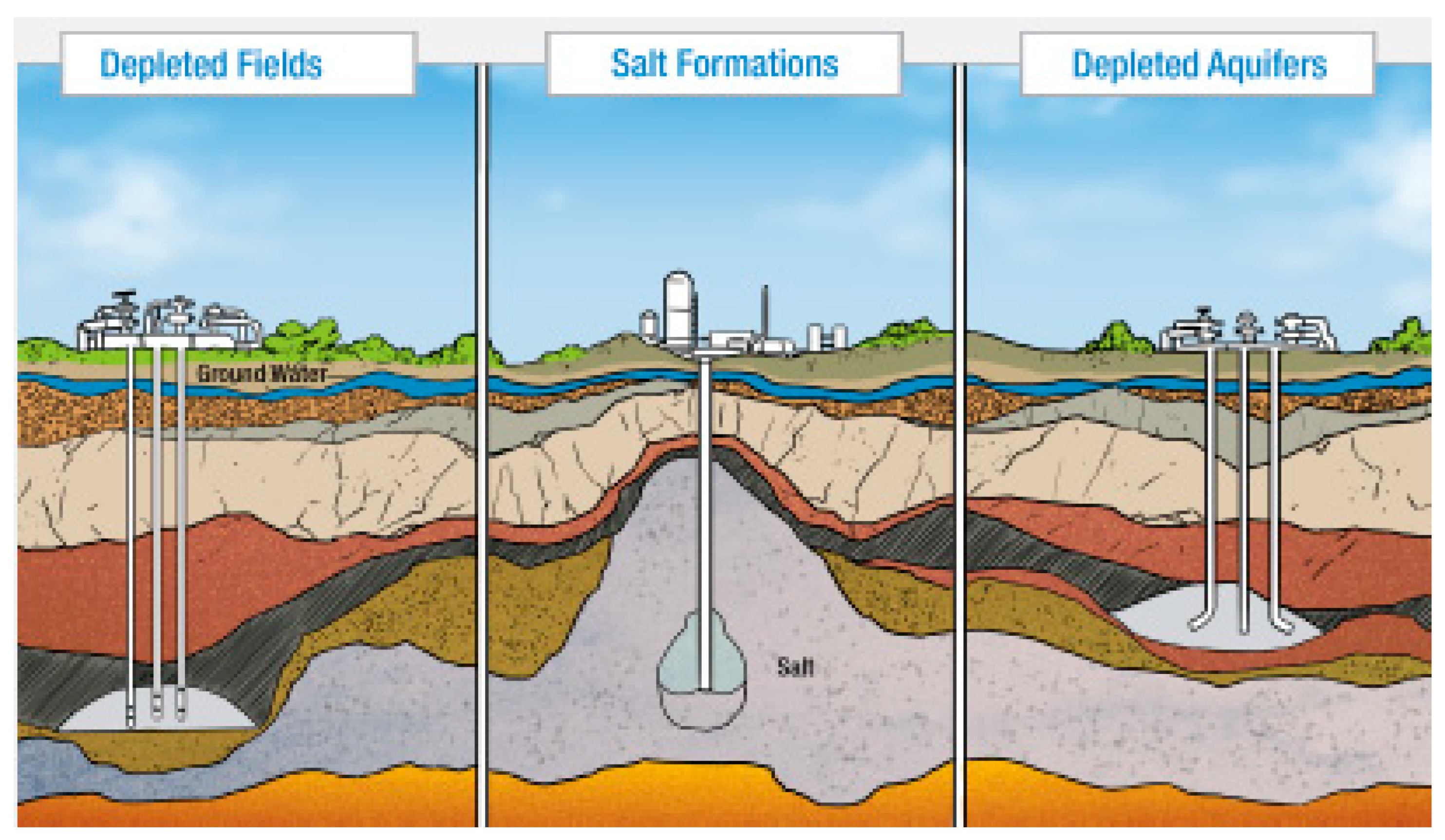
the following: (a) underground geological formations; (b) transmission pipelines; and (c) refrigerated tanks as liquid (LNG). As shown in
Figure 2, the geological formations consist of depleted oil fields, salt cavern formations, and depleted aquifers [
24].
The available volumes of natural gas storage in the USA are summarized in
Table 6. The gross volume of all the underground storage is just about 140 billion cubic meters in over 400 geological formations in 30 states [
26,
27]. Of the total volume of underground storage in the USA, 80% is in depleted oil fields, 10% is in salt formations, and 10% is in aquifers [
27,
28]. The length of the natural gas pipeline network in the USA is about 4.72 million km; it delivers almost 775 billion m
3 of natural gas annually, and it has an inherent storage capacity of about 3.5 billion m
3 [
26,
27,
28]. In 2022, there were some 180 LNG storage facilities in the USA with a total capacity of just under 10 million tons, or about 24 billion m
3 [
29]. The available volumes of natural gas storage in Germany are also summarized in
Table 6. Since Germany does not produce oil, underground storage takes place in salt caverns and depleted aquifers. The total storage capacity in 40 geological locations across the country is over 23 billion m
3 [
30]. The length of the natural gas pipeline network in Germany is about 0.53 million km, which delivered in 2023 over 77 billion m
3 of natural gas—down from 96 billion m
3 in 2021 due to the war in Ukraine—and has an estimated storage capacity of about 0.5 billion m
3 [
31,
32]. The LNG storage capacity in Germany is expected to reach 37 billion m
3 in 2024 and double by 2028 [
33].
The results in
Table 7 are instructive, as under the circumstances of the two wind and solar penetration scenarios in both the USA and Germany, there is more than an adequate existing storage capacity for natural gas to accommodate in the future the storage and transport of excess power in the form of methane. In fact, the available underground and pipeline storage capacities alone are sufficient to accommodate the 80% methane and 20% hydrogen gaseous storage mediums in both countries.
We may also note that as heat pumps gain acceptance as three- to four-times more efficient electric systems to replace natural gas for space heating and domestic hot water supply, the demand for natural gas is decreasing [
27,
34], Moreover, heat pumps are under development that can deliver (a) process heat to industrial applications at high temperatures, such as pulp, paper, and food processing, and (b) preheating for even higher temperature processes, such as cement, glass, and chemical manufacturing. A recent study suggests that electricity can supply 78% of process heat in industries using currently available technologies and up to 99% with technologies under development [
35,
36]. Thus, the use of electricity in the future will supplant the current use of natural gas in residential, commercial, and industrial applications, thereby liberating more natural gas storage capacity to be used in the future for the storage of renewable methane along with green hydrogen.
3. The Storage of Green Hydrogen in Biomethane
The utilization of fossil fuels starting in the late 19th century, coal initially, then crude oil, and now natural gas, has resulted in an increased concentration of carbon dioxide in the atmosphere. The increase in carbon dioxide concentration has accelerated in recent decades and now stands at over 400 ppm, up from a pre-industrial revolution estimated concentration of about 280 ppm [
37]. The increased atmospheric carbon dioxide concentration along with increased concentrations of methane and nitrous oxides, all classified as anthropogenic emissions, are believed to contribute to the observed increase in the average temperature of the earth (global warming) and the attendant climate change. One promising solution to reduce anthropogenic carbon dioxide emissions is methanation, i.e., the reaction of carbon dioxide with hydrogen to produce methane. The interest in carbon dioxide methanation is being advanced as a power-to-gas (PtG) technology with concurrent carbon capture and storage (CCS). Hydrogen will be produced through water electrolysis employing wind and solar power. Producing an energy carrier, i.e., methane, via the methanation of a suitable carbon source and green hydrogen is an exceptionally effective method of capturing energy generated by intermittent renewable energy sources, as we have already discussed.
The conversion of hydrogen to methane via carbon dioxide methanation occurs through the already highly developed Sabatier process. This process was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897 [
38]. It proceeds according to Equation (2) at an elevated temperature of 300 °C to 400 °C and an elevated pressure of about 30 atm in the presence of a nickel catalyst [
39,
40,
41]. A more efficient and longer lasting, albeit more expensive, ruthenium on alumina catalyst has also been proposed [
39,
42].
The conversion reaction per Equation (2) is exothermic, releasing 165 kJ/mol, or 17%, of the energy in the hydrogen, while 83% of the energy in the hydrogen is converted into energy in the methane. Thus, efficient utilization of that released heat energy for another application becomes necessary, attaining a practical 97% conversion efficiency of the energy in the hydrogen as methane fuel and process heat [
43].
The question arises as to how to obtain the carbon dioxide necessary for the reaction with hydrogen. An obvious option is to remove it from the atmosphere. However, the extremely low concentration of carbon dioxide, i.e., >400 ppm, makes the process rather unlikely to be used on a large scale. A promising option is to extract the carbon dioxide from the flue gases of power plants, where the carbon dioxide concentration may be in the range of 4 to 15% by volume. And the most effective option to obtain carbon dioxide would be to separate it from biogas (volumetric composition: ~60% CH
4, ~40% CO
2) produced from the anaerobic digestion of organic wastes, agricultural residues, energy crops, and even forestry wastes. In all these cases, the carbon dioxide can be removed via the so-called “amine gas treating or scrubbing process” [
44]. It entails the passing of the carbon dioxide containing gas through an aqueous solution of a variety of alkylamines, referred to simply as amines, that selectively capture about 90% of the carbon dioxide molecules in the passing gas. The charged carbon dioxide amine is then heated to release it and regenerate itself to be used again. Amine carbon dioxide, as well as hydrogen sulfide scrubbing, is widely used in refineries, petrochemical plants, and natural gas processing plants. However, a major drawback of the amine scrubbing process is its high energy requirements to regenerate the amine. Consequently, solid sorbents for carbon dioxide capture in power plant flue gases are being developed as more energy-efficient alternatives to amines [
45]. A promising technology nearing commercialization consists of metal –organic frameworks (MOFs), which consist of one-, two-, or three-dimensional structures comprising metallic ions, e.g., Cr, connected with polymer links. For biogas, which has a very high carbon dioxide concentration, other processes are used besides amine scrubbing, such as pressure swing adsorption (PSA) and membrane separation, to separate out an even higher percentage of carbon dioxide in the mid-nineties and above while producing a pipeline-quality natural gas with a 97+ to 99+ percent methane content. The energy required for biogas upgrading, thereby separating the methane from the carbon dioxide, is on the order of 0.50 kWh per cubic meter of the produced methane for a triple-stage membrane separation, or about 5% of the energy content of the methane [
46]. It should also be noted that the purity of the carbon dioxide, which is important for the methanation process, can exceed 95% and approach 100% content with the aforementioned two energy-efficient processes for biogas upgrading [
47].
The first industry-scale, power-to-methane plant was developed by ETOGAS G.m.b.H for Audi AG in Werlte, Germany, in 2013 [
48,
49]. The plant uses carbon dioxide separated from biogas produced in a waste-to-energy plant and hydrogen from intermittent wind power to produce carbon-neutral methane, or synthetic natural gas (SNG), as it is also called. The renewable methane, or SNG, is then fed directly into the existing natural gas grid. This plant employs 6.3 MW of wind power as an input and annually recycles 2800 tons of carbon dioxide from the biogas production. The total conversion efficiency of input wind power to methane output is estimated to be 54% and includes the production of hydrogen via electrolysis: the separation of carbon dioxide from the biogas and the conversion of both via the Sabatier process to, on average, three million cubic meters of methane annually, presumably including the methane from the waste-to-energy plant [
49,
50].
The methanation process involving green hydrogen with carbon dioxide can also take place via a biological process, whereby methanogenic archaea effect the conversion process according to Equation (2). The green hydrogen and the carbon dioxide are introduced into an anaerobic trickle bed reactor operating at a thermophilic temperature of 55 °C. Hydrogenothrophic archaea predominated by
Methanothermobacter thermoautotrophicus convert almost 99% of the carbon dioxide into a higher than 98% methane gas output [
51,
52]. The biological conversion of carbon dioxide with green hydrogen into green methane is offered commercially [
53]. One commercial facility is already in operation in Denmark [
52]. Most organic waste, which is actually a resource, along with energy crops, can be used to produce biomethane fuel via anaerobic digestion, with the carbon dioxide sequestered to be used as an industrial gas or employed, as just mentioned, to produce more biomethane [
54]. To this end, the conversion of properly treated waste wood into bio-methane could supply 25% of the current energy use globally [
55,
56].
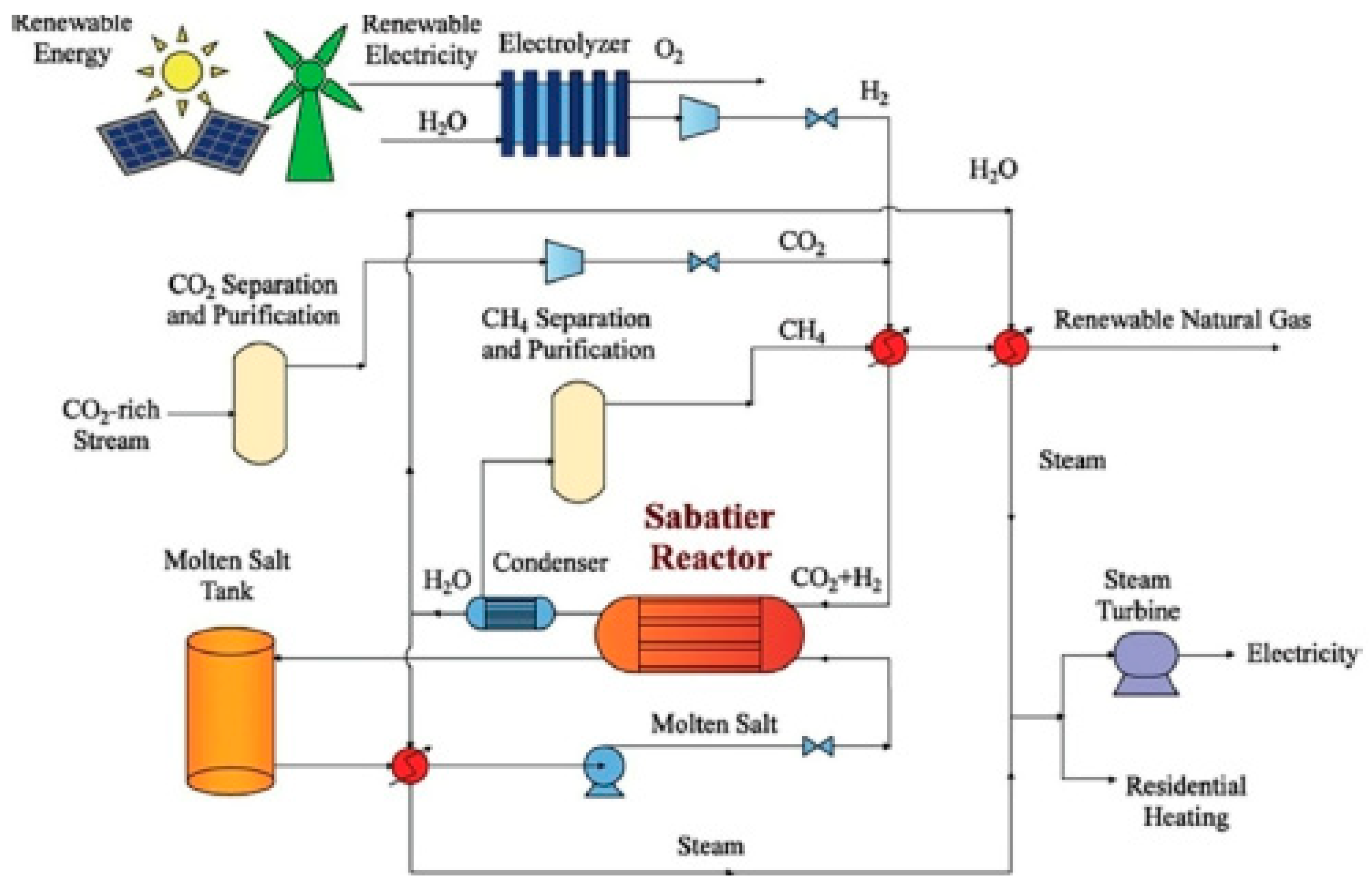
A schematic of the methanation process is shown in
Figure 3 for illustrative purposes [
57]. The carbon dioxide-rich stream would come from biogas production or power plant flue gas emissions. As already indicated, the methanation process is highly exothermic, and it is imperative that the generated heat be captured and used in a productive fashion. The generated biomethane or renewable natural gas may be used to generate power and heat as well as be used as a transportation fuel in plug-in electric vehicles [
25]. Power can be generated at the present time in a combined cycle system comprising a gas turbine operating on a mixture of biomethane with up to 20% hydrogen and a steam turbine in a second stage to obtain a conversion electrical efficiency of at least 60%, while industrial steam turbines capturing the exhaust heat as process heat can attain efficiencies of 90%. All the major gas turbine manufacturers, such as GE, Siemens, and Mitsubishi, already have systems that can operate on an 80–20 percent mixture of methane–hydrogen, respectively.
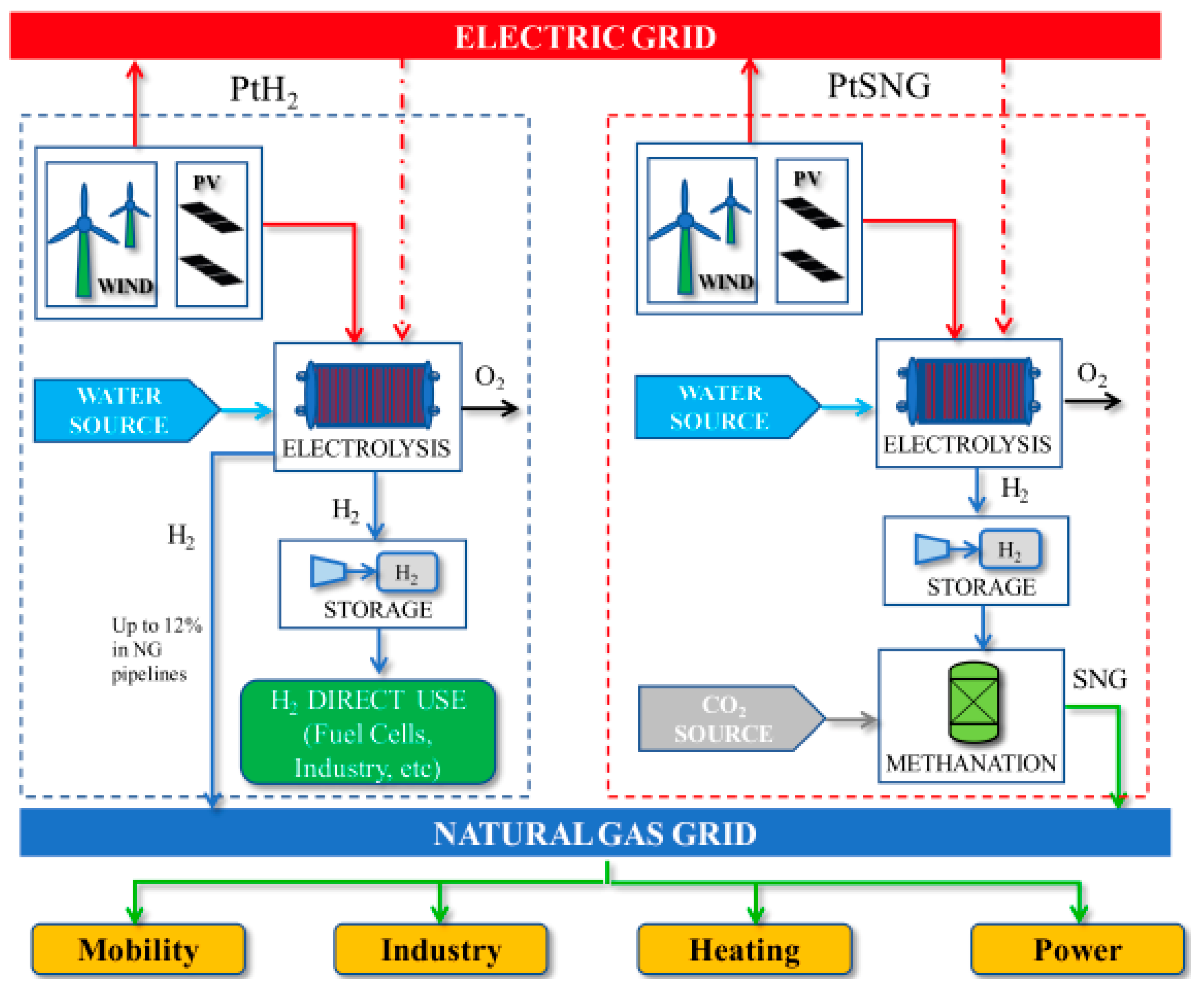
The application of the power-to-gas (PtG), generically, or the power-to-SNG (PtSNG), specifically, technology as it is known now addresses the intermittent power supply of wind and solar along with the attendant grid stability by converting electricity into green hydrogen via electrolysis and subsequent methanation of the hydrogen with sequestered carbon dioxide into RNG/SNG that is stored in the existing natural gas system. In addition, hydrogen can be directly used for industrial applications as a power-to-hydrogen (PtH2) application. These two options are summarized schematically in
Figure 4. Moreover, the sequestered carbon dioxide in the PtSNG case should be mostly separated out of the biogas obtained from the biological conversion of biomass, which comprises a large segment of the renewable energy supply, while additional renewable methane is produced. The entire process, as summarized in
Figure 4, would then be the ideal way to combine wind and solar power with biomass in the 100% or even 75% renewable energy scenario in a national economy, as discussed earlier. We may also note that while a mixture of up to 50% hydrogen with 50% methane in the existing natural gas pipeline system is feasible, safety risk concerns as well as an increased leakage rate of hydrogen would limit the amount of hydrogen up to 20% by volume [
58]. In addition, current natural gas appliances can operate with up to a 20% hydrogen content without any modifications [
58]. Lastly, hydrogen also has a high global warming potential of 37.3 over 20 years and 11.6 over 100 years compared to that of methane, which is 82.0 over 20 years and 28.6 over 100 years, on average [
59,
60]. Carbon dioxide has, by definition, a fixed global warming potential of 1.0 over 100 years [
60].
A very important consideration, to which we alluded briefly in the previous section, deals with the efficiencies involved in the various steps of the PtH2 and PtSNG processes. We have discussed some of the conversion efficiencies involved, and a comprehensive summary is presented in
Table 8 of the range of efficiencies of the current technologies [
61,
62].
The results in
Table 8 show that the current power-to-gas-storage-to power technology conversion efficiency is well below 50%, with the hydrogen-only path being slightly better than the methane-only path, while the 20% hydrogen and 80% methane mixture (RHYME) path provides a range of 31% to 40%, depending on the water electrolysis technology used and assuming a combined cycle gas turbine-generating power system. The inclusion of co-generation with the capture of waste heat raises the overall efficiency in the 43% to 56% range. But even then, the overall conversion efficiency of wind and solar intermittent renewable power is well below that of pumped hydro or battery storage, both of which have their own limitations in a large-scale application. Obviously, the two weak points in the current conversion technologies that are commercially available involving hydrogen are as follows: (a) electrolysis of water to hydrogen; and (b) hydrogen and methane fuel to power. Given that methane or SNG offers the best and only choice available for the large-scale storage of intermittent wind and solar power via the hydrogen/methane path, as we have already discussed, a new conversion technology must be developed to increase the total conversion process efficiency substantially; otherwise, the prospects for the large-scale implementation of wind and solar are highly diminished [
7].
Fortunately, there is potential to increase the efficiency of power-to-gas storage. In 2015, a study found that by using high-temperature pressurized reversible solid oxide fuel cells as solid oxide electrolysis cells (SOEC) for the electrolysis of water along with carbon dioxide into the methane/SNG process and the employment of solid oxide fuel cells (SOFC) for the conversion of methane/SNG into electricity, electricity-to-electricity round-trip efficiencies exceeding 70% can be reached at a low cost [
63,
64]. In addition, a 2019 study using high-temperature pressurized reversible solid oxide cells and a similar methodology found that round-trip efficiencies (power-to-power) of up to 80% might be feasible [
65]. Essentially, the employment of SOEC and SOFC bypasses the step of having an external production of hydrogen that traditional electrolysis and fuel cells require [
63]. Equation (3) summarizes the steps involved in the use of SOEC to produce methane/SNG from the electrolysis of carbon dioxide with water [
66,
67,
68]. Thus, a possible efficiency of 75% for the storage and recovery of intermittent wind and solar power by implementing future technology based on solid oxide electrolytes is also shown in
Table 8.
The solid oxide technology offers several advantages, as follows: (a) highest electrolysis efficiency; (b) low capital costs; and (c) integration with methanation or SNG production via waste heat recycling. However, certain disadvantages must be overcome, as follows: (a) advance technology beyond proof of concept; (b) improve solid electrolyte lifetime and stability due to the high operating temperature ranging from 500 °C to 1000 °C; and (c) address flexibility with respect to power input [
63]. Given the potential of solid oxide electrolyte technology, the European Union supported the HELMETH (Integrated
High-temperature
ELectrolysis and
METHanation for effective power to gas conversion) research project starting in 2014 under the direction of KIT (Karlsruhe Institute of Technology) in Germany [
69]. This project entailed the proof-of-concept of a highly efficient power-to-gas technology by thermally integrating high-temperature SOEC electrolysis with CO
2 methanation. The process consisted of pressurized high-temperature steam electrolysis and a pressurized CO
2 methanation module. The thermal integration of exothermal methanation and steam generation for high-temperature steam electrolysis has a theoretical conversion efficiency of over 85%, and the project achieved an efficiency of 76% for the prototype completed in 2017 with a potential 80% efficiency in industrial-scale plants [
69,
70]. The operating conditions of CO
2 methanation comprise a gas pressure of 10–30 bar, an SNG production of 1–5.4 Nm
3/h, and a conversion of reactants that produced SNG with less than 2% hydrogen and methane with over 97% volumetric composition. Thus, the generated synthetic natural gas could be injected into the natural gas network without limitations. As a cooling medium for the exothermic methanation reaction, boiling water was used up to 300 °C, the SOEC operated with a pressure of up to 15 bar, stream conversions of up to 90% were attained, and generated hydrogen with an energy input of 3.37 kWh of electricity per standard cubic meter was used as feed for the methanation. Significant improvements have been attained in the commercialization of SOEC in recent years, with an industrial-scale system already in operation as of the end of 2013 [
71,
72]. A 4 MW unit, representing just the step per Equation (3b), has been installed at NASA’s Ames Research Center in California and is generating 2400 kg of hydrogen daily at an efficiency of 89% of input electricity: 37.5 kWh per kg of H
2 versus 52 to 54 kWh per kg of H
2 for PEM and alkaline electrolysis. This installation is being monitored by the Idaho National Laboratory of US DOE to determine the durability, reliability, and overall performance of this SOEC. Obviously, the next step could be to couple this SOEC with a CO
2 source to produce SNG in accordance with the HELMETH project per Equations (3a) and (3c) to attain a projected industrial-scale 80% conversion efficiency. Alternatively, this SOEC can just provide hydrogen via Equation (3b) to be coupled with carbon dioxide via the traditional Sabatier methanation process for an increased process efficiency of around 76%, obtain the 20% hydrogen and 80% methane RHYME fuel at a combined efficiency of 0.78 to store and transmit through the natural gas storage and pipeline systems, and convert the RHYME fuel into electricity with an SOFC, obtaining a total conversion efficiency of 62% of the PtGtP process. This efficiency is composed of 88% of electricity to H
2, 78% of H
2 with CO
2 to 80 bar RHYME fuel with utilization of the exothermic heat, and 89% conversion of the latter fuel to electricity. Consequently, the necessary technology to store and transport intermittent wind and solar electricity as a synthetic or renewable natural gas fuel is within reach, as on-going research and development activities will commercialize the SOEC and SOEF technology within this decade and concurrently with the increasing deployment of wind and solar power.
4. Discussion and Recommendations
The preceding analysis has demonstrated that the high degree of penetration of renewable but intermittent wind and solar electricity, on the order of 50% to 75% of the primary energy consumed in a national economy, requires a certain amount of storage. Existing or proposed storage technologies such as pumped hydropower and compressed air storage, although highly efficient, are limited because of the lack of suitable geological sites. The other alternative of storage in electrochemical batteries, even though highly efficient, suffers from a constraint in the availability of certain materials on a global scale and would also be costly. This leads to the use of a chemical compound of some type to act as a storage as well as a transportation medium from the generation site to the end-user site of the excess renewable power—a mismatch between demand and supply. Hydrogen has been suggested as the chemical of choice, given that it contains no carbon. However, hydrogen suffers from an extremely low energy density, and no compact and efficient large-scale storage has ever been developed. Methane, on the other hand, has all the right attributes as a storage and transportation medium for excess renewable wind and solar power because of its high volumetric energy density relative to hydrogen and the ability to use the existing infrastructure of natural gas storage and pipeline systems. Consequently, an analysis of the required storage as methane of the excess wind and solar electricity in the economies of the USA and of Germany, two countries with the most advanced utilization of natural gas in their respective energy systems at the present time, was carried out, assuming that 75% of the supply of the respective primary energy needs would be met through wind and solar power and the remainder 25% by biomass. The conclusion is that both these economies already have more than adequate capacity in underground gas storage, along with developing LNG storage to meet the excess wind and solar electricity. The on-going global shift toward natural gas, along with the developing transport and storage of LNG, bodes very well for the storage and transport of excess wind and solar power as renewable electricity from these two sources makes inroads in the supply of primary energy globally. Lastly, the conversion of hydrogen to methane through the well-established and highly efficient Sabatier process would utilize carbon dioxide separated from biogas, comprising a major component of the aforementioned 25% biomass primary energy source.
The major drawback of this power-to-gas-to-power process is its relatively low conversion efficiency, ranging from at most 40% with only a power recovery to 50% with power and heat recovery employing current conversion technology. Such low conversion efficiencies do not support the penetration of wind and typically exceed 20% to 30% of electricity demand. The factors causing this low conversion efficiency are as follows: (a) the use of commercial alkaline or PEM electrolysis systems with practical electricity to hydrogen efficiencies in the low 60%; and (b) the conversion of either hydrogen or methane or hydrogen–methane mixtures to electricity via combined cycle gas turbines with efficiencies around 60% as well. A practical solution for a higher efficiency in electrolysis as well as a higher efficiency in the conversion of fuel to electricity would be the commercialization of high-temperature, pressurized solid oxide electrolysis cells (SOEC) and solid oxide fuel cells (SOFC), the former operating in the reverse mode of the latter, whereby conversion efficiencies of power-to-gas-to power of 75% can be obtained. In the past decade, extensive research both in Germany and in the USA has resulted in the advancement of SOECs. An industrial-scale 4 MW unit with an 89% electrolysis efficiency became operational in 2013. Continued commercialization efforts of solid oxide electrolysis and fuel cells are imperative if intermittent wind- and solar-obtained power is going to become, over time, the mainstream source of energy in a renewable-energy-based economy, along with the realization that renewable natural gas should be the means by which the mismatch between renewable power supply and power demand can be addressed in terms of storage and transportation.