Aluminum Cation Doping in Ruddlesden-Popper Sr2TiO4 Enables High-Performance Photocatalytic Hydrogen Evolution
Abstract
1. Introduction
2. Materials and Methods
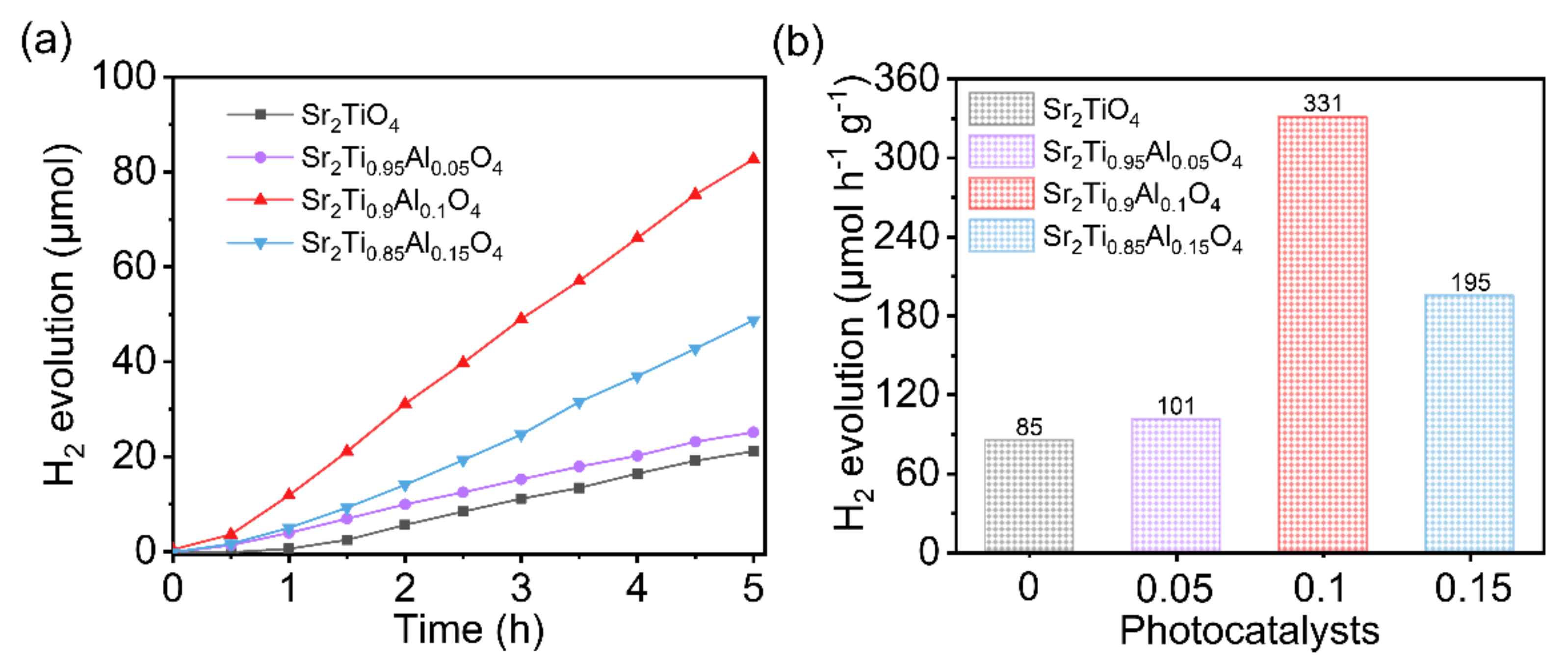
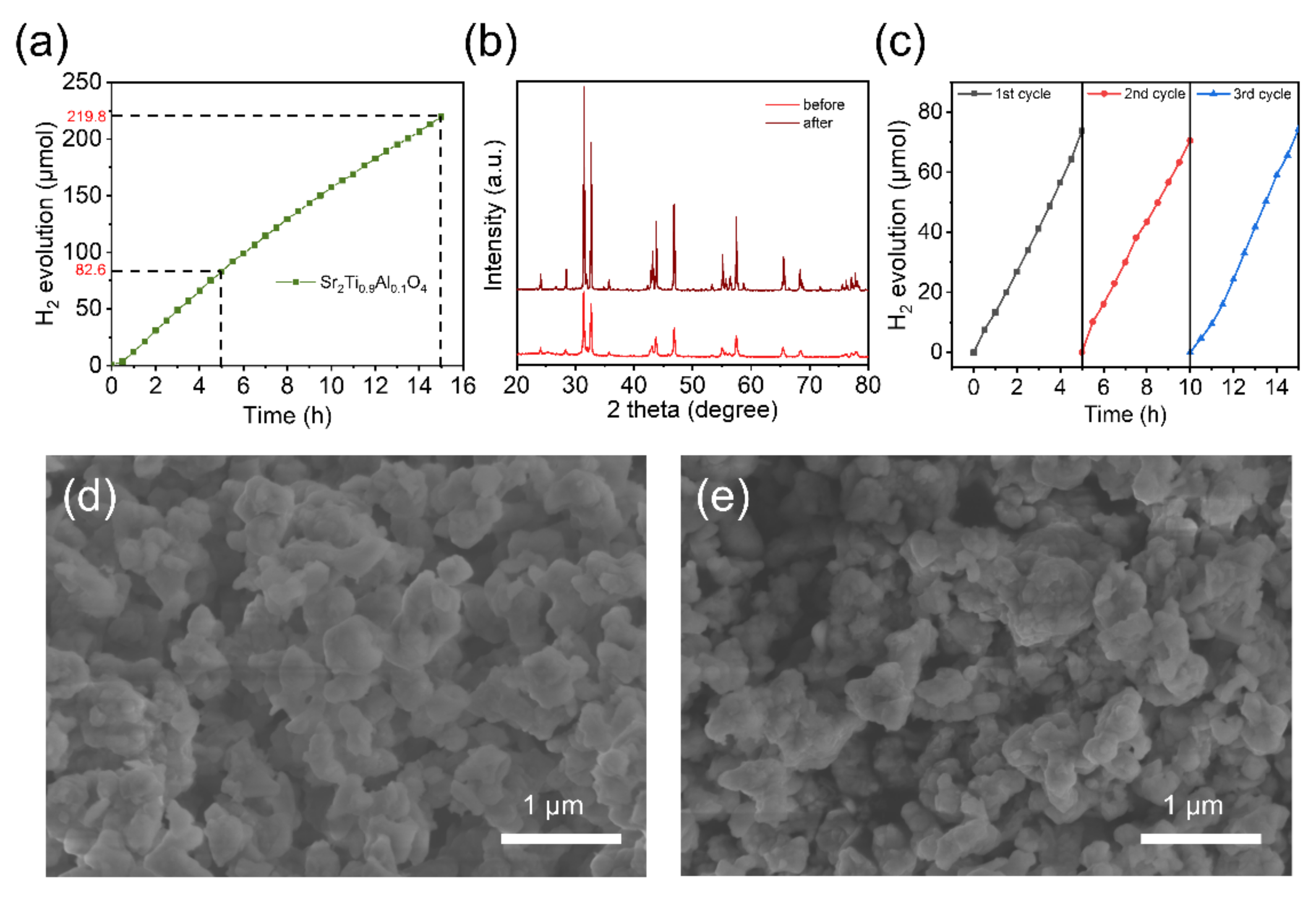
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable development using renewable energy technology. Renew. Energy 2020, 146, 2430–2437. [Google Scholar] [CrossRef]
- Razmjoo, A.; Gakenia Kaigutha, L.; Vaziri Rad, M.A.; Marzband, M.; Davarpanah, A.; Denai, M. A technical analysis investigating energy sustainability utilizing reliable renewable energy sources to reduce CO2 emissions in a high potential area. Renew. Energy 2021, 164, 46–57. [Google Scholar] [CrossRef]
- Saavedra, M.M.R.; de O. Fontes, C.H.; Freires, M.F.G. Sustainable and renewable energy supply chain: A system dynamics overview. Renew. Sust. Energy Rev. 2018, 82, 247–259. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sust. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. A review on solar energy use in industries. Renew. Sust. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A.G. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef]
- Solangi, K.H.; Islam, M.R.; Saidur, R.; Rahim, N.A.; Fayaz, H. A review on global solar energy policy. Renew. Sust. Energy Rev. 2011, 15, 2149–2163. [Google Scholar] [CrossRef]
- Tosti, S.; Pozio, A.; Farina, L.; Santucci, A. Hydrogen and oxygen production via water splitting in a solar-powered membrane reactor-A conceptual study. Hydrogen 2021, 2, 18–32. [Google Scholar] [CrossRef]
- He, J.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Single-atom catalysts for high-efficiency photocatalytic and photoelectrochemical water splitting: Distinctive roles, unique fabrication methods and specific design strategies. J. Mater. Chem. A 2022, 10, 6835–6871. [Google Scholar] [CrossRef]
- Huang, D.; Xiang, H.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in nanostructured inorganic hole-transporting materials for perovskite solar cells. Nanomaterials 2022, 12, 2592. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, M.; Xu, X.; Zhou, W.; Shao, Z. Perovskite oxide based electrodes for high-performance photoelectrochemical water Splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Two-dimensional Dion-Jacobson halide perovskites as new-generation light absorbers for perovskite solar cells. Renew. Sust. Energy Rev. 2022, 166, 112614. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Recent advances in Cs2AgBiBr6-based halide double perovskites as lead-free and inorganic light absorbers for perovskite solar cells. Energy Fuels 2020, 34, 10513–10528. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yuan, J.; Xiang, W.; Xin, X.; Liu, H.; Luo, H.; Li, L.; Chen, M.; Zhong, D.; et al. Photocatalytic optical fibers for degradation of organic pollutants in wastewater: A review. Environ. Chem. Lett. 2021, 19, 1335–1346. [Google Scholar] [CrossRef]
- Xiang, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Sodium fluoride sacrificing layer concept enables high-efficiency and stable methylammonium lead iodide perovskite solar cells. J. Mater. Sci. Technol. 2022, 113, 138–146. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, H.; Huang, J.; Zhou, C.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Thiourea with sulfur-donor as an effective additive for enhanced performance of lead-free double perovskite photovoltaic cells. J. Colloid Interface Sci. 2022, 628, 476–485. [Google Scholar] [CrossRef]
- Ahmad, K.; Ghatak, H.R.; Ahuja, S.M. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: A sustainable approach. Environ. Technol. Innov. 2020, 19, 100893. [Google Scholar] [CrossRef]
- Nasir, A.M.; Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yajid, M.A.M. Recent progress on fabrication and application of electrospun nanofibrous photocatalytic membranes for wastewater treatment: A review. J. Water Process. Eng. 2021, 40, 101878. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent progress on fullerene-based materials: Synthesis, properties, modifications, and photocatalytic applications. Materials 2020, 13, 2924. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Liu, X.; Chi, J.; Dong, B.; Sun, Y. Recent progress in decoupled H2 and O2 production from electrolytic water splitting. ChemElectroChem 2019, 6, 2157–2166. [Google Scholar] [CrossRef]
- Miseki, Y.; Sayama, K. Photocatalytic water splitting for solar hydrogen production using the carbonate effect and the Z-Scheme reaction. Adv. Energy Mater. 2019, 9, 1801294. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Recent progress in single-atom catalysts for photocatalytic water splitting. Sol. RRL 2020, 4, 2000283. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Y.-R.; Yang, H.-C. Recent developments in graphitic carbon nitride based hydrogels as photocatalysts. ChemSusChem 2019, 12, 1794–1806. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Zhang, G.; Wang, L.; Liu, Y.; Chen, W.; Chen, L. 2D covalent organic frameworks toward efficient photocatalytic hydrogen evolution. ChemSusChem 2022, 15, e202200901. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Reddy, K.R.; Shetti, N.P.; Shim, J.; Aminabhavi, T.M.; Dionysiou, D.D. Hetero-nanostructured metal oxide-based hybrid photocatalysts for enhanced photoelectrochemical water splitting—A review. Int. J. Hydrogen Energy 2020, 45, 18331–18347. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Fan, R.; Li, Z.; Zou, Z. Promotion effect of metal phosphides towards electrocatalytic and photocatalytic water splitting. EcoMat 2021, 3, e12097. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Z.; Wang, X. Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew. Chem. Int. Ed. 2019, 58, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Soto Morillo, E.; Mota Toledo, N.; García Fierro, J.L.; Navarro Yerga, R.M. Role of the sulphur source in the solvothermal synthesis of Ag-CdS photocatalysts: Effects on the structure and photoactivity for hydrogen production. Hydrogen 2020, 1, 64–89. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic water oxidation on ZnO: A review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties-A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite oxide based materials for energy and environment-oriented photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Abdi, F.F.; Berglund, S.P. Recent developments in complex metal oxide photoelectrodes. J. Phys. D Appl. Phys. 2017, 50, 193002. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; He, H.; Yang, S.; Duan, X.; Wang, S. Bismuth-based complex oxides for photocatalytic applications in environmental remediation and water splitting: A review. Sci. Total Environ. 2022, 804, 150215. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sust. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden-Popper perovskite oxides for photocatalysis-based water splitting and wastewater treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Hu, C.; Chen, T.-S.; Huang, H.-X. Heterojunction of n-type Sr2TiO4 with p-type Bi5O7I with enhanced photocatalytic activity under irradiation of simulated sunlight. Appl. Surf. Sci. 2017, 426, 536–544. [Google Scholar] [CrossRef]
- Sun, X.; Xu, X. Efficient photocatalytic hydrogen production over La/Rh co-doped Ruddlesden-Popper compound Sr2TiO4. Appl. Catal. B Environ. 2017, 210, 149–159. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Mao, M.M.; Chen, X.M.; Liu, X.Q. Structure and microwave dielectric properties of solid solution in SrLaAlO4-Sr2TiO4 System. J. Am. Ceram. Soc. 2011, 94, 3948–3952. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, K.; Li, Y.; Qi, W.; Ruan, C.; Wu, Y. Composite cathode based on Mn-doped perovskite niobate-titanate for efficient steam electrolysis. Chin. J. Chem. Phys. 2014, 27, 457–464. [Google Scholar] [CrossRef][Green Version]
- Noguera, C. Theoretical investigation of the Ruddlesden-Popper compounds Srn+1TinO3n+1 (n = 1–3). Philos. Mag. Lett. 2000, 80, 173–180. [Google Scholar] [CrossRef]
- Sun, X.; Mi, Y.; Jiao, F.; Xu, X. Activating layered perovskite compound Sr2TiO4 via La/N codoping for visible light photocatalytic water splitting. ACS Catal. 2018, 8, 3209–3221. [Google Scholar] [CrossRef]
- Shang, P.; Ye, Z.; Ding, Y.; Zhu, Z.; Peng, X.; Ma, G.; Li, D. Nanosponge-like solid solution of NiMo with a high hydrogen evolution reaction performance over a wide range of current densities. ACS Sustain. Chem. Eng. 2020, 8, 10664–10672. [Google Scholar] [CrossRef]
- She, S.; Yu, J.; Tang, W.; Zhu, Y.; Chen, Y.; Sunarso, J.; Zhou, W.; Shao, Z. Systematic study of oxygen evolution activity and stability on La1-xSrxFeO3-δ perovskite electrocatalysts in alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 11715–11721. [Google Scholar] [CrossRef]
- Kwak, B.S.; Do, J.Y.; Park, N.K.; Kang, M. Surface modification of layered perovskite Sr2TiO4 for improved CO2 photoreduction with H2O to CH4. Sci. Rep. 2017, 7, 16370. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Wang, B.; Li, S.; Jiang, B.; Yang, L.; Fu, W.; Fu, H.; Sun, J. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Han, X.; Xiang, H.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Aluminum Cation Doping in Ruddlesden-Popper Sr2TiO4 Enables High-Performance Photocatalytic Hydrogen Evolution. Hydrogen 2022, 3, 501-511. https://doi.org/10.3390/hydrogen3040032
He J, Han X, Xiang H, Ran R, Wang W, Zhou W, Shao Z. Aluminum Cation Doping in Ruddlesden-Popper Sr2TiO4 Enables High-Performance Photocatalytic Hydrogen Evolution. Hydrogen. 2022; 3(4):501-511. https://doi.org/10.3390/hydrogen3040032
Chicago/Turabian StyleHe, Jingsheng, Xiao Han, Huimin Xiang, Ran Ran, Wei Wang, Wei Zhou, and Zongping Shao. 2022. "Aluminum Cation Doping in Ruddlesden-Popper Sr2TiO4 Enables High-Performance Photocatalytic Hydrogen Evolution" Hydrogen 3, no. 4: 501-511. https://doi.org/10.3390/hydrogen3040032
APA StyleHe, J., Han, X., Xiang, H., Ran, R., Wang, W., Zhou, W., & Shao, Z. (2022). Aluminum Cation Doping in Ruddlesden-Popper Sr2TiO4 Enables High-Performance Photocatalytic Hydrogen Evolution. Hydrogen, 3(4), 501-511. https://doi.org/10.3390/hydrogen3040032






