First Hydrogenation of TiFe with Addition of 20 wt.% Ti
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
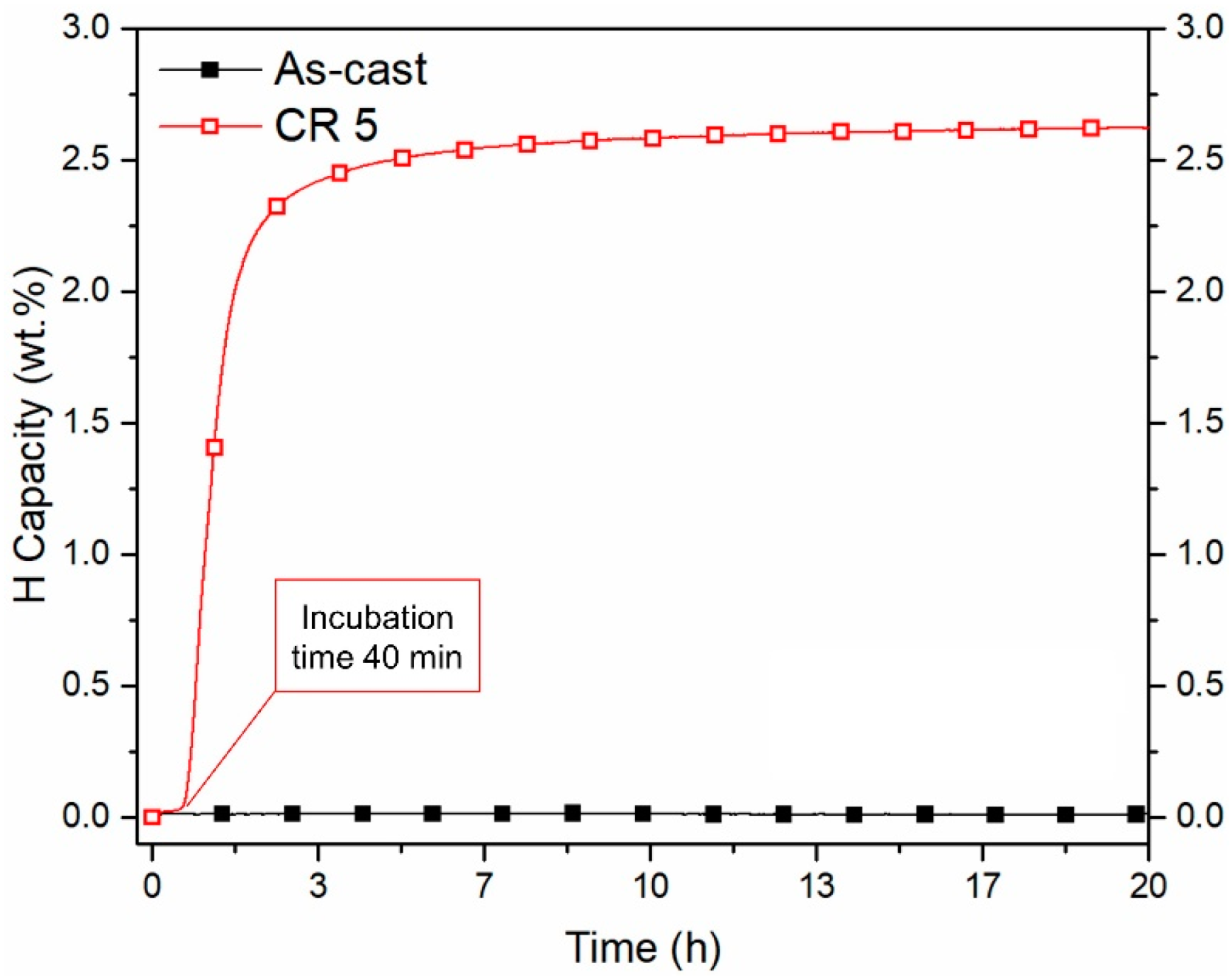
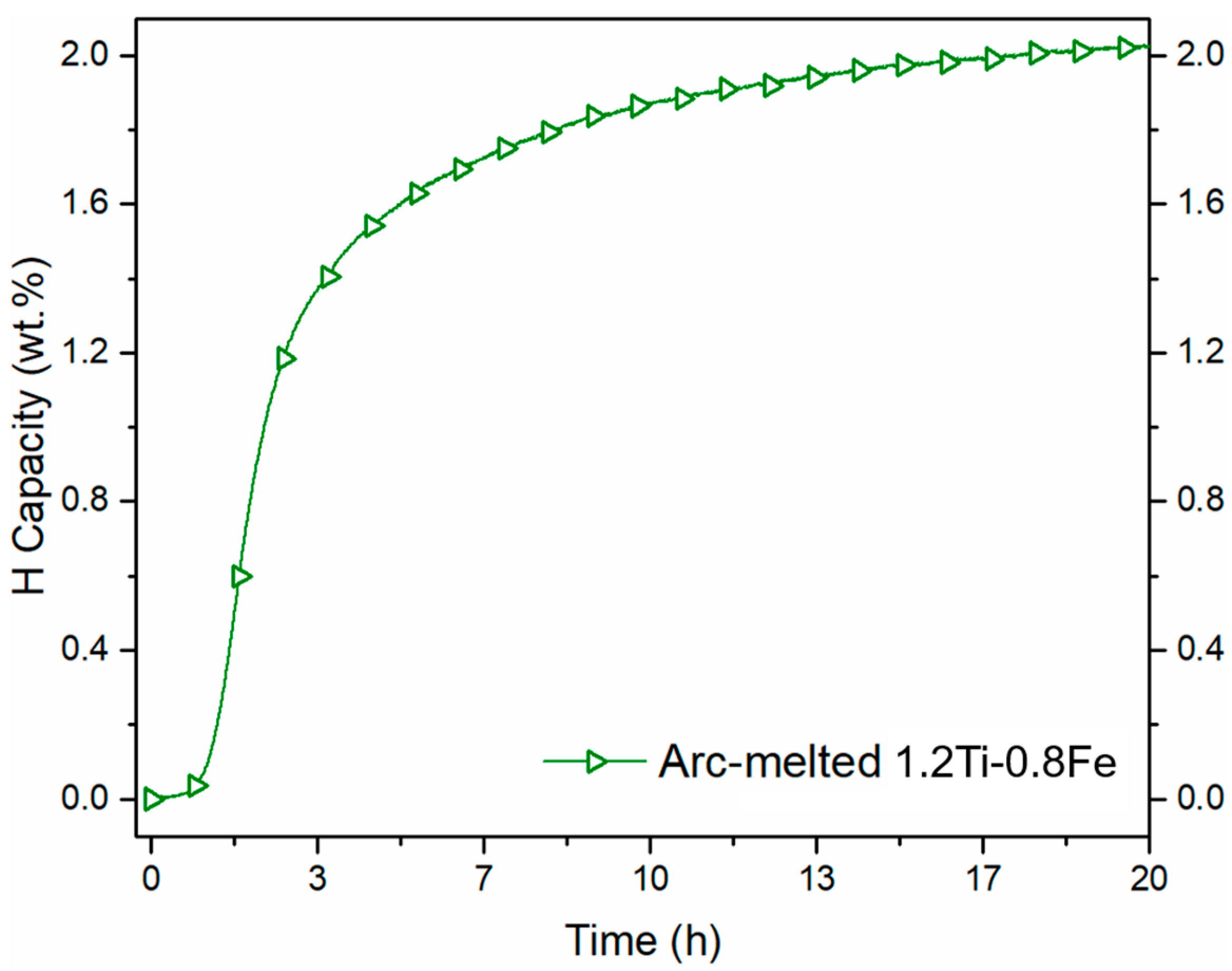
- The synthesis of a 9 kg 1.2Ti-0.8Fe ingot produced by induction casting results in an alloy that can activate after cold rolling in air without the need for an activation heat treatment.
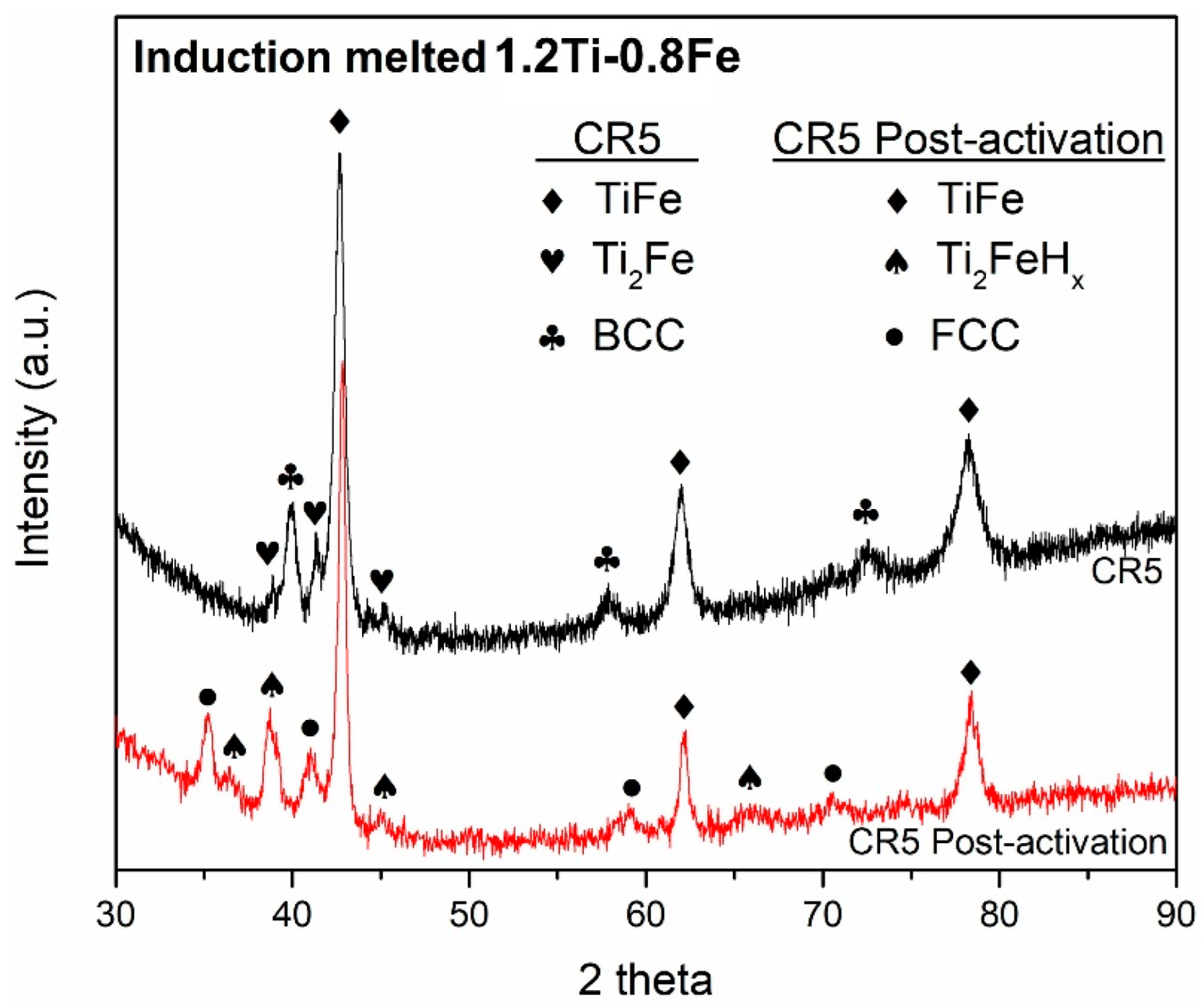
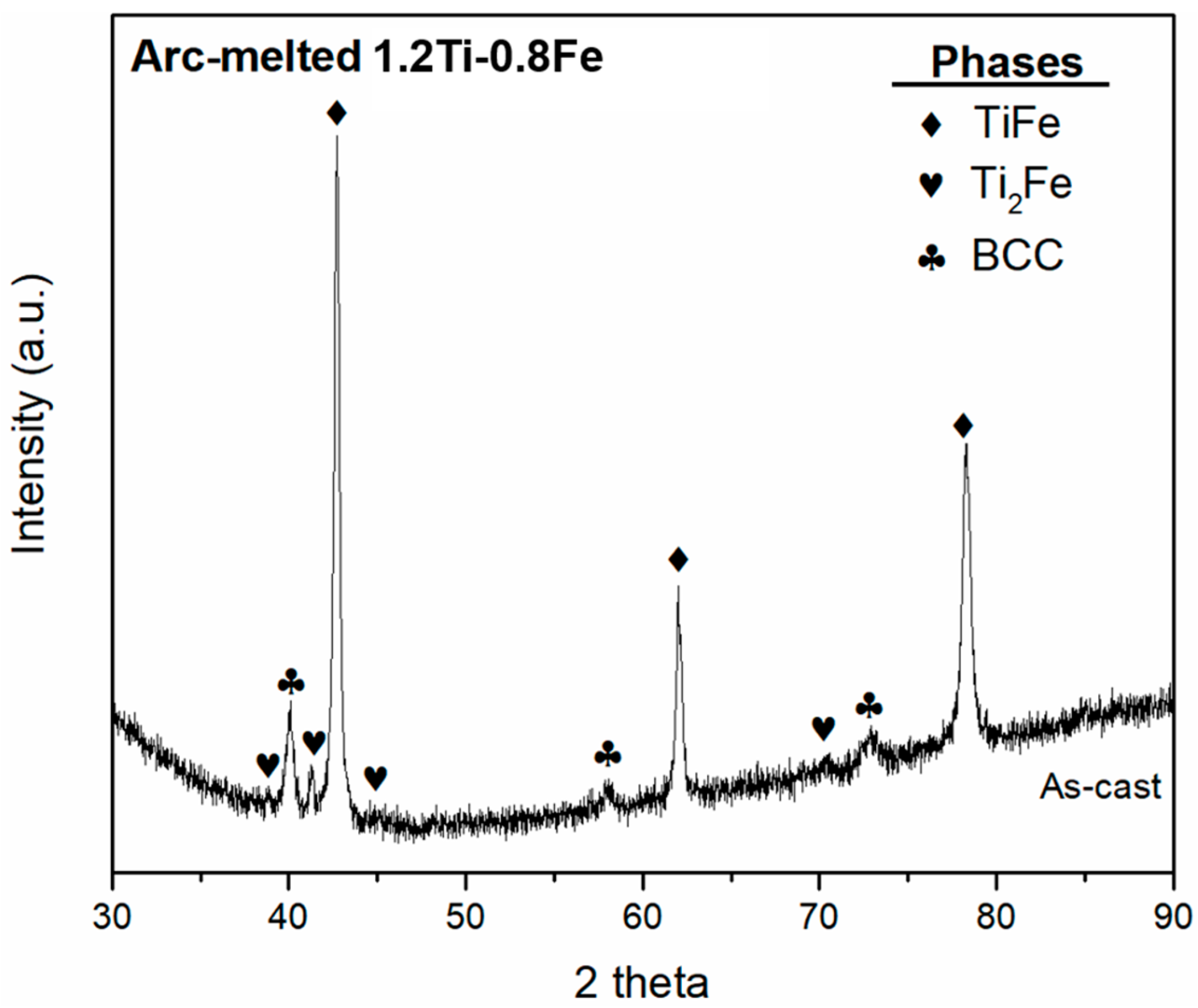
- The phases formed by synthesizing 1.2Ti-0.8Fe match the crystal structures (TiFe, Ti2Fe and BCC) of the phases previously reported when alloying TiFe with Zr and Mn. Similar to the addition of these more expensive alloying elements, the addition of Ti to TiFe resulted in the improvement of first hydrogenation kinetics.
- The finer distribution of secondary phases (Ti2Fe and BCC) in a TiFe-based alloy present a superior activation ability than that of the same composition with a coarse microstructure. This is thought to be due to the larger surface interphase between the matrix and the secondary phases.
- The three-phase system (TiFe, Ti2Fe and BCC) activates as a single-phase system regardless of the formation of two stable hydrides (Ti2FeHx and FCC).
Author Contributions
Funding
Conflicts of Interest
References
- von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Sujan, G.K.; Pan, Z.; Li, H.; Liang, D.; Alam, N. An overview on TiFe intermetallic for solid-state hydrogen storage: Microstructure, hydrogenation and fabrication processes. Crit. Rev. Solid State Mater. Sci. 2020, 45, 410–427. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Lv, P.; Guzik, M.N.; Sartori, S.; Huot, J. Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of TiFe+4 wt.% Zr alloy. J. Mater. Res. Technol. 2019, 8, 1828–1834. [Google Scholar] [CrossRef]
- Silva, R.; Lean Neto, R.L.; Leiva, D.R.; Ishikawa, T.T.; Kiminami, C.S.; Jorge, A.M., Jr.; Botta, W.J. Room temperature hydrogen absorption by Mg and Mg TiFe nanocomposites processed by high-energy ball milling. Int. J. Hydrogen Energy 2018, 43, 12251–12259. [Google Scholar] [CrossRef]
- Falcão, R.B.; Dammann, E.D.; Rocha, C.J.; Durazzo, M.; Ichikawa, R.U.; Martinez, L.G.; Botta, W.J.; Neto, R.M.L. An alternative route to produce easily activated nanocrystalline TiFe powder. Int. J. Hydrogen Energy 2018, 43, 16107–16116. [Google Scholar] [CrossRef]
- Guo, F.; Namba, K.; Miyaoka, H.; Jain, A.; Ichikawa, T. Hydrogen storage behavior of TiFe alloy activated by different methods. Mater. Lett. X 2021, 9, 100061. [Google Scholar] [CrossRef]
- Ulate-Kolitsky, E.; Tougas, B.; Neumann, B.; Schade, C.; Huot, J. First hydrogenation of mechanically processed TiFe-based alloy synthesized by gas atomization. Int. J. Hydrogen Energy 2021, 46, 7381–7389. [Google Scholar] [CrossRef]
- Vega, L.E.R.; Leiva, D.R.; Neto, R.M.L.; Silva, W.B.; Silva, R.A.; Ishikawa, T.T.; Kiminami, C.S.; Botta, W.J. Mechanical activation of TiFe for hydrogen storage by cold rolling under inert atmosphere. Int. J. Hydrogen Energy 2018, 43, 2913–2918. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion: Similarities and differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Arita, M.; Daio, T.; Akiba, E.; Horita, Z. Mechanism of activation of TiFe intermetallics for hydrogen storage by severe plastic deformation using high-pressure torsion. Appl. Phys. Lett. 2013, 103, 143902. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Beatrice, C.A.G.; Neto, R.M.L.; Silva, W.B.; Pessan, L.A.; Botta, W.J.; Leiva, D.R. Hydrogen Absorption/Desorption Behavior of a Cold-Rolled TiFe Intermetallic Compound. Mater. Res. 2021, 24, e20210204. [Google Scholar] [CrossRef]
- Gosselin, C.; Huot, J. Hydrogenation Properties of TiFe Doped with Zirconium. Materials 2015, 8, 7864–7872. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, C.; Huot, J. First Hydrogenation Enhancement in TiFe Alloys for Hydrogen Storage Doped with Yttrium. Metals 2019, 9, 242. [Google Scholar] [CrossRef]
- Lv, P.; Liu, Z.; Dixit, V. Improved hydrogen storage properties of TiFe alloy by doping (Zr+2V) additive and using mechanical deformation. Int. J. Hydrogen Energy 2019, 44, 27843–27852. [Google Scholar] [CrossRef]
- Ali, W.; Li, M.; Gao, P.; Wu, C.; Li, Q.; Lu, X.; Li, C. Hydrogenation properties of Ti-Fe-Mn alloy with Cu and Y as additives. Int. J. Hydrogen Energy 2017, 42, 2229–2238. [Google Scholar] [CrossRef]
- Leng, H.; Yu, Z.; Luo, Q.; Yin, J.; Miao, N.; Li, Q.; Chou, K.-C. Effect of cobalt on the microstructure and hydrogen sorption performances of TiFe0.8Mn0.2 alloy. Int. J. Hydrogen Energy 2020, 45, 19553–19560. [Google Scholar] [CrossRef]
- Leng, H.; Yu, Z.; Yin, J.; Li, Q.; Wu, Z.; Chou, K.-C. Effects of Ce on the hydrogen storage properties of TiFe 0.9 Mn 0.1 alloy. Int. J. Hydrogen Energy 2017, 42, 23731–23736. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.; Liu, N.; Liang, C.; Yin, F.; Li, Q. Effect of chromium, manganese and yttrium on microstructure and hydrogen storage properties of TiFe-based alloy. Int. J. Hydrogen Energy 2020, 45, 12071–12081. [Google Scholar] [CrossRef]
- Park, K.B.; Ko, W.-S.; Fadonougbo, J.O.; Na, T.-W.; Im, H.-T.; Park, J.-Y.; Kang, J.-W.; Kang, H.-S.; Park, C.-S.; Park, H.-K. Effect of Fe substitution by Mn and Cr on first hydrogenation kinetics of air-exposed TiFe-based hydrogen storage alloy. Mater. Charact. 2021, 178, 111246. [Google Scholar] [CrossRef]
- Faisal, M.; Suh, J.-Y.; Lee, Y.-S. Understanding first cycle hydrogenation properties of Ti–Fe–Zr ternary alloys. Int. J. Hydrogen Energy 2021, 46, 4241–4251. [Google Scholar] [CrossRef]
- Zeng, L.; Xu, G.; Liu, L.; Bai, W.; Zhang, L. Experimental investigation of phase equilibria in the Ti-Fe-Zr system. Calphad 2018, 61, 20–32. [Google Scholar] [CrossRef]
- Tamara, V.; Kostyantyn, K. Iron-Titanium-Zirconium. In Iron Systems, Part 5, (Landolt-Börnstein - Group IV Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; Chapter 34; pp. 702–720. [Google Scholar]
- Zhou, G.; Zeng, D.; Liu, Z. Phase equilibria in the Fe–Ti–Zr system at 1023K. J. Alloys Compd. 2010, 490, 463–467. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Schade, C.; Sharma, P.; Huot, J. Microstructure and first hydrogenation properties of TiFe alloy with Zr and Mn as additives. Int. J. Hydrogen Energy 2020, 45, 787–797. [Google Scholar] [CrossRef]
- Ulate-Kolitsky, E.; Tougas, B.; Huot, J. Hydrogenation of TixFe2-x-based alloys with overstoichiometric Ti ratio (x = 1.1, 1.15 and 1.2). Int. J. Hydrogen Energy 2021, 46, 38363–38369. [Google Scholar] [CrossRef]
- Manna, J.; Tougas, B.; Huot, J. First hydrogenation kinetics of Zr and Mn doped TiFe alloy after air exposure and reactivation by mechanical treatment. Int. J. Hydrogen Energy 2020, 45, 11625–11631. [Google Scholar] [CrossRef]
- Christ, H.J.; Schmidt, P. Influence of Beta Stability on Hydrogen Diffusion in Various Beta Titanium Alloys. Defect Diffus. Forum 2009, 289–292, 87–94. [Google Scholar] [CrossRef]
- Marczewski, M.; Miklaszewski, A.; Jurczyk, M. Structure evolution analysis in ultrafine-grained Zr and Nb-based beta titanium alloys. J. Alloys Compd. 2018, 765, 459–469. [Google Scholar] [CrossRef]
- Mehjabeen, A.; Xu, W.; Qiu, D.; Qian, M. Redefining the β-Phase Stability in Ti-Nb-Zr Alloys for Alloy Design and Microstructural Prediction. JOM 2018, 70, 2254–2259. [Google Scholar] [CrossRef]
- Dong, C.; Hei, Z.K.; Wang, L.B.; Song, Q.H.; Wu, Y.K.; Kuo, K.H. A new icosahedral quasicrystal in rapidly solidified FeTi2. Scr. Metall. 1986, 20, 1155–1158. [Google Scholar] [CrossRef]
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. Binary Alloy Phase Diagrams. In Asm Handbook; ASM International Materials Park: Materials Parks, OH, USA, 2016. [Google Scholar]
- Park, K.B.; Na, T.-W.; Kim, Y.D.; Park, J.-Y.; Kang, J.-W.; Kang, H.-S.; Park, K.; Park, H.-K. Characterization of microstructure and surface oxide of Ti1.2Fe hydrogen storage alloy. Int. J. Hydrogen Energy 2021, 46, 13082–13087. [Google Scholar] [CrossRef]
- Schlapbach, L.; Riesterer, T. The activation of FeTi for hydrogen absorption. Appl. Phys. A 1983, 32, 169–182. [Google Scholar] [CrossRef]
- Balcerzak, M.; Wagstaffe, M.; Robles, R.; Pruneda, M.; Noei, H. Effect of Cr on the hydrogen storage and electronic properties of BCC alloys: Experimental and first-principles study. Int. J. Hydrogen Energy 2020, 45, 28996–29008. [Google Scholar] [CrossRef]
- Ćirić, K.D.; Kocjan, A.; Gradišek, A.; Koteski, V.J.; Kalijadis, A.; Ivanovski, V.; Laušević, Z.V.; Stojić, D.L. A study on crystal structure, bonding and hydriding properties of Ti–Fe–Ni intermetallics—Behind substitution of iron by nickel. Int. J. Hydrogen Energy 2012, 37, 8408–8417. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E.; Hocker, S.; Schmauder, S. Influence of substitutional impurities on hydrogen diffusion in B2-TiFe alloy. Int. J. Hydrogen Energy 2014, 39, 12213–12220. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Park, K.B.; Na, T.-W.; Park, C.-S.; Park, H.-K.; Ko, W.-S. An integrated computational and experimental method for predicting hydrogen plateau pressures of TiFe1-xMx-based room temperature hydrides. Int. J. Hydrogen Energy 2022, 47, 17673–17682. [Google Scholar] [CrossRef]
- TOPAS. Bruker, Alan A. Coelho, V6. 2021. Available online: http://www.topas-academic.net/ (accessed on 20 September 2022).
- Buchner, H.; Gutjahr, M.A.; Beccu, K.D.; Saeufferer, H. Hydrogen in intermetallic phases: The system titanium--nickel--hydrogen. Wasserstoff in intermetallischen phasen am beispiel des systems titan-nickel-wasserftoff. Int. J. Mater. Res. 1972, 63, 497–500. [Google Scholar] [CrossRef]
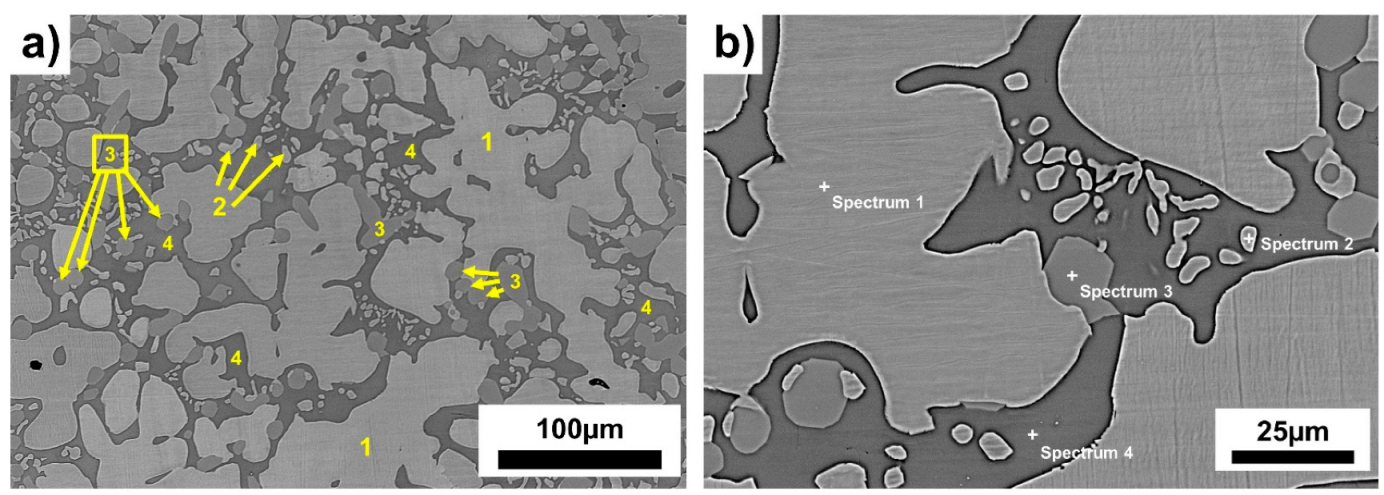


| Region | Ti wt.% | Fe wt.% | |
|---|---|---|---|
| Nominal composition | N/A | 56 | 44 |
| Overall composition | N/A | 59 | 41 |
| Spectra 1 and 2 | Bright | 49 | 51 |
| Spectrum 3 | Grey | 63 | 37 |
| Spectrum 4 | Dark-grey | 79 | 21 |
| Phase | Phase Fraction Image Analysis (area%) | Phase Fraction Rietveld Refinement (wt.%) | Lattice Parameters “a” (Å) |
|---|---|---|---|
| TiFe | 58 | 64 | 2.9904 (2) |
| Ti2Fe | 12 | 10 | 11.328 (1) |
| BCC | 30 | 26 | 3.1850 (3) |
| CR5 | CR5 Post-Activation | ||||||
|---|---|---|---|---|---|---|---|
| Phase | Lattice parameters (Å) | Unit cell volume (Å3) | Micro strain | Phase | Lattice parameters (Å) | Unit cell Volume (Å3) | Micro strain |
| Ti2Fe | 11.320 (3) | 1451 (1) | 0.13 (8)% | Ti2FeHx | 12.064 (5) | 1756 (2) | 0.44 (1)% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulate-Kolitsky, E.; Tougas, B.; Huot, J. First Hydrogenation of TiFe with Addition of 20 wt.% Ti. Hydrogen 2022, 3, 379-388. https://doi.org/10.3390/hydrogen3040023
Ulate-Kolitsky E, Tougas B, Huot J. First Hydrogenation of TiFe with Addition of 20 wt.% Ti. Hydrogen. 2022; 3(4):379-388. https://doi.org/10.3390/hydrogen3040023
Chicago/Turabian StyleUlate-Kolitsky, Elena, Bernard Tougas, and Jacques Huot. 2022. "First Hydrogenation of TiFe with Addition of 20 wt.% Ti" Hydrogen 3, no. 4: 379-388. https://doi.org/10.3390/hydrogen3040023
APA StyleUlate-Kolitsky, E., Tougas, B., & Huot, J. (2022). First Hydrogenation of TiFe with Addition of 20 wt.% Ti. Hydrogen, 3(4), 379-388. https://doi.org/10.3390/hydrogen3040023









