Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
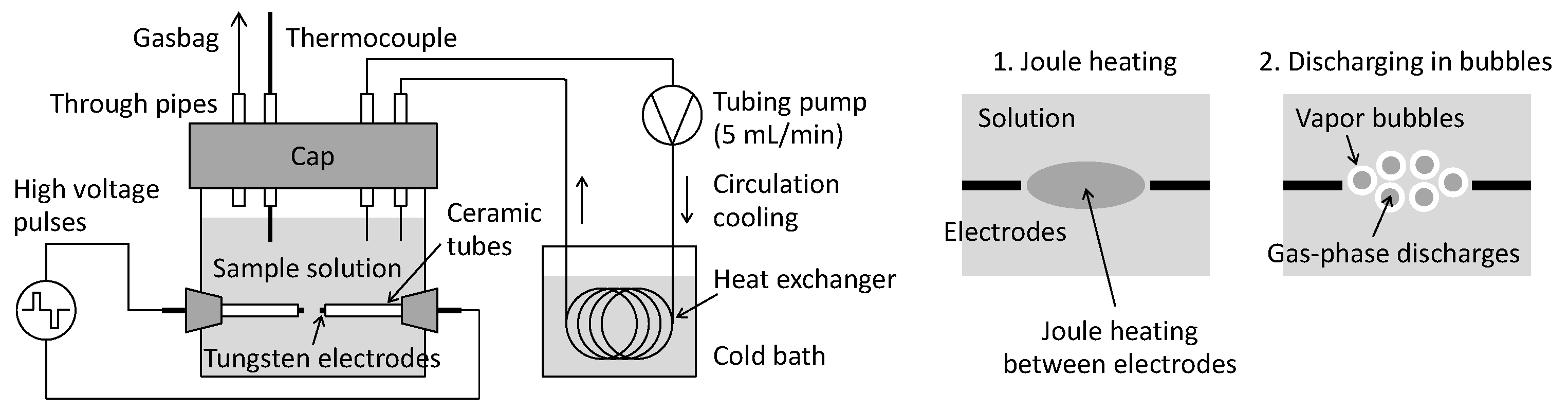
2.2. Solution-Plasma Treatment
2.3. Analytical Methods
3. Results and Discussion
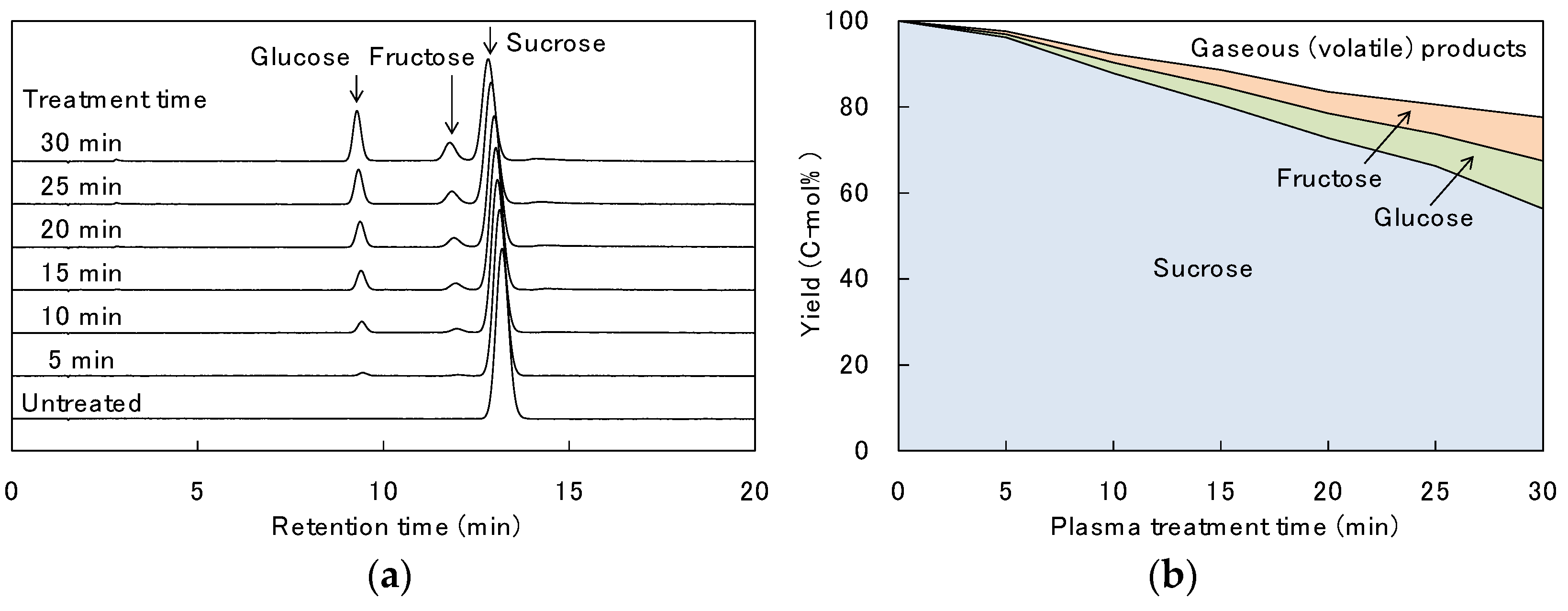
3.1. Decomposition Behavior of Sucrose
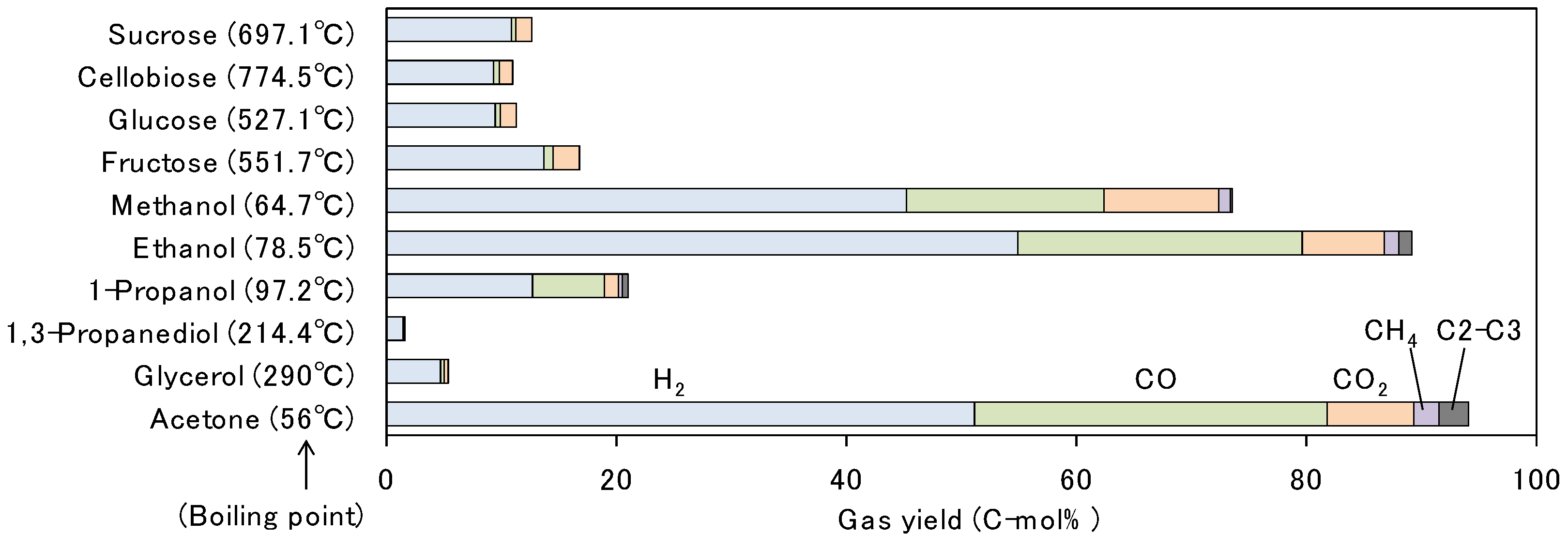
3.2. Comparison of Various Saccharides and Alcohols
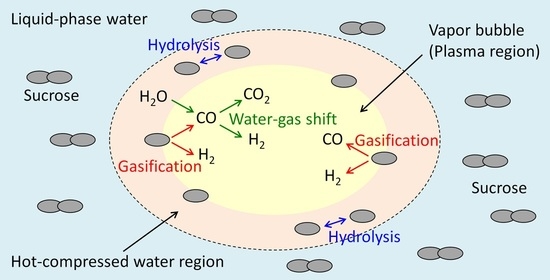
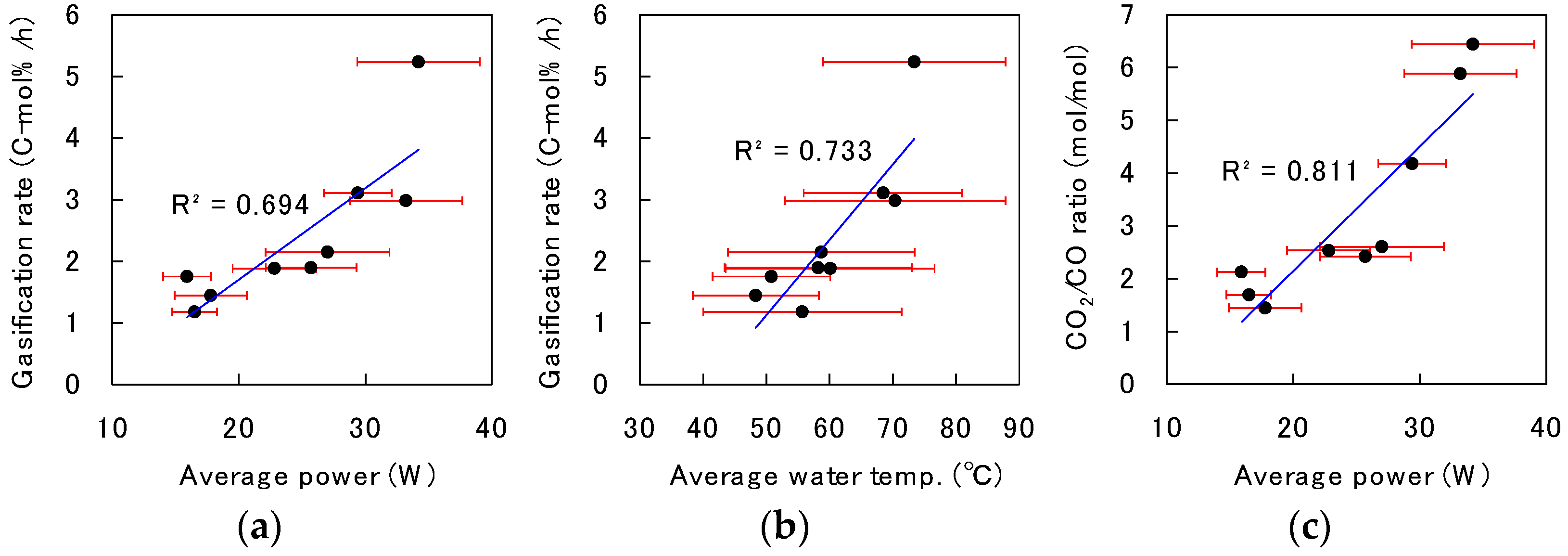
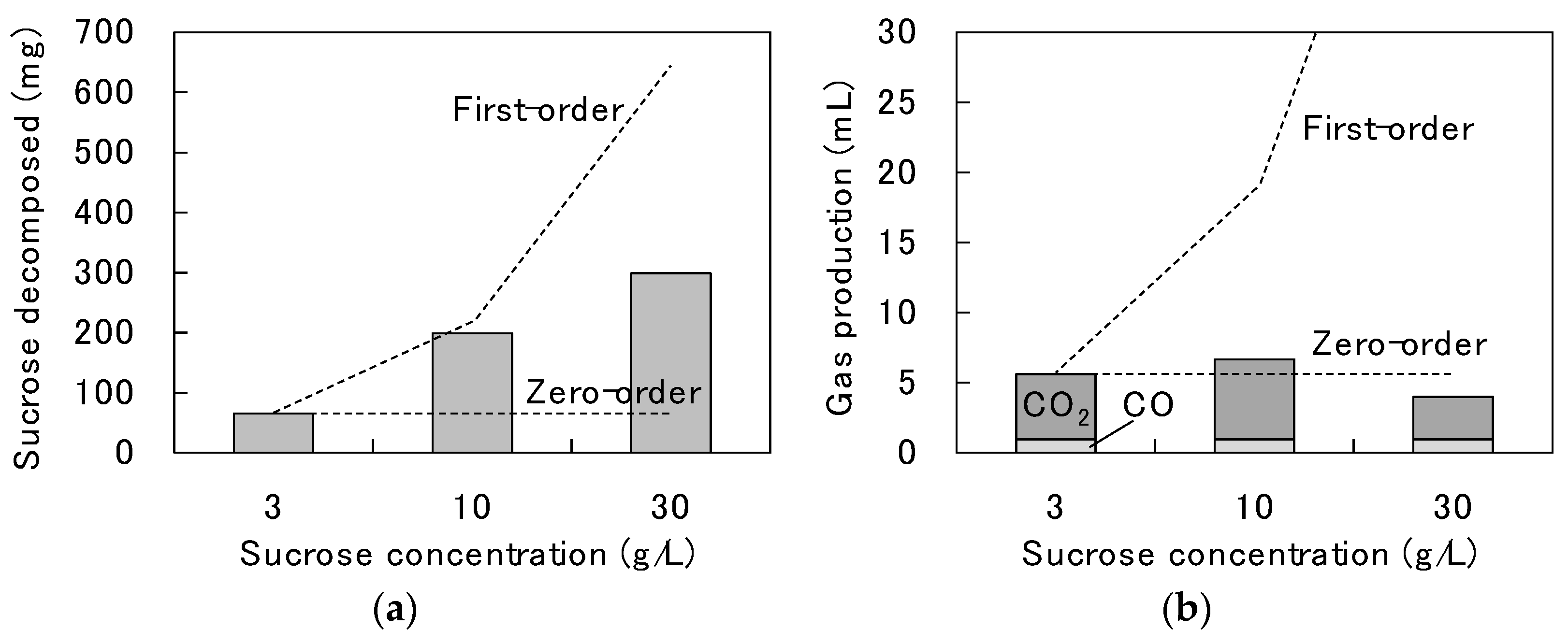
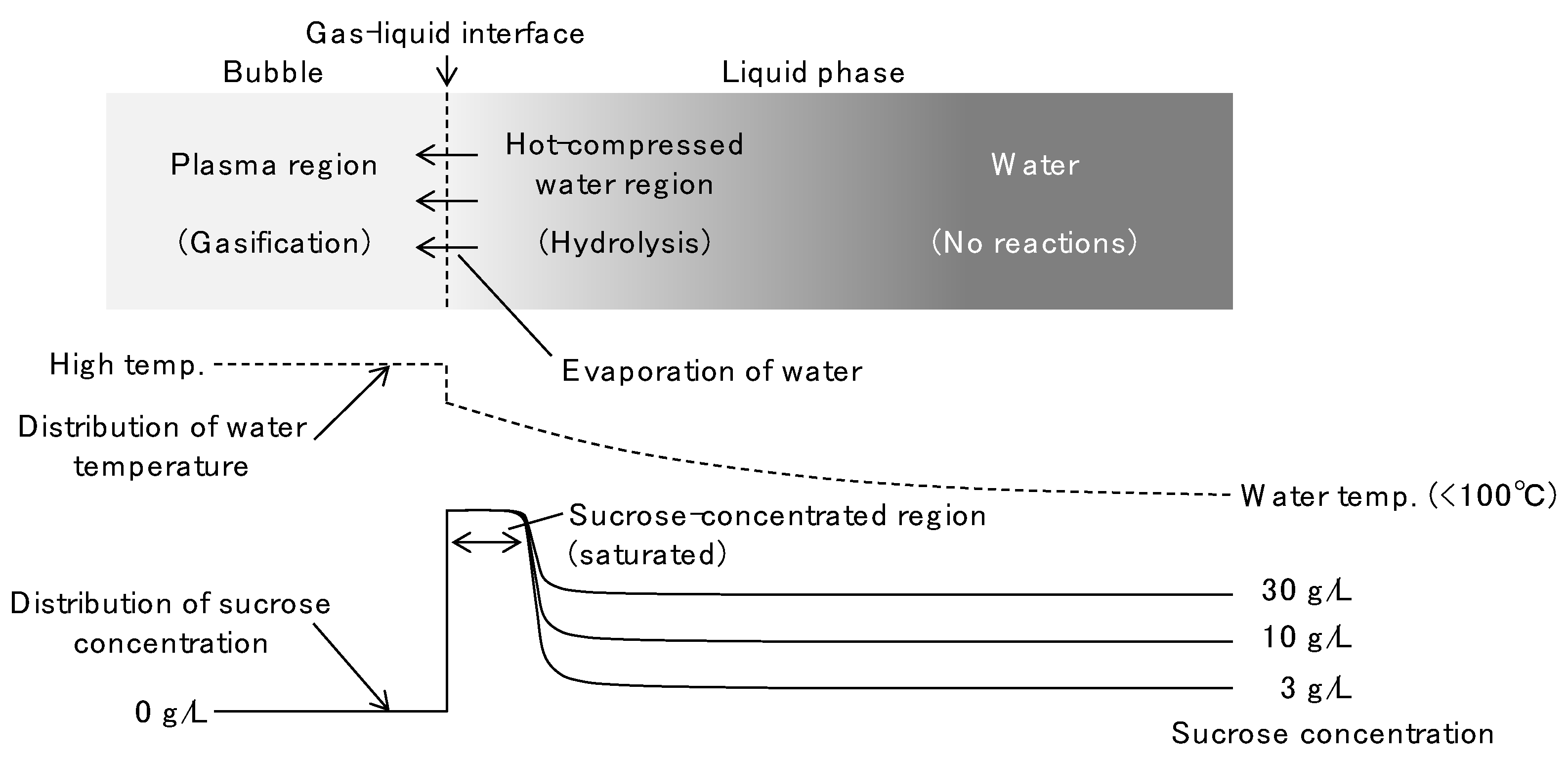
3.3. Discussion of the Decomposition Behavior in Solution Plasma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

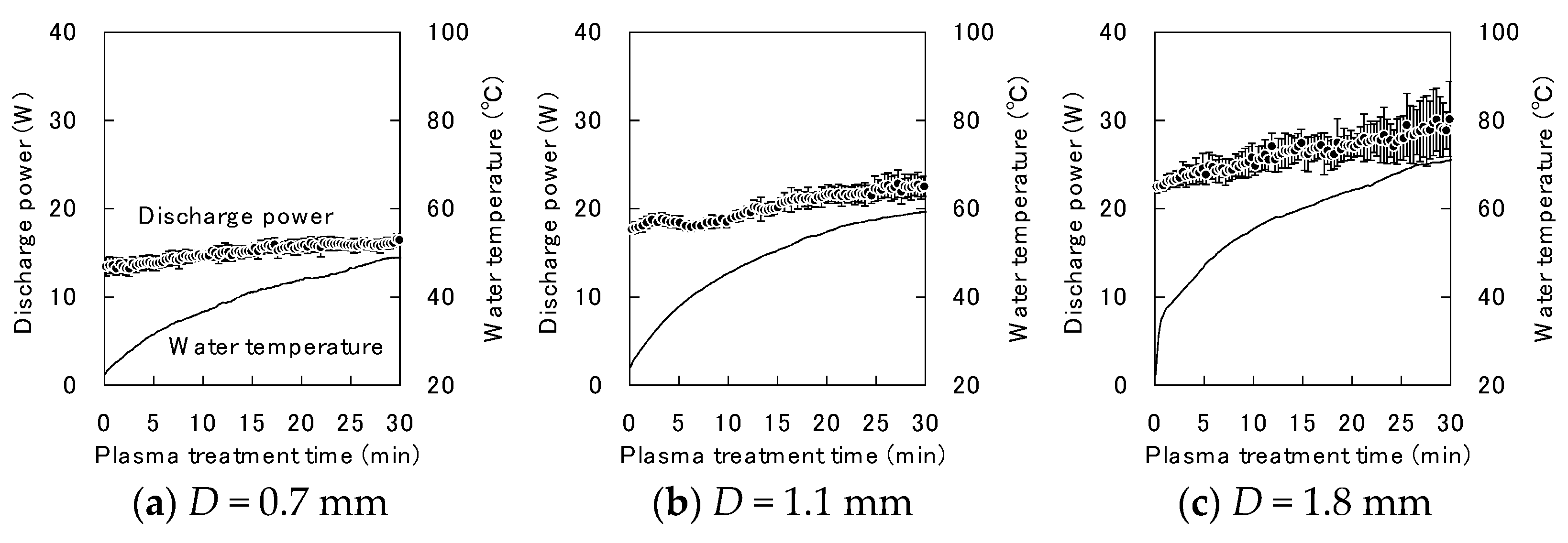
Appendix B
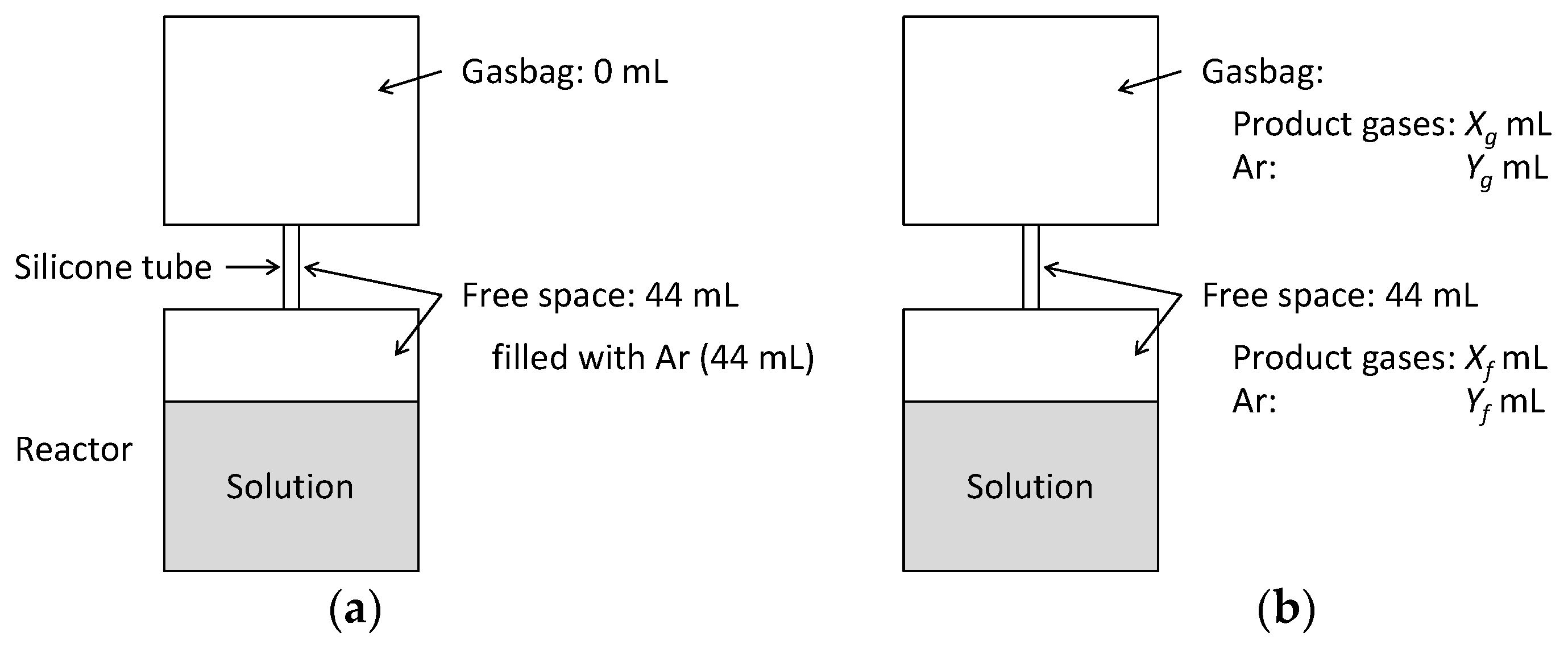
Appendix C
Appendix D
| Electrode Distance D, mm | 0.7 | 1.1 | 1.8 |
|---|---|---|---|
| H2, mL | 3.7 | 11.6 | 16.9 |
| CO2, mL | 0.011 | 0.014 | 0.023 |
References
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.; Hacker, V.; Cobo, M. Impact of bioethanol impurities on steam reforming for hydrogen production: A review. Int. J. Hydrog. Energy 2020, 45, 11923–11942. [Google Scholar] [CrossRef]
- Cao, L.; Iris, K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- REN21. Renewables 2022 Global Status Report; REN21: Paris, France, 2022; pp. 1–312. [Google Scholar]
- Esmaeili, S.A.H.; Sobhani, A.; Szmerekovsky, J.; Dybing, A.; Pourhashem, G. First-generation vs. second-generation: A market incentives analysis for bioethanol supply chains with carbon policies. Appl. Energy 2020, 277, 115606. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities and Ecosystems, 2nd ed.; The Macmillan Company: New York, NY, USA, 1975; pp. 1–352. [Google Scholar]
- Lee, Y.; Iyer, P.; Torget, R.W. Dilute-acid hydrolysis of lignocellulosic biomass. Recent Prog. Bioconversion Lignocellul. 1999, 65, 93–115. [Google Scholar]
- Khare, S.K.; Pandey, A.; Larroche, C. Current perspectives in enzymatic saccharification of lignocellulosic biomass. Biochem. Eng. J. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Abdullah, R.; Ueda, K.; Saka, S. Hydrothermal decomposition of various crystalline celluloses as treated by semi-flow hot-compressed water. J. Wood Sci. 2014, 60, 278–286. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first-and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Origin, impact and control of lignocellulosic inhibitors in bioethanol production—A review. Energies 2020, 13, 4751. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Piel, A.; Brown, M. Plasma physics: An introduction to laboratory, space, and fusion plasmas. Phys. Today 2011, 64, 55. [Google Scholar]
- Konno, K.; Onodera, H.; Murata, K.; Onoe, K.; Yamaguchi, T. Comparison of cellulose decomposition by microwave plasma and radio frequency plasma. Green Sustain. Chem. 2011, 1, 85–91. [Google Scholar] [CrossRef]
- Vecten, S.; Wilkinson, M.; Bimbo, N.; Dawson, R.; Herbert, B.M. Hydrogen-rich syngas production from biomass in a steam microwave-induced plasma gasification reactor. Bioresour. Technol. 2021, 337, 125324. [Google Scholar] [CrossRef]
- Sasujit, K.; Dussadee, N.; Tippayawong, N. Overview of tar reduction in biomass-derived producer gas using non-thermal plasma discharges. Maejo Int. J. Sci. Technol. 2019, 13, 42–61. [Google Scholar]
- Hlina, M.; Hrabovský, M.; Kopecký, V.; Konrad, M.; Kavka, T.; Skoblja, S. Plasma gasification of wood and production of gas with low content of tar. Czechoslov. J. Phys. 2006, 56, B1179–B1184. [Google Scholar] [CrossRef]
- Takai, O. Solution plasma processing (SPP). Pure Appl. Chem. 2008, 80, 2003–2011. [Google Scholar] [CrossRef]
- MubarakAli, D.; Park, J.; Han, T.; Srinivasan, H.; Lee, S.-Y.; Kim, J.-W. Solution plasma process: An option to degrade bisphenol A in liquid-phase to non-toxic products. J. Mol. Liq. 2019, 276, 605–610. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Wang, X.; Niu, J.; Fan, T.; Saito, N. Fundamentals of solution plasma for advanced materials synthesis. Mater. Today Adv. 2022, 14, 100244. [Google Scholar] [CrossRef]
- Dithi, A.B.; Nezu, T.; Nagano-Takebe, F.; Hasan, M.R.; Saito, T.; Endo, K. Application of solution plasma surface modification technology to the formation of thin hydroxyapatite film on titanium implants. Coatings 2018, 9, 3. [Google Scholar] [CrossRef]
- Prasertsung, I.; Chutinate, P.; Watthanaphanit, A.; Saito, N.; Damrongsakkul, S. Conversion of cellulose into reducing sugar by solution plasma process (SPP). Carbohydr. Polym. 2017, 172, 230–236. [Google Scholar] [CrossRef]
- Prasertsung, I.; Aroonraj, K.; Kamwilaisak, K.; Saito, N.; Damrongsakkul, S. Production of reducing sugar from cassava starch waste (CSW) using solution plasma process (SPP). Carbohydr. Polym. 2019, 205, 472–479. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.-K. Hydrogen production from a solution plasma process of bio-oil. Int. J. Hydrog. Energy 2020, 45, 20210–20215. [Google Scholar] [CrossRef]
- Tange, K.; Nomura, S.; Mukasa, S.; Kitahara, T. Cellulose decomposition in electrolytic solution using in-liquid plasma method. J. Jpn. Inst. Energy 2019, 98, 265–271. [Google Scholar] [CrossRef]
- Syahrial, F.; Mukasa, S.; Toyota, H.; Okamoto, K.; Nomura, S. Hydrogen production from glucose and cellulose using radio frequency in-liquid plasma and ultrasonic irradiation. J. Jpn. Inst. Energy 2014, 93, 1207–1212. [Google Scholar] [CrossRef]
- Ismail, R.; Nomura, S.; Mukasa, S.; Toyoda, H. Fuel gas production from biomass sources by radio frequency in-liquid plasma method. J. Power Energy Eng. 2015, 3, 28–35. [Google Scholar]
- Phaiboonsilpa, N.; Yamauchi, K.; Lu, X.; Saka, S. Two-step hydrolysis of Japanese cedar as treated by semi-flow hot-compressed water. J. Wood Sci. 2010, 56, 331–338. [Google Scholar] [CrossRef]
- Nomura, T.; Mizuno, H.; Minami, E.; Kawamoto, H. Fast pyrolysis of cellulose by infrared heating. Energies 2021, 14, 1842. [Google Scholar] [CrossRef]
- Gao, D.; Kobayashi, T.; Adachi, S. Kinetics of sucrose hydrolysis in a subcritical water-ethanol mixture. J. Appl. Glycosci. 2013, 61, 9–13. [Google Scholar] [CrossRef]


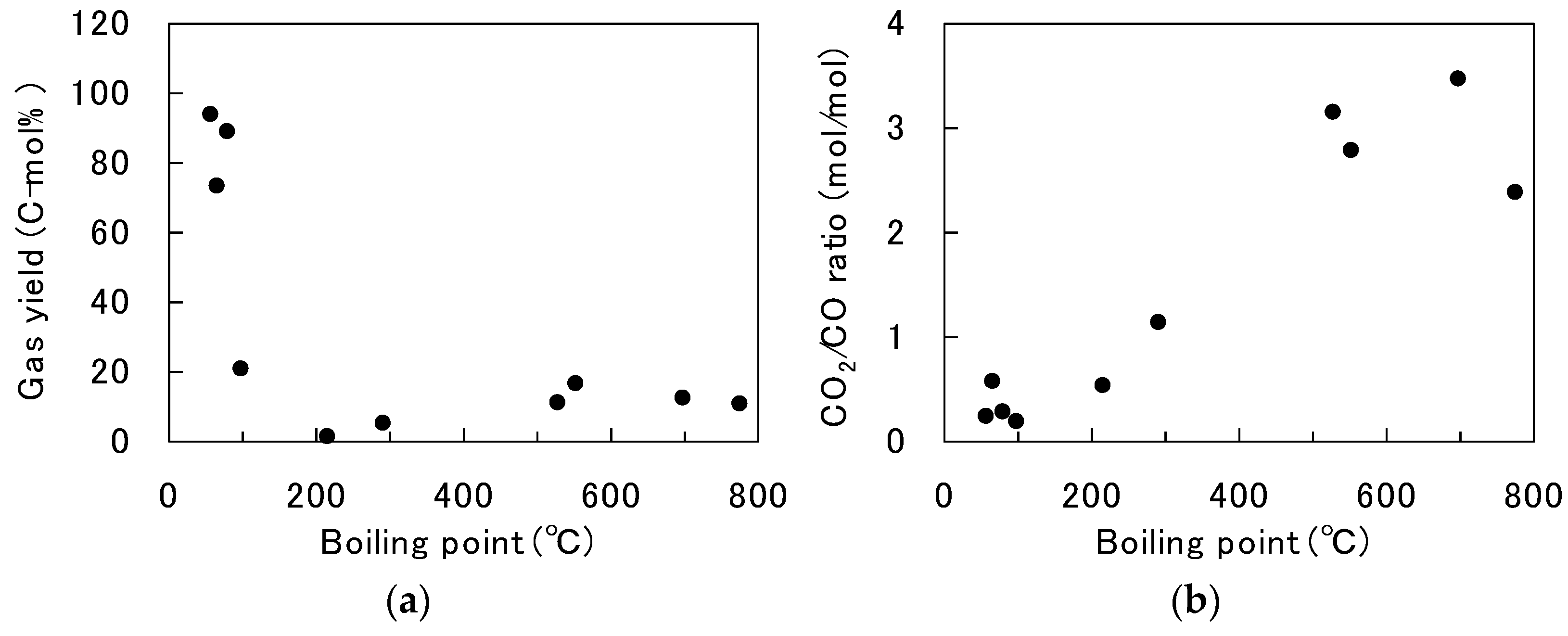
| H2 | CO | CO2 * | |
|---|---|---|---|
| Volume (mL) | 134 | 2.8 | 17.8 |
| Yield (C-mol%) ** | 17.1 | 0.35 | 2.27 |
| Entry No. | D, mm | Cooling Method | Average Power, W | Average Temp., °C | Time, min | Gas Yield, C-mol% * | ||
|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 ** | ||||||
| 1 | 0.9 | no cooling | 16.5 | 55.7 | 35 | 4.8 | 0.26 | 0.43 |
| 2 | 0.8 | R.T. water | 15.9 | 50.8 | 60 | 9.5 | 0.56 | 1.19 |
| 3 | 0.7 | ice water | 17.8 | 48.3 | 60 | 9.8 | 0.59 | 0.85 |
| 4 | 1.3 | no cooling | 22.8 | 60.1 | 25 | 6.1 | 0.22 | 0.56 |
| 5 | 1.5 | ice water | 27.0 | 58.7 | 30 | 7.4 | 0.30 | 0.78 |
| 6 | 1.5 | R.T. water | 25.7 | 58.2 | 25 | 6.4 | 0.23 | 0.56 |
| 7 | 1.9 | ice water | 29.4 | 68.5 | 30 | 7.5 | 0.30 | 1.26 |
| 8 | 2.3 | ice water | 33.2 | 70.4 | 17 | 6.5 | 0.12 | 0.72 |
| 9 | 2.4 | ice water | 34.2 | 73.4 | 30 | 17.1 | 0.35 | 2.27 |
| Continuous | 1 min on/1 min off | 1 min on/3 min off | |
|---|---|---|---|
| Avg. discharge power, W | 25.2 | 26.2 | 24.4 |
| Total discharge time, min | 45 | 45 | 45 |
| Total treatment time, min | 45 | 90 | 180 |
| Total electric energy, Wh | 18.9 | 19.7 | 18.3 |
| Avg. water temp., °C | 63.7 | 47.5 | 35.3 |
| Sucrose recovery, C-mol% * | 46.5 | 51.5 | 73.4 |
| CO and CO2 yield, C-mol% * | 1.8 | 1.9 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, E.; Miyamoto, T.; Kawamoto, H. Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production. Hydrogen 2022, 3, 333-347. https://doi.org/10.3390/hydrogen3030020
Minami E, Miyamoto T, Kawamoto H. Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production. Hydrogen. 2022; 3(3):333-347. https://doi.org/10.3390/hydrogen3030020
Chicago/Turabian StyleMinami, Eiji, Takaki Miyamoto, and Haruo Kawamoto. 2022. "Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production" Hydrogen 3, no. 3: 333-347. https://doi.org/10.3390/hydrogen3030020
APA StyleMinami, E., Miyamoto, T., & Kawamoto, H. (2022). Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production. Hydrogen, 3(3), 333-347. https://doi.org/10.3390/hydrogen3030020