Spontaneous Ignition of Cryo-Compressed Hydrogen in a T-Shaped Channel System
Abstract
:1. Introduction
2. Problem Formulation and Numerical Details
2.1. Problem Formulation
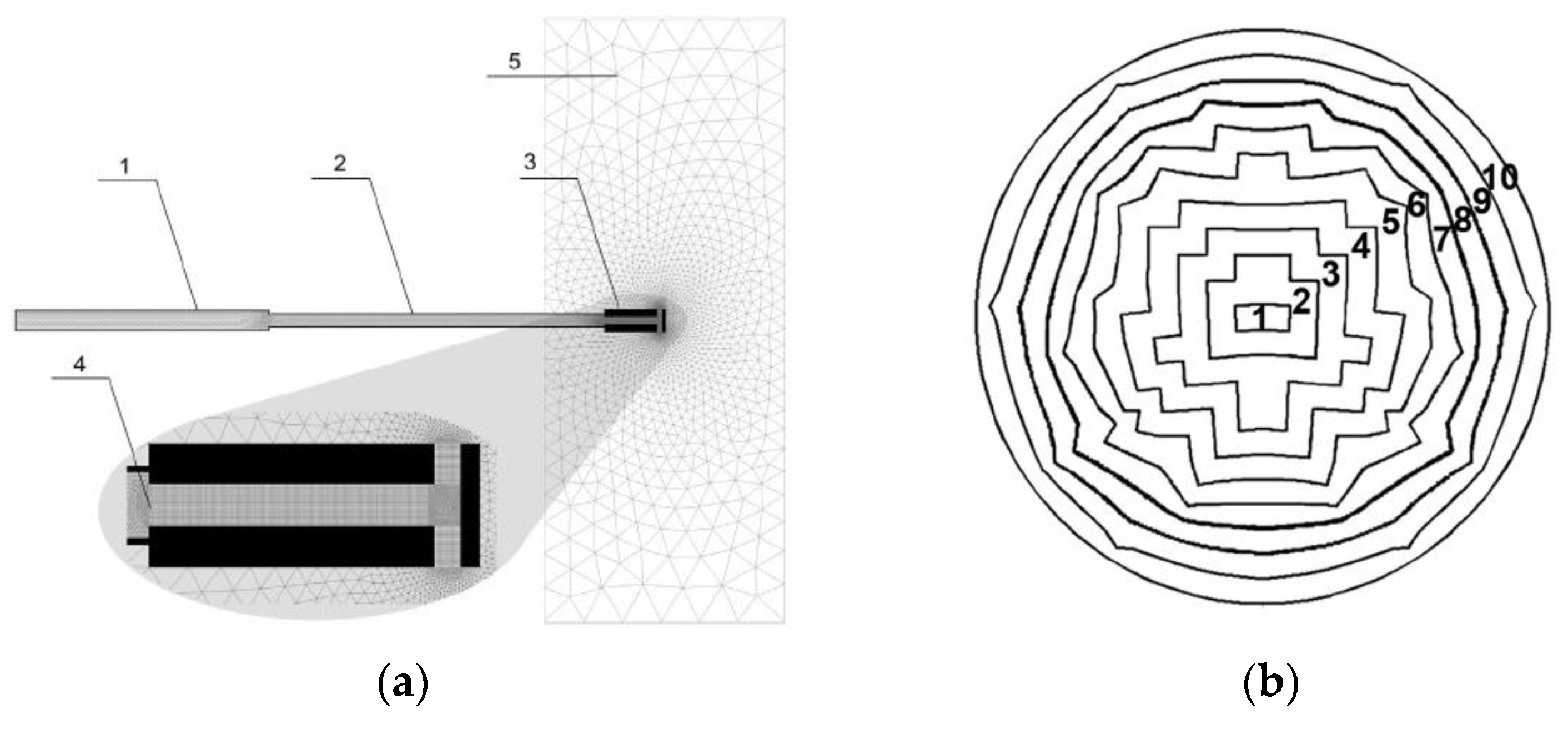
2.2. CFD Model and Numerical Details
3. Results and Discussion
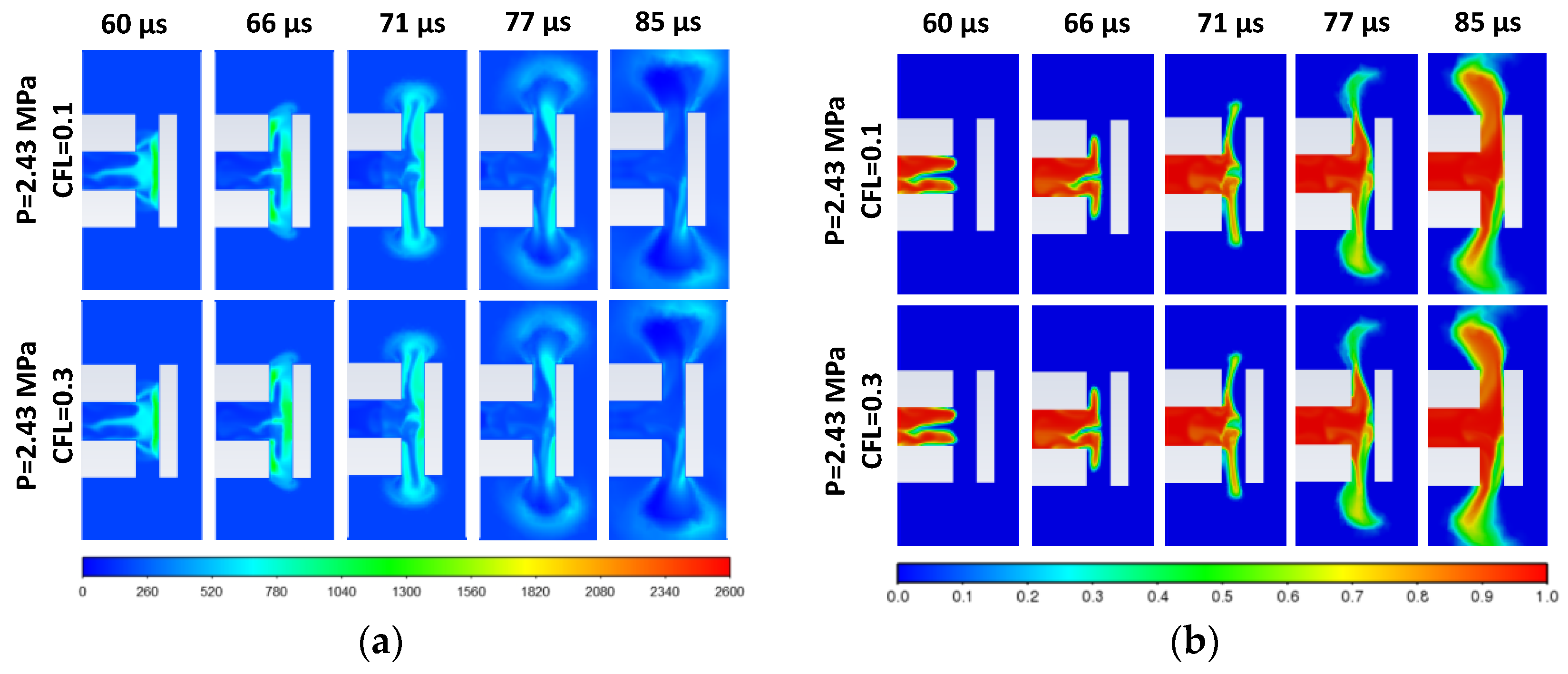
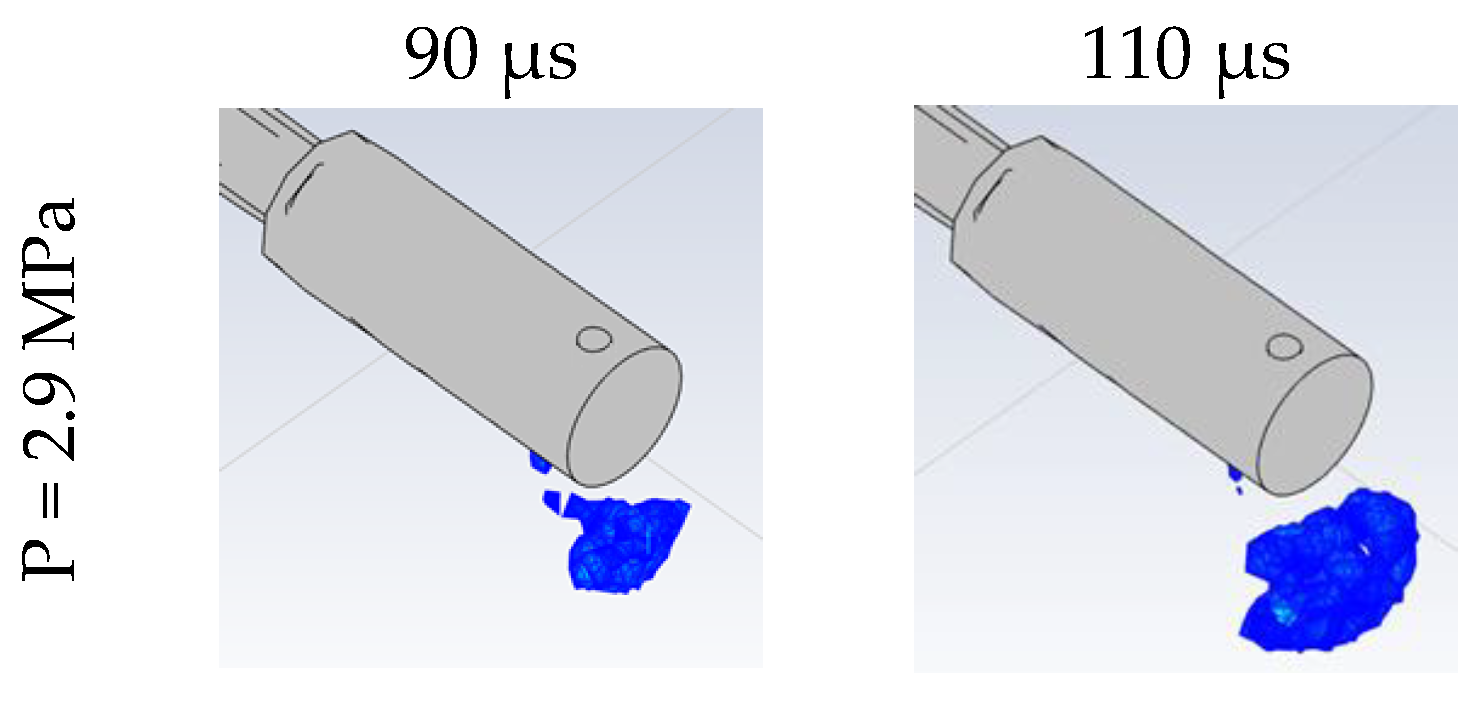
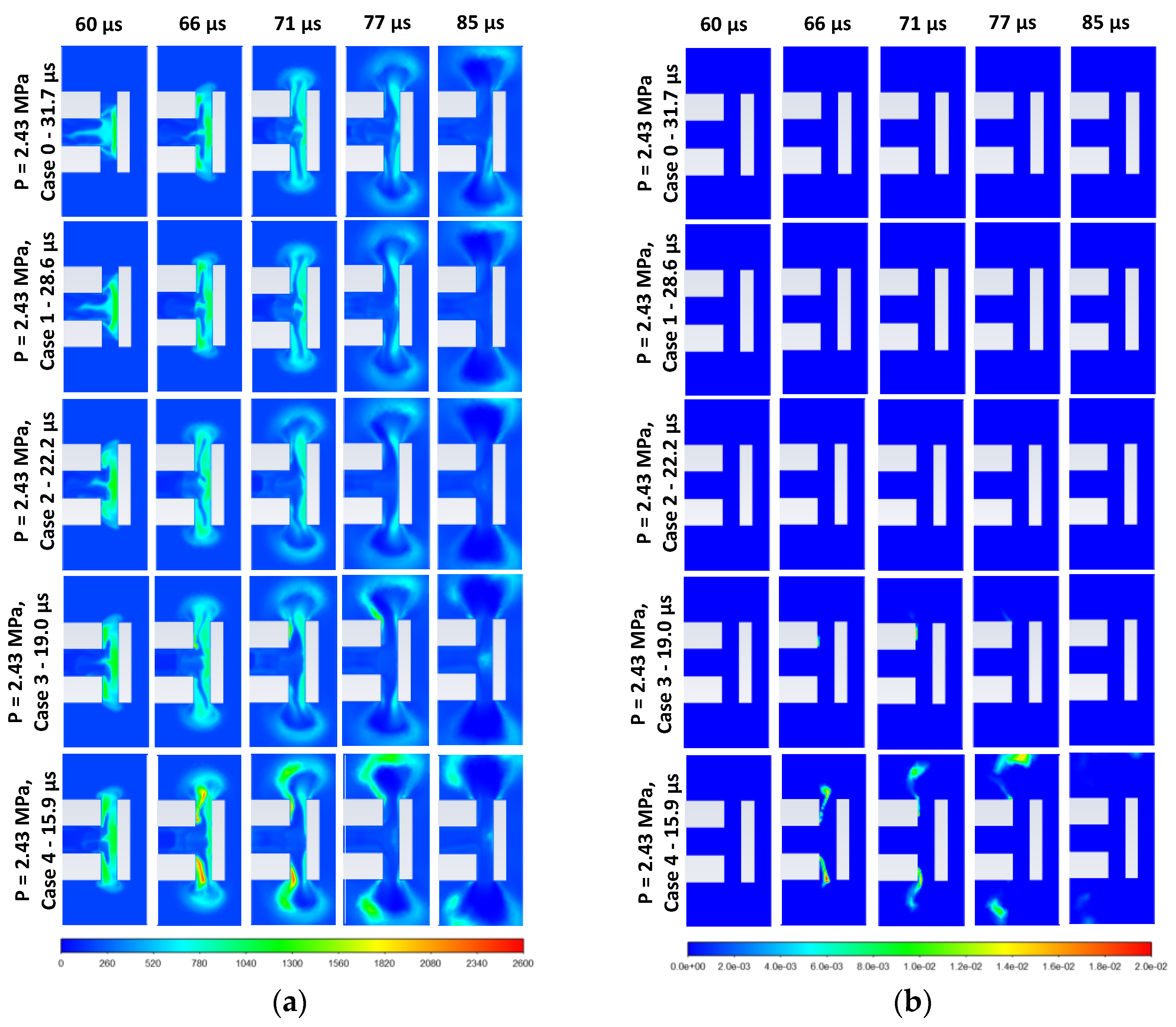
3.1. Effect of Diaphragm Opening Time on Spontaneous Ignition for Pressure 2.43 MPa
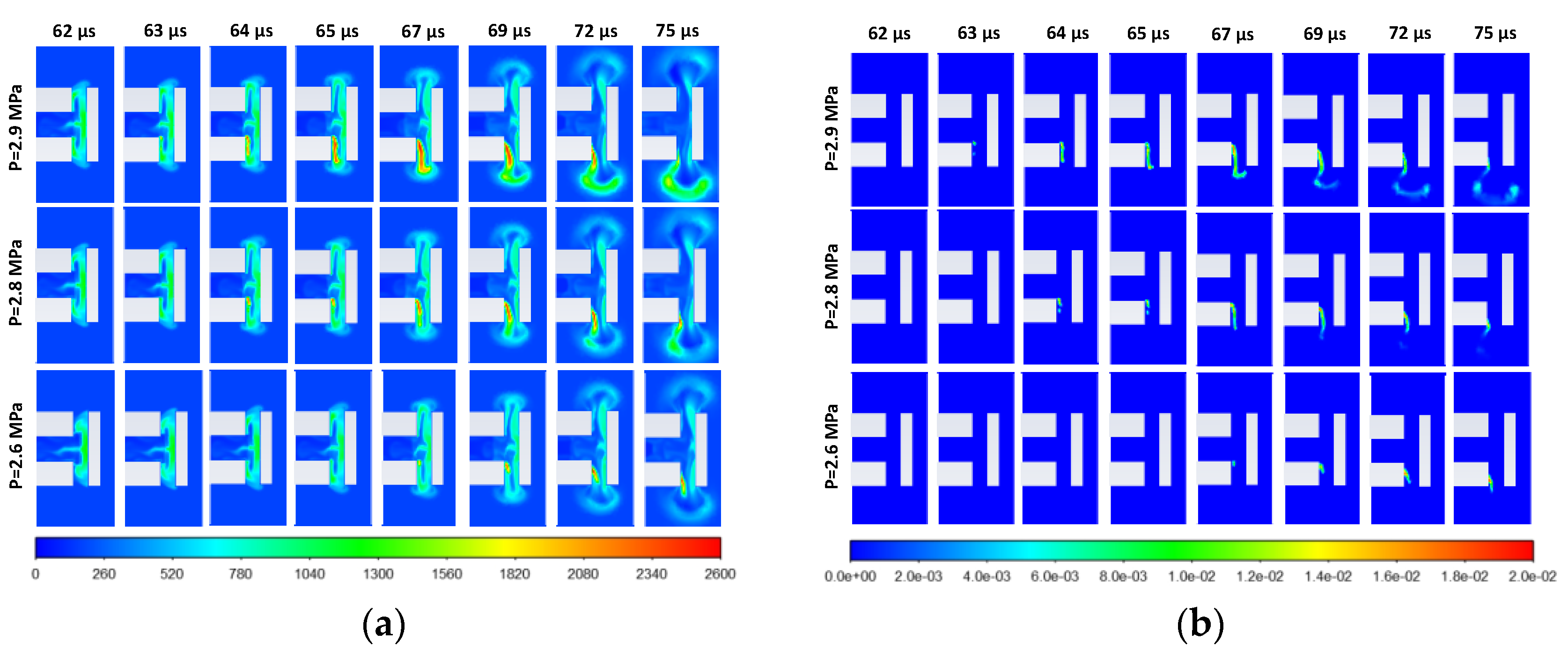
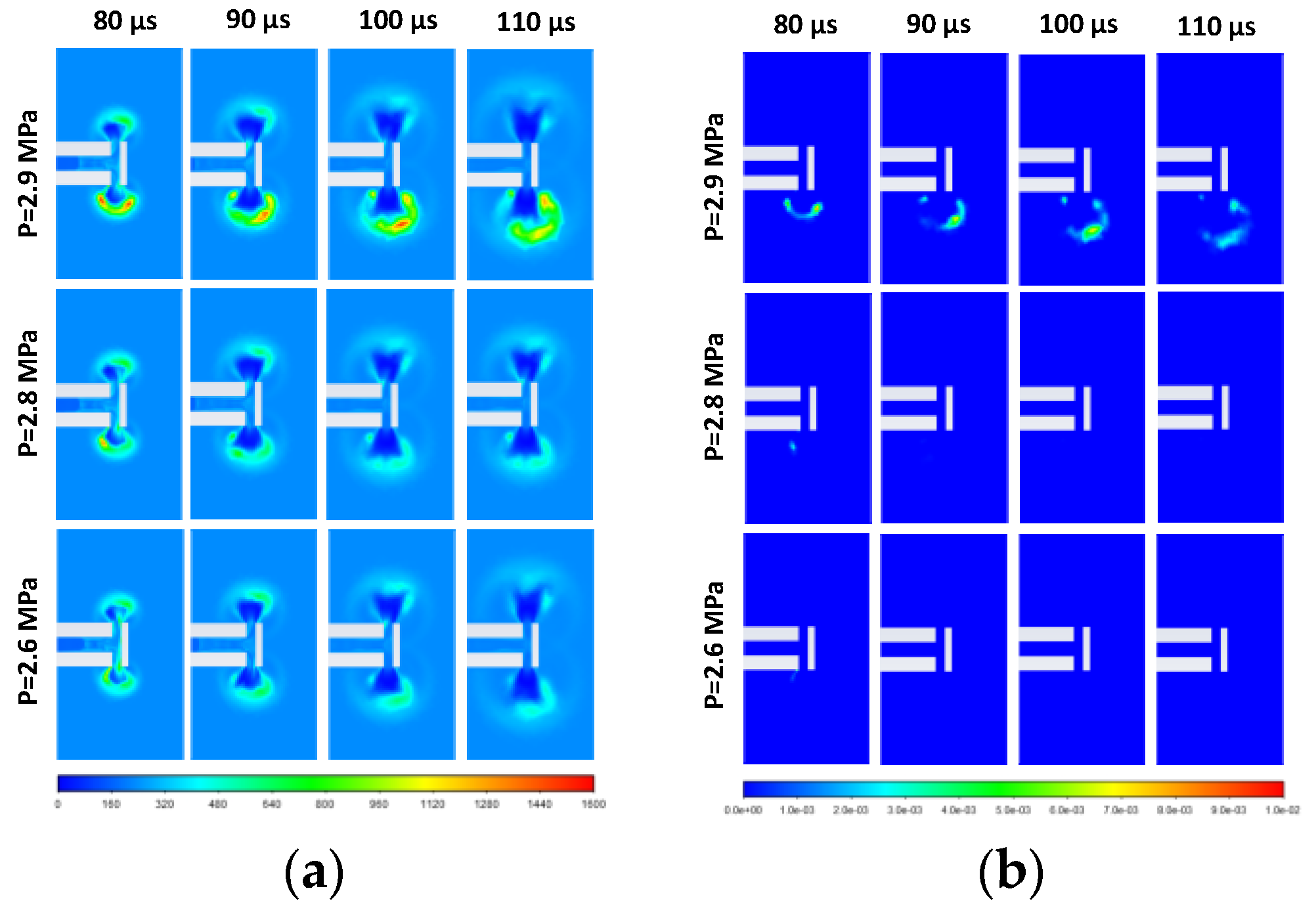
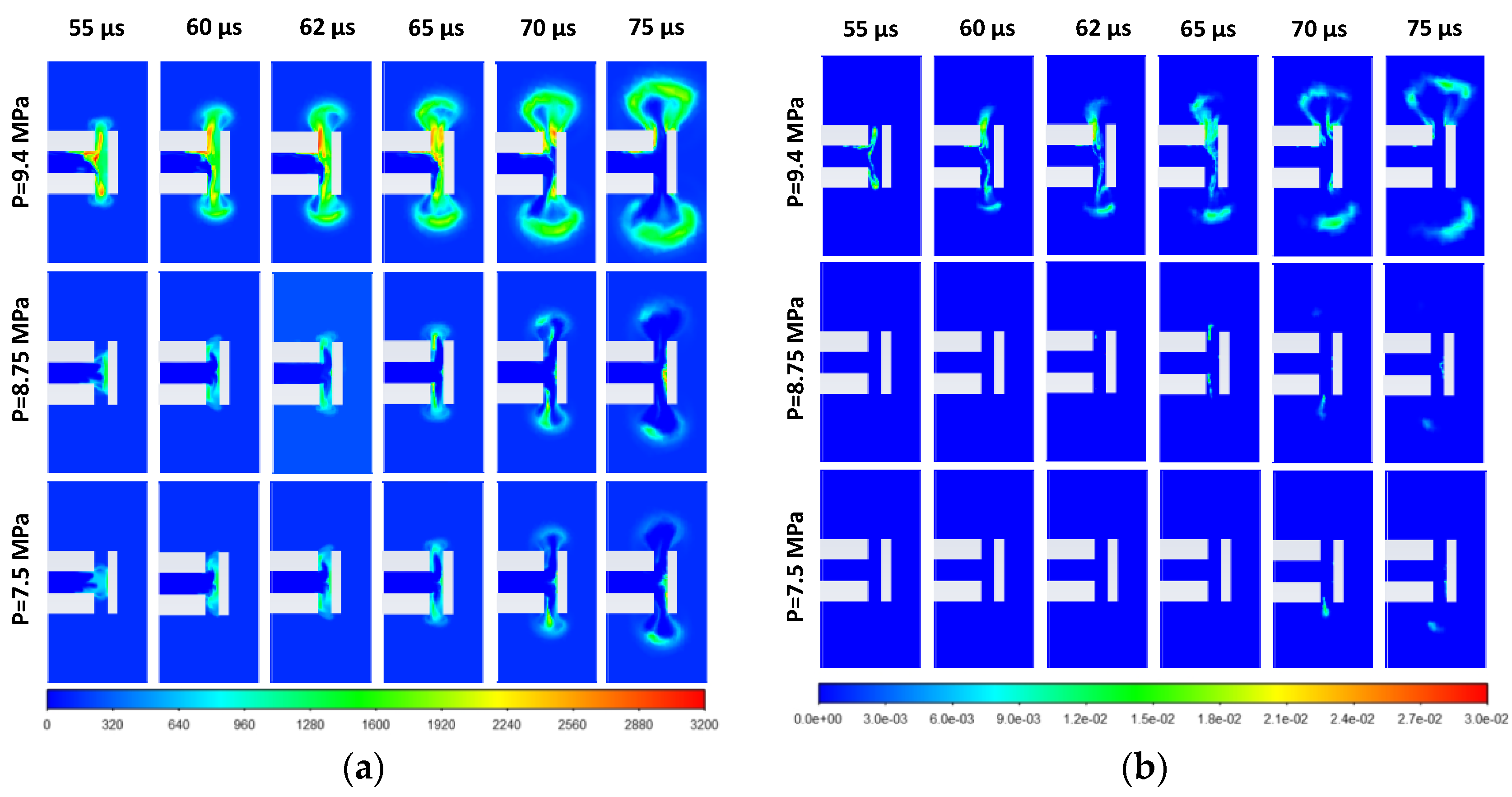
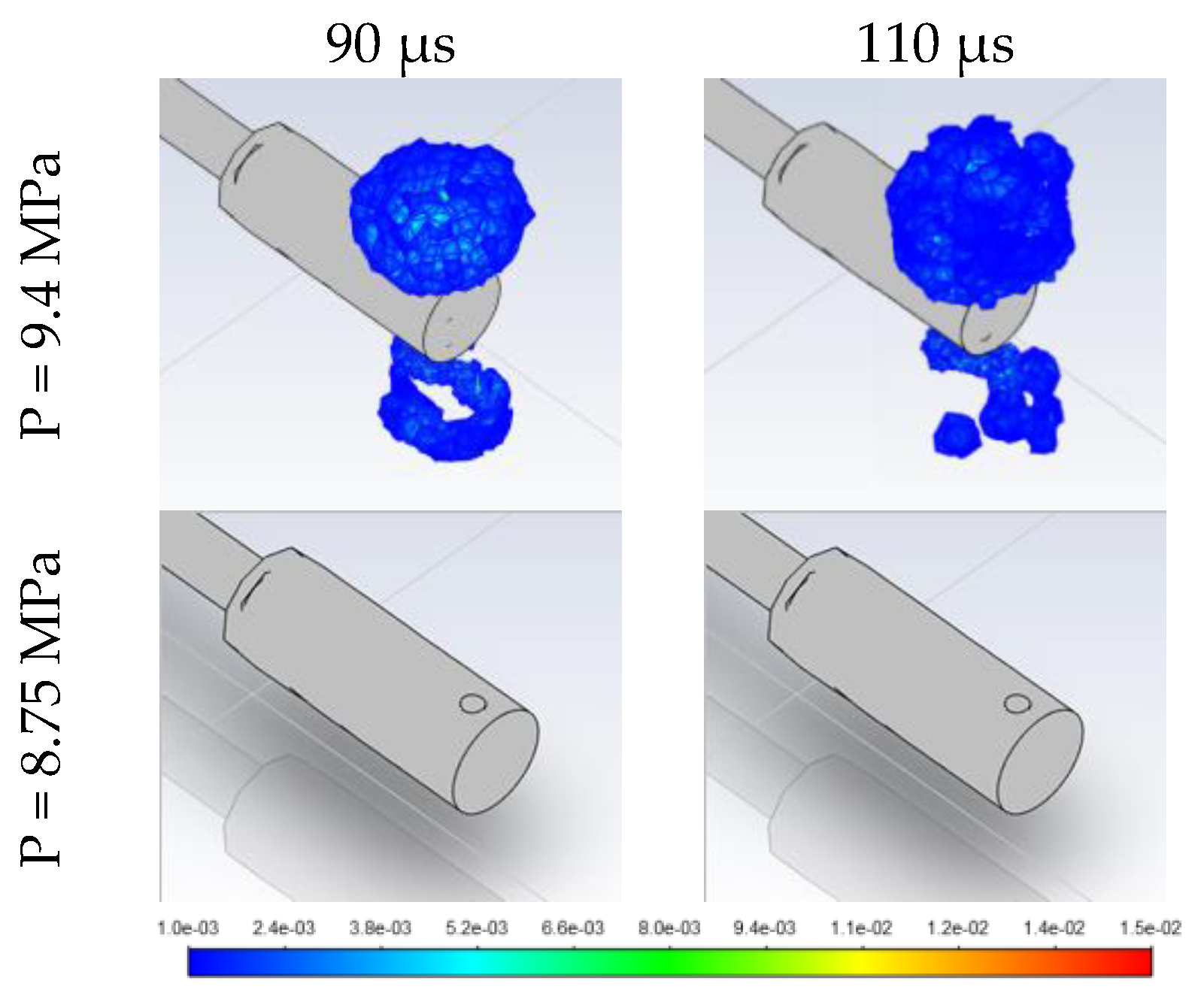
3.2. Effect of Stored Hydrogen Temperature on Pressure Limit for Spontaneous Ignition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| thickness of the diaphragm (m) | |
| diameter of the diaphragm (m) | |
| constant (0.92) | |
| diaphragm burst pressure (Pa) | |
| diaphragm opening time (s) | |
| density of the diaphragm material (kg/m3) |
References
- Wolanski, P.; Wojcicki, S. Investigation into the Mechanism of Diffusion Ignition of a Combustible Gas Flowing into Oxidizing Atmosphere. In Proceedings of the Fourteenth Symposium (International) on Combustion, Pittsburgh, PA, USA, 20–25 August 1972; pp. 1217–1223. [Google Scholar]
- Bazhenova, T.; Bragin, M.; Golub, V.; Ivanov, M. Self-ignition of a fuel gas upon pulsed efflux into an oxidative medium. Tech. Phys. Lett. 2006, 32, 269–271. [Google Scholar] [CrossRef]
- Golub, V.V.; Baklanov, D.I.; Bazhenova, T.V.; Bragin, M.V.; Golovastov, S.V.; Ivanov, M.F.; Volodin, V.V. Shock-induced ignition of hydrogen gas during accidental or technical opening of high-pressure tanks. J. Loss Prev. Process Ind. 2007, 20, 439–446. [Google Scholar] [CrossRef]
- Dryer, F.L.; Chaos, M.; Zhao, Z.; Stein, J.N.; Alpert, J.Y.; Homer, C.J. Spontaneous ignition of pressurized releases of hydrogen and natural gas into air. Combust. Sci. Technol. 2007, 179, 663–694. [Google Scholar] [CrossRef]
- Golovastov, S.; Bocharnikov, V. The influence of diaphragm rupture rate on spontaneous self-ignition of pressurized hydrogen: Experimental investigation. Int. J. Hydrogen Energy 2012, 37, 10956–10962. [Google Scholar] [CrossRef]
- Kaneko, W.; Ishii, K. Effects of diaphragm rupturing conditions on self-ignition of high-pressure hydrogen. Int. J. Hydrogen Energy 2016, 41, 10969–10975. [Google Scholar] [CrossRef]
- Xu, B.P.; El Hima, L.; Wen, J.X.; Dembele, S.; Tam, V.H.Y.; Donchev, T. Numerical study on the spontaneous ignition of pressurized hydrogen release through a tube into air. J. Loss Prev. Process Ind. 2008, 21, 205–213. [Google Scholar] [CrossRef]
- Xu, B.P.; Wen, J.X.; Dembele, S.; Tam, V.H.Y.; Hawksworth, S.J. The effect of pressure boundary rupture rate on spontaneous ignition of pressurized hydrogen release. J. Loss Prev. Process Ind. 2009, 22, 279–287. [Google Scholar] [CrossRef]
- Golub, V.V.; Baklanov, D.I.; Golovastov, S.V.; Ivanov, M.F.; Laskin, I.N.; Saveliev, A.S.; Semin, N.V.; Volodin, V.V. Mechanisms of high-pressure hydrogen gas self-ignition in tubes. J. Loss Prev. Process Ind. 2008, 21, 185–198. [Google Scholar] [CrossRef]
- Bragin, M.V.; Molkov, V.V. Physics of spontaneous ignition of high-pressure hydrogen release and transition to jet fire. Int. J. Hydrogen Energy 2011, 36, 2589–2596. [Google Scholar] [CrossRef]
- Bragin, M.V.; Makarov, D.V.; Molkov, V.V. Pressure limit of hydrogen spontaneous ignition in a T-shaped channel. Int. J. Hydrogen Energy 2013, 38, 8039–8052. [Google Scholar] [CrossRef]
- Golub, V.; Volodin, V.; Baklanov, D.; Golovastov, S.; Lenkevich, D. Experimental investigation of hydrogen ignition at the discharge into channel filled with air. Phys. Extrem. States Matter 2010, 110–113. [Google Scholar]
- Gong, L.; Duan, Q.; Suna, J.; Molkov, V. Similitude analysis and critical conditions for spontaneous ignition of hydrogen release into the atmosphere through a tube. Fuel 2019, 245, 413–419. [Google Scholar] [CrossRef]
- Gong, L.; Li, Z.; Jin, K.; Gao, Y.; Duan, Q.; Zhang, Y.; Sun, J. Numerical study on the mechanism of spontaneous ignition of high-pressure hydrogen during its sudden release into a tube. Saf. Sci. 2020, 129, 104807. [Google Scholar] [CrossRef]
- Gong, L.; Jin, K.; Yang, S.; Yang, Z.; Li, Z.; Gao, Y.; Zhang, Y. Numerical study on the mechanism of spontaneous ignition of high-pressure hydrogen in the L-shaped tube. Int. J. Hydrogen Energy 2020, 45, 32730–32742. [Google Scholar] [CrossRef]
- Jin, K.; Yang, S.; Gong, L.; Han, Y.; Yang, X.; Gao, Y.; Zhang, Y. Numerical study on the spontaneous ignition of pressurized hydrogen during its sudden release into the tube with varying lengths and diameters. J. Loss Prev. Process Ind. 2021, 72, 104592. [Google Scholar] [CrossRef]
- Jin, K.; Yang, S.; Gong, L.; Mo, T.; Gao, Y.; Zhang, Y. Mechanism of spontaneous ignition of high-pressure hydrogen during its release through a tube with local contraction: A numerical study. Int. J. Hydrogen Energy 2022, 47, 6421–6436. [Google Scholar] [CrossRef]
- Radebaugh, R. Cryogenics. MacMillan Encycl. Chem. 2002, 1–3. [Google Scholar]
- Ahluwalia, R.K.; Peng, J.K.; Hua, T.Q. Cryo-Compressed Hydrogen Storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2016; pp. 119–145. [Google Scholar]
- Stetson, N.T.; McWhorter, S.; Ahn, C.C. Introduction to Hydrogen Storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2016; pp. 3–25. [Google Scholar]
- Aceves, S.M.; Berry, G.D.; Rambach, G.D. Insulated pressure vessels for hydrogen storage on vehicles. Int. J. Hydrogen Energy 1998, 23, 583–591. [Google Scholar] [CrossRef]
- Petitpas, G.; Bénard, P.; Klebanoff, L.E.; Xiao, J.; Aceves, S. A comparative analysis of the cryo-compression and cryo-adsorption hydrogen storage methods. Int. J. Hydrogen Energy 2014, 39, 10564–10584. [Google Scholar] [CrossRef]
- Brunner, T. BMW Hydrogen. In Hydrogen Storage Workshop; Cryo-compressed Hydrogen Storage: Washington, DC, USA, 2011. [Google Scholar]
- Yakhot, V.; Orszag, S. Renormalization group analysis of turbulence. I. Basic theory. J. Sci. Comput. 1986, 1, 3–51. [Google Scholar] [CrossRef]
- Gutheil, E.; Balakrishnan, G.; Williams, F. Structure and Extinction of Hydrogen-Air Diffusion Flames. In Reduced Kinetic Mechanisms for Applications in Combustion Systems; Peters, N., Rogg, B., Eds.; Springer: New York, NY, USA, 1993; p. 179. [Google Scholar]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spence, D.; Woods, B. A review of theoretical treatments of shock-tube attenuation. J. Fluid Mech. 1964, 19, 161–174. [Google Scholar] [CrossRef]
- Bulewicz, E.M.; Sugden, T.M. The recombination of hydrogen atoms and hydroxyl radicals in hydrogen flame gases. Trans. Faraday Soc. 1958, 54, 1855–1860. [Google Scholar] [CrossRef]


| Number of Simulations | Storage Temperature, K | Storage Pressure, MPa |
|---|---|---|
| 6 | 300 | 1.35, 1.65, 2.43, 2.60, 2.80, 2.90 |
| 5 | 80 | 5.00, 7.50, 8.75, 9.40, 10.00 |
| Burst Disk Section No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pressure, MPa | Opening Times, μs | |||||||||
| 1.35 | 0 | 4.7 | 9.5 | 14.2 | 18.9 | 23.7 | 28.4 | 33.1 | 37.9 | 42.6 |
| 1.65 | 0 | 4.2 | 8.6 | 12.8 | 17.1 | 21.4 | 25.7 | 29.9 | 34.3 | 38.5 |
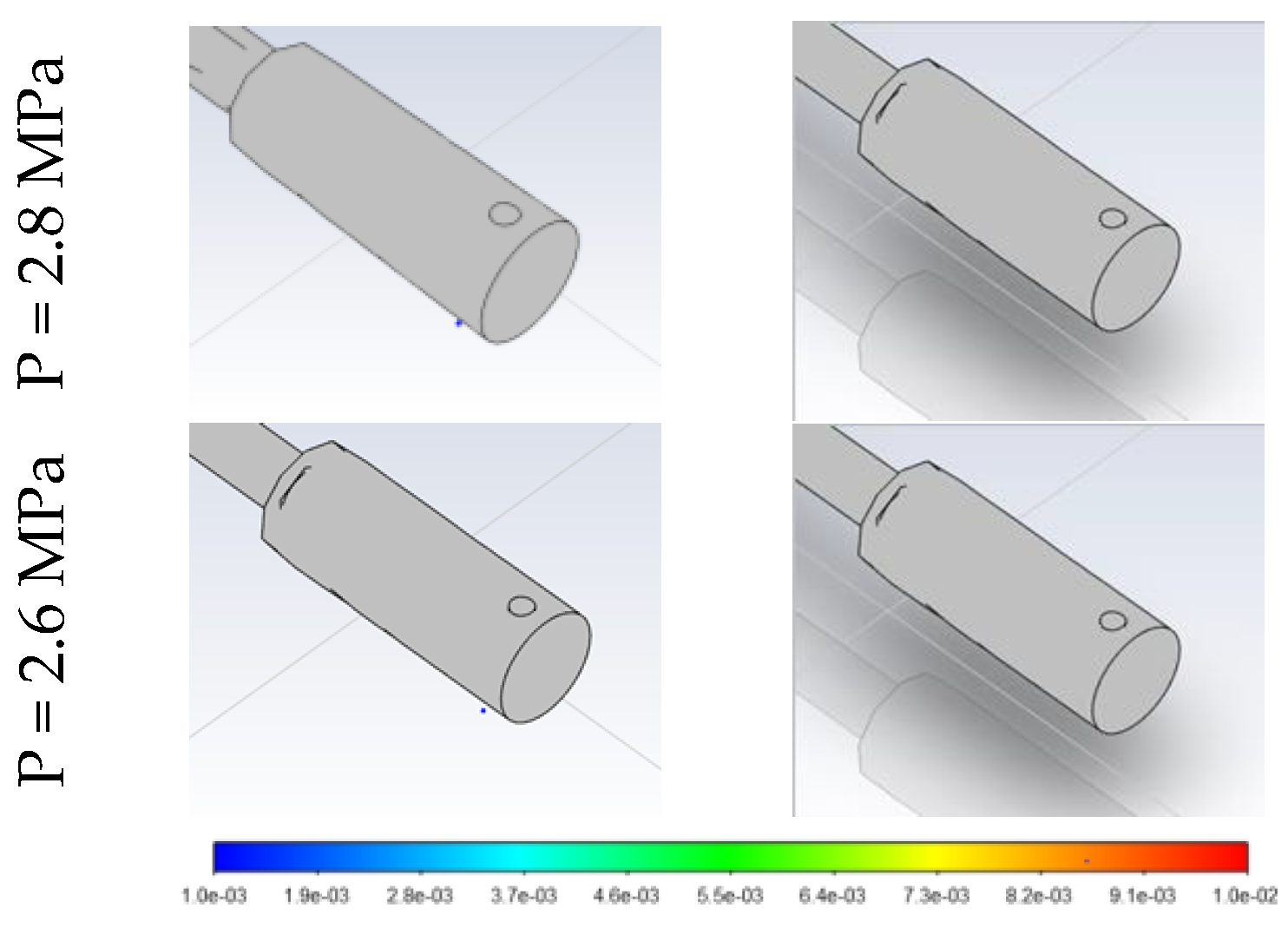
| 2.43 | 0 | 3.5 | 7.1 | 10.6 | 14.1 | 17.7 | 21.2 | 24.7 | 28.3 | 31.7 |
| 2.6 | 0 | 3.4 | 6.9 | 10.2 | 13.6 | 17.1 | 20.5 | 23.8 | 27.3 | 30.7 |
| 2.8 | 0 | 3.3 | 6.6 | 9.9 | 13.1 | 16.5 | 19.7 | 23.0 | 26.3 | 29.6 |
| 2.9 | 0 | 3.2 | 6.5 | 9.7 | 12.9 | 16.2 | 19.4 | 22.6 | 25.9 | 29.1 |
| 5.0 | 0 | 2.4 | 4.9 | 7.4 | 9.8 | 12.3 | 14.8 | 17.2 | 19.7 | 22.1 |
| 7.5 | 0 | 2.0 | 4.0 | 6.0 | 8.0 | 10.1 | 12.0 | 14.0 | 16.1 | 18.1 |
| 8.75 | 0 | 1.8 | 3.7 | 5.6 | 7.4 | 9.3 | 11.2 | 13.0 | 14.9 | 16.7 |
| 9.4 | 0 | 1.8 | 3.6 | 5.4 | 7.2 | 9.0 | 10.8 | 12.5 | 14.4 | 16.1 |
| 10.0 | 0 | 1.7 | 3.5 | 5.2 | 6.9 | 8.7 | 10.4 | 12.2 | 13.9 | 15.6 |
| Burst Disk Section No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Thickness, m | Opening Times, μs | |||||||||
| 0 | 5.0 × 10−5 | 0 | 3.5 | 7.1 | 10.6 | 14.1 | 17.7 | 21.2 | 24.7 | 28.3 | 31.7 |
| 1 | 4.1 × 10−5 | 0 | 3.1 | 6.4 | 9.5 | 12.7 | 15.9 | 19.0 | 22.2 | 25.4 | 28.6 |
| 2 | 2.5 × 10−5 | 0 | 2.4 | 5.0 | 7.4 | 9.8 | 12.4 | 14.8 | 17.3 | 19.8 | 22.2 |
| 3 | 1.8 × 10−5 | 0 | 2.1 | 4.3 | 6.3 | 8.4 | 10.6 | 12.7 | 14.8 | 17.0 | 19.0 |
| 4 | 1.3 × 10−5 | 0 | 1.7 | 3.5 | 5.3 | 7.0 | 8.8 | 10.6 | 12.3 | 14.1 | 15.9 |
| Storage Temperature, K | 80 | 300 |
|---|---|---|
| Storage pressure leading to spontaneous ignition followed by self-extinction, MPa | 8.75 | 2.60 |
| Storage pressure leading to spontaneous ignition and likely transition into a hydrogen jet flame, MPa | 9.40 | 2.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirrone, D.; Makarov, D.; Molkov, V. Spontaneous Ignition of Cryo-Compressed Hydrogen in a T-Shaped Channel System. Hydrogen 2022, 3, 348-360. https://doi.org/10.3390/hydrogen3030021
Cirrone D, Makarov D, Molkov V. Spontaneous Ignition of Cryo-Compressed Hydrogen in a T-Shaped Channel System. Hydrogen. 2022; 3(3):348-360. https://doi.org/10.3390/hydrogen3030021
Chicago/Turabian StyleCirrone, Donatella, Dmitriy Makarov, and Vladimir Molkov. 2022. "Spontaneous Ignition of Cryo-Compressed Hydrogen in a T-Shaped Channel System" Hydrogen 3, no. 3: 348-360. https://doi.org/10.3390/hydrogen3030021
APA StyleCirrone, D., Makarov, D., & Molkov, V. (2022). Spontaneous Ignition of Cryo-Compressed Hydrogen in a T-Shaped Channel System. Hydrogen, 3(3), 348-360. https://doi.org/10.3390/hydrogen3030021







