Abstract
Our study was conducted in the Valle Nuevo National Park and included four habitat classes: tussock grass (Sabapa), pine forest (Pinoc), broadleaf forest (Boslat), and agricultural ecosystem (Ecoag). We had two main objectives: to comparatively describe millipede communities and to determine the relationships between population density/diversity and soil physicochemical variables. The research was cross-sectional and non-manipulative, with a descriptive and correlational scope; sampling followed a stratified systematic design, with eight transects and 32 quadrats of 1 m2, covering 21.7 km. We found a sandy loam soil with an extremely acidic pH. The highest population density of millipedes was recorded in Sabapa, and the lowest in Ecoag. The highest alpha diversity was shared between Boslat (Margalef = 1.72) and Pinoc (Shannon = 2.53); Sabapa and Boslat showed the highest Jaccard similarity (0.56). The null hypothesis test using the weighted Shannon index revealed a statistically significant difference in diversity between the Boslat–Sabapa and Pinoc–Sabapa pairs. Two of the species recorded highly significant indicator values (IndVal) for two habitat classes. We found significant correlations (p < 0.05) between various soil physicochemical variables and millipede density and diversity.
1. Introduction
Numerous studies on the ecology of millipedes (Myriapoda: Diplopoda) have been published, mainly since the second half of the 20th century. These investigations have addressed various objectives and approaches: their role in the decomposition of dead plant matter as facilitators of microbial activity, population dynamics, pest status in some cases, dispersal and migration, temporal activity, responses to anthropogenic interventions, and competition [1]. Key aspects highlighted include their fundamental ecological role in the soil nutrient cycle through the establishment of a complex network of interactions [2,3]; along with other soil meso- and macroinvertebrates, their abundance concerning soil type and leaf litter [4]; the dynamics of their distribution among spatially related ecosystems [5]; and ecological niche modeling to predict the distribution of specific taxa [6].
However, researchers’ attention to the ecological functions of millipedes remains much lower than that devoted to their taxonomy and systematics, despite their potential as indicators of diversity in tropical or subtropical forests [3,7]. Ecological research on groups of soil meso- and macroinvertebrates, including millipedes, should be encouraged, as they respond to environmental disturbances and are significantly related to all indicators of soil quality. This aspect supports their value as indicators of ecosystem services [8]. More specifically, this soil fauna allows for the monitoring of changes and intensification in land use, as well as the progress of rehabilitation in degraded or contaminated soils [9].
In Hispaniola (comprising the Dominican Republic and Haiti), the taxonomy of millipedes began to be documented in the second decade of the 20th century, with the first major contribution published by Ralph V. Chamberlin in 1918, according to Pérez-Asso and Pérez-Gelabert [10]. Since then, numerous studies have been published, with a comprehensive bibliography and commentary available in a recent publication [11]. However, in terms of ecological studies, the situation mirrors what has been described internationally. It is limited to our previous research in Valle Nuevo National Park (VNNP) [12], analyses of soil macrofauna (including millipedes) affected by fire [13], and in cocoa agroecosystems [14]. Our study gains added importance due to its location in a protected area that, unfortunately, faces serious anthropogenic threats. Since VNNP was declared a protected area, many land ownership conflicts have arisen and persist in part, leading to problems derived from land invasions and illegal practices; in addition to the advance of the agricultural frontier, habitat fragmentation, fire, and the introduction of invasive species are factors that threaten the conservation status of their ecosystems, their valuable resources, and their ecosystem services [15].
This research had two main objectives: (1) to describe and compare millipede communities across four habitat classes, each corresponding to a distinct vegetation type; and (2) to determine the relationships between millipede diversity and population density with a set of soil physicochemical variables, and to characterize these relationships in connection with vegetation type.
2. Materials and Methods
2.1. Study Area, Research Design, and Sampling Design
The habitat classes compared in this study—referred to using acronyms valid only for this work—are as follows: (1) agricultural ecosystem (Ecoag), represented by two localities—one with short-cycle crops such as tomato, carrot, cabbage, and bean, among others, and another area previously used for strawberry and flower cultivation; (2) broadleaf forest (Boslat), consisting of a patch of secondary forest with Cecropia schreberiana, Tabebuia berteri, Ocotea leucoxylon, O. patens, Trema micrantha, and Cyathea spp., among other plant species; (3) tussock grass (Sabapa), a formation that extends between pine forests and is dominated by the native grass Danthonia domingensis (Pajon), associated with various shrubs such as Baccharis myrsinites, Rubus sp., Lyonia heptamera, and Myrica cerifera, among others; and (4) pine forest (Pinoc) of Pinus occidentalis, a species endemic to Hispaniola, with an understory of pajon and shrubs such as Garrya fadyenii, Rubus sp., and Fuchsia spp. A comprehensive description of the vegetation throughout the VNNP is available [15,16]. The study approach focused not only on millipede communities (metacommunities) but also on the abiotic factors and limitations that may influence the selected ecosystems, thus supporting an investigation grounded in the concept of the meta-ecosystem, which has already been applied to other groups of organisms [17].
A cross-sectional, non-manipulative study was conducted, with a descriptive and correlational scope. The research focused on comparing the diversity and abundance of millipedes, as well as their relationship with soil physicochemical characteristics, habitat conditions, and vegetation type. The study areas are composed of a metamorphic base with inclusions of igneous and volcanic rocks of Cretaceous origin, surrounded by Tertiary sediments. The soils have been classified as clayey at high altitude and non-calcareous clayey; based on their aridity index, they fall within the dry-humid and semi-humid categories; slope values are predominantly found within the range of 32% to 64%, and greater than 64% in the highest areas [18]. For our southern habitats, the average annual precipitation is 1075.5 mm/year, with a dry season from December to March and a rainy season from May to November (peaking in May), and an average annual temperature of 23.5 °C. In contrast, for the northern habitats of the study, precipitation is 1026.4 mm (with peak values in May), and the average annual temperature is 18.2 °C [15].
The sampling design was stratified systematic [19]: a random starting point was selected for each transect, and from there, the quadrats (sampling units) were repeatedly delineated within the habitat classes being compared. Transects of 20 m in length were established, two in each habitat type. In each transect, four quadrats or sampling units of 1 m2 each were delineated, spaced 5 m apart, leaving a 0.5 m margin from the starting end and before the end (a total of 32 quadrats, 8 in each included ecosystem or habitat type) (Figure 1). The unit of effort consisted of three trained individuals collecting simultaneously for 21 min in each quadrat, using the same stopwatch for timing. In this manner, with some modifications, a methodological procedure was followed based on similar studies of soil macrofauna in the Dominican Republic [14,15,20]. For soil analysis in each transect, four subsamples were taken at a depth of 10–40 cm, at points adjacent to the corresponding quadrats (no more than 2 m away), and were mixed to obtain a composite sample. The transects were distributed along a 21.7 km mountainous route, following the road that crosses the VNNP (Figure 2; Table A1 in Appendix A). The map was produced using QGIS version 3.34.13 “Prizren”, utilizing the vector layer of the park included in the World Database on Protected Areas (WDPA) of the UN Environment Programme World Conservation Monitoring Centre.
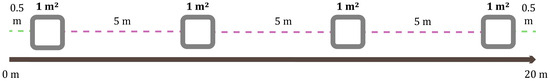
Figure 1.
Sampling transect design. Length of 20 m, divided into four sampling units of 1 m2.

Figure 2.
Study area. Map of Valle Nuevo National Park (VNNP) showing the eight sampling transects, which were located along the road (map created with QGIS 3.34.13 ‘Prizren’ based on the UNEP-WCMC vector layer of the protected area). Ecoag: agricultural ecosystem; Boslat: broadleaf forest; Sabapa: tussock grass; Pinoc: pine forest.
2.2. Field and Laboratory Work Execution
Two prospecting expeditions were carried out in the study areas (10–11 July and 7 August 2020). Millipede and soil sampling continued during three expeditions in the dry season of these mountains: 21–24 November 2020; 7–8 December 2020; and 18–22 January 2021, only once in each sampling unit.
The study habitats were properly characterized: georeferencing and altitude were recorded using a GPS device (Garmin Marine, model 76). Vegetation was documented based on the published literature for the region [10,17,21], supported by photographs and direct consultation with the Botany Department of the National Botanical Garden “Rafael María Moscoso.” To delineate the transects, a measuring tape and flagging tape were used. Millipede sampling always began at 8:00 a.m. and continued until 3:00 p.m. Before collection, each transect underwent a basic physicochemical characterization following both our methodological procedures and a partially modified version of a previous protocol [5]. Temperature (°C) above vegetation (TOV) and below vegetation (TUV) were measured using a Forestry Suppliers digital thermometer, model SN89212. TOV was recorded by placing the probe on the tallest shrub or grass, while TUV was measured at the level of their most superficial roots (without penetrating the soil). Soil temperature (BGT) was measured using a Durac Plus thermometer, model 3811, with the probe inserted to a depth of 5 cm to spatially differentiate it from the TUV variable. Relative air humidity (RH%) was measured with a Testo 608-H1 hygrometer approximately 10 cm above the soil surface.
Millipede collection was conducted manually, using hand shovels and garden hand rakes, as well as forceps and jars (both empty and containing alcohol). Before initiating the standardized 21 min search period within each quadrat, individuals observed moving on the vegetation were first collected. The area was subsequently cleared of superficial debris. If any sizable rock or thick root was encountered after the timing had begun, the stopwatch was momentarily paused to allow for the removal of such obstructions. For the collection of soil subsamples, only new, rust-free instruments were used. The surface layer was first cleared using a flat-edged spade, and then an opening approximately 60 cm wide and 40 cm deep was excavated [22].
The preservation and transportation of the millipedes from the sampling areas followed the methodology of Pérez-Asso [23]. Specimen sorting and identification were carried out in the laboratories of the National Museum of Natural History “Prof. Eugenio de Jesús Marcano” (MNHN), using the institution’s scientific reference collections and the relevant taxonomic literature corresponding to the separated taxa [24,25,26,27].
Soil subsamples from each transect were sieved, then homogenized and placed in labeled plastic bags for transport to the Dominican Agro-Business Laboratory (LAD) of the Dominican Agro-Business Board, Inc. (JAD), located in Santo Domingo, D.N. All physicochemical analyses were conducted in this laboratory: soil texture (%) (sand, clay, and silt) was determined using the Bouyoucos hydrometer method; pH in water (1:2); organic matter (%) was quantified via wet combustion with potassium dichromate (Walkley–Black method); Ca (meq/100 g), Mg (meq/100 g), and K (meq/100 g) were extracted using ammonium acetate at pH 7.0, and potassium chloride 1 N for Ca and Mg; P (meq/100 g) was determined using the modified Olsen method; Fe (mg/kg), Cu (mg/kg), Zn (mg/kg), Mn (mg/kg), and Pb (mg/kg) (bioavailable fraction only, excluding potential accumulation) were extracted using EDTA + sodium bicarbonate at pH 8; exchangeable Al was quantified via acid-base titration (using KCl, NaOH, and HCl); electrical conductivity (EC) (µmhos/cm), extractable acidity (EA) (Al + H+) (meq/100g), and effective cation exchange capacity (CEC) (meq/100g) were also measured.
2.3. Statistical Data Analysis
IBM SPSS Statistics 27 was used for two analyses: (1) Normality and correlation coefficients between the population density/species richness of millipedes and the soil physicochemical variables. The Shapiro–Wilk test was applied to assess normality due to the sample size (<50; 32 in total). Based on the result of this preliminary test, the appropriate statistical path was chosen: parametric (p-value ≥ 0.05) Pearson’s correlation coefficient or non-parametric (p-value ˂ 0.05) Spearman’s rank correlation coefficient. (2) Principal Component Analysis (PCA) for the characterization of the soil in the four habitat classes (20 numerical variables and two nominal ones) to considerably reduce the number of variables and to better visualize, identify, and interpret the phenomena involved. To assist the PCA and reduce the multivariate statistical tool, multidimensional scaling was used, which allowed us to scale the set of soil variables to a multidimensional plane, and analyze the proximities between them.
INFOSTAT 2020 was used to perform a variance analysis of species among the compared habitat classes. R (version 4.4.2) and R Studio (version 2024.12.0+467) were used with the multipatt function from the indicspecies package to calculate the indicator value (IV) of species across the habitat classes, based on the original IndVal index [28,29] and subsequent modifications [30], using 999 permutations to assess significance. For all statistical analyses, a 95% confidence level and a significance threshold of α = 0.05 (two-tailed) were used.
For the comparison of habitat classes, based on their millipede fauna and soil variables, a non-metric multidimensional scaling (NMDS) analysis was performed based on a matrix of the abundance of families for each sampled habitat, and the average of the values of the soil variables using the Bray–Curtis distance and two dimensions, with the vegan package in its version 2.7-1 for R.
Diversity indices were manually calculated for each habitat class from data matrices in Excel. The hypothesis testing for Shannon’s diversity index weighted in pairwise comparisons of habitat classes followed the procedure of Hutcheson [31] and the Satterthwaite approximation for degrees of freedom and the critical t value from the t-Student distribution, implemented using SciPy 1.10+. For this Hutcheson procedure (using Excel), the Shannon index matrix developed for the four habitat classes was used as a starting point, comparing them two-by-two and testing the null hypothesis that their diversities were equal. For each of the two classes, the weighted index was calculated, followed by its variance and the difference between these variances, obtained as the square root of their sum. The t value was obtained by dividing the difference in the weighted diversity of both classes by this latter value. The degrees of freedom were then calculated (using SciPy, level 0.05 two-tailed test), and for these, the value of the t distribution was found in a statistical table. If the t value obtained was greater than the t value in the table, it was concluded that the diversity between both sites was different, and the null hypothesis was rejected. For this analysis, log2 was used [Supplementary Materials].
The theoretical framework supporting the diversity analysis, as well as the selection of indices, estimators, and their respective formulas, followed the approaches of Moreno and Magurran [31,32] (Table A2 in Appendix A). To address alpha diversity, both species richness and community structure were assessed. Based on the records of millipedes collected in each of the four habitat classes, richness was measured as the number of species (S), and Margalef’s index was applied. To evaluate community structure, emphasis was placed on species proportionality, using Simpson’s dominance index and Shannon’s index. Considering the relatively small sample sizes, the non-parametric estimators Chao 2 and first-order Jackknife were applied to explore potential discrepancies between observed and estimated species richness based on the presence of rare species in the samples. The degree of similarity/dissimilarity was assessed to characterize beta diversity, or diversity among habitat classes. For qualitative data, Jaccard’s similarity index was used, and for quantitative data, Sørensen’s quantitative similarity coefficient was applied. Species turnover, or beta diversity in the strict sense, was evaluated using Magurran’s beta diversity index [32].
3. Results
3.1. Characterization of the Sampling Transects
The elevation of the transects was similar: 2003–2384 m a.s.l. (Table A1). Above vegetation (TOV), the lowest temperature value (°C) was recorded in Pinoc (7.5), with a mean and standard deviation of 13.33 ± 3.85, while the highest was in Sabapa (TOV: 25.9; 20.3 ± 4.48). Below ground vegetation (BGT), the lowest temperature was recorded in Pinoc (8; 12.1 ± 3.01), while the highest value was in Sabapa (22.5; 13.84 ± 5.24). The coefficient of variation (CV), with attention to the BGT variable, recorded the lowest value in Boslat (5.24), while the highest value corresponded to the Sabapa habitat class (37.86), followed by Ecoag (26.7). Sabapa was also the habitat with the highest variability in relative air humidity (%RH), with a range of 22.6–89 (64.18 ± 25), and the highest CV in the records (38.95%) (Table S1 in Supplementary Material).
3.2. Population Density, Species Richness, and Distribution of Millipedes
A total of 359 millipede specimens were collected, corresponding to six families, six genera, and 12 species (all endemic to Hispaniola), classified into four orders (Table 1). This number includes 120 specimens identified only at the family level (Chelodesmidae Cook, 1895), as they were early-stage juveniles that could not be identified to lower taxonomic levels.

Table 1.
Identified taxa and their distribution across the four habitat classes in the 32 sampling units.
Millipede density per m2 showed the highest values in Sabapa, with a mean and standard deviation of 28.75 ± 18.87 (Tables S2 and S3). In this habitat class, the median is 30, and its minimum and maximum values are 0–54, with no outliers (Figure 3). The number of specimens from this site was 230 (64.07% of the total). In Ecoag, only one species was found, while in Boslat, Pinoc, and Sabapa, seven species were recorded in each (Table 1). The distribution of density values in Ecoag is strongly concentrated at zero, with only one score outside the normal range (4), which should be considered an outlier. Although the total number of different species was the same in Sabapa, Pinoc, and Boslat, the distribution of their values per sampling unit differed (Figure 4).

Figure 3.
Millipede density per m2 in the habitat classes. Data are based on the eight sampling units from each site. Sabapa: Median, 30; Q1, 14; Q3, 43.5; IQR, 29.5; 0–54. Pinoc: Median, 6.5; Q1, 1.5; Q3, 15; IQR, 13.5; 0–37. Boslat: Median, 5; Q1, 3; Q3, 8; IQR, 5; 0–10. Ecoag: Median, 0; Q1, 0; Q3, 0; IQR, 0; 0–4. Outliers: 4, in Ecoag. Sabapa: tussock grass; Pinoc: pine forest; Boslat: broadleaf forest; Ecoag: agricultural ecosystem. The cross represents the average value for the total transects considered.

Figure 4.
Number of species per sampling unit (1 m2) in the habitat classes. Data are based on the eight sampling units from each site. Sabapa: Median, 2.5; Q1, 2; Q3, 3.5; IQR, 1.5; 0–5. Pinoc: Median, 3; Q1, 1; Q3, 5; IQR, 4; 0–5. Boslat: Median, 3; Q1, 2; Q3, 3.5; IQR, 1.5; 0–5. Ecoag: Median, 0; Q1, 0; Q3, 0; IQR, 0; 0–1. The cross represents the average value for the total transects considered.
The family Chelodesmidae was the most abundant in the overall count (84.96%), with 305 specimens, and recorded the highest number of individuals per quadrat or sampling unit (54) in Sabapa. The chelodesmids corresponded to six species, all belonging to the genus Achromoporus Loomis, 1936, with A. andujari Pérez-Asso 2009 being the most abundant, not only within the family but in the overall count (Table 1). Four of the six families were represented by a single taxon, and two of these by only three and four specimens, respectively. The species with the widest distribution were present in three of the four habitat classes: A. platyurus (Boslat, Pinoc, and Sabapa), Prostemmiulus sp. 1 (Ecoag, Boslat, and Sabapa), and Prostemmiulus sp. 2 (Boslat, Pinoc, and Sabapa). Five species were found exclusively in one habitat class: A. concolor, A. vallenuevo, and Siphonophora sp. (in Pinoc); A. pallidus and Spirobolellus sp. (in Boslat).
The greatest family representation was found in Boslat, with five of the six recorded families, while the lowest was in Ecoag. In the latter, where evident signs of greater anthropogenic impact were observed (tillage and pesticide use), only the family Stemmiulidae was found, represented by one morphospecies of the genus Prostemmiulus. Ecoag was the habitat class with the lowest abundance; only four specimens were collected in a single sampling unit. Stemmiulidae was the most widely distributed family, present in all habitat classes compared. It was also recorded as slightly dominant in Boslat, with a total of 16 specimens, and a maximum score of five. This result was slightly ahead of Chelodesmidae in Boslat, which totaled 15 specimens at the site, also with a maximum score of five (Table 2; Supplementary Materials). The family Siphonophoridae was only found in Pinoc, with a total of seven specimens distributed across two sampling units, and a maximum score of four.

Table 2.
Distribution of families among habitat classes (sum of specimens; minimum–maximum; mean).
Analysis of the variance of species based on the density of specimens per m2 across sampling units revealed significantly different means (p < 0.05) among the habitat classes for seven out of the twelve species: two in Sabapa (A. andujari and A. occultus), three in Pinoc (A. concolor, Siphonophora sp., and Prostemmiulus sp. 2), and two in Boslat (Spirobolellus sp. and Prostemmiulus sp. 1). The two best-fitting models correspond to A. andujari in Sabapa and Siphonophora sp. in Pinoc, with 63% and 62% of their respective variability explained by the model (R2). Moreover, the adjustment for the number of predictors and sample size (adjusted R2) retained a higher predictive power, 40% and 38%, respectively. These two species also showed the highest F-statistic values: 2.70 for A. andujari (model p-value = 0.0260) and 2.58 for Siphonophora sp. (model p-value = 0.0314), indicating a stronger relationship between the variability explained by the model and the residual variability. This suggests that the explanatory variables were more relevant in these particular cases. Additionally, the model showed the greatest overall stability for A. andujari, as this species exhibited the lowest relative data dispersion (CV = 172.54%) (Table A3 in Appendix B). These latest results partially coincide with the values obtained from the determination of species indicator values (IV) by habitat classes. A total of four species showed high indicator values with a p-value < 0.05 (Table 3): three species were associated with a single class, one species with two habitat classes, and no species were associated with all three habitat classes. Spirobolellus sp. and Prostemmiulus sp. 1 were associated with the Boslat class, A. occultus with the Sabapa class, and A. andujari with the combined Pinoc + Sabapa habitat classes. The highest indicator value corresponded to the chelodesmid A. andujari (79.1%, p-value = 0.005), followed by Spirobolellus sp. (70.7%, p-value = 0.007). The statistically significant p-values for these four species, each with an IV above 65% for the specified habitat classes, indicate that their associations are not random. These species may therefore be considered indicators of the respective habitat classes in their current condition, although a detailed assessment of site health remains pending for potential future monitoring.

Table 3.
Indicator values (IV) of species by habitat classes. Only species with statistically significant values at a 95% confidence level (α = 0.05) are included.
3.3. Species Richness and Population Structure
In Ecoag, only one species was found in a single sampling unit, with zero recorded in all other units; as a result, both the Margalef and Shannon indices equal zero (0). In contrast, its Simpson dominance index was the highest (1). Boslat and Pinoc showed the highest values for the Margalef and Shannon indices, respectively (Table 4).

Table 4.
Estimation of species richness and population structure.
The results obtained with the non-parametric estimators were very similar for Boslat and Pinoc: 8 and 7.67 with Chao 2, and identical values with Jackknife 1 (8.75). At the same time, the number of estimated species in both ecosystems was very close to the number of observed species (7): 8 and 7.67 with Chao 2, and 8.75 with Jackknife 1. The greatest discrepancy between estimated and observed species values occurred in Sabapa: 15 and 10.5 estimated with Chao 2 and Jackknife 1, respectively.
For Boslat–Sabapa and Pinoc–Sabapa (Table 5), the Shannon index results are statistically significant at an alpha level of 0.05 (two-tailed test). In both comparisons, the calculated t value, following Hutcheson’s procedure, exceeds the critical t value from Student’s t distribution table. In contrast, diversity is not statistically significant in the Boslat–Pinoc comparison. The Ecoag habitat class was excluded from these comparisons, as its weighted diversity is zero, rendering the Shannon metric and hypothesis testing meaningless in this context.

Table 5.
Hypothesis testing of Shannon diversity for habitat pairs 1.
3.4. Similarity/Dissimilarity Indices and Species Replacement: Beta Diversity
The highest estimated beta diversity value, based on Magurran’s index, was found between the Boslat and Pinoc ecosystems (11.62), with a Jaccard similarity of 0.17, lower than that of the Boslat–Sabapa (0.56) and Sabapa–Pinoc (0.4) pairs, and only higher than the pairs involving Ecoag: Boslat–Ecoag (0.14), Sabapa–Ecoag (0.14), and Pinoc–Ecoag (0) (Table 6).

Table 6.
Similarity, dissimilarity, and beta diversity.
The correspondence between these opposing indices is further confirmed by observing that the lowest Magurran value (6.16) corresponds to the Boslat–Sabapa pair, which also has the highest Jaccard similarity (0.56). This pattern holds for the other combinations, except for the Sabapa–Pinoc pair, which shows both the second-highest Magurran value (8.4) and the second-highest Jaccard similarity (0.4). The presence of Sabapa vegetation is strongly pronounced across the entire plateau of the VNNP, and its spatial connection with Pinoc is consistently high, though it also maintains some connection with Boslat to a lesser extent.
3.5. Analysis of Habitats Based on the Composition of Their Communities with NMDS
Analysis of the distance scores between habitat classes, based on the matrix of millipede families (F1-F6) in two-dimensional space, using NMDS (with R), generated a clear representation of the ecological relationships, achieving a stress value < 0.05 (approximately 0.0001) with 100 permutations, based on the sum of the abundance values of F1-F6 in each habitat class (Figure 5). This achieved stress value constitutes an excellent representation of the real distance between the values of the sampling units. The distinct locations and distances of the habitats are linked to the representative vectors of the families (F1–F6), which are projected according to their relationships (Figure 5). Additionally, the figure was edited to highlight, in parentheses, key data associated with each vector within the habitat class where its trend is most evident.
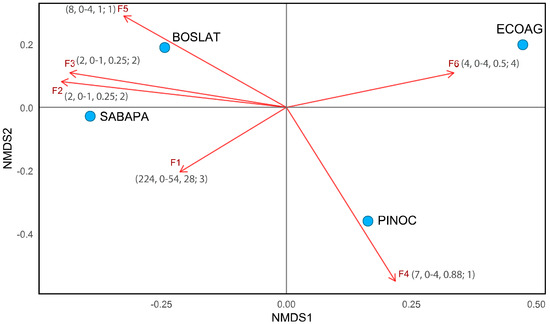
Figure 5.
Two-dimensional graphical representation (NMDS) of the relationships between millipede families and their relationships with habitat classes. F1, Chelodesmidae; F2, Fuhrmannodesmidae; F3, Pyrgodesmidae; F4, Siphonophoridae; F5, Spirobolellidae; F6, Stemmiulidae. The axis values represent the relative positions of the values in two-dimensional space. Aggregate data in parentheses: total number of specimens, minimum–maximum range, mean; number of habitat classes in which the species occurs.
Ecological segregation is evident between habitat classes. First, the separation of Ecoag from the other sites is notable; it hosts the community with the lowest diversity and abundance, limited to the presence of the Stemmiulidae family, represented clearly by the projected vector (F6). Four specimens found in a single sampling unit make this an atypical value for this habitat. The F1 vector points toward Sabapa because of the dominance of the family it represents, Chelodesmidae, in this habitat class. However, Chelodesmidae is not exclusive to this habitat, as it is shared with Pinoc, followed in abundance by Boslat. The Sabapa and Boslat habitat classes are more similar to each other than to Pinoc in terms of family composition. These two habitat classes share four families: Chelodesmidae (F1), Fuhrmannodesmidae (F2), Pyrgodesmidae (F3), and Stemmiulidae (F6). Vector F4, directed toward Pinoc, represents Siphonophoridae, a family found only in this habitat class, similar to Spirobolellidae (F5), found only in Boslat.
The same type of analysis was also conducted using only the three families with the highest abundance values (in the same order): Chelodesmidae, Stemmiulidae, and Spirobolellidae (Figure 6). Only non-zero values with comparatively significant weights were considered. With 100 permutations, a stress value of 0.064 was obtained, indicating a very good ordination (>0.05 and ˂0.10) with minimal distortion in the representation of dissimilarities (with Bray–Curtis index in R). Chelodesmidae (F1) was present in three habitat classes but predominated in Sabapa and Pinoc. The family Stemmiulidae (F6) predominated in Boslat, had a notable presence in Pinoc, and was the only taxon recorded in Ecoag. It was also detected in Sabapa, although not graphically highlighted due to the low scores derived from just two individuals (see matrix in Supplementary Material). Finally, the family Spirobolellidae (F5), previously noted as exclusive to Boslat, is represented by a single point in the graph, corresponding to one of four scores in the matrix (=4), while the remaining three scores are 2, 1, and 1.

Figure 6.
Two-dimensional graphical representation (NMDS) of the relationships between millipedes of the families Chelodesmidae (F1), Stemmiulidae (F6), and Spirobolellidae (F5) and their relationships with habitat classes. The axis values represent the relative positions of the values in two-dimensional space.
3.6. Soil Physicochemical Variables: Characteristics and Correlation with Millipede Density and Richness
The textural analysis of soils in the study classifies them as predominantly sandy loam, with the highest mean sand content recorded in Boslat (86.25%) (Table 7). The soil pH values recorded in this study, with an overall mean of 4.18, fall within the extremely acidic category, with an ultra-acidic value of 3.45 in Boslat—the lowest among all habitat classes [33]. The highest pH was recorded in Sabapa (4.59), corresponding to the very strongly acidic category [33]. The Al saturation (mean) in Boslat was 37.99%, classified as strongly limiting or toxic [34], while in the agricultural ecosystem (Ecoag), the recorded mean was 6.73%, only slightly limiting according to the same source.

Table 7.
Soil physicochemical variables considered in the study for the four compared ecosystems. (mean–standard deviation–coefficient of variation).
Correlation was found between the response variables, density and species richness, and soil variables in 18 statistically significant pairs (α < 0.05)—eight combinations for the response variable Density and ten for Species richness. These significant correlations were distributed as follows: two in Ecoag, four in Boslat, nine in Pinoc, and three in Sabapa. (D = density, R = species richness): Ecoag, D–Cu (+) and R–Cu (+); Boslat, D–K (–), R–Cu (–), R–Ca (+), and R–N (–); Pinoc, D–K (–), D–P (–), D–Ca (–), D–Mg (–), D–CEC (–), D–Zn (–), R–P (–), R–Ca (–), and R–CEC (–); Sabapa, R–N (+), R–EC (+), and R–Fe (+) (Table A4 in Appendix B). All tests were conducted at a 95% confidence level, with alpha = 0.05 (two-tailed). Among all significant correlation coefficients, only the R–N (–) relationship in Boslat was highly significant (–0.847) with p = 0.0080, meeting the threshold for significance at the 0.01 level. A two-dimensional representation using NMDS, based on a matrix of the mean values, allows us to more clearly visualize the relationship between these values and the four habitat classes (Figure 7). One hundred permutations were performed, and a Stress < 0.05 was reached.

Figure 7.
Two-dimensional graphical representation (NMDS) of the relationships between soil variables and their relationships with habitat classes. The axis values represent the relative positions of the values in two-dimensional space. The abbreviations and symbols are the same as those used in Table 7.
The largest cluster of vectors associated with Pinoc and Sabapa is notable, reflecting the affinity of these habitats and their relationship with Boslat; of these, the pH vector stands out in the opposite projection to Cu, Fe, EA, Sat Al (%), N, and OM. As seen above, Chelodesmidae is the dominant family in Sabapa and Pinoc; Spirobolellidae characterizes the Boslat habitat. The organic matter (OM) and nitrogen (N) vectors project strongly toward Boslat, but the main characterization of this habitat is aluminum saturation, the Sat. Al (%) vector, to which we should link extractable acidity (EA), also projected toward this site. Likewise, Sat. Al appears closely associated with Fe and Cu. The P, K, and Ca vectors are associated with Ecoag, where their highest values would be associated with the application of fertilizers and lime to crops. However, cation exchange capacity (CEC) and lead are also projected toward this habitat class; the CEC vector appears to be associated with K. Boslat and Ecoag share the projection of the EC vector, which is slightly inclined toward the former. The metals Fe and Cu appear to be associated with each other and projected toward Boslat. These results, displayed in the two-dimensional space of NMDS, correspond to those shown in Table 7.
3.7. Principal Components Analysis (PCA) and Multidimensional Scaling
The PCA managed to reduce the space of the original set of variables to five (5) principal components, with an explanatory variance of 94.32%. However, the representation in space of the five principal components obtained is complex, so in this work, only the first three (3) principal components were considered, which managed to describe 81.03% of the variability of the data (Table 8). The first principal component represents a phenomenon that can be described by the variables (OM, pH, N, AE, and Fe), describing 43.55% of the total variability of the data. The second principal component represents a phenomenon that involves the variables (D, Ca, Mg, P, K, and CEC), which describes 24.69% of the total variability of the data. For its part, the third component, with a level of description of the total variability of the data of 12.79% (Table 8), relatively lower than the first two principal components, explains a phenomenon that fundamentally involves the variables (S, St, C, OM, pH, EC, EA, Ca, Al, Pb, Cu, and Zn). The level of contribution of each variable in each principal component can be corroborated (Table 9).

Table 8.
Total explained variance obtained in the Principal Components Analysis.

Table 9.
Component scoring coefficient matrix.
The three-dimensional representation of the principal components makes the process of identifying and analyzing them considerably more difficult, which is why the variable space was reduced using multidimensional scaling analysis. The model obtained recorded a raw normalized stress value of 0.00083, a considerably low value, confirming the quality of the model fit (values below 0.15 are considered quite good). The quality of the model obtained is also corroborated by the value of the Tucker coefficient of congruence (0.99959), very close to 1. The obtained model recorded a raw normalized Stress value of 0.00083, a considerably low value, confirming the quality of the model fit (values lower than 0.15 are considered quite good). The quality of the obtained model is also corroborated by the value of the Tucker Congruence Coefficient (0.99959), very close to 1. According to the results obtained in the multidimensional scaling analysis (Figure 8), it can be clearly seen that Dimension 1, related to the first principal component (PCA), explains a phenomenon in which iron (Fe) differs considerably from the rest of the variables considered.

Figure 8.
Multidimensional scaling analysis of the relationships between soil variables. D, distance in the transect; MO (organic matter); arc (clay); CE (electrical conductivity); AE (extractable acidity); arc (clay); L (silt); A (sand).
This phenomenon, in turn, is more closely linked to the Boslat habitat class, where the Spirobolellidae family dominates (Figure 5), as we saw previously. A similar phenomenon occurs with Dimension 2 and principal component 2, represented by a phenomenon where P (phosphorus) differs considerably from the rest of the variables studied. This phenomenon is related to the Ecoag habitat class, with the sole presence of the Stemmiulidae family (Figure 5). Dimension 3, for its part, represents significant differences between the variables C and (S and St), which are represented in the third principal component and are contrasted, with the phenomenon being common across the different habitats. These behaviors can help identify the phenomena associated with these dimensions by conducting new studies, some of which could be manipulative.
4. Discussion
The millipede representativeness found in our study, viewed overall, could be considered high when compared with the historical taxonomic record of the VNNP region and nearby localities [10,27]. This is a broad region that, in addition to the four habitat classes included in this study, geographically encompasses many other habitat types: 100% of the orders (4), 86% of the families (6 out of 7; except for Rhinocricidae), 67% of the genera (6 out of 9; missing were Anadenobolus, Henicomus, and Jeekelia), and finally 52.2% of the species (12 out of 23), assuming that our five morphospecies identified only to the genus level would correspond to an equal number of species from that genus in the historical record (Supplementary material). The absence of the family Rhinocricidae—which accounts for one genus and one species in the comparative record—can be explained by the lack of dead tree trunks within the delimited transects, a habitat where these millipedes are typically found in Dominican forests.
These general data could be compared with the results of some studies conducted outside of Hispaniola, but this would necessarily be an indirect comparison, as they differ in methodology and in the unique characteristics of the millipede fauna in each location: Puerto Rico [35], Brazil [3], Hungary [5], Swiss cities [36], and Colombia [37].
In the same region of the VNNP, we have a study conducted ten years ago [12]. That research included the same habitat classes, except for the agricultural ecosystem, that we incorporated and was carried out during the rainy season (ours was conducted in the dry season). Furthermore, their sampling design differed, and in the current research, the analysis of variance of the species and their indicator value in the respective habitat classes are introduced, as well as the correlation with 19 physicochemical variables of the soil and NMDS analysis.
The alpha and beta diversity results of these two studies show differences that may not be due exclusively to the sampling methodology and the unit of effort, but perhaps to the influence of the rain/no rain factor due to the season of the year; it would be interesting to follow up on this topic in subsequent studies of the millipedes of these mountains. Nonetheless, in a study of spiders conducted exclusively in Sabapa, no significant differences were found between seasons for the Shannon and Pielou indices [38]. However, considering the confirmed aggregation of Chelodesmidae millipedes beneath the roots of Danthonia domingensis in Sabapa, as shown by our study during the dry season, rainfall may represent a limiting factor in this ecosystem. At the same time, it may promote greater millipede density in the adjacent pine forest during that season, in line with the findings of both studies, although for the moment this is nothing more than conjecture. This connection could be explored further by including other zoological groups within a metacommunity or meta-ecosystem research framework [17].
The relationship between diversity and species composition of soil-dwelling millipedes and landscape structure was studied in 12 forest patches in northeastern Puerto Rico [35], revealing a positive correlation between these response variables and both the amount of forest in the surrounding matrix and the connectivity among patches—rather than with specific characteristics such as patch shape and area. In southeastern Brazil, a relationship was found between millipede diversity and habitat heterogeneity; however, species composition appeared to be independent of this heterogeneity [3]. In our case, with different objectives, methodology, and not directly addressing vegetation structure, we confirmed that the evident anthropogenic impacts in Ecoag correspond to the very poor results found there—only one species and just four specimens (Section 3.2). Sabapa, the second most impacted ecosystem and with lower vegetation cover than Boslat and Pinoc, recorded by far the highest millipede abundance. Studies from other latitudes have reported significantly lower species richness and abundance in pine forests compared to primary and secondary forests, along with higher dissimilarity values estimated using the Jaccard index [3]. When comparing grasslands (partially comparable to our Tussock grass or Danthonia Savannah) with forests, the number of species in forests was significantly higher [5].
It was to be expected that Ecoag would present the lowest alpha diversity values [37,38,39], a situation further aggravated in our case by the presence of short-cycle crops involving tillage and pesticide use. Changes in land use negatively affect the diversity of diplopods. It has been shown that pesticides reduce both the abundance and diversity of soil macrofauna [40]. These two factors—pesticide use and tillage—are likely responsible for the very low diversity and abundance observed in this ecosystem compared to the other three, which is consistent with its lowest soil organic matter value.
5. Conclusions
The soil in the compared habitats is predominantly sandy loam, with low clay and silt content, good aeration and drainage, and an extremely acidic pH with a mean below 5.5. Soil acidity may be contributing to plant growth deficiencies due to the low availability of nutrients such as Ca2+ and Mg2+, as well as increased toxicity and reduced organic matter decomposition caused by decreased microbial activity. The low concentrations of these two cations may be associated with the toxic levels of Al3+ and Mn2+ found in the soils. Among the four compared ecosystems, Mn2+ values were very high, with the lowest level recorded precisely in Sabapa, the habitat class exhibiting the highest population density of millipedes.
The positive and negative correlations between a significant number of physicochemical variables and the population density and species richness of millipedes, justify special monitoring and more precise characterization of these high mountain soils concerning their macroinvertebrate fauna in general.
Analysis of the composition and population density of the four habitat classes compared reveals obvious ecological segregation. The Sabapa and Boslat habitats are most similar in terms of their millipede taxonomic composition, but the Pinoc and Sabapa habitats are most similar in terms of abundance. These three habitat classes show marked differences in composition and abundance compared to Ecoag, with the presence of only four specimens, which correspond to the same family (Stemmiulidae) and were found in the same sampling unit. The soil in both sampling transects within Ecoag exhibits signs of degradation, likely resulting from excessive fertilizer application, continuous tillage, and intensive pesticide use. These results are evidenced by markedly lower concentrations of nitrogen and organic matter, accompanied by elevated levels of phosphorus and potassium. Beyond these analytical indicators, additional physical evidence includes nearby deforestation, construction activities, erosion, and soil compaction.
Shannon diversity was statistically significant (95% confidence level and α = 0.05, two-tailed) for the Sabapa–Boslat and Sabapa–Pinoc pairs. The highest indicator value scores found for several species in specific habitat classes could be closely linked to somewhat favorable conditions in the respective environments. The taxa with the highest indicator values for the habitat classes considered were Achromoporus andujari (Chelodesmidae), associated with Sabapa and Pinoc (p = 0.005), and Spirobolellus sp., associated with Boslat (p = 0.007). The Sabapa ecosystem, with the lowest estimated vegetation cover (only higher than that of Ecoag) and the greatest variability in relative humidity and temperature values, accounted for 64.07% of the total specimens collected, predominantly from the Chelodesmidae family. This ecosystem presents unique morphological features that favor millipede aggregation beneath the roots of Danthonia domingensis, and it is likely to play important ecological roles as a corridor for the remaining ecosystems during the dry and rainy seasons in the VNNP mountains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies6030055/s1: Supplementary Materials S1. This file contains nine sheets: 1, Table S1—temperature (°C) and relative humidity (RH, %) recorded in the two transects of each ecosystem type; 2, General list and proportions—list of families and species with their relative abundances in each ecosystem and overall; 3, Shannon—Shannon evenness index and results of hypothesis testing; 4, Density—Tables S2 and S3 show the number of specimens per sampling unit, organized by family and by species; 5, Diplo and soil (Table S4)—abundance, species richness, and results in relation to physicochemical soil variables; 6, Normality test—preliminary normality tests to determine the appropriate correlation coefficient; 7, Variance—analysis of variance of species by ecosystem; 8, Correlation—summary of correlation results between the response variables (species density and richness) and each physicochemical parameter, using Pearson or Spearman coefficients according to normality; 9, Taxonomic ordering and species list, and 10) NMDS coordinate matrix.
Author Contributions
C.S.: conceptualization, methodology, field investigation, writing—original draft preparation, review, and editing; U.J.J.-H.: advice, supervision, review, editing, and corrections; J.B.-V.: advice, supervision, review, and corrections. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Higher Education, Science and Technology (MESCYT), through the National Fund for Innovation and Scientific and Technological Development (FONDOCYT), which granted a scholarship to the first author (C.S.) for doctoral studies in Environmental Sciences at Technological Institute of Santo Domingo (INTEC), Santo Domingo, Dominican Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in this article and in the Supplementary Materials.
Acknowledgments
The research that supports this article was carried out as part of the Doctorate in Environmental Sciences at INTEC, Santo Domingo. The Ministry of Higher Education, Science and Technology (MESCYT) partially funded the doctoral studies of the first author. A. Cordero Rivera (Universidade de Vigo, ECOEVO LAB, Spain) provided valuable suggestions and corrections to the manuscript. The National Museum of Natural History “Eugenio de Jesús Marcano” (MNHN, Dominican Republic) facilitated fieldwork activities. Biologists (MNHN) A. León, E. Gabot, and J. Almonte assisted the first author with millipede and soil sampling, and O. Álvarez provided support in figure editing. A. Pimentel (IDIAF, Santo Domingo, Dominican Republic) supported the use of INFOSTAT 2020 software for the analysis of species variance. Chat GPT-4 (Open AI, 2025) was used to check and correct the English in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Georeferencing of Transects and Their Locations
The eight sampling transects, located along the San José de Ocoa–Constanza road, extended from La Nuez (in the south) to Rancho en Medio (in the north), and encompassed the four habitat classes under comparison: Ecoag 1–2, both situated less than 50 m from the roadside, with estimated vegetation cover of 5% and 10%, respectively; Boslat 1–2, located 50 and 75 m from the road, respectively, with vegetation cover estimated at 95–100%; Sabapa 1–2, located 50 and 75 m from the road, respectively, with vegetation cover estimated at 80–90%; and Pinoc 1–2, located 100 and 150 m from the road, with estimated vegetation cover ranging from 85% to 95%.

Table A1.
Sampling Transects: Georeferencing and Locations.
Table A1.
Sampling Transects: Georeferencing and Locations.
| Transects | Coordinates | Altitude (m a.s.l) | Locations |
|---|---|---|---|
| Ecoag 1 | 18°40′55.5″ N, 70°35′20.2″ O | 2003 | La Nuez |
| Ecoag 2 | 18°47′14.6″ N, 70°38′53.7″ O | 2333 | Rancho en Medio |
| Boslat 1 | 18°41′49.7″ N, 70°35′27.6″ O | 2252 | Vuelta de la Culebra |
| Boslat 2 | 18°41′47.5″ N, 70°35′32.3″ O | 2243 | Vuelta de la Culebra |
| Sabapa 1 | 18°42′24.5″ N, 70°36′08.8″ O | 2375 | Sabana Los Frailes |
| Sabapa 2 | 18°45′39.6″ N, 70°38′14.0″ O | 2278 | Sabana Quéliz |
| Pinoc 1 | 18°42′31.9″ N, 70°36′04.5″ O | 2384 | North of the Sabana Los Frailes |
| Pinoc 2 | 18°45′39.6″ N, 70°38′13.7″ O | 2279 | Sabana Quéliz |

Table A2.
Formulas Used for Diversity and Explanatory Breakdown of Their Terms According to Magurran [31] and Moreno [32].
Table A2.
Formulas Used for Diversity and Explanatory Breakdown of Their Terms According to Magurran [31] and Moreno [32].
| Indices and Estimators | Formulas Formula | Components |
|---|---|---|
| Margalef diversity index | S = Number of species N = Total number of individuals ln = natural logarithm | |
| Simpson index | pi = Proportional abundance of species i in the sample | |
| Shannon index | * | pi = as above; ln = natural logarithm |
| Chao 2 | Chao 2 = S est = Estimated number of species; S obs = Observed number of species L = Number of species occurring in only one sample (“uniques”); M = Number of species occurring in exactly two samples | |
| Jackknife 1 | m = Number of samples; S and L as defined in the Margalef index and Chao 2 estimator, respectively. | |
| Jaccard Similarity Coefficient | a = Number of species present at site A; b = Number of species present at site B; c = Number of species shared by both sites A and B | |
| Dissimilarity | 1-S | S = Jaccard Coefficient value |
| Quantitative Sørensen Similarity Coefficient | aN = Total number of individuals at site A; bN = Total number of individuals at site B; pN = Sum of the lowest abundances for each species shared between both sites | |
| Beta Diversity Index (β) | a = Number of species present at site A; b = Number of species present at site B; Ij = Jaccard index value |
* For these index log2 was used.
Appendix B
Analysis of Variance of the Species and Correlation Coefficient Results

Table A3.
Analysis of variance of species population density across all sampling units (N = 32; R2, adjusted R2, and CV%), and comparison of their means across the four separate habitats 1.
Table A3.
Analysis of variance of species population density across all sampling units (N = 32; R2, adjusted R2, and CV%), and comparison of their means across the four separate habitats 1.
| Species | R2 | R2 Aj | CV (%) | F | p-Value |
|---|---|---|---|---|---|
| Achromoporus andujari | 0.63 | 0.4 | 172.54 | 2.70 | 2Sig: Sabapa |
| A. concolor | 0.57 | 0.29 | 330.87 | 2.07 | Sig: Pinoc |
| A. occultus | 0.55 | 0.27 | 269.45 | 1.95 | Sig: Sabapa |
| A. platyurus | 0.45 | 0.1 | 259.31 | 1.27 | No sig |
| A. pallidus | 0.43 | 0.07 | 545.02 | 1.20 | No sig |
| A. vallenuevo | 0.42 | 0.06 | 549.87 | 1.15 | No sig |
| Chilaphrodesmus sp. | 0.3 | 0 | 337.04 | 0.69 | No sig |
| Docodesmus angustus | 0.38 | 0 | 337.55 | 0.99 | No sig |
| Siphonophora sp. | 0.62 | 0.38 | 313.2 | 2.58 | Sig: Pinoc |
| Spirobolellus sp. | 0.41 | 0.04 | 314.8 | 1.11 | Sig: Boslat |
| Prostemmiulus sp. 1 | 0.52 | 0.21 | 196.86 | 1.69 | Sig: Boslat |
| Prostemmiulus sp. 2 | 0.55 | 0.27 | 249.53 | 1.96 | Sig: Pinoc |
1 A 95% confidence level is used (p ≥ 0.05, not significant; p < 0.05, significant). 2 Sig: significant difference.

Table A4.
Correlation between the response variables (density–richness) and the physicochemical variables of the soil. Tests were performed at a 95% confidence level (alpha: 0.05, two-tailed), including only statistically significant values. The correlation coefficient selected in each case was based on the Shapiro–Wilk normality test 1.
Table A4.
Correlation between the response variables (density–richness) and the physicochemical variables of the soil. Tests were performed at a 95% confidence level (alpha: 0.05, two-tailed), including only statistically significant values. The correlation coefficient selected in each case was based on the Shapiro–Wilk normality test 1.
| Response Variable | Soil Variables | Ecoag | Boslat | Pinoc | Sabapa |
|---|---|---|---|---|---|
| Density (individuals/m2) | Cu | S: 0.756, p-0.0300 | - | - | - |
| K | - | P: −0.721, p-0.0435 | S: −0.755, P-0.0305 | - | |
| P | - | - | S: −0.805, p-0.0159 | - | |
| Ca | - | - | P: −0.781, p-0.0221 | - | |
| Mg | - | - | S: −0.766, p-0.0267 | - | |
| ECEC 2 | - | - | S: −0.738, P-0.0366 | - | |
| Zn | - | - | S: -0.762, p-0.0280 | - | |
| Richness (species/m2) | Cu | S: 0.756, p-0.0300 | S: −0.717, p-0.0453 | - | - |
| P | - | - | S: −0.823, p-0.0121 | - | |
| Ca | - | S: 0.710, p-0.0484 | P: −0.822, p-0.0123 | - | |
| ECEC | - | - | S: −0.766, p-0.0267 | - | |
| N | - | S: −0.847 **, p-0.0080 | - | S: 0.741, p-0.0356 | |
| EC | - | - | - | P: 0.758, p-0.0294 | |
| Fe | - | - | - | P: 0.744, p-0.0344 |
1 S = Spearman’s coefficient; P = Pearson’s coefficient. 2 ECEC = effective cation exchange capacity; EC = electrical conductivity. ** Significant correlation at the 0.01 level (two-tailed).
References
- Hopkin, S.P.; Read, H.J. The Biology of Millipedes; Reprinted; Oxford University Press: New York, NY, USA, 2002; pp. 158–182. [Google Scholar]
- Bueno-Villegas, J.; Sierwald, P.; Bond, J.E. Diplopoda. In Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México: Hacia una Síntesis de su Conocimiento; Llorente Bousquets, J.E., Morrone, J.J., Yáñez Ordóñez, O., Vargas Fernández, I., Eds.; Conabio: Mexico D.F., Mexico, 2004; Volume IV, pp. 569–599. Available online: https://www.biodiversidad.gob.mx/publicaciones/versiones_digitales/Artropodos4_links.pdf (accessed on 15 July 2024).
- Rodrigues, P.E.; Costa-Schmidt, L.E.; Ott, R.; Rodrigues, E.N.L. Influence of forest structure upon the diversity and composition of edaphic diplopods. J. Insect Conserv. 2017, 21, 297–306. [Google Scholar] [CrossRef]
- Adis, J.; de Morais, J.W.; Ribeiro, E.F. Vertical distribution and abundance of arthropods in the soil of a neotropical secondary forest during the dry season. Trop. Ecol. 1987, 28, 174–181. Available online: https://museum.wa.gov.au/catalogues/pseudoscorpions/bibliography/vertical-distribution-and-abundance-arthropods-soil-neotropical (accessed on 19 April 2025).
- Bogyó, D.; Magura, T.; Nagy, D.D.; Tóthmérész, B. Distribution of millipedes (Myriapoda, Diplopoda) along a forest interior–forest edge–grassland habitat complex. ZooKey 2015, 510, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, F.M.; Bouzan, R.S.; Rodrigues, P.E.S.; Almeida, T.M.; Ott, R.; Brescovit, A.D. Ecological niche modeling predicting the potential invasion of the non-native millipede Oxidus gracilis (C. L. Koch, 1847) (Polydesmida: Paradoxosomatidae) in Brazilian Atlantic Forest. Ann. Soc. Entomol. Fr. 2020, 56, 387–394. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Velasquez, E.; Lavelle, P. Soil macrofauna as an indicator for evaluating soil based ecosystem services in agricultural landscapes. Acta Oecol. Int. J. Ecol. 2019, 100, 103446. [Google Scholar] [CrossRef]
- Cabrera-Dávila, G.D.L.C. Evaluación de la Macrofauna Edáfica Como Bioindicador del Impacto del uso y Calidad del Suelo en el Occidente de Cuba. Ph.D. Thesis, Universidad de Alicante-Instituto de Ecología y Sistemática, La Habana, Cuba, 9 January 2019. Available online: https://rua.ua.es/dspace/handle/10045/88889 (accessed on 10 July 2024).
- Pérez-Asso, A.R.; Pérez-Gelabert, D.E. Checklist of the millipeds (Diplopoda) of Hispaniola. Bol. SEA 2001, 28, 67–80. Available online: http://sea-entomologia.org/PDF/BOLETIN_28/B28-009-067.pdf (accessed on 7 February 2023).
- Suriel, C.; Bueno-Villegas, J.; Means, J.C.; Bouzan, R.S. A bibliographic review of the Chelodesmidae of the Antilles and Bahamas (Diplopoda: Polydesmida). Zootaxa 2024, 5538, 247–263. [Google Scholar] [CrossRef]
- Rodríguez-Soto, K.; Suriel, C. Comparative study of millipedes communities (Arthropoda: Diplopoda) present in highland savanna, pine forest, and broadleaf forest of Valle Nuevo National Park, Dominican Republic. Novit. Caribaea 2015, 8, 50–60. [Google Scholar] [CrossRef]
- Sánchez-Ruiz, A.; Suriel, C.; de los Santos, G. Soil arthropods post-fire sampling in pine forests from the National Park José del Carmen Ramírez, Dominican Republic. Novit. Caribaea 2009, 2, 30–39. [Google Scholar] [CrossRef]
- Guittonneau, M. Study of the Soil Macrofauna in Tropical Cacao-Based Agroforestry Systems in the Dominican Republic. Master’s Thesis, Isara, Agro School for Life, Norwegian University of Life Sciences, Ås, Norway, September 2021. Available online: https://nmbu.brage.unit.no/nmbu-xmlui/bitstream/handle/11250/2832359/Master%20thesis_Marie%20Guittonneau_NMBU.pdf?sequence=1&isAllowed=y (accessed on 21 September 2024).
- Núñez, F.; Ramírez, N.; McPherson, M.; Portorreal, F. Conservation Plan for the Juan B. Pérez Rancier National Park (Valle Nuevo); Secretariat of State for the Environment and Natural Resources and the Moscoso Puello Foundation: Santo Domingo, Dominican Republic, 2002; pp. 9–87. Available online: https://bvearmb.do/handle/123456789/5257 (accessed on 5 February 2024).
- Guerrero, A.; Ramírez, N.; Veloz, A.; Peguero, B. Vegetation and Flora of Juan Bautista Pérez Rancier National Park (Valle Nuevo). In Integrated Ecological Assessment of Juan Bautista Pérez Rancier National Park (Valle Nuevo); Núñez, F., Ed.; Secretariat of State for the Environment and Natural Resources and the Moscoso Puello Foundation: Santo Domingo, Dominican Republic, 2002; pp. 34–56. Available online: https://bvearmb.do/handle/123456789/2077 (accessed on 5 February 2024).
- Rivas-Torres, A.; Cordero-Rivera, A. A review of the density, biomass, and secondary production of odonates. Insects 2024, 15, 510. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment and Natural Resources. Atlas de Biodiversidad y Recursos Naturales de la República Dominicana, 2nd ed.; Ministry of Environment and Natural Resources: Santo Domingo, Dominican Republic, 2011. [Google Scholar]
- Cruz Flores, D.D.; Martínez Borrego, D.; Fontenla, J.L.; Mancina, C.A. Inventarios y estimaciones de la biodiversidad. In Diversidad Biológica de Cuba: Métodos de Inventario, Monitoreo y Colecciones Biológicas; Mancina, C.A., Cruz, D.D., Eds.; Editorial AMA, La Habana, Cuba; Instituto de Ecología y Sistemática: La Habana, Cuba, 2017; pp. 26–43. Available online: https://ruffordorg.s3.amazonaws.com/media/project_reports/Cap%C3%ADtulo%2015.%20Invertebrados%20Cavern%C3%ADcolas.pdf (accessed on 24 June 2024).
- De los Santos, G. Araneofauna en Sabanas de Montañas Altas del Valle de Lilís y Sabana de Macutico, República Dominicana. Master’s Thesis, Instituto Tecnológico de Santo Domingo (Intec), Santo Domingo, Dominican Republic, 2019. [Google Scholar]
- Peguero, B. Vegetation Diversity and Structure in the Tussock grass of Valle Nuevo, Central Mountain Range, Dominican Republic. Moscosoa 2013, 18, 137–153. Available online: https://bvearmb.do/handle/123456789/636 (accessed on 5 February 2024).
- Calderón-Medina, C.L.; Bautista-Mantilla, G.P.; Rojas-González, S. Propiedades químicas, físicas y biológicas del suelo, indicadores del estado de diferentes ecosistemas en una terraza alta del departamento del Meta. Orinoquía 2018, 22, 141–157. [Google Scholar] [CrossRef]
- Pérez-Asso, A.R. Colecta y conservación de diplópodos. Cocuyo 1995, 2, 2–3. [Google Scholar]
- Loomis, H.F. Millipeds of the West Indies and Guiana, collected by the Allison V. Armour Expedition in 1932 (with four plates). Smithson. Misc. Collect. 1934, 89, 1–69. Available online: https://repository.si.edu/handle/10088/23919 (accessed on 10 March 2021).
- Loomis, H.F. The millipeds of Hispaniola, with descriptions of a new family, new genera, and new species. Bull. Mus. Comp. Zool. 1936, 80, 3–197. Available online: https://www.biodiversitylibrary.org/part/146395 (accessed on 10 March 2021).
- Loomis, H.F. Millipeds collected in Puerto Rico and the Dominican Republic by Dr. P. J. Darlington in 1938. Bull. Mus. Comp. Zool. 1941, 88, 17–80. Available online: https://www.biodiversitylibrary.org/part/6184 (accessed on 10 March 2021).
- Pérez-Asso, A.R. El género Achromoporus (Diplopoda: Polydesmida: Chelodesmidae) en República Dominicana: Especies nuevas y sinonimias. Solenodon 2009, 8, 33–81. Available online: https://archive.org/details/solenodon-8-033-081 (accessed on 15 March 2021).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Bakker, J.D. Increasing the utility of Indicator Species Analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement, 1st ed.; Croom Helm Ltd.: London, UK, 1988; pp. 1–179. Available online: http://www.bio-nica.info/Biblioteca/Magurran2004MeasuringBiological.pdf (accessed on 1 December 2024).
- Moreno, C.E. Métodos Para Medir la Biodiversidad, 1st ed.; Cyted, Orcyt-UNESCO, SEA: Zaragoza, España, 2001; pp. 1–83. Available online: http://entomologia.rediris.es/sea/manytes/metodos.pdf (accessed on 2 September 2024).
- Soil Science Division Staff. Examination and Description of Soil Profiles, Chapter 3. In Soil Survey Manual. USDA Handbook 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017; pp. 83–233. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/SSM-ch3.pdf (accessed on 27 January 2025).
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: Columbus, OH, USA, 2016; pp. 374–461. [Google Scholar]
- Galanes, I.T.; Thomlinson, J.R. Soil millipede diversity in tropical forest patches and its relation to landscape structure in northeastern. Biodivers. Conserv. 2011, 20, 2967–2980. [Google Scholar] [CrossRef]
- Vilisics, F.; Bogyó, D.; Sattler, T.; Moretti, M. Occurrence and assemblage composition of millipedes (Myriapoda, Diplopoda) and terrestrial isopods (Crustacea, Isopoda, Oniscidea) in urban areas of Switzerland. ZooKeys 2012, 176, 199–214. [Google Scholar] [CrossRef]
- Ruiz-Cobo, D.H.; Bueno-Villegas, J.; Feijoo-Martinez, A. Uso de la tierra y diversidades alfa, beta y gamma de diplópodos en la cuenca del río Otún, Colombia. Univ. Sci. 2010, 15, 59–67. Available online: https://www.redalyc.org/pdf/499/49913062006.pdf (accessed on 21 June 2024). [CrossRef]
- Carrero-Jiménez, S.; de los Santos, G. Araneofauna (Arachnida: Araneae) de la sabana de pajón en el Parque Nacional Valle Nuevo, República Dominicana y su comparación entre dos temporadas del año. Novit. Caribaea 2014, 7, 61–71. [Google Scholar] [CrossRef]
- Pompozzi, G.; Ferretti, N.; Copperi, S.; Simó, M.; Ferrero, A.A. Arthropod fauna of winter wheat of southwest Buenos Aires province, Argentina. Mun. Ent. Zool. 2014, 9, 182–190. Available online: https://www.munisentzool.org/Issue/2014-vol-9-number-1-18 (accessed on 16 January 2025).
- Nare, R.W.A.; Savadogo, P.W.; Traore, M.; Gountan, A.; Nacro, H.B.; Sedogo, M.P. Soil macrofauna behaviour in the presence of pesticides and organic amendments. J. Geosci. Environ. Prot. 2017, 5, 202–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).