Forest Management Effects on Breeding Bird Communities in Apennine Beech Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bird Survey
2.3. Data Analysis
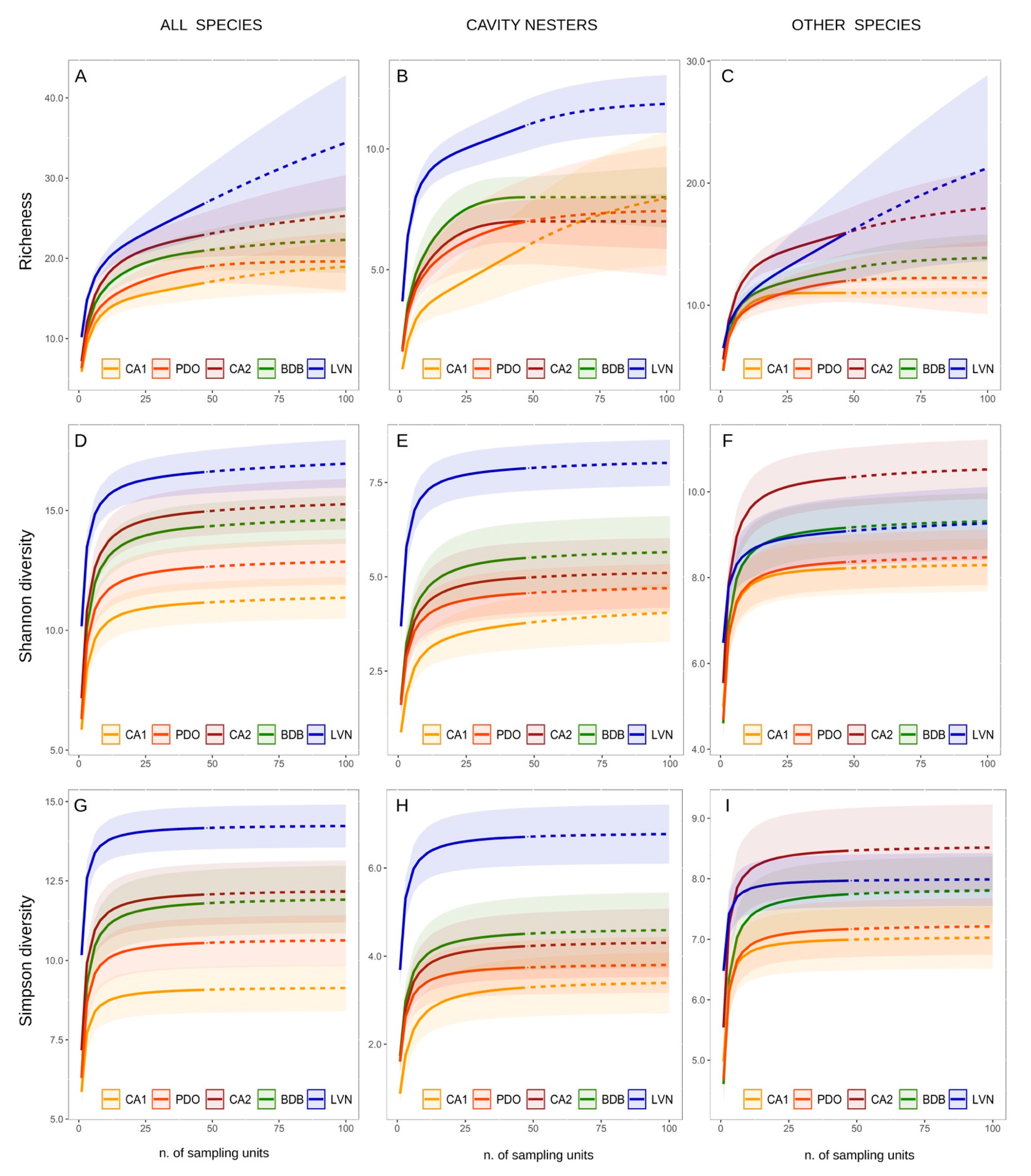
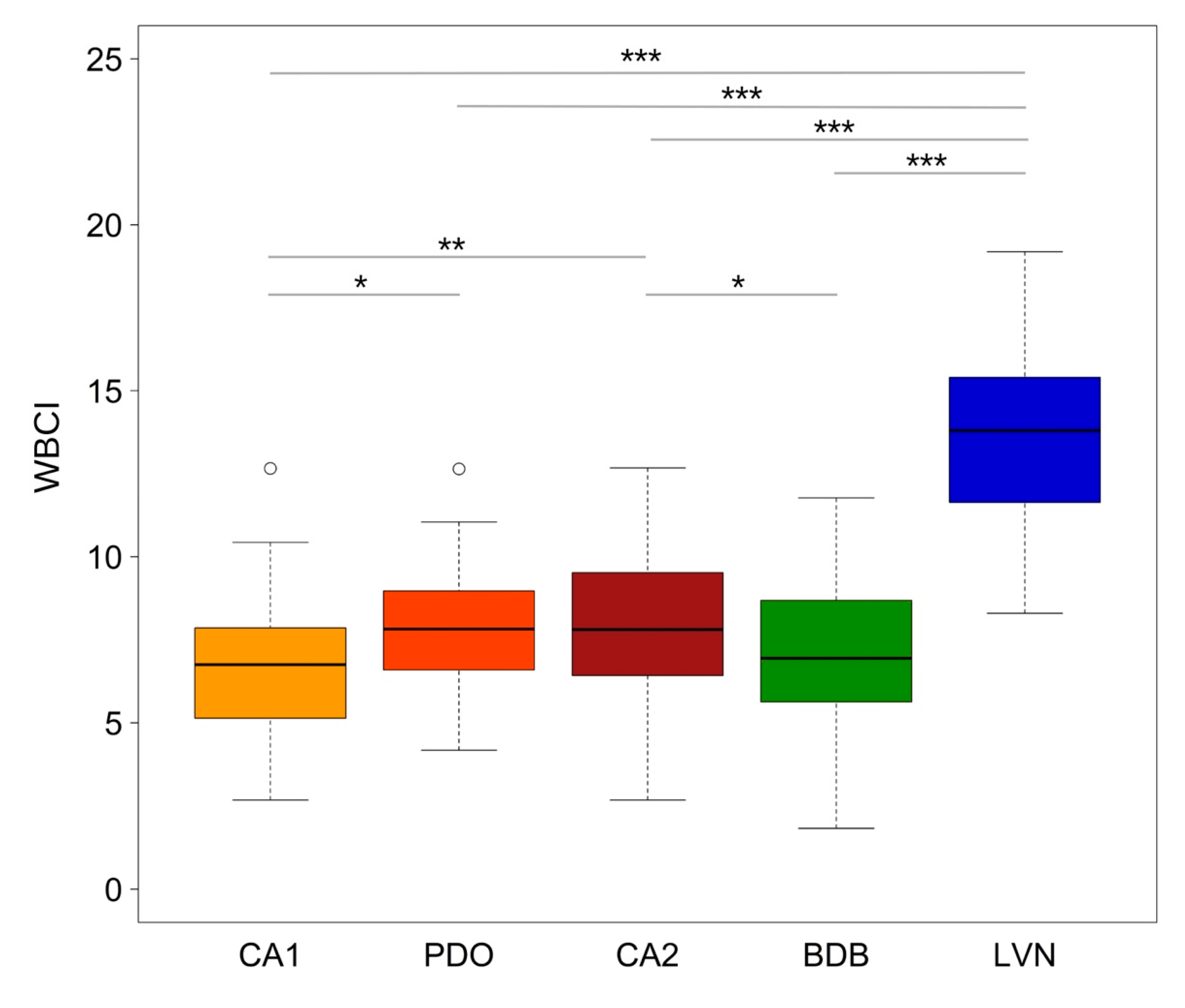
3. Results
3.1. Bird Community Structure
3.2. Bird Community Composition
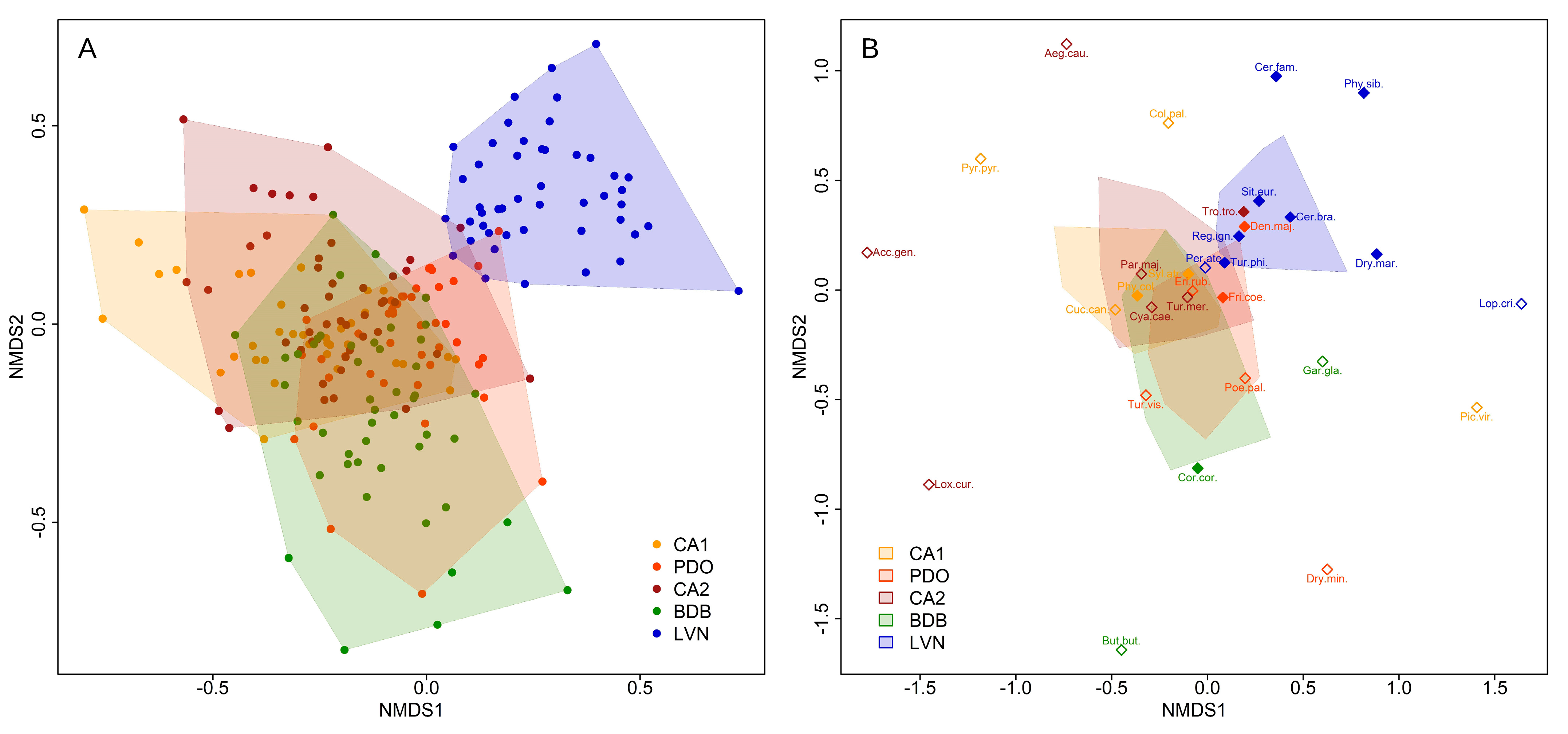
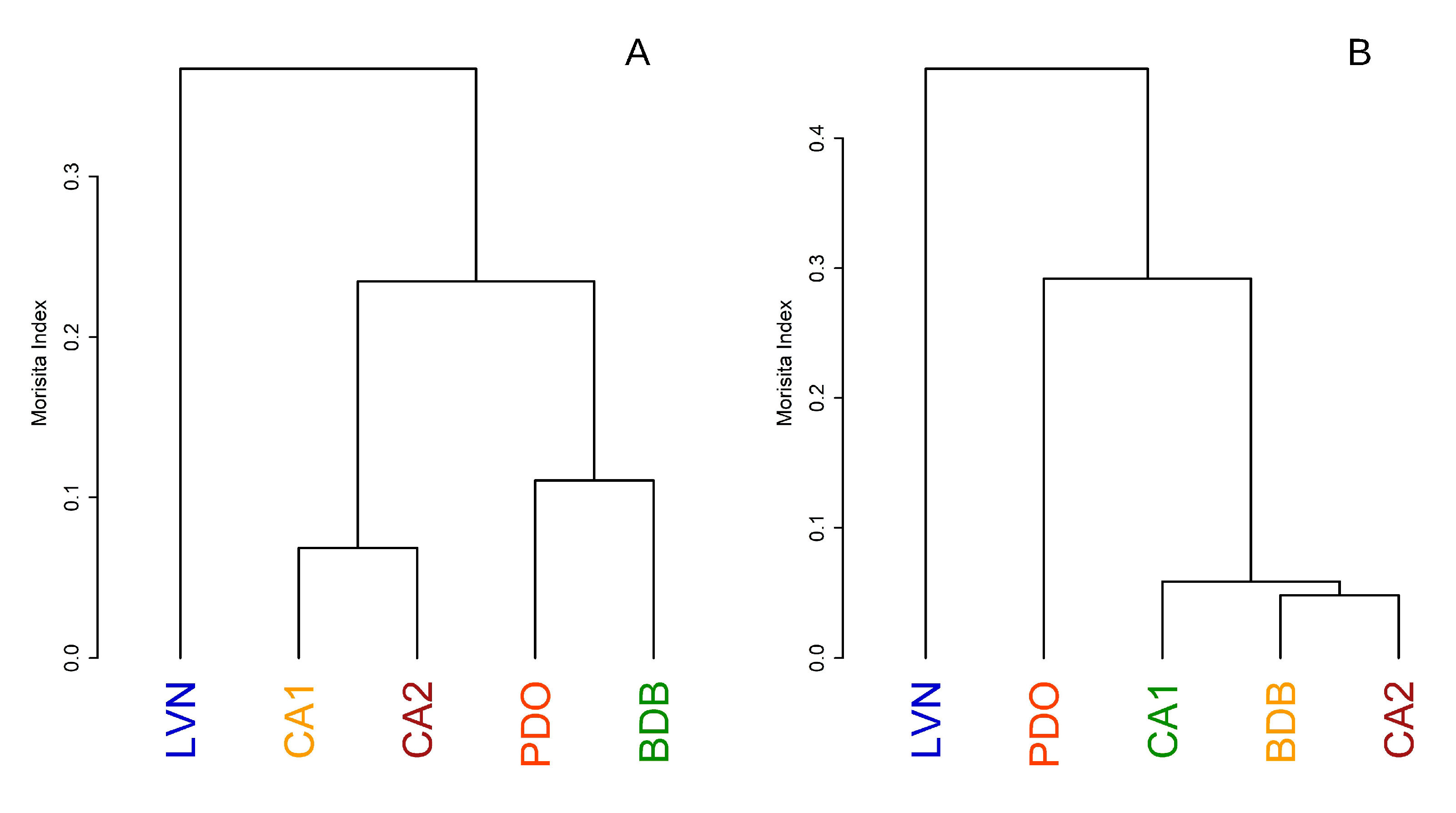
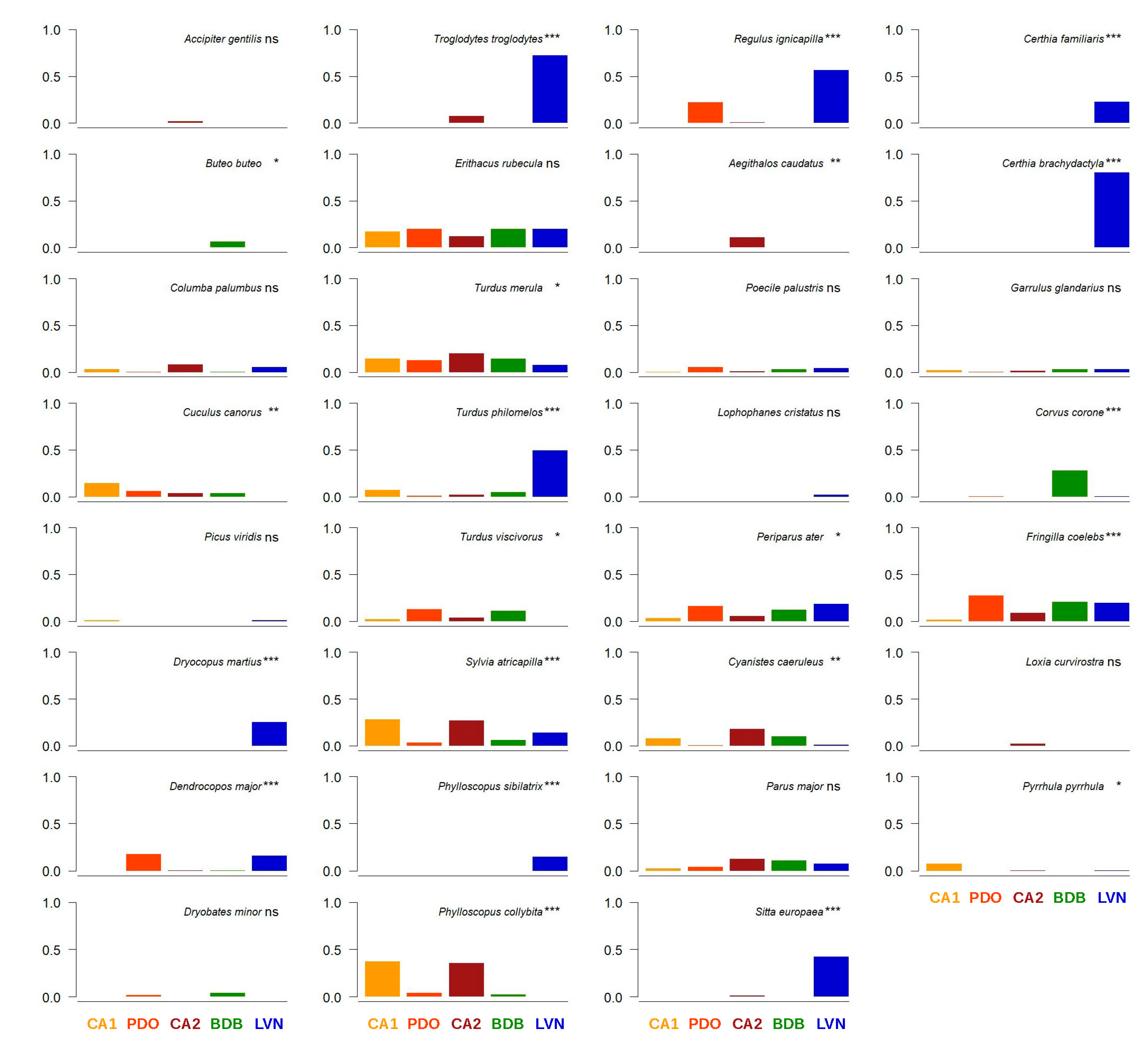
3.3. Bird Community Specialization
4. Discussion
4.1. Bird Community Structure
4.2. Bird Community Composition
4.3. Ecological Specialization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Clements, T.J.; Mountford, E.P.; Pakenham, R.; Hahn, K.; Fanta, J.; Diaci, J.; Abruda, I.V. Contemporary Beech Forest M Anagement in Europe. NAT-M AN Working Report 1; Hahan, K., Fanta, J., Eds.; Nat-Man Project (Nature-Based Management of Beech in Europe); Forest & Landscape Denmark: Copenhagen, Denmark, 2001. [Google Scholar]
- Burrascano, S.; Chianucci, F.; Trentanovi, G.; Kepfer-Rojas, S.; Sitzia, T.; Tinya, F.; Doerfler, I.; Paillet, Y.; Nagel, T.A.; Mitic, B.; et al. Where Are We Now with European Forest Multi-Taxon Biodiversity and Where Can We Head To? Biol. Conserv. 2023, 284, 110176. [Google Scholar] [CrossRef]
- Walentowski, H.; Müller-Kroehling, S.; Bergmeier, E.; Bernhardt-Römermann, M.; Gossner, M.M.; Reif, A.; Schulze, E.-D.; Bußler, H.; Strätz, C.; Adelmann, W. Fagus sylvatica Forests and Their Faunal Diversity: A Regional and European Perspective. Ann. For. Res. 2014, 57, 2015–2231. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömöry, D.; Latałowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A New Scenario for the Quaternary History of European Beech Populations: Palaeobotanical Evidence and Genetic Consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef]
- Gasparini, P.; Di Cosmo, L.; Floris, A.; De Laurentis, D. (Eds.) Italian National Forest Inventory-Methods and Results of the Third Survey. Inventario Nazionale Delle Foreste e dei Serbatoi Forestali di Carbonio-Metodi e Risultati Della Terza Indagine; Springer Tracts in Civil Engineering; Springer International Publishing: Cham, Germany, 2022; ISBN 978-3-030-98677-3. [Google Scholar]
- Nocentini, S. Structure and Management of Beech (Fagus sylvatica L.) Forests in Italy. IForest-Biogeosci. For. 2009, 2, 105–113. [Google Scholar] [CrossRef]
- Marchetti, M.; Blasi, C. Old-Growth Forests in Italy: Towards a First Network. Ital. For. E Mont. 2010, 65, 679–698. [Google Scholar] [CrossRef]
- Vacchiano, G.; Garbarino, M.; Lingua, E.; Motta, R. Forest Dynamics and Disturbance Regimes in the Italian Apennines. For. Ecol. Manag. 2017, 388, 57–66. [Google Scholar] [CrossRef]
- Agnoletti, M.; Piras, F.; Venturi, M.; Santoro, A. Cultural Values and Forest Dynamics: The Italian Forests in the Last 150 Years. For. Ecol. Manag. 2022, 503, 119655. [Google Scholar] [CrossRef]
- Malandra, F.; Vitali, A.; Urbinati, C.; Garbarino, M. 70 Years of Land Use/Land Cover Changes in the Apennines (Italy): A Meta-Analysis. Forests 2018, 9, 551. [Google Scholar] [CrossRef]
- Tellini Florenzano, G. Birds as Indicators of Recent Environmental Changes in the Apennines (Foreste Casentinesi National Park, Central Italy). Ital. J. Zool. 2004, 71, 317–324. [Google Scholar] [CrossRef]
- Keller, V.; Herrando, S.; Voříšek, P.; Martí, F.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V.; et al. (Eds.) European Breeding Bird Atlas 2. Distribution, Abundance and Change, Lynx ed.; European Bird Census Council: Beek, The Netherlands, 2020. [Google Scholar]
- Borghi, C.; Francini, S.; McRoberts, R.E.; Parisi, F.; Lombardi, F.; Nocentini, S.; Maltoni, A.; Travaglini, D.; Chirici, G. Country-Wide Assessment of Biodiversity, Naturalness and Old-Growth Status Using National Forest Inventory Data. Eur. J. For. Res. 2024, 143, 271–303. [Google Scholar] [CrossRef]
- Fabbio, G.; Merlo, M.; Tosi, V. Silvicultural Management in Maintaining Biodiversity and Resistance of Forests in Europe—The Mediterranean Region. Maint. For. Biodivers. 2003, 67, 67–76. [Google Scholar] [CrossRef]
- Barbati, A.; Marchetti, M.; Chirici, G.; Corona, P. European Forest Types and Forest Europe SFM Indicators: Tools for Monitoring Progress on Forest Biodiversity Conservation. Mech. Predict. Ecol. Change Manag. For. Sel. Pap. Second Int. Conf. Biodivers. For. Ecosyst. Landsc. 2014, 321, 145–157. [Google Scholar] [CrossRef]
- Voříšek, P.; Schwartz, M.; Rastislav, R. Pilot Study: Common Forest Bird Species Indicator; FOREST EUROPE Liaison Unit: Bratislava, Slovakia, 2019. [Google Scholar]
- Amori, G.; Mazzei, A.; Storino, P.; Urso, S.; Luzzi, G.; Aloise, G.; Gangale, C.; Ouzounov, D.; Luiselli, L.; Pizzolotto, R.; et al. Forest Management and Conservation of Faunal Diversity in Italy: A Review. Plant Biosyst. 2022, 155, 1226–1239. [Google Scholar] [CrossRef]
- Redolfi De Zan, L.; Battisti, C.; Carpaneto, G.M. Bird and Beetle Assemblages in Relict Beech Forests of Central Italy: A Multi-Taxa Approach to Assess the Importance of Dead Wood in Biodiversity Conservation. Community Ecol. 2014, 15, 235–245. [Google Scholar] [CrossRef]
- Parisi, F.; Innangi, M.; Tognetti, R.; Lombardi, F.; Chirici, G.; Marchetti, M. Forest Stand Structure and Coarse Woody Debris Determine the Biodiversity of Beetle Communities in Mediterranean Mountain Beech Forests. Glob. Ecol. Conserv. 2021, 28, e01637. [Google Scholar] [CrossRef]
- Balestrieri, R.; Basile, M.; Posillico, M.; Altea, T.; De Cinti, B.; Matteucci, G. A Guild-Based Approach to Assessing the Influence of Beech Forest Structure on Bird Communities. For. Ecol. Manag. 2015, 356, 216–223. [Google Scholar] [CrossRef]
- De Zan, L.R.; De Gasperis, S.r.; Fiore, L.; Battisti, C.; Carpaneto, G.M. The Importance of Dead Wood for Hole-Nesting Birds: A Two Years Study in Three Beech Forests of Central Italy. Isr. J. Ecol. Evol. 2016, 63, 19–27. [Google Scholar] [CrossRef]
- Parisi, F.; Vangi, E.; Francini, S.; D’Amico, G.; Chirici, G.; Marchetti, M.; Lombardi, F.; Travaglini, D.; Ravera, S.; De Santis, E.; et al. Sentinel-2 Time Series Analysis for Monitoring Multi-Taxon Biodiversity in Mountain Beech Forests. Front. For. Glob. Change 2023, 6, 1020477. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Jones, G.; Mazzoleni, S. Roost Selection by Barbastelle Bats (Barbastella Barbastellus, Chiroptera: Vespertilionidae) in Beech Woodlands of Central Italy: Consequences for Conservation. Biol. Conserv. 2004, 117, 73–81. [Google Scholar] [CrossRef]
- Russo, D.; Cistrone, L.; Garonna, A.P.; Jones, G. Reconsidering the Importance of Harvested Forests for the Conservation of Tree-Dwelling Bats. Biodivers. Conserv. 2010, 19, 2501–2515. [Google Scholar] [CrossRef]
- Fraixedas, S.; Lindén, A.; Piha, M.; Cabeza, M.; Gregory, R.; Lehikoinen, A. A State-of-the-Art Review on Birds as Indicators of Biodiversity: Advances, Challenges, and Future Directions. Ecol. Indic. 2020, 118, 106728. [Google Scholar] [CrossRef]
- Turner, A.; Fischer, M.; Tzanopoulos, J. Sound-Mapping a Coniferous Forest-Perspectives for Biodiversity Monitoring and Noise Mitigation. PLoS ONE 2018, 13, e0189843. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Pieretti, N. Sonic Environment and Vegetation Structure: A Methodological Approach for a Soundscape Analysis of a Mediterranean Maqui. Ecol. Acoust. 2014, 21, 120–132. [Google Scholar] [CrossRef]
- Farina, A.; Mullet, T.C. Sonotope Patterns within a Mountain Beech Forest of Northern Italy: A Methodological and Empirical Approach. Front. Ecol. Evol. 2024, 12, 1341760. [Google Scholar] [CrossRef]
- Sugai, L.S.M.; Silva, T.S.F.; Ribeiro, J.W.; Llusia, D. Terrestrial Passive Acoustic Monitoring: Review and Perspectives. BioScience 2019, 69, 15–25. [Google Scholar] [CrossRef]
- Alcocer, I.; Lima, H.; Sugai, L.S.M.; Llusia, D. Acoustic Indices as Proxies for Biodiversity: A Meta-analysis. Biol. Rev. 2022, 97, 2209–2236. [Google Scholar] [CrossRef]
- Bradfer-Lawrence, T.; Desjonqueres, C.; Eldridge, A.; Johnston, A.; Metcalf, O. Using Acoustic Indices in Ecology: Guidance on Study Design, Analyses and Interpretation. Methods Ecol. Evol. 2023, 14, 2192–2204. [Google Scholar] [CrossRef]
- Farina, A.; Lattanzi, E.; Malavasi, R.; Pieretti, N.; Piccioli, L. Avian Soundscapes and Cognitive Landscapes: Theory, Application and Ecological Perspectives. Landsc. Ecol. 2011, 26, 1257–1267. [Google Scholar] [CrossRef]
- Parisi, F.; Mazziotta, A.; Chirici, G.; D’amico, G.; Vangi, E.; Francini, S.; Travaglini, D. Effects of Forest Management on Beetle (Coleoptera) Communities in Beech Forests (Fagus sylvatica) in the Apennines of Central Italy (Tuscany). Forests 2024, 15, 1085. [Google Scholar] [CrossRef]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and Mapping of the Ecoregions of Italy. Plant Biosyst. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Rempel, R.S.; Francis, C.M.; Robinson, J.N.; Campbell, M. Comparison of Audio Recording System Performance for Detecting and Monitoring Songbirds. J. Field Ornithol. 2013, 84, 86–97. [Google Scholar] [CrossRef]
- Farina, A.; Ceraulo, M.; Bobryk, C.; Pieretti, N.; Quinci, E.; Lattanzi, E. Spatial and Temporal Variation of Bird Dawn Chorus and Successive Acoustic Morning Activity in a Mediterranean Landscape. Bioacoustics 2015, 24, 269–288. [Google Scholar] [CrossRef]
- Voříšek, P.; Klvaňová, A.; Wotton, S.; Gregory, R.D. (Eds.) A Best Practice Guide for Wild Bird Monitoring Schemes; CSO/RSPB: Sandy, UK, 2008; p. 151. ISBN 978-80-903554-3-9. [Google Scholar]
- Balestrieri, R.; Basile, M.; Posillico, M.; Altea, T.; Matteucci, G. Survey Effort Requirements for Bird Community Assessment in Forest Habitats. Acta Ornithol. 2017, 52, 1–9. [Google Scholar] [CrossRef]
- Hujatulatif, A.; Jumadi, J.; Kuswanto, H.; Ilma, A.Z. Analyzing and Comparing Frequency of the Birds Sound Spectrum Using Audacity Software in Practicum Activity. J. Penelit. Pendidik. IPA 2022, 8, 2586–2592. [Google Scholar] [CrossRef]
- Cook, A.; Hartley, S. Efficient Sampling of Avian Acoustic Recordings: Intermittent Subsamples Improve Estimates of Single Species Prevalence and Total Species Richness. Avian Conserv. Ecol. 2018, 13, art21. [Google Scholar] [CrossRef]
- Yip, D.A.; Mahon, C.L.; MacPhail, A.G.; Bayne, E.M. Automated Classification of Avian Vocal Activity Using Acoustic Indices in Regional and Heterogeneous Datasets. Methods Ecol. Evol. 2021, 12, 707–719. [Google Scholar] [CrossRef]
- Diaz, S.D.U.; Gan, J.L.; Tapang, G.A. Acoustic Indices as Proxies for Bird Species Richness in an Urban Green Space in Metro Manila. PLoS ONE 2023, 18, e0289001. [Google Scholar] [CrossRef] [PubMed]
- Budka, M.; Sokołowska, E.; Muszyńska, A.; Staniewicz, A. Acoustic Indices Estimate Breeding Bird Species Richness with Daily and Seasonally Variable Effectiveness in Lowland Temperate Białowieża Forest. Ecol. Indic. 2023, 148, 110027. [Google Scholar] [CrossRef]
- Erdelen, M. Bird Communities and Vegetation Structure: I. Correlations and Comparisons of Simple and Diversity Indices. Oecologia 1984, 61, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, T.; Martin, K. Tree Holes and Hole-Nesting Birds in European and North American Forests. In Ecology and Conservation of Forest Birds; Mikusiński, G., Roberge, J.-M., Fuller, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 79–134. ISBN 978-1-107-42072-4. [Google Scholar]
- Zurr, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R. Statistics for Biology and Health; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (HIll Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Smith, J.M.; Mather, M.E. Using Assemblage Data in Ecological Indicators: A Comparison and Evaluation of Commonly Available Statistical Tools. Ecol. Indic. 2012, 13, 253–262. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T. A New Statistical Approach for Assessing Similarity of Species Composition with Incidence and Abundance Data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Morisita, M. Measuring of Interspecific Association and Similarity between Assemblages. Mem. Fac. Sci. Kyushu. Univ. Ser. E Biol. 1959, 3, 65–80. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Community Ecology Package. R Package ‘Vegan’, Version 2.6-8; Comprehensive R Archive Network; 2022. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 14 June 2025).
- Dufrene, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D.W. Package ‘Labdsv’, version: 2.1-0; Comprehensive R Archive Network (CRAN), R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Londi, G.; Tellini Florenzano, G.; Mini, L.; Caliendo, M.F.; Campedelli, T.; De Carli, E. Assessing Woodland Ecological Characters Through a New Objective Bird Community Index, the WBCI. Avocetta 2009, 33, 107–114. [Google Scholar]
- Phillips, J.N.; Cooper, W.J.; Luther, D.A.; Derryberry, E.P. Territory Quality Predicts Avian Vocal Performance Across an Urban-Rural Gradient. Front. Ecol. Evol. 2020, 8, 587120. [Google Scholar] [CrossRef]
- Grava, T.; Otter, K.A.; Grava, A. Vocal Performance Varies with Habitat Quality in Black-Capped Chickadees (Poecile atricapillus). Behaviour 2012, 149, 35–50. [Google Scholar] [CrossRef]
- Kuznetzova, A.; Brockhoff, P.B.; Christensen, R.H.B.; Jensen, S.P. Package ‘lmerTest’, lmerTest: Tests in Linear Mixed Effects Models (R Package Version 3.1-3) [Computer Software]; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.5.1; Comprehensive R Archive Network: Vienna, Austria, 2024. [Google Scholar]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Begehold, H.; Rzanny, M.; Flade, M. Forest Development Phases as an Integrating Tool to Describe Habitat Preferences of Breeding Birds in Lowland Beech Forests. J. Ornithol. 2015, 156, 19–29. [Google Scholar] [CrossRef]
- Birčák, T.; Reif, J. The Effects of Tree Age and Tree Species Composition on Bird Species Richness in a Central European Montane Forest. Biologia 2015, 70, 1528–1536. [Google Scholar] [CrossRef]
- Moning, C.; Müller, J. Critical Forest Age Thresholds for the Diversity of Lichens, Molluscs and Birds in Beech (Fagus sylvatica L.) Dominated Forests. Ecol. Indic. 2009, 9, 922–932. [Google Scholar] [CrossRef]
- Gil-Tena, A.; Saura, S.; Brotons, L. Effects of Forest Composition and Structure on Bird Species Richness in a Mediterranean Context: Implications for Forest Ecosystem Management. For. Ecol. Manag. 2007, 242, 470–476. [Google Scholar] [CrossRef]
- Basile, M.; Storch, I.; Mikusiński, G. Abundance, Species Richness and Diversity of Forest Bird Assemblages—The Relative Importance of Habitat Structures and Landscape Context. Ecol. Indic. 2021, 133, 108402. [Google Scholar] [CrossRef]
- Du Bus De Warnaffe, G.; Deconchat, M. Impact of Four Silvicultural Systems on Birds in the Belgian Ardenne: Implications for Biodiversity in Plantation Forests. Biodivers. Conserv. 2008, 17, 1041–1055. [Google Scholar] [CrossRef]
- Mentil, L.; Battisti, C.; Carpaneto, G.M. The Older the Richer: Significant Increase in Breeding Bird Diversity Along an Age Gradient of Different Coppiced Woods. Web Ecol. 2018, 18, 143–151. [Google Scholar] [CrossRef]
- Franklin, J.F.; Van Pelt, R. Spatial Aspects of Structural Complexity in Old-Growth Forests. J. For. 2004, 102, 22–28. [Google Scholar] [CrossRef]
- Motta, R.; Garbarino, M.; Berretti, R.; Bono, A.; Curovic, M.; Dukić, V.; Nola, P. Monastic Silviculture Legacies and Current Old-Growthness of Silver Fir (Abies alba) Forests in the Northern Apennines (Italy). Front. For. Glob. Change 2023, 6, 1252462. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On Bird Species Diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, J.; Fan, C.; Von Gadow, K. Structure Complexity Is the Primary Driver of Functional Diversity in the Temperate Forests of Northeastern China. For. Ecosyst. 2022, 9, 100048. [Google Scholar] [CrossRef]
- De La Montaña, E.; Rey-Benayas, J.M.; Carrascal, L.M. Response of Bird Communities to Silvicultural Thinning of Mediterranean Maquis. J. Appl. Ecol. 2006, 43, 651–659. [Google Scholar] [CrossRef]
- Versluijs, M.; Hekkala, A.-M.; Lindberg, E.; Lämås, T.; Hjältén, J. Comparing the Effects of Even-Aged Thinning and Selective Felling on Boreal Forest Birds. For. Ecol. Manag. 2020, 475, 118404. [Google Scholar] [CrossRef]
- Bottalico, F.; Brundu, P.; Ciancio, O.; Nocentini, S.; Puletti, N.; Travaglini, D. “Baldo’s Forest”: Selection felling based on traditional knowledge in a beech stand in the Tuscan Appenines. For.-Riv. Selvic. Ed Ecol. For. 2010, 7, 58–72. [Google Scholar] [CrossRef]
- Savilaakso, S.; Johansson, A.; Häkkilä, M.; Uusitalo, A.; Sandgren, T.; Mönkkönen, M.; Puttonen, P. What Are the Effects of Even-Aged and Uneven-Aged Forest Management on Boreal Forest Biodiversity in Fennoscandia and European Russia? A Systematic Review. Environ. Evid. 2021, 10, 1. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The Impact of Even-aged and Uneven-aged Forest Management on Regional Biodiversity of Multiple Taxa in European Beech Forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Bouvet, A.; Paillet, Y.; Archaux, F.; Tillon, L.; Denis, P.; Gilg, O.; Gosselin, F. Effects of Forest Structure, Management and Landscape on Bird and Bat Communities. Environ. Conserv. 2016, 43, 148–160. [Google Scholar] [CrossRef]
- Laiolo, P.; Rolando, A.; Valsania, V. Responses of Birds to the Natural Re-Establishment of Wilderness in Montane Beechwoods of North-Western Italy. Acta Oecologica 2004, 25, 129–136. [Google Scholar] [CrossRef]
- Czeszczewik, D.; Zub, K.; Stanski, T.; Sahel, M.; Kapusta, A.; Walankiewicz, W. Effects of Forest Management on Bird Assemblages in the Bialowieza Forest, Poland. IForest-Biogeosci. For. 2015, 8, 377–385. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences Between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Lewandowski, P.; Przepióra, F.; Ciach, M. Single Dead Trees Matter: Small-Scale Canopy Gaps Increase the Species Richness, Diversity and Abundance of Birds Breeding in a Temperate Deciduous Forest. For. Ecol. Manag. 2021, 481, 118693. [Google Scholar] [CrossRef]
- Martin, K.; Aitken, K.E.H.; Wiebe, K.L. Nest Sites and Nest Webs for Cavity-Nesting Communities in Interior British Columbia, Canada: Nest Characteristics and Niche Partitioning. Condor 2004, 106, 5–19. [Google Scholar] [CrossRef]
- Angelstam, P.K. Maintaining and Restoring Biodiversity in European Boreal Forests by Developing Natural Disturbance Regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar] [CrossRef]
- Wilson, M.W.; Pithon, J.; Gittings, T.; Kelly, T.C.; Giller, P.S.; O’Halloran, J. Effects of Growth Stage and Tree Species Composition on Breeding Bird Assemblages of Plantation Forests. Bird Study 2006, 53, 225–236. [Google Scholar] [CrossRef]
- Sweeney, O.F.M.; Wilson, M.W.; Irwin, S.; Kelly, T.C.; O’Halloran, J. Breeding Bird Communities of Second-rotation Plantations at Different Stages of the Forest Cycle. Bird Study 2010, 57, 301–314. [Google Scholar] [CrossRef]
- Schall, P.; Heinrichs, S.; Ammer, C.; Ayasse, M.; Boch, S.; Buscot, F.; Fischer, M.; Goldmann, K.; Overmann, J.; Schulze, E.; et al. Can Multi-taxa Diversity in European Beech Forest Landscapes Be Increased by Combining Different Management Systems? J. Appl. Ecol. 2020, 57, 1363–1375. [Google Scholar] [CrossRef]
- Bani, L.; Massimino, D.; Bottoni, L.; Massa, R. A Multiscale Method for Selecting Indicator Species and Priority Conservation Areas: A Case Study for Broadleaved Forests in Lombardy, Italy. Conserv. Biol. 2006, 20, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Mikusiński, G.; Villero, D.; Herrando, S.; Brotons, L. Macroecological Patterns in Forest Bird Diversity in Europe. In Ecology and Conservation of Forest Bird; Mikusiński, G., Roberge, J.-M., Fuller, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 137–182. ISBN 978-1-107-42072-4. [Google Scholar]
- Boncina, A. Comparison of Structure and Biodiversity in the Rajhenav Virgin Forest Remnant and Managed Forest in the Dinaric Region of Slovenia. Glob. Ecol. Biogeogr. 2000, 9, 201–211. [Google Scholar] [CrossRef]
- Tomiałojć, L.; Wesołowski, T. Diversity of the Białowieża Forest Avifauna in Space and Time. J. Ornithol. 2004, 145, 81–92. [Google Scholar] [CrossRef]
- Kornan, M. Breeding Bird Assemblage Dynamics of a Primaeval Temperate Mixed Forest in the Western Carpathians (Slovakia): Support for Pluralistic Community Concept. Ornis Fenn. 2013, 90, 151–177. [Google Scholar] [CrossRef]
- Kouki, J.; Väänänen, A. Impoverishment of Resident Old-Growth Forest Bird Assemblages along an Isolation Gradient of Protected Areas in Eastern Finland. Ornis Fenn. 2000, 77, 145–154. [Google Scholar]
- Rosenvald, R.; Lõhmus, A.; Kraut, A.; Remm, L. Bird Communities in Hemiboreal Old-Growth Forests: The Roles of Food Supply, Stand Structure, and Site Type. For. Ecol. Manag. 2011, 262, 1541–1550. [Google Scholar] [CrossRef]
- Kebrle, D.; Zasadil, P.; Hošek, J.; Barták, V.; Šťastný, K. Large Trees as a Key Factor for Bird Diversity in Spruce-Dominated Production Forests: Implications for Conservation Management. For. Ecol. Manag. 2021, 496, 119460. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; Du Puy, S.; Bouget, C.; Boulanger, V.; Debaive, N.; Gilg, O.; Gosselin, F.; Guilbert, E. The Indicator Side of Tree Microhabitats: A Multi-taxon Approach Based on Bats, Birds and Saproxylic Beetles. J. Appl. Ecol. 2018, 55, 2147–2159. [Google Scholar] [CrossRef]
- Parisi, F.; D’Amico, G.; Vangi, E.; Chirici, G.; Francini, S.; Cocozza, C.; Giannetti, F.; Londi, G.; Nocentini, S.; Borghi, C.; et al. Tree-Related Microhabitats and Multi-Taxon Biodiversity Quantification Exploiting ALS Data. Forests 2024, 15, 660. [Google Scholar] [CrossRef]
- Angelstam, P.K.; Bütler, R.; Lazdinis, M.; Mikusiński, G.; Roberge, J.M. Habitat Thresholds for Focal Species at Multiple Scales and Forest Biodiversity Conservation—Dead Wood as an Example. Ann. Zool. Fenn. 2003, 40, 473–482. [Google Scholar]
- Lõhmus, A.; Kinks, R.; Soon, M. The Importance of Dead-Wood Supply for Woodpeckers in Estonia. Balt. For. 2010, 16, 76–86. [Google Scholar]
- Siitonen, J.; Jonsson, B.G. Other Association with Dead Woody Material. In Biodiversity in Dead Wood; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 58–81. [Google Scholar]
- Zasadil, P.; Romportl, D.; Horák, J. Disentangling the Roles of Topography, Patch, and Land Use on Conservation Trait Status of Specialist Birds in Marginal Forest Land Use Types. Forests 2020, 11, 103. [Google Scholar] [CrossRef]
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of Tree Characteristics and Forest Management on Tree Microhabitats. Biol. Conserv. 2011, 144, 441–450. [Google Scholar] [CrossRef]
- Bruun, H.H.; Heilmann-Clausen, J. What Is Unmanaged Forest and How Does It Sustain Biodiversity in Landscapes with a Long History of Intensive Forestry? J. Appl. Ecol. 2021, 58, 1813–1816. [Google Scholar] [CrossRef]
- Tozer, D.C.; Burke, D.M.; Nol, E.; Elliott, K.A. Short-Term Effects of Group-Selection Harvesting on Breeding Birds in a Northern Hardwood Forest. For. Ecol. Manag. 2010, 259, 1522–1529. [Google Scholar] [CrossRef]
- Arcilla, N.; Strazds, M. Ten Principles for Bird-Friendly Forestry: Conservation Approaches in Natural Forests Used for Timber Production. Birds 2023, 4, 245–261. [Google Scholar] [CrossRef]
- Cordeiro Pereira, J.M.; Mikusiński, G.; Storch, I. A Systematic Review of the Effects of Multi-Purpose Forest Management Practices on the Breeding Success of Forest Birds. Curr. For. Rep. 2024, 10, 175–195. [Google Scholar] [CrossRef]
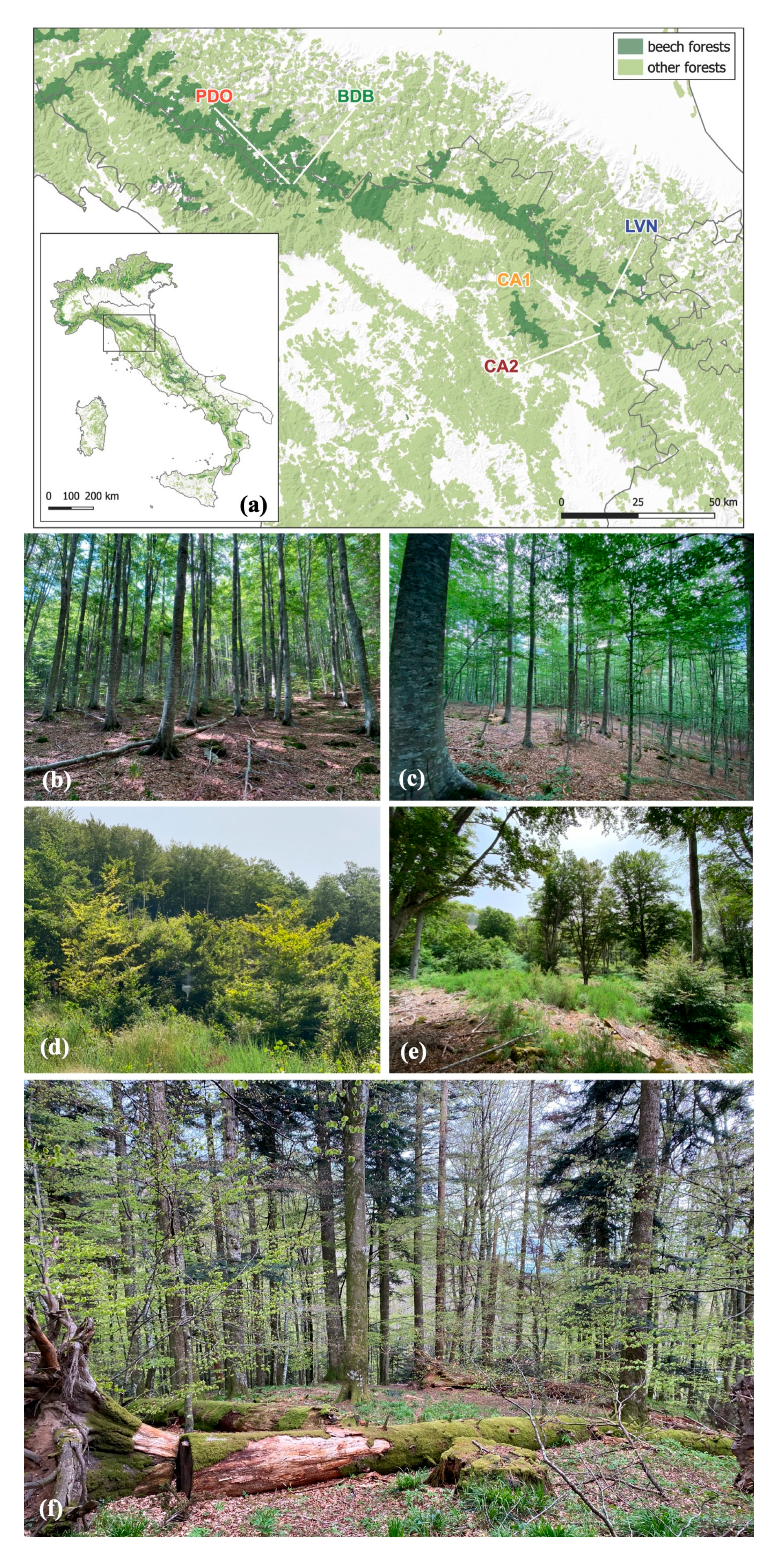
| Site Code | Site Name | Coordinate | Management | Age Class Structure | Volume (m3 ha−1) | Deadwood Volume (m3 ha−1) | Elevation (m a.s.l.) | Exposure (°N) | Forest Cover (Proportion) Within 1 km Buffer | Forest Cover (Proportion) Within 5 km Buffer |
|---|---|---|---|---|---|---|---|---|---|---|
| CA1 | Casella 1 | 43°39′37.34″ N, 11°55′11.7″ E | Uniform shelterwood system | Even-aged (ca. 20-years-old) | - | 5.5 | 1125 | 228 | 0.97 | 0.81 |
| PDO | Pian degli Ontani | 44°6′26.52″ N, 10°41′40.14″ E | Uniform shelterwood system | Even-aged (ca. 60-years-old) | 528.2 | 6.4 | 1229 | 26 | 0.97 | 0.88 |
| CA2 | Casella 2 | 44°6′33.13″ N, 10°41′49.6″ E | Uniform shelterwood system | Even-aged (ca. 100-years-old) | 204.4 | 14.9 | 1102 | 279 | 0.97 | 0.77 |
| BDB | Bosco di Baldo | 44°6′33.13″ N, 10°41′49.6″ E | Single-tree selection system | Uneven-aged | 363.4 | 4.4 | 1189 | 61 | 0.97 | 0.87 |
| LVN | La Verna | 43°42′32.48″ N, 11°55′51.67″ E | Unmanaged | Uneven-aged | 997.8 | 426 | 1165 | 142 | 0.86 | 0.85 |
| Euring | Species | Breeding Site | CA1 | PDO | CA2 | BDB | LVN | |
|---|---|---|---|---|---|---|---|---|
| 1 | Northern Goshawk | Accipiter gentilis | other | 0.02 | ||||
| 2 | Eurasian Buzzard | Buteo buteo | other | 0.06 | ||||
| 3 | Common Woodpigeon | Columba palumbus | other | 0.13 | 0.02 | 0.21 | 0.02 | 0.17 |
| 4 | Common Cuckoo | Cuculus canorus | other | 0.40 | 0.25 | 0.21 | 0.21 | 0.02 |
| 5 | Eurasian Green Woodpecker | Picus viridis | CAV | 0.02 | 0.02 | |||
| 6 | Black Woodpecker | Dryocopus martius | CAV | 0.25 | ||||
| 7 | Great Spotted Woodpecker | Dendrocopos major | CAV | 0.42 | 0.08 | 0.08 | 0.40 | |
| 8 | Lesser Spotted Woodpecker | Dryobates minor | CAV | 0.04 | 0.06 | |||
| 9 | Northern Wren | Troglodytes troglodytes | other | 0.31 | 0.96 | |||
| 10 | European Robin | Erithacus rubecula | other | 0.88 | 0.94 | 0.73 | 0.94 | 0.94 |
| 11 | Eurasian Blackbird | Turdus merula | other | 0.71 | 0.67 | 0.83 | 0.71 | 0.52 |
| 12 | Song Thrush | Turdus philomelos | other | 0.38 | 0.13 | 0.21 | 0.31 | 1.00 |
| 13 | Mistle Thrush | Turdus viscivorus | other | 0.15 | 0.38 | 0.21 | 0.35 | 0.02 |
| 14 | Eurasian Blackcap | Sylvia atricapilla | other | 0.98 | 0.33 | 0.96 | 0.46 | 0.69 |
| 15 | Wood Warbler | Phylloscopus sibilatrix | other | 0.15 | ||||
| 16 | Common Chiffchaff | Phylloscopus collybita | other | 0.96 | 0.31 | 0.94 | 0.23 | 0.02 |
| 17 | Common Firecrest | Regulus ignicapilla | other | 0.63 | 0.13 | 0.02 | 1.00 | |
| 18 | Long-tailed Tit | Aegithalos caudatus | other | 0.13 | 0.02 | |||
| 19 | Marsh Tit | Poecile palustris | CAV | 0.02 | 0.17 | 0.06 | 0.13 | 0.15 |
| 20 | Crested Tit | Lophophanes cristatus | CAV | 0.02 | ||||
| 21 | Coal Tit | Periparus ater | CAV | 0.29 | 0.65 | 0.38 | 0.56 | 0.69 |
| 22 | Eurasian Blue Tit | Cyanistes caeruleus | CAV | 0.33 | 0.06 | 0.50 | 0.38 | 0.13 |
| 23 | Great Tit | Parus major | CAV | 0.19 | 0.25 | 0.46 | 0.42 | 0.35 |
| 24 | Eurasian Nuthatch | Sitta europaea | CAV | 0.02 | 0.02 | 0.10 | 0.04 | 0.56 |
| 25 | Eurasian Treecreeper | Certhia familiaris | CAV | 0.23 | ||||
| 26 | Short-toed Treecreeper | Certhia brachydactyla | CAV | 0.04 | 0.06 | 0.90 | ||
| 27 | Eurasian Jay | Garrulus glandarius | other | 0.10 | 0.04 | 0.08 | 0.13 | 0.13 |
| 28 | Carrion Crow | Corvus corone | other | 0.04 | 0.33 | 0.02 | ||
| 29 | Common Chaffinch | Fringilla coelebs | other | 0.21 | 0.96 | 0.54 | 0.83 | 0.81 |
| 30 | Red Crossbill | Loxia curvirostra | other | 0.02 | ||||
| 31 | Eurasian Bullfinch | Pyrrhula pyrrhula | other | 0.10 | 0.02 | 0.02 |
| Site | CA1 | PDO | CA2 | BDB | LVN |
|---|---|---|---|---|---|
| Models for total richness | |||||
| CA1 | - | 0.93 | 0.82 * | 0.92 | 0.57 *** |
| PDO | 1.07 | - | 0.88 | 0.99 | 0.62 *** |
| CA2 | 1.22 * | 1.14 | - | 1.12 | 0.70 *** |
| BDB | 1.09 | 1.01 | 0.89 | - | 0.63 *** |
| LVN | 1.74 *** | 1.62 *** | 1.42 *** | 1.60 *** | - |
| Models for cavity-nesting richness | |||||
| CA1 | - | 0.54 ** | 0.54 ** | 0.50 *** | 0.24 *** |
| PDO | 1.84 ** | - | 1 | 0.92 | 0.44 *** |
| CA2 | 1.84 ** | 1 | - | 0.92 | 0.44 *** |
| BDB | 2.01 *** | 1.09 | 1.09 | - | 0.48 *** |
| LVN | 4.22 *** | 2.30 *** | 2.29 *** | 2.10 *** | - |
| Models for other species richness | |||||
| CA1 | - | 1.06 | 0.9 | 1.08 | 0.77 ** |
| PDO | 0.94 | - | 0.85 | 1.02 | 0.72 *** |
| CA2 | 1.11 | 1.18 | - | 1.20 * | 0.86 |
| BDB | 0.92 | 0.98 | 0.83 * | - | 0.71 *** |
| LVN | 1.3 | 1.38 | 1.17 | 1.14 *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londi, G.; Parisi, F.; Vangi, E.; D’Amico, G.; Travaglini, D. Forest Management Effects on Breeding Bird Communities in Apennine Beech Stands. Ecologies 2025, 6, 54. https://doi.org/10.3390/ecologies6030054
Londi G, Parisi F, Vangi E, D’Amico G, Travaglini D. Forest Management Effects on Breeding Bird Communities in Apennine Beech Stands. Ecologies. 2025; 6(3):54. https://doi.org/10.3390/ecologies6030054
Chicago/Turabian StyleLondi, Guglielmo, Francesco Parisi, Elia Vangi, Giovanni D’Amico, and Davide Travaglini. 2025. "Forest Management Effects on Breeding Bird Communities in Apennine Beech Stands" Ecologies 6, no. 3: 54. https://doi.org/10.3390/ecologies6030054
APA StyleLondi, G., Parisi, F., Vangi, E., D’Amico, G., & Travaglini, D. (2025). Forest Management Effects on Breeding Bird Communities in Apennine Beech Stands. Ecologies, 6(3), 54. https://doi.org/10.3390/ecologies6030054









