Abstract
Semi-natural grasslands are key biodiversity reservoirs in Mediterranean mountain ecosystems. Grazing pressure may significantly influence plant communities and soil conditions, with potential effects on ecosystem functioning. This study evaluated the impact of grazing intensity on floristic diversity, community structure, and soil physico-chemical and microbiological properties across eight grasslands in the Jbel Bouhachem massif (northern Morocco). Species richness, Shannon diversity, and floristic composition were assessed using PERMANOVA and NMDS ordination. Soil parameters and microbial groups were analyzed through laboratory measurements, with statistical comparisons based on Wilcoxon and t-tests. No significant differences were found in species richness or alpha diversity between grazing intensities, although floristic dispersion was higher under intensive grazing. Soil texture, potassium, iron, zinc, and electrical conductivity differed significantly between treatments. Among microbial groups, only yeasts and molds showed higher abundance under intensive grazing, while sulfite-reducing clostridia were exclusively detected in these plots. These results suggest that grazing intensity has a selective impact on soil properties and microbial communities, while plant diversity remains relatively stable.
1. Introduction
Grazing critically modulates the structure and function of semi-natural grasslands through three interconnected mechanisms: selective forage removal, soil compaction via trampling, and heterogeneous nutrient redistribution through dung deposition [1]. These processes drive spatiotemporal heterogeneity in plant community composition, with outcomes contingent upon intensity. Moderate grazing enhances floristic diversity by suppressing competitive dominance and creating microhabitat complexity [2], whereas overgrazing induces soil degradation, fertility loss, and biodiversity erosion [3]. Shifts in grazing regimes—whether intensification or abandonment—fundamentally restructure ecosystem dynamics [4,5].
In Mediterranean landscapes, grazing exhibits a dual ecological role: it maintains open habitats and herbaceous diversity when properly managed, yet drives ecosystem degradation under maladaptive practices. As a key ecological driver [6], it shapes both landscape structure and traditional agro-silvo-pastoral systems. This functional dichotomy necessitates adaptive management strategies integrating biodiversity indicators with disturbance metrics to reconcile conservation and land-use objectives [7].
In Mediterranean mountain regions, grasslands, typically dominated by Poaceae, Cyperaceae, and Juncaceae, develop under moderate water availability and support plant communities adapted to local edaphic and climatic constraints [8,9]. However, these ecosystems are increasingly vulnerable to multiple pressures, including agricultural intensification, habitat fragmentation, and altered grazing regimes [10,11].
In the Rif Mountains of northern Morocco, the grasslands of Jbel Bouhachem exemplify these challenges. Despite their high ecological value [12], these ecosystems face growing anthropogenic pressures, especially due to the overexploitation of water resources for cannabis cultivation [13,14]. This threatens both traditional pastoral systems and regional biodiversity [15,16]. Paradoxically, these grasslands, despite their hydrological regulatory role and cultural significance (e.g., medicinal resources, pastoral livelihoods), remain poorly studied. There is a noticeable lack of empirical research investigating the interactions between biotic, abiotic, and anthropogenic factors, which limits the development of conservation strategies adapted to local realities.
The main objective of this study is to assess the impact of grazing intensity on the floristic diversity and edaphic characteristics of semi-natural grasslands in the Jbel Bouhachem massif. By comparing grasslands subjected to moderate versus intensive grazing, we aim to highlight how this anthropogenic pressure affects vegetation composition and soil properties, both physico-chemical and microbiological.
We hypothesize that grazing intensity has a significant influence on species richness and diversity indices of plant communities. We also expect floristic composition to vary according to grazing levels, reflecting species filtering associated with land-use pressure. In addition, we assume that physico-chemical soil characteristics, such as texture, nutrient concentrations, pH, and electrical conductivity, are affected by grazing intensity. Likewise, the structure of soil microbial communities, represented by distinct functional groups, is expected to respond to this disturbance.
2. Materials and Methods
2.1. Study Area
The study area lies within the Jbel Bouhachem massif in the western part of the Rif mountain range in northern Morocco (Figure 1). This territory is of exceptional natural, archeological, historical, and religious value. Due to its ecological richness, it has been granted several national and international conservation statuses. It is entirely included within the Mediterranean Intercontinental Biosphere Reserve (MIBR), officially recognized by UNESCO on 25 October 2006 [17]. The site also hosts a priority conservation area under the Important Plant Areas (IPAs) of the Mediterranean [18]. At the national level, it has been designated as a first-priority Site of Biological and Ecological Interest (SIBE), as identified in the Master Plan for Protected Areas [19].
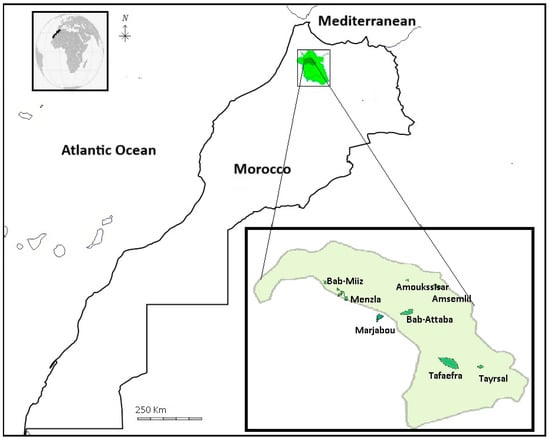
Figure 1.
Location of the study area and distribution of grasslands within the Jbel Bouhachem massif (Northern Morocco).
The landscape of Bouhachem is highly heterogeneous (Figure 2). In addition to semi-natural grasslands, the region encompasses various ecosystems, including heritage wetlands (peat bogs, temporary ponds) and relict vegetation types such as cedar, tauz, zéne oak, and cork oak forests.
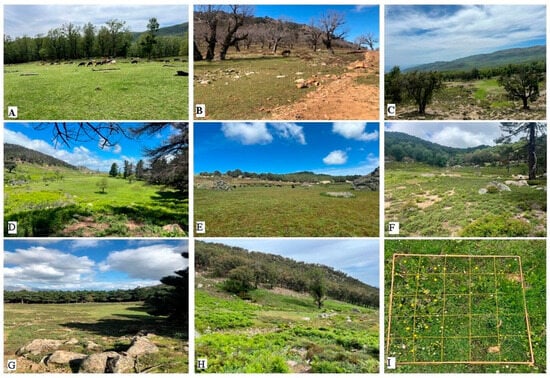
Figure 2.
Photographic overview of the surveyed grasslands ((A): Menzla; (B): Bab-Miiz; (C): Mrjabou; (D): Amsemlil; (E): Tafaefra; (F): Tayrsal; (G): Amoukssisar; (H): Bab-Ataba) and the quadrat used for floristic surveys (I).
Additionally, the massif’s geomorphological and ecological context is shaped by acidic sedimentary substrates such as flysch, Mauretanian, and Numidian sandstones, which are highly prone to erosion due to historical deforestation. The elevation gradient (575–1658 m) creates distinct bioclimatic zones, ranging from sub-humid to per-humid. This topographic and climatic stratification supports three major vegetation belts: evergreen Quercus rotundifolia woodlands in lower zones, deciduous oak stands (Q. faginea, Q. pyrenaica) at mid-elevations, and high-altitude coniferous forests (Pinus pinaster subsp. maghrebiana, Cedrus atlantica). These forests coexist with semi-natural pastures, peat bogs, and ephemeral wetlands, forming a complex mosaic of habitats.
2.2. Pastoral Context and Grazing Dynamics
Grazing pressure in the Jbel Bouhachem massif is primarily exerted by goats, sheep, and cattle, with a more occasional presence of equines. According to field surveys and semi-structured interviews conducted with local pastoralists, livestock densities vary depending on the proximity of villages, grassland accessibility, and herd size. Most grazing activity occurs year-round near inhabited areas, while traditional seasonal transhumance (July to September) persists in remote high-elevation pastures such as Menzla and Bab Miiz. Across the massif, peak grazing activity is observed in spring and early summer, when forage productivity reaches its maximum.
2.3. Grazing Intensity Assessment and Classification
Given the historical and continuous nature of pastoralism throughout the massif, it was not possible to identify ungrazed control sites. All accessible grasslands are subject to some level of grazing, and exclusion experiments were not feasible during the study period. Consequently, a gradient-based approach was adopted, consistent with methodologies commonly applied in Mediterranean pastoral ecosystems.
Grazing intensity was estimated in livestock units per hectare (LU/ha) through the triangulation of three complementary data sources: (i) official livestock census data provided by ONSSA (2021); (ii) GIS analysis of village proximity to grasslands; and (iii) semi-structured interviews with local herders regarding herd size, grazing frequency, and historical land-use. Based on this multi-source assessment, grasslands were classified into two distinct levels of grazing intensity (moderate and intensive), reflecting significant differences in grazing pressure.
This classification was further validated through multivariate analysis. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) performed on 19 abiotic and anthropozoogenic variables revealed two main groups of grasslands. These results empirically support the ecological coherence and reproducibility of the two-level typology used in this study.
2.4. Site Selection and Sampling Design
Eight semi-natural grasslands were selected between May 2022 and June 2024, following the aforementioned classification framework and a preliminary ecological survey conducted in 2021–2022. Five of these grasslands (Amsemlil, Bab-Miiz, Amokssisar, Menzla, and Marjabou) were under intensive grazing, and three (Bab Ataba, Tayrsal, and Tafaefra) were under moderate grazing (Figure 2A–H). This 5:3 imbalance reflects the actual distribution of grazing pressure across the massif, as confirmed by field observations and interviews.
Four of the grasslands (Bab-Miiz, Amokssisar, Tayrsal, and Bab Ataba) were ecologically homogeneous, each requiring a single 20 m2 sampling unit. The remaining four grasslands displayed internal heterogeneity and thus required multiple homogeneous sampling units: four at Amsemlil, five at Menzla, three at Tafaefra, and two at Marjabou. Homogeneous units were defined as plots exhibiting consistent slope, vegetation structure, and soil conditions. Heterogeneous grasslands, showing variation in microtopography and floristic composition, required stratified sampling to ensure representative data.
2.5. Data Collection
2.5.1. Floristic Data Collection
Floristic sampling combined a quadrat-based quantitative assessment with complementary visual inspection. A 1 m2 quadrat, subdivided into 25 sub-quadrats of 20 × 20 cm, was used within each homogeneous (Figure 2I) unit following standard protocols for Mediterranean grasslands [20]. This approach enabled the following:
- 1.
- Calculation of species frequency (based on the number of sub-quadrats occupied per species) [21];
- 2.
- Detection of fine-scale spatial distribution patterns;
- 3.
- Identification of microhabitats potentially generated by grazing activity.
Minimum area analysis confirmed that the 1 m2 quadrat size was sufficient, with species richness reaching an asymptote at 6400 cm2 (0.64 m2). Visual scanning across each unit was also performed to ensure the detection of rare or clustered species [22]. Collected specimens were identified using Flore pratique du Maroc [23] and verified by Professor Kadiri; vouchers were deposited in the herbarium of the Faculty of Sciences, Tetouan.
2.5.2. Soil Sampling and Analysis
Within each homogeneous unit, four soil cores, 30 cm deep, were collected using a steel auger and pooled to form one representative composite sample. Physico-chemical analyses were performed in a private agro-environmental laboratory based in Larache (Morocco). The parameters analyzed included soil texture (percentages of clay, silt, and sand), major nutrients such as total nitrogen (N), organic matter (OM), calcium (Ca), magnesium (Mg), potassium (K), and phosphorus (P), as well as pH and the carbon-to-nitrogen (C/N) ratio. Additional analyses covered trace and general soil properties, including iron (Fe), sodium (Na), electrical conductivity (EC), zinc (Zn), manganese (Mn), copper (Cu), boron (Br), chromium (Cr), and cation exchange capacity (CEC).
2.5.3. Microbiological Analysis
Microbiological analyses were conducted at the Biology and Health Laboratory of the Faculty of Sciences of Tetouan. The enumeration of soil microorganisms was performed using the suspension–dilution method as described by [24]. Briefly, 10 g of air-dried and sieved soil (2 mm mesh) was suspended in 90 mL of 0.9% sterile physiological saline solution and homogenized by vortexing. Serial decimal dilutions were prepared, and 1 mL aliquots from the 10−2 dilution were plated in triplicate on selective media.
- -
- Total aerobic mesophilic bacteria (TAM) were cultured on Plate Count Agar (PCA) and incubated aerobically at 30 °C for 72 h.
- -
- Total coliforms (TC) were assessed on Violet Red Bile Lactose Agar (VRBL) and incubated at 37 °C for 24–48 h.
- -
- Yeasts and molds (Y&M) were enumerated on Sabouraud Chloramphenicol Agar and incubated at 30 °C for 3–7 days.
- -
- Sulfite-reducing clostridia (SRC) were cultured anaerobically in Sulfite Polymyxin Sulfadiazine (SPS) agar tubes at 30 °C for 48–72 h.
Colony-forming units (CFU) were counted and expressed per gram of dry soil. For statistical analysis, values were log-transformed using the formula log10 (CFU/g + 1).
2.6. Statistical Analyses
Alpha diversity was assessed using species richness (the number of species per homogeneous unit) and the Shannon diversity index, which accounts for both richness and evenness. These indices were calculated from species frequency data collected within 1 m2 quadrats. Data normality was evaluated using the Shapiro–Wilk test. Since species richness slightly deviated from a normal distribution (p = 0.0501), comparisons between grazing intensities were made using the non-parametric Wilcoxon rank-sum test. The Shannon index, which followed a normal distribution (p = 0.4807), was compared using a Student’s t-test.
Floristic composition was analyzed using permutational multivariate analysis of variance (PERMANOVA), based on a Bray–Curtis dissimilarity matrix. The analysis was conducted using 999 permutations, and homogeneity of multivariate dispersion was verified beforehand through ANOVA on distances to centroids. Non-Metric Multidimensional Scaling (NMDS) was performed using the same dissimilarity index, retaining two dimensions for graphical ordination.
For each soil physico-chemical parameter, the choice of test was based on the distribution of values. When data met the assumption of normality, differences between grazing treatments were assessed using Student’s t-test; otherwise, the Wilcoxon rank-sum test was applied. Results are reported as mean ± standard deviation for each treatment.
Microbial abundance data were log-transformed using the formula log10 (CFU/g + 1) to account for skewness and the presence of zeros. Normality was reassessed for each transformed variable, and most comparisons were carried out using Wilcoxon rank-sum tests due to persistent non-normality.
A Canonical Correspondence Analysis (CCA) was also explored to examine potential relationships between floristic composition and environmental variables. However, the model was overfitted and did not reveal patterns beyond those identified in the primary analyses.
All statistical analyses and visualizations were performed in R (version 4.4.3), with statistical significance set at p < 0.05. The analyses relied on the vegan package for multivariate statistics (e.g., adonis2, betadisper, metaMDS) and ggplot2 for graphical representations. Only functions from these packages and base R were used.
3. Results
3.1. Floristic Composition and Taxonomic Distribution
A total of 70 plant species were recorded across the 18 homogeneous sampling units. These species belong to 21 botanical families, with Fabaceae (10 species), Asteraceae (9 species), and Poaceae (6 species) being the most represented. Caryophyllaceae, Apiaceae, and Lamiaceae each accounted for four species. This taxonomic structure reflects typical Mediterranean mountain grassland communities, where legumes, composites, and grasses commonly dominate.
Among these species, 35 were shared under both grazing intensities, while the remaining 35 were exclusive to the intensively grazed grasslands. No species were exclusive to the moderately grazed units. Mean species richness was higher under intensive grazing (20.6 ± 7.4 species) compared to moderate grazing (15.4 ± 3.8 species), although this difference was not statistically significant (Wilcoxon rank-sum test, p = 0.1019).
Beyond taxonomic enumeration, we analyzed the ecological profiles of dominant and grazing-indicator species. Intensively grazed grasslands harbored ruderal and disturbance-adapted taxa such as Filago pyramidata, Trifolium repens, Ranunculus bulbosus, and Cistus crispus, which are favored by trampling and nutrient enrichment. Conversely, moderately grazed units were characterized by more mesophilous and competitive species, including Poa annua, Bellis sylvestris, Lotus corniculatus, and Plantago coronopus. These contrasting syndromes point to two divergent functional assemblages: (i) open, heterogeneous communities shaped by localized disturbances and (ii) continuous, structured grasslands maintained by intermediate grazing pressure. These patterns contribute to a more functionally informed interpretation of the floristic composition.
3.2. Inter-Site Variability in Species Richness
A site-level analysis revealed marked variability in species richness between grasslands. Intensively grazed sites exhibited the highest richness, with Bab-Miiz reaching a maximum of 26 species, followed by Menzla (21.6 ± 3.85) and Amsemlil (20.0 ± 13.44), the latter showing notable internal heterogeneity (min = 10; max = 39). In contrast, moderately grazed sites such as Tayrsal (14 species) and Bab Ataba (15 species) had the lowest richness values. Tafaefra, also moderately grazed, had an intermediate richness (16.0 ± 5.2). These observations support the pattern observed across grazing categories, while emphasizing within-group variability.
3.3. Alpha Diversity Patterns Across Grazing Intensities
No statistically significant differences were found in alpha diversity indices between the two grazing intensities. Species richness tended to be higher under intensive grazing (median = 20.5) than under moderate grazing (median = 17), though the difference was not significant (Wilcoxon test, p = 0.1019). The Shannon diversity index was slightly higher in intensely grazed units (median = 2.60) compared to moderately grazed units (2.45), but again not significantly so (Student’s t-test, p = 0.1362). These results are illustrated in Figure 3. Despite visible differences, especially in Shannon diversity, grazing intensity did not significantly affect plant diversity at the plot level.
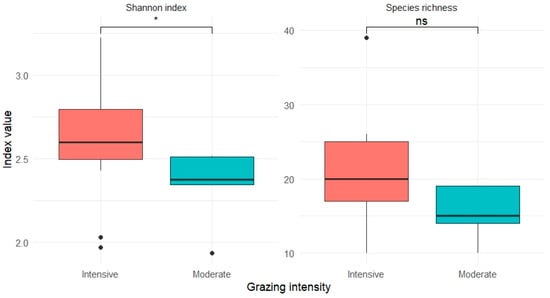
Figure 3.
Variation in species richness and Shannon diversity index across grazing intensities. Boxplots represent the median and interquartile range; outliers are shown as dots. * p < 0.05; ns = not significant (p ≥ 0.05). A non-significant difference was observed for richness (Wilcoxon test, p = 0.102, ns), while the difference in Shannon index was also non-significant (Student’s t-test, p = 0.136, ns), despite a slight visual trend.
The slight increase in species richness under intensive grazing appears to be driven by the emergence of ruderal species adapted to high disturbance, such as Filago pyramidata, Trifolium repens, and Ranunculus bulbosus. These species exploit microhabitats generated by trampling and nutrient hotspots, enhancing spatial heterogeneity within plots. This pattern is consistent with the concept of grazing-induced patchiness, which has been shown to promote diversity in low-input grasslands [25]. Consequently, our findings align with the intermediate disturbance hypothesis, whereby increased diversity under intense grazing reflects structural variability rather than improved ecological integrity.
3.4. Community Structure and Compositional Heterogeneity
The analysis of plant community composition revealed that grazing intensity did not significantly affect the overall species composition between treatments, as indicated by the non-significant PERMANOVA results (R2 = 0.086; F = 1.51; p = 0.101). However, the test for homogeneity of multivariate dispersion showed a highly significant difference between groups (F = 17.29; p = 0.0007), highlighting greater within-group variability under intensive grazing.
This result suggests that while the floristic composition remains globally comparable between moderately and intensively grazed grasslands, the spatial heterogeneity of species assemblages is markedly enhanced by intensive grazing. Such increased dispersion could reflect localized disturbance effects, including trampling, selective grazing, and nutrient redistribution by livestock, creating a mosaic of microhabitats with varying environmental conditions.
The NMDS ordination (stress = 0.223) supported these observations by illustrating a wider spread of sample units under intensive grazing along the ordination space, without clear separation between the two grazing intensities. The ellipses representing one standard deviation around group centroids emphasize this greater compositional heterogeneity in intensively grazed plots (Figure 4).
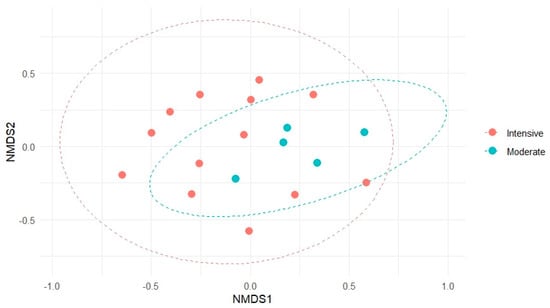
Figure 4.
Non-metric multidimensional scaling (NMDS) of floristic composition in grassland communities under moderate and intensive grazing. Each point represents a homogeneous sampling unit. Ellipses indicate one standard deviation around the centroid of each group. No significant compositional differences were detected between grazing intensities (PERMANOVA, p = 0.101), but multivariate dispersion was significantly higher under intensive grazing (p = 0.0007).
3.5. Soil Physico-Chemical Responses to Grazing
The comparison of soil properties revealed several significant differences between grazing intensities (Figure 5). Clay and silt contents were significantly higher in intensely grazed plots, while sand content was greater under moderate grazing. Potassium (K) and iron (Fe) concentrations were also higher in intensively grazed soils, whereas zinc (Zn) levels and electrical conductivity (EC) were significantly higher under moderate grazing.
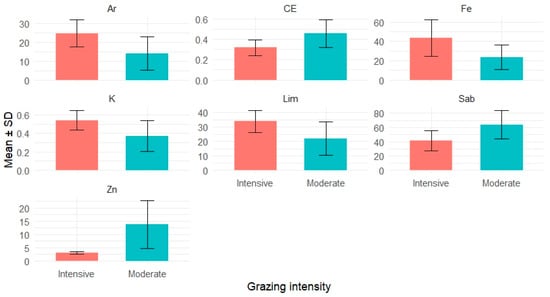
Figure 5.
Variation in key physico-chemical soil parameters under moderate and intensive grazing. Bars represent mean values ± standard deviation. Only variables with statistically significant differences between grazing intensities are shown (p < 0.05). Abbreviations: Ar = clay (%), Lim = silt (%), Sab = sand (%), K = potassium (meq/100 g), Fe = iron (ppm), Zn = zinc (ppm), CE = electrical conductivity (dS/m).
No significant differences were observed for total nitrogen (N), organic matter (OM), C/N ratio, calcium (Ca), magnesium (Mg), phosphorus (P), or pH. These results suggest that grazing intensity modulates soil texture and alters nutrient availability, with potential ecological implications for plant–soil interactions.
3.6. Microbial Community Dynamics Under Grazing Pressure
Grazing intensity selectively influenced certain soil microbial groups, particularly fungal communities. Yeasts and molds exhibited significantly higher abundance under intensive grazing (Wilcoxon test, p < 0.01), with a log-transformed mean of 3.41 ± 0.31 log10 (CFU/g + 1), compared to 2.78 ± 0.58 in moderately grazed units. This suggests that these fungal groups may be more responsive to soil disturbances associated with grazing, such as compaction, salinity changes, and nutrient redistribution.
Sulfite-reducing clostridia were exclusively detected in intensively grazed plots, although their abundance showed high variability and did not reach statistical significance. The presence of SRC only under intensive grazing may reflect localized anaerobic microsites resulting from trampling and reduced soil aeration. In contrast, total aerobic mesophilic bacteria did not exhibit significant differences between grazing intensities, although greater variability was observed in moderately grazed plots.
These results suggest that grazing disturbance does not uniformly affect all microbial groups but may selectively promote stress-tolerant fungi such as yeasts and molds. This differentiated microbial response highlights the need to consider functional group-specific dynamics when evaluating the ecological impacts of grazing on soil biological communities (Figure 6).
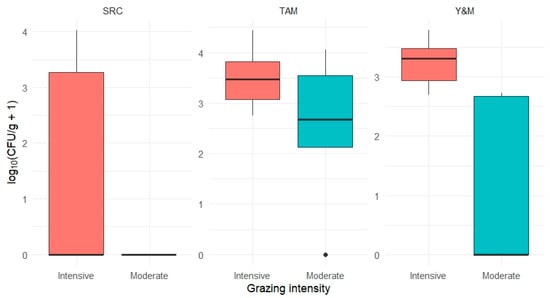
Figure 6.
Log-transformed abundance (log10[CFU/g + 1]) of soil microbial groups under moderate and intensive grazing. Each panel shows the distribution of one group: TAM = total aerobic mesophilic bacteria, Y&M = yeasts and molds, SRC = sulfite-reducing clostridia. Asterisks indicate the significance level of the Wilcoxon rank-sum test (p < 0.05; ns = not significant).
4. Discussion
4.1. Effects of Grazing Intensity on Species Richness and Floristic Diversity
Although no statistically significant differences in species richness or Shannon diversity were detected between grazing intensities, a slight trend toward higher values under intensive grazing was observed. This tendency suggests that grazing may help limit the dominance of competitive species and foster coexistence among a broader range of plant taxa. These dynamics are well-documented in Mediterranean grasslands, where moderate to high grazing pressure can prevent competitive exclusion and sustain floristic diversity by enhancing spatial heterogeneity and generating disturbance-driven microhabitats [1,26,27].
Among the 70 recorded species, several are of patrimonial or conservation interest, including Ophioglossum lusitanicum, Isoetes histrix, and Ranunculus ficaria. Their presence across grazing regimes reinforces the ecological value of Bouhachem’s grasslands. Importantly, no clear evidence was found for local extinctions due to overgrazing, which remains a marginal pressure in the massif. Nevertheless, the potential for long-term compositional shifts under cumulative disturbance cannot be ruled out. We thus recommend the establishment of biodiversity monitoring programs to assess the persistence and trajectories of these plant communities over time.
The marked variability observed among sampled units, particularly in intensively grazed sites, highlights the influence of microenvironmental heterogeneity. Trampling, localized compaction, and soil moisture gradients likely shape the microsite conditions that drive community assembly. These results are consistent with previous studies [5,28] emphasizing the importance of fine-scale abiotic variation in maintaining species with differing ecological requirements in grazed landscapes.
A key limitation of the present study lies in the absence of ungrazed control plots, which restricts direct comparisons with undisturbed baselines. This constraint stems from the lack of exclosures or formal grazing restrictions in the study area. Future work should prioritize the implementation of such reference sites to better evaluate the full gradient of grazing impacts.
4.2. Grazing Effects on Floristic Diversity and Community Structure
The NMDS analysis revealed greater dispersion of plant communities under intensive grazing, suggesting enhanced floristic heterogeneity within this regime. However, PERMANOVA results showed no significant differences in average community composition between grazing intensities. Together, these findings suggest that grazing acts more as a driver of within-group variability than as a directional filter altering overall species assemblages.
This interpretation is supported by the frequency data for Poa annua (Figure 7). While it was recorded in all quadrats from moderately grazed sites (100% frequency), it appeared in only 40% of intensively grazed plots and was entirely absent from three of them. This variation indicates that P. annua, despite being a disturbance-tolerant species, may be excluded under more extreme grazing conditions, possibly due to stronger trampling or altered soil properties.
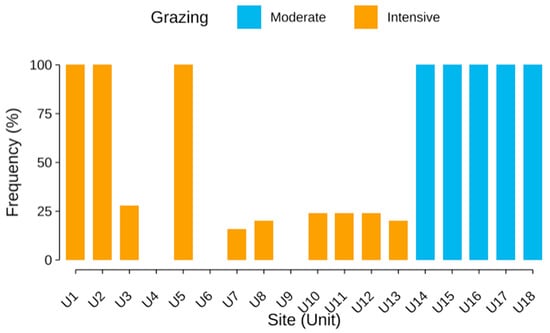
Figure 7.
Frequency of Poa annua (%) across homogeneous sampling units under moderate and intensive grazing. Each bar represents one unit (U1–U18), with blue indicating moderately grazed sites and orange indicating intensively grazed sites. Poa annua was present in all moderately grazed units (100% frequency), but showed variable and often low frequency under intensive grazing, with complete absence in three units.
P. annua, known for its early successional role, forms dense mats that influence soil moisture and shading dynamics. Its prevalence under moderate grazing and decline under intensive regimes illustrates how species responses can vary along a disturbance gradient, even within tolerant functional groups. These patch-level dynamics support the hypothesis that grazing contributes to a mosaic of ruderal and competitive species coexisting through localized disturbance effects.
Similar mechanisms have been documented in Mediterranean pastures and temporary wetlands, where grazing prevents the dominance of perennial competitors and promotes species turnover [29,30]. Moreover, the interaction between grazing pressure and site productivity, as discussed by Bakker et al. (2006) [31], may further explain the context-specific nature of plant responses observed in Bouhachem.
4.3. Impact of Grazing on Soil Physico-Chemical Properties
In contrast to the modest floristic responses, grazing intensity had a significant effect on multiple soil physico-chemical parameters. Notably, concentrations of potassium, iron, and zinc, as well as soil texture (clay and sand proportions) and electrical conductivity, differed significantly between grazing regimes. These patterns suggest that grazing modifies nutrient distribution and soil structure, likely through livestock trampling and manure deposition [32,33].
Other parameters, such as total nitrogen, organic matter, phosphorus, calcium, magnesium, and pH, did not vary significantly, indicating that certain soil attributes are more resistant to grazing-induced disturbance. This selective response may be linked to the buffering capacity of the soil, differences in underlying bedrock, or historical land use.
These findings echo results from other Mediterranean grazing systems [34,35], where trampling and nutrient deposition primarily affect physical soil attributes and exchangeable elements rather than total nutrient pools. Additionally, local edaphic conditions, including baseline fertility and water retention, likely modulate the direction and magnitude of grazing effects [4,36]. The observed variability in soil responses thus emphasizes the importance of site-specific assessments in evaluating grazing impacts.
4.4. Microbial Community Response to Grazing Intensity
Grazing intensity also influenced the structure of soil microbial communities, with significant differences observed in the abundance of yeasts and molds. These fungal groups were more prevalent under intensive grazing, likely due to increased organic matter inputs, localized salinity changes, and modified aeration caused by trampling and animal waste. Their elevated abundance suggests that yeasts and molds are sensitive indicators of soil disturbance and ecological stress [28,37].
In contrast, no significant differences were found in the abundance of total aerobic mesophilic bacteria between grazing regimes. Although sulfite-reducing clostridia were detected exclusively in intensively grazed plots, their overall abundance was low and highly variable, preventing statistical confirmation of grazing effects.
These findings point to a differential sensitivity among microbial functional groups. Fungal communities, particularly those adapted to disturbed or enriched environments, respond more markedly to grazing than generalist bacterial taxa. This underlines the importance of integrating microbial bioindicators in future monitoring programs to better understand the belowground consequences of grazing practices.
5. Conclusions
This study provides new insights into the ecological effects of grazing intensity on semi-natural grasslands in the Jbel Bouhachem massif. Although grazing did not significantly affect plant species richness or alpha diversity, it influenced the spatial structure of plant communities and selectively modified key soil physico-chemical parameters, particularly those related to soil texture and nutrient availability.
These findings highlight the complexity of ecological responses to grazing pressure, showing that vegetation, soil properties, and disturbance regimes interact in a non-linear and compartment-specific manner. Rather than inducing homogenization, intensive grazing maintained a heterogeneous plant assemblage, while contributing to significant shifts in certain edaphic characteristics.
Such results emphasize the importance of adopting grazing practices that balance ecosystem conservation and pastoral use. Promoting adaptive management strategies, based on local ecological variability, appears essential for sustaining both biodiversity and soil functions in Mediterranean mountain ecosystems.
Although this study does not currently propose setting up grazing exclosures, it emphasizes the need for a differentiated management strategy tailored to local realities. A complete ban on grazing is not considered feasible, as these grasslands are vital resources for traditional pastoral systems in the Rif. The “Agdal” system, a customary-managed land model used in the High Atlas, could inspire a future pastoral management strategy for the Bouhachem Park, which currently lacks a specific regulatory framework.
Further research integrating long-term monitoring and functional approaches would enhance our understanding of these interactions and support more effective biodiversity conservation policies in these fragile environments [26].
Author Contributions
Conceptualization, S.C. (Saïd Chakri) and A.T.; methodology, S.C. (Saïd Chakri); software, S.C. (Saïd Chakri) and A.T.; validation, S.C. (Saïd Chakri) and M.M.A.; formal analysis, S.C. (Saïd Chakri) and A.T.; investigation, S.C. (Saïd Chakri), M.K. and F.E.L.; resources, S.C. (Saïd Chakri); writing-original draft preparation, S.C. (Saïd Chakri); writing-review and editing, S.C. (Saïd Chakri), A.T. and S.C. (Susan Canavan); supervision, M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fleurance, G.; Duncan, P.; Farruggia, A.; Dumont, B.; Leconte, T. Impact du pâturage équin sur la diversité floristique et faunistique des milieux pâturés. Fourrages 2011, 207, 189–199. [Google Scholar]
- Pykälä, J. Cattle Grazing Increases Plant Species Richness of Most Species Trait Groups in Mesic Semi-Natural Grasslands. Plant Ecol. 2005, 175, 217–226. [Google Scholar] [CrossRef]
- Lavorel, S.; de Bello, F.; Grigulis, K.; Lepš, J.; Garnier, E.; Castro, H.; Dolezal, J.; Godolets, C.; Quétier, F.; Thébault, A. Response of Herbaceous Vegetation Functional Diversity to Land Use Change across Five Sites in Europe and Israel. Isr. J. Ecol. Evol. 2011, 57, 53–72. [Google Scholar] [CrossRef]
- Ribeiro, S.; Fernandes, J.P. Diversity and Floristic Patterns of Mediterranean Grasslands: The Relative Influence of Environmental and Land Management Factors. Biodivers. Conserv. 2014, 23, 2903–2921. [Google Scholar] [CrossRef]
- Marion, B. Impact du pâturage sur la structure de la végétation: Interactions biotiques, traits et conséquences fonctionnelles. Ph.D. Thesis, Université Rennes-I, Rennes, France, 2010. [Google Scholar]
- Papanastasis, V.P.; Bautista, S.; Chouvardas, D.; Mantzanas, K.; Papadimitriou, M.; Mayor, A.G.; Koukioumi, P.; Papaioannou, A.; Vallejo, R.V. Comparative Assessment of Goods and Services Provided by Grazing Regulation and Reforestation in Degraded Mediterranean Rangelands. Land Degrad. Dev. 2017, 28, 1178–1187. [Google Scholar] [CrossRef]
- Moinardeau, C.; Mesléard, F.; Dutoit, T. Using Different Grazing Practices for Increasing Plant Biodiversity in the Dykes and Embankments Along the Rhône River (Southern France). Environ. Manag. 2016, 58, 984–997. [Google Scholar] [CrossRef]
- Schirpke, U.; Kohler, M.; Leitinger, G.; Fontana, V.; Tasser, E.; Tappeiner, U. Future Impacts of Changing Land-Use and Climate on Ecosystem Services of Mountain Grassland and Their Resilience. Ecosyst. Serv. 2017, 26, 79–94. [Google Scholar] [CrossRef]
- Chollet, S.; Rambal, S.; Fayolle, A.; Hubert, D.; Foulquié, D.; Garnier, E. Combined Effects of Climate, Resource Availability, and Plant Traits on Biomass Produced in a Mediterranean Rangeland. Ecology 2014, 95, 737–748. [Google Scholar] [CrossRef]
- Hempson, G.P.; Parr, C.L.; Lehmann, C.E.R.; Archibald, S. Grazing Lawns and Overgrazing in Frequently Grazed Grass Communities. Ecol. Evol. 2022, 12, e9268. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and Productivity in a Long-Term Grassland Experiment. Sci. Am. Assoc. Adv. Sci. 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Benabid, A. Etude Phytoécologique Des Peuplements Forestiers et Préforestiers Du Rif Centro-Occidental (Maroc). Tech. Rep. Inst. Sci. Univ. Mohammed V Rabat Moroc. 1984. Available online: https://agris.fao.org/search/en/providers/122621/records/647396a768b4c299a3fb6154 (accessed on 22 June 2025).
- Gatchui, H.; Smektala, G.; Smektala, G.; Solano, D.; Solano, D.; Taiqui, L.; Taiqui, L.; Ngoment, A.; Ngoment, A. Cannabis Cultivation and Deforestation in the Site of Bio Ecological Interest (SIBE) of Bouhachem, Morocco. Int. J. Biol. Chem. Sci. 2014, 8, 1179. [Google Scholar] [CrossRef]
- Bouahim, S.; Rhazi, L.; Amami, B.; Sahib, N.; Rhazi, M.; Waterkeyn, A.; Zouahri, A.; Mesleard, F.; Muller, S.D.; Grillas, P. Impact of grazing on the species richness of plant communities in Mediterranean temporary pools (western Morocco). C. R. Biol. 2010, 333, 670–679. [Google Scholar] [CrossRef]
- Bachar, M.; Zidane, L.; Rochdi, A. Ethno-medicinal and traditional Phytotherapy of plants used in Bouhachem Natural Regional Park “Rif of Morocco”—Case of Tazroute district. J. Mater. Environ. Sci. 2016, 11, 4175–4204. [Google Scholar]
- Ftaïta, T.; Daïde, H.; Chaouki, M.; Tribak, A.; Taous, A.; Perez, S. Environnements et sociétés dans les basses montagnes du Rif (Maroc): Des potentialités peu exploitées. SHS Web Conf. 2012, 3, 02008. [Google Scholar] [CrossRef]
- Vázquez, F.M.; Díaz, A.V. La reserva de biosfera intercontinental de Mediterráneo Andalucía (España)-Marruecos como instrumento de cooperación. Ecosistemas 2008, 17, 117–123. [Google Scholar]
- Radford, E.A.; World Conservation Union. Zones Importantes Pour les Plantes en Méditerranée Méridionale et Orientale: Sites Prioritaires Pour la Conservation; IUCN: Gland, Switzerland, 2011; ISBN 978-2-8317-1372-4. [Google Scholar]
- MPPA. Master Plan for Protected Areas (1996–2006), Jbel Bouhachem, N° 05; Department of Water and Forests: Rabat, Morocco, 1996. [Google Scholar]
- Braun-Blanquet, J.; Plant Sociology. The Study of Plant Communities. 1932. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19331600801 (accessed on 22 June 2025).
- Gilbertson, D.D.; Kent, M.; Pyatt, F.B. Floristic Methods for Describing Vegetation. In Practical Ecology for Geography and Biology; Springer: Boston, MA, USA, 1985; pp. 75–98. ISBN 978-1-4684-1415-8. [Google Scholar]
- Smart, S.M. Ecological Assessment of Vegetation from a Nature Reserve Using Regional Reference Data and Indicator Scores. Biodivers. Conserv. 2000, 9, 811–832. [Google Scholar] [CrossRef]
- Fennane; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Pratique Du Maroc. Manuel de Détermination Des Plantes Vasculaires. Volume 1: Pteridophyta, Gymnospermae, Angiospermae (Lauraceae–Neuradaceae); Travaux de l’Institut Scientifique, Série Botanique, n° 36; Institut Scientifique, Université Mohammed V—Agdal: Rabat, Maroc, 1999. [Google Scholar]
- Yusuf, A.B.; Mohammed, S.S.D.; Ijah, U.J.J. Microbiological And Physicochemical Assessment of Tannery Polluted Soil Inoculated With Pure And Mixed Fungi Slurry. FUDMA J. Sci. 2020, 4, 732–743. [Google Scholar]
- Tonn, B.; Densing, E.M.; Gabler, J.; Isselstein, J. Grazing-induced Patchiness, Not Grazing Intensity, Drives Plant Diversity in European Low-input Pastures. J. Appl. Ecol. 2019, 56, 1624–1636. [Google Scholar] [CrossRef]
- Vidaller, C.; Malik, C.; Dutoit, T. Grazing Intensity Gradient Inherited from Traditional Herding Still Explains Mediterranean Grassland Characteristics despite Current Land-Use Changes. Agric. Ecosyst. Environ. 2022, 338, 108085. [Google Scholar] [CrossRef]
- Noy-Meir, I.; Gutman, M.; Kaplan, Y. Responses of Mediterranean Grassland Plants to Grazing and Protection. J. Ecol. 1989, 77, 290–310. [Google Scholar] [CrossRef]
- Hanafi, A.; Aridhi, O. La Végétation Méditerranéene Aride Dans Les Aires de Transition: Typologie, Richesse et Défis de Valorisation. Cas Des Monts de Matmata (Sud Tunisien). Analele Univ. Bucuresti Geogr. Univ. Buchar.–Geogr. Ser. 2021, 70, 85–130. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, L.; Rhazi, M.; Grillas, P.; Khyari, D.E. Richness and Structure of Plant Communities in Temporary Pools from Western Morocco: Influence of Human Activities. Hydrobiologia 2006, 570, 197–203. [Google Scholar] [CrossRef]
- Zedler, P.H. The Ecology of Southern California Vernal Pools: A Community Profile; Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1987; Volume 85. [Google Scholar]
- Bakker, E.S.; Ritchie, M.E.; Olff, H.; Milchunas, D.G.; Knops, J.M.H. Herbivore Impact on Grassland Plant Diversity Depends on Habitat Productivity and Herbivore Size. Ecol. Lett. 2006, 9, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Hastings, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical Review of the Impacts of Grazing Intensity on Soil Organic Carbon Storage and Other Soil Quality Indicators in Extensively Managed Grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef]
- Oustani, M.; Halilat, M.T.; Chelloufi, H. Essai d’optimisation de La Fertilisation Organique de La Pomme de Terre Sous Les Conditions Salinesdes Regions Arides. Rev. Bioressources 2014, 4, 53–67. [Google Scholar] [CrossRef]
- Erktan, A.; Coq, S.; Blanchart, E.; Chevallier, T.; Trap, J.; Bernard, L.; Nahmani, J.; Hartmann, C.; Hedde, M.; Ganault, P.; et al. Biodiversité et Structure Physique Des Sols: Une Vision Spatialisée Du Fonctionnement Des Sols. Étude Gest. Sols 2022, 29, 153–167. [Google Scholar]
- Gamoun, M.; Ouled Belgacem, A.; Hanchi, B.; Neffati, M.; Gillet, F. Effet Du Pâturage Sur La Diversité Floristique Des Parcours Arides Du Sud Tunisien. Rev. DÉcologie 2012, 67, 271–282. [Google Scholar] [CrossRef]
- Loisel, R.; Gomila, H.; Rolando, C. Déterminisme écologique de la diversité des pelouses dans la plaine de la Crau (France méridionale). Ecol. Mediterr. 1990, 16, 255–277. [Google Scholar] [CrossRef]
- Vázquez, E.; Benito, M.; Espejo, R.; Teutscherova, N. Response of Soil Properties and Microbial Indicators to Land Use Change in an Acid Soil under Mediterranean Conditions. Catena 2020, 189, 104486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).