Fragmented Habitats, Fragmented Functions: Unveiling the Role of Habitat Structure in Andean Bird Communities
Abstract
1. Introduction
2. Materials and Methods
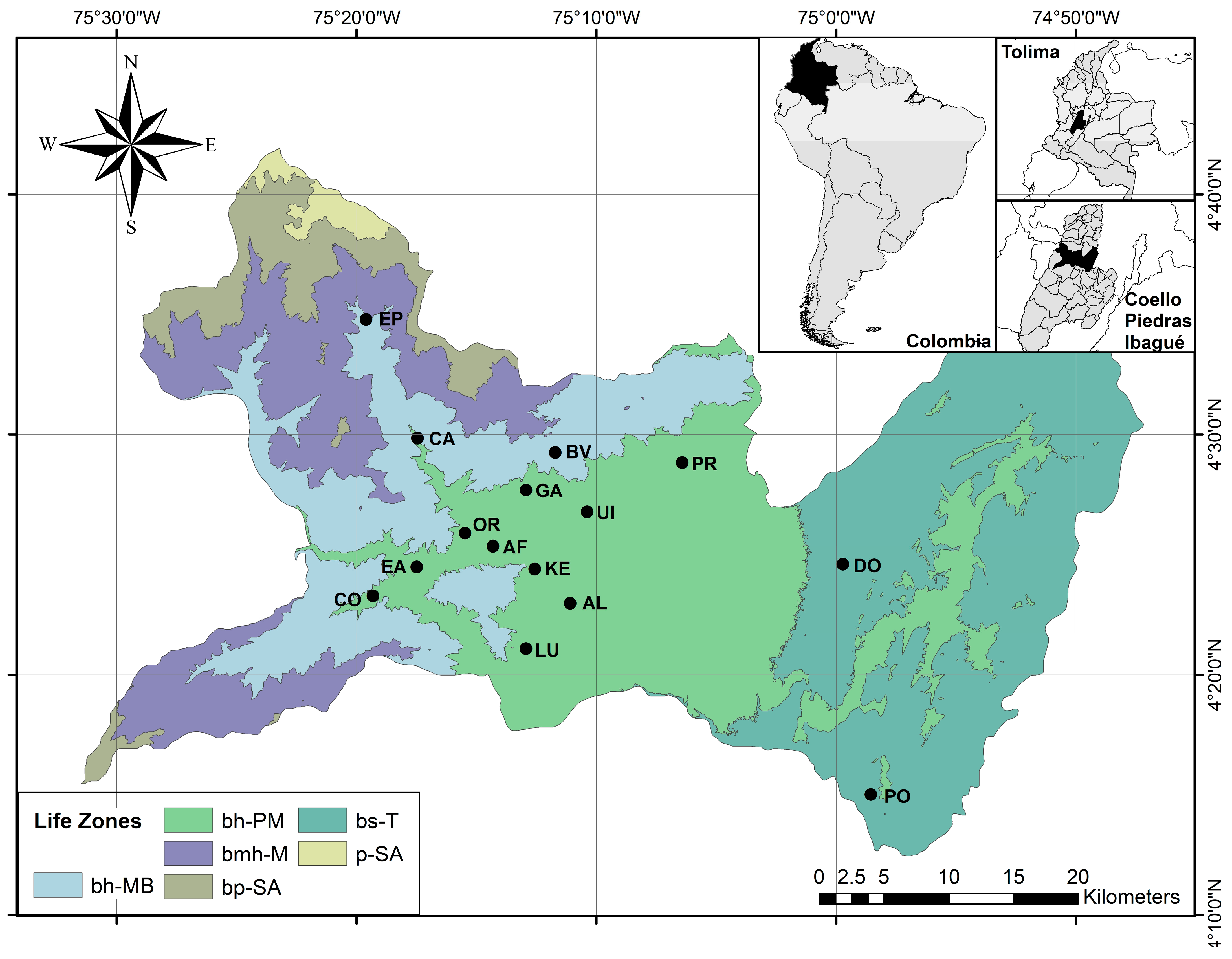
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.3.1. Landscape Metrics
2.3.2. Functional Diversity
2.3.3. Effect of Landscape Structure on Bird Functional Diversity
3. Results
3.1. Landscape Metrics
3.2. Functional Diversity
3.3. Effect of Landscape Structure on Bird Functional Diversity
4. Discussion
4.1. Landscape Metrics
4.2. Functional Alpha Diversity
4.3. Functional Beta Diversity
4.4. Effect of Landscape Structure on Bird Functional Diversity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banks-Leite, C.; Ewers, R.M.; Folkard-Tapp, H.; Fraser, A. Countering the effects of habitat loss, fragmentation, and degradation through habitat restoration. One Earth 2020, 3, 672–676. [Google Scholar] [CrossRef]
- Fan, Q.; Liang, Z.; Liang, L.; Ding, S.; Zhang, X. Landscape pattern analysis based on optimal grain size in the core of the Zhengzhou and Kaifeng integration area. Pol. J. Environ. 2018, 27, 1229–1237. [Google Scholar]
- Quintero-Ángel, A.; Osorio-Domínguez, D.; Valenzuela, L. Algunas reflexiones sobre fragmentación y sus retos para la investigación. Rev. Biodivers. Neotrop. 2012, 2, 15–20. [Google Scholar] [CrossRef]
- Salazar, E.; Mendoza, J.; Ochoa-Gaona, S.; Ku-Quej, V.; Hidalgo-Mihart, M. Evaluación de la conectividad del paisaje en la región Puuc-Chenes, México, con base en los requerimientos de hábitat del jaguar (Panthera onca). Investig. Geogr. Bol. Inst. Geogr. 2017, 17, 101–115. [Google Scholar] [CrossRef]
- Vitar-Mendoza, J.J.; Sandoval-Parra, K.X.; Ortiz-Moreno, M.L. Land-cover change in the Department of Vichada, Colombia, from 1985 to 2017. Rev. Investig. Agrar. Ambient. 2022, 13, 149–174. [Google Scholar] [CrossRef]
- Jasso-del Toro, C.; Márquez-Valdelamar, L.; Mondragón-Ceballos, R. Diversidad genética en grupos de monos aulladores de manto (Alouatta palliata mexicana) en la Reserva de la Biosfera Los Tuxtlas (Veracruz, México). Rev. Mex. Biodiv. 2016, 87, 1069–1079. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.L. Effects of habitat fragmentation on bird behavior and extinction mechanisms. Int. J. Mol. Zool. 2024, 14, 97–110. [Google Scholar] [CrossRef]
- Velastegui-Montoya, A.; Montalván-Burbano, N.; Peña-Villacreses, G.; de Lima, A.; Herrera-Franco, G. Land Use and Land Cover in Tropical Forest: Global Research. Forests 2022, 13, 1709. [Google Scholar] [CrossRef]
- Rurangwa, M.L.; Aguirre-Gutiérrez, J.; Matthews, T.J.; Niyigaba, P.; Wayman, J.P.; Tobias, J.A.; Whittaker, R.J. Effects of land-use change on avian taxonomic, functional and phylogenetic diversity in a tropical montane rainforest. Divers. Distrib. 2021, 27, 1732–1746. [Google Scholar] [CrossRef]
- Bełcik, M.; Lenda, M.; Amano, T.; Skórka, P. Different response of the taxonomic, phylogenetic and functional diversity of birds to forest fragmentation. Sci. Rep. 2020, 10, 20320. [Google Scholar] [CrossRef]
- Uezu, A.; Metzger, J.P.; Vielliard, J. Effects of structural and functional connectivity and patch size on the abundance of seven Atlantic Forest bird species. Biol. Conserv. 2005, 123, 507–519. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity conservation in agriculture requires a multi-scale approach. Proc. R. Soc. Lond. B Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef]
- Rangel, J. La biodiversidad de Colombia: Significado y distribución regional. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2015, 39, 176–200. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.; Guo, J.; Ma, S.; Zhang, W. The taxonomic, functional and phylogenetic diversity of birds in Xiaohongxiang Wetland, southwest China. Biodivers. Data J. 2024, 12, e136248. [Google Scholar] [CrossRef]
- Mariano-Neto, E.; Santos, R.A.S. Changes in the Functional Diversity of Birds Due to Habitat Loss in the Brazil Atlantic Forest. Front. For. Glob. Change 2023, 6, 1041268. [Google Scholar] [CrossRef]
- Nunes, C.A.; Braga, R.F.; Figueira, J.E.C.; De Siqueira, F.; Fernandes, G.W. Dung beetles along a tropical altitudinal gradient: Environmental filtering on taxonomic and functional diversity. PLoS ONE 2016, 11, e0157442. [Google Scholar] [CrossRef]
- Ausprey, I.J.; Newell, F.L.; Robinson, S.K. Functional response traits and altered ecological niches drive the disassembly of cloud forest bird communities in tropical montane countrysides. J. Anim. Ecol. 2022, 91, 2314–2328. [Google Scholar] [CrossRef]
- Bain, G.C.; MacDonald, M.A.; Hamer, R.; Gardiner, R.; Johnson, C.N.; Jones, M.E. Changing bird communities of an agricultural landscape: Declines in arboreal foragers, increases in large species. R. Soc. Open Sci. 2020, 7, 200076. [Google Scholar] [CrossRef]
- Castro-Navarro, J.; Sahagún Sánchez, F.J.; Reyes-Hernández, H. Dinámica de fragmentación en la Sierra Madre Oriental y su impacto sobre la distribución potencial de la avifauna. Madera Y Bosques 2017, 23, 99–117. [Google Scholar] [CrossRef]
- Willson, M.F.; Armesto, J.J. Efectos de la fragmentación de bosques para las aves de los bosques australes chilenos. Ambiente Desarro. CIPMA 2003, 19, 54–59. [Google Scholar]
- Carranza-Quiceno, J.A.; Henao-Isaza, J.R.; Castaño, J.H. Avifauna de un paisaje rural heterogéneo en Risaralda, cordillera Central de Colombia. Biota Colomb. 2018, 19, 92–104. [Google Scholar] [CrossRef]
- Liu, X.; Yang, G.; Que, Q.; Wang, Q.; Zhang, Z.; Huang, L. How Do Landscape Heterogeneity, Community Structure, and Topographical Factors Contribute to the Plant Diversity of Urban Remnant Vegetation at Different Scales? Int. J. Environ. Res. Public Health 2022, 19, 14302. [Google Scholar] [CrossRef]
- Katayama, N.; Amano, T.; Naoe, S.; Yamakita, T.; Komatsu, I.; Takagawa, S.-I.; Sato, N.; Ueta, M.; Miyashita, T. Land-scape Heterogeneity-Biodiversity Relationship: Effect of Range Size. PLoS ONE 2014, 9, e93359. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental Heterogeneity as a Universal Driver of Species Richness across Taxa, Biomes and Spatial Scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- De Stefano, K.; Merler, J.A.; Magnano, A.L.; Nanni, A.S.; Kandus, P.; Quintana, R.D. Relación entre la heterogeneidad ambiental y el patrón de distribución y la riqueza de aves en dos unidades de paisajes del delta del Paraná, Argentina. Ornitol. Neotrop. 2012, 23, 169–184. [Google Scholar]
- Zheng, Z.; Zhang, F.; Lin, Z.; Yuan, L.; Yao, H.; Duan, G.; Yuan, L.; Liu, Y.; Shi, H.; Wen, Z.; et al. Using the response–effect trait framework to disentangle the effects of environmental change on the ecosystem services. J. Plant Ecol. 2024, 17, rtae024. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- de Luna, A.G.; Link, A. Distribution, population density and conservation of the critically endangered brown spider monkey (Ateles hybridus) and other primates of the inter-andean forests of Colombia. Biodivers. Conserv. 2018, 27, 3469–3511. [Google Scholar] [CrossRef]
- Montes-Rojas, A.; Delgado-Morales, N.A.J.; Escucha, R.S.; Siabatto, R.S.; Link, A. Recovering connectivity through restoration corridors in a fragmented landscape in the Magdalena River’s valley in Colombia. Biodivers. Conserv. 2024, 33, 3171–3185. [Google Scholar] [CrossRef]
- Ramos-Mosquera, V.; López-Delgado, E.; Losada-Prado, S.; Moreno-Palacios, M. Análisis multi-temporal de la biodiversidad aviar en el Centro Universitario Regional del Norte (Tolima, Colombia). Rev. Biol. Trop. 2024, 72, e58133. [Google Scholar] [CrossRef]
- Romero-Duque, L.P.; Rosero-Toro, J.H.; Fernández-Lucero, M.; Simbaqueba-Gutierrez, A.; Pérez, C. Trees and shrubs of the tropical dry forest of the Magdalena river upper watershed (Colombia). Biodivers. Data J. 2019, 7, e36191. [Google Scholar] [CrossRef]
- Salgado, J.; Jaramillo-Monroy, C.; Link, A.; Lopera-Congote, L.; Velez, M.I.; Gonzalez-Arango, C.; Yang, H.; Panizzo, V.N.; McGowan, S. Riverine connectivity modulates elemental fluxes through a 200-year period of intensive anthropic change in the Magdalena River floodplains, Colombia. Water Res. 2025, 268, 122633. [Google Scholar] [CrossRef]
- Albornoz-Garzón, J.G.; Conde-Saldaña, C.C.; López-Delgado, E.O.; García-Melo, J.E.; Villa-Navarro, F.A. Fishes from the Río Alvarado drainage, Upper Río Magdalena Basin, Colombia. Check List 2020, 16, 1181–1198. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Santini, L.; Spake, R.; Bowler, D.E. Population abundance estimates in conservation and biodiversity research. Trends Ecol. Evol. 2024, 39, 515–523. [Google Scholar] [CrossRef]
- Ralph, C.J.; Geupel, G.R.; Pyle, P.; Martin, T.E.; DeSante, D.F.; Milá, B. Handbook of Field Methods for Monitoring Landbirds; General Technical Report PSW-GTR-144; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1993.
- eBird. eBird: An Online Database of Bird Distribution and Abundance. Available online: http://www.ebird.org (accessed on 1 June 2023).
- Ulrich, W.; Gotelli, N.J. Null model analysis of species associations using abundance data. Ecology 2010, 91, 3384–3397. [Google Scholar] [CrossRef]
- Tobias, J. AVONET: Morphological, Ecological and Geographical Data for All Birds, Figshare. 2022. Available online: https://doi.org/10.6084/m9.figshare.16586228.v7 (accessed on 1 December 2023).
- Tobias, J.A.; Sheard, C.; Pigot, A.L.; Devenish, A.J.M.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, Ecological and Geographical Data for All Birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Jacoboski, L.I.; Debastiani, V.J.; de Mendonça-Lima, A.; Duarte, L. How do diversity and functional nestedness of bird communities respond to changes in the landscape caused by eucalyptus plantations? Community Ecol. 2016, 17, 107–113. [Google Scholar] [CrossRef]
- Díaz-Cháux, J.T.; Velasquez-Valencia, A.; Martínez-Salinas, A.; Casanoves, F. Functional Diversity and Ecosystem Services of Birds in Productive Landscapes of the Colombian Amazon. Diversity 2025, 17, 305. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Chown, S.L. Land-use change promotes avian diversity at the expense of species with unique traits. Ecol. Evol. 2016, 6, 7610–7622. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; MacGregor, H.E.A.; Darski, B.; de Moura, N.G.; Aleixo, A.; Barlow, J.; Tobias, J.A. Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. B 2016, 283, 20161289. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Castro, A.F.; Maron, M.; Mitchell, M.G.E.; Rhodes, J.R. Disentangling direct and indirect effects of landscape structure on urban bird richness and functional diversity. Ecol. Appl. 2022, 32, e2713. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.H.; Bedoya-Durán, M.J.; Colorado, Z.G.J.; Bohlman, S.A.; Robinson, W.D. Dietary and habitat specialization, eye size, clutch size, and aerial lifestyle predict avian fragmentation sensitivity in an Andean biodiversity hotspot. Biodivers. Conserv. 2023, 32, 4057–4081. [Google Scholar] [CrossRef]
- Layman, C.A.; Quattrochi, J.P.; Peyer, C.M.; Allgeier, J.E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 2007, 10, 937–944. [Google Scholar] [CrossRef]
- Suárez-Parra, K.V.; Cély-Reyes, G.E.; Forero-Ulloa, F.E. Validación de la metodología Corine Land Cover (CLC) para determi- nación espacio-temporal de coberturas: Caso microcuenca de la quebrada Mecha (Cómbita, Boyacá), Colombia. Biota Colomb. 2016, 17, 1–15. [Google Scholar] [CrossRef][Green Version]
- McGarigal, K.; Marks, B. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; General Technical Report PNW-351; USDA Forest Service: Corvallis, OR, USA, 1995.
- Basile, M.; Storch, I.; Mikusiński, G. Abundance, species richness and diversity of forest bird assemblages—The relative importance of habitat structures and landscape context. Ecol. Indic. 2021, 133, 108402. [Google Scholar] [CrossRef]
- Adler, K.; Jedicke, E. Landscape metrics as indicators of avian community structures—A state of the art review. Ecol. Indic. 2022, 145, 109575. [Google Scholar] [CrossRef]
- Salas-Correa, A.; Mancera-Rodríguez, N. Aves como indicadoras ecológicas de etapas sucesionales en un bosque secundario, Antioquia, Colombia. Rev. Biol. Trop. 2020, 68, 23–39. [Google Scholar] [CrossRef]
- Santos, B.A.; Alvarado, F.; Morante-Filho, J.C. Impacts of Urbanization on Multiple Dimensions of Bird Diversity in Atlantic Forest Landscapes. Glob. Ecol. Conserv. 2024, 54, e03078. [Google Scholar] [CrossRef]
- Bonfim, F.C.G.; Dodonov, P.; Cazetta, E. Landscape composition is the major driver of the taxonomic and functional diversity of tropical frugivorous birds. Landsc. Ecol. 2021, 36, 2535–2547. [Google Scholar] [CrossRef]
- Carrara, E.; Arroyo-Rodríguez, V.; Vega-Rivera, J.H.; Schondube, J.E.; de Freitas, S.M.; Fahrig, L. Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol. Conserv. 2015, 184, 117–126. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. The Corrplot Package, R Package Version 0.95. 2024. Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 5 February 2024).
- Harrell, F. The Hmisc Package, R Package Version 4.2-0. 2019. Available online: https://cran.r-project.org/package=Hmisc (accessed on 5 February 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 20 June 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. The Vegan Package, R Package Version 2.5-6. 2019. Available online: https://cran.r-project.org/package=vegan (accessed on 5 February 2024).
- Gómez, C.; Tenorio, E.A.; Cadena, C.D. Change in Avian Functional Fingerprints of a Neotropical Montane Forest over 100 Years as an Indicator of Ecosystem Integrity. Conserv. Biol. 2022, 35, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Maire, E.; Grenouillet, G.; Brosse, S.; Villéger, S. How Many Dimensions Are Needed to Accurately Assess Functional Diversity? A Pragmatic Approach for Assessing the Quality of Functional Spaces. Glob. Ecol. Biogeogr. 2015, 24, 728–740. [Google Scholar] [CrossRef]
- Poveda-Cuellar, J.; López-Delgado, E.; Zúñiga-Upegui, P.; Villa-Navarro, F. What Controls Fish Functional Diversity Patterns in Colombian Andean Streams? Ecol. Freshw. Fish 2021, 31, 87–101. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Leclerc, C.; Villéger, S.; Marino, C.; Bellard, C. Global Changes Threaten Functional and Taxonomic Diversity of Insular Species Worldwide. Divers. Distrib. 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A Functional Approach Reveals Community Responses to Disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. FD: Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://cran.r-project.org/web/packages/FD/index.html (accessed on 5 February 2024).
- Gamboa-Soto, F.; Bautista-García, R.; Llanes-Gil López, D.I.; Bermea, J.E.; Tinoco Mendiola, R.; Olive-Méndez, S.F.; González-Hernández, A. Heat Treatment-Driven Structural and Morphological Transformation Under Non-Parametric Tests on Metal–Ceramic-Sputtered Coatings. Ceramics 2025, 8, 25. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing Functional β-Diversity Reveals that Low Functional β-Diversity is Driven by Low Functional Turnover in European Fish Assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Baselga, A. The Relationship Between Species Replacement, Dissimilarity Derived from Nestedness, and Nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5.6. 2024. Available online: https://CRAN.R-project.org/package=betapart (accessed on 5 February 2024).
- García-Álvarez, D.; Fuente, M.J. Estudio Comparativo de Técnicas de Detección de Fallos Basadas en el Análisis de Componentes Principales (PCA). Rev. Iberoam. Automat. Informa. Ind. 2011, 8, 182–195. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining with R. R Package Version 1.25. 2013. Available online: http://CRAN.R-project.org/package=FactoMineR (accessed on 10 February 2024).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://cran.r-project.org/package=factoextra (accessed on 10 February 2024).
- Arroyo, I.; Bravo, L.C.; Llinás, H.; Muñoz, F. Distribuciones Poisson y Gamma: Una Discreta y Continua Relación. Prospect 2014, 12, 99–107. [Google Scholar]
- Sutherland, C.; Hare, D.; Johnson, P.J.; Linden, D.W.; Montgomery, R.A.; Droge, E. Practical Advice on Variable Selection and Reporting Using Akaike Information Criterion. Proc. R. Soc. Lond. B Biol. Sci. 2023, 290, 20231261. [Google Scholar] [CrossRef] [PubMed]
- Hamner, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning. R Package Version 0.1.4. 2018. Available online: https://cran.r-project.org/package=Metrics (accessed on 10 February 2024).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; Borchers, H.W. DescTools: Tools for Descriptive Statistics. R Package Version 0.99. 2019. Available online: https://cran.r-project.org/web/packages/DescTools/index.html (accessed on 10 February 2024).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.7. 2024. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 10 February 2024).
- Legendre, P.; Anderson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Clappe, S.; Dray, S.E.; Peres-Neto, P.R. Beyond Neutrality: Disentangling the Effects of Species Sorting and Spurious Correlations in Community Analysis. Ecology 2018, 99, 1737–1747. [Google Scholar] [CrossRef]
- Dray, S.; Blanchet, G.; Borcard, D.; Guénard, G.; Jombart, T.; Legendre, P.; Wagner, H. Adespatial: Multivariate Multiscale Spatial Analysis; R Package Version 0.0–9. 2017. Available online: https://rdrr.io/cran/adespatial/ (accessed on 10 February 2024).
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef]
- Ribeiro, M.D.; Teresa, F.B.; Casatti, L. Use of functional traits to assess changes in stream fish assemblages across a habitat gradient. Neotrop. Ichthyol. 2016, 14, e140185. [Google Scholar] [CrossRef]
- Baldasso, M.C.; de Oliveira, A.G.; Kotz Kliemann, B.C.; Delariva, R.L. Habitat modification driven by land use as an environmental filter on the morphological traits of neotropical stream fish fauna. Neotrop. Ichthyol. 2024, 22, e230119. [Google Scholar] [CrossRef]
- Beukhof, E.; Frelat, R.; Pecuchet, L.; Maureaud, A.; Dencker, T.S.; Sólmundsson, J.; Punzón, A.; Primicerio, R.; Hidalgo, M.; Möllmann, C.; et al. Marine fish traits follow fast-slow continuum across oceans. Sci. Rep. 2019, 9, 17878. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Göthe, E.; Riis, T.; O’Hare, M. Functional Trait Composition of Aquatic Plants Can Serve to Disentangle Multiple Interacting Stressors in Lowland Streams. Sci. Total Environ. 2016, 543, 230–238. [Google Scholar] [CrossRef]
- Tai, D.; Chen, C.; Song, Y.; Tan, X.; Yang, X.; Wang, Y. Ecological Traits and Landscape Characteristics Predicting Bird Sensitivity to Urbanization in City Parks. Basic Appl. Ecol. 2022, 58, 110–120. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Meza-Joya, F.L.; Ramos, E.; Cardona, D. Forest fragmentation erodes mammalian species richness and functional diversity in a human-dominated landscape in Colombia. Mastozool. Neotrop. 2020, 27, 338–348. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I. Origin matters: Widely distributed native and non-native species benefit from different functional traits. Ecol. Lett. 2012, 15, 696–703. [Google Scholar] [CrossRef]
- Seress, G.; Liker, A. Habitat urbanization and its effects on birds. Acta Zool. Acad. Sci. Hung. 2015, 61, 373–408. [Google Scholar] [CrossRef]
- Reddy, C.S.; Sreelekshmi, S.; Jha, C.S.; Dadhwal, V.K. National assessment of forest fragmentation in India: Landscape indices as measures of the effects of fragmentation and forest cover change. Ecol. Eng. 2013, 60, 453–464. [Google Scholar] [CrossRef]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits? Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; MacGregor-Fors, I. Living in the big city: Effects of urban land-use on bird community structure, diversity, and composition. Landsc. Urban Plan. 2009, 90, 189–195. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Burch, W.R.; Dalton, S.E.; Timonthy, F.; Morgan, G.; Rowntree, R. A conceptual framework for the study of human ecosystems in urban areas. Urban Ecosyst. 1997, 1, 185–199. [Google Scholar] [CrossRef]
- Callaghan, C.; Major, R.; Wilshire, J.; Martin, J.; Kingsford, R.; Cornwell, W. Generalists are the most urban-tolerant of birds: A phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 2019, 128, 845–858. [Google Scholar] [CrossRef]
- Del Barco-Trillo, J. Shyer and larger bird species show more reduced fear of humans when living in urban environments. Biol. Lett. 2018, 14, 20170730. [Google Scholar]
- Pena, J.C.; Ovaskainen, O.; MacGregor-Fors, I.; Teixeira, C.P.; Ribeiro, M.C. The relationships between urbanization and bird functional traits across the streetscape. Landsc. Urban Plan. 2023, 232, 104685. [Google Scholar] [CrossRef]
- Ikin, K.; Knight, E.; Lindenmayer, D.B.; Fischer, J.; Manning, A.D. Linking bird species traits to vegetation characteristics in a future urban development zone: Implications for urban planning. Urban Ecosyst. 2012, 15, 961–977. [Google Scholar] [CrossRef]
- Culbert, P.D.; Radeloff, V.C.; Flather, C.H.; Kellndorfer, J.M.; Rittenhouse, C.D.; Pidgeon, A.M. The influence of vertical and horizontal habitat structure on nationwide patterns of avian biodiversity. Auk 2013, 130, 656–665. [Google Scholar] [CrossRef]
- Gumede, S.T.; Smith, D.A.E.; Ngcobo, S.P.; Sosibo, M.; Smith, Y.C.E.; Downs, C.T. The influence of forest characteristics on avian species richness and functional diversity in Southern Mistbelt Forests of South Africa. Glob. Ecol. Conserv. 2022, 34, e02047. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Remeš, V.; Remešová, E.; Friedman, N.R.; Matysioková, B.; Rubáčová, L. Functional diversity of avian communities increases with canopy height: From individual behavior to continental-scale patterns. Ecol. Evol. 2021, 11, 11839–11851. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, R.; Leon, E.; Juani, M.; Beltzer, A.; Peltzer, P.; Lajmanovich, R.; Attademo, A.M. Can agroecological management increase functional diversity of birds in rice fields? Rev. Biol. Trop. 2020, 68, 873–883. [Google Scholar] [CrossRef]
- Santillán, V.; Quitián, M.; Tinoco, B.; Zarate, E.; Schleuning, M.; Böhning-Gaese, K.; Neuschulz, E.L. Different responses of taxonomic and functional bird diversity to forest fragmentation across an elevational gradient. Oecologia 2019, 189, 863–873. [Google Scholar] [CrossRef]
- Jernakoff, M.G.; Knowlton, J.L.; Vásquez-Ávila, B.; Espinosa, C.I.; Tinoco, B.A. Effects of land use change on the functional diversity and composition of mixed species avian flocks in the high tropical Andes of southern Ecuador. J. Field Ornithol. 2023, 94, e103. [Google Scholar] [CrossRef]
- Sitters, H.; Di Stefano, J.; Christie, F.J.; Swan, M.; York, A. Bird functional diversity decreases with time since disturbance: Does patchy prescribed fire enhance ecosystem function? Ecol. Appl. 2016, 26, 115–127. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Ibáñez-Álamo, J.D.; Tryjanowski, P.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Pérez-Contreras, T.; Sprau, P.; Suhonen, J.; Yosef, R.; et al. Insurance for the future? Potential avian community resilience in cities across Europe. Clim. Chang. 2020, 159, 195–214. [Google Scholar] [CrossRef]
- Luck, G.W.; Carter, A.; Smallbone, L. Changes in Bird Functional Diversity across Multiple Land Uses: Interpretations of Functional Redundancy Depend on Functional Group Identity. PLoS ONE 2013, 8, e63671. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Terlizzi, A. Nestedness and Turnover Unveil Inverse Spatial Patterns of Compositional and Functional β-Diversity at Varying Depth in Marine Benthos. Divers. Distrib. 2020, 26, 743–757. [Google Scholar] [CrossRef]
- Bender, M.G.; Leprieur, F.; Mouillot, D.; Kulbicki, M.; Parravicini, V.; Pie, M.R.; Barneche, D.R.; Oliveira-Santos, L.G.R.; Floeter, S.R. Isolation Drives Taxonomic and Functional Nestedness in Tropical Reef Fish Faunas. Ecography 2017, 40, 425–435. [Google Scholar] [CrossRef]
- Stuart, C.T.; Brault, S.; Rowe, G.T.; Wei, C.L.; Wagstaff, M.; McClain, C.R.; Rex, M.A. Nestedness and species replacement along bathymetric gradients in the deep sea reflect productivity: A test with polychaete assemblages in the oligotrophic north-west Gulf of Mexico. J. Biogeogr. 2017, 44, 548–555. [Google Scholar] [CrossRef]
- Matthews, T.J.; Sheard, C.; Cottee-Jones, H.E.W.; Bregman, T.P.; Tobias, J.A.; Whittaker, R.J. Ecological traits reveal functional nestedness of bird communities in habitat islands: A global survey. Oikos 2015, 124, 817–826. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Z.; Yang, G.; Feng, G. Dominated Taxonomic and Phylogenetic Turnover but Functional Nestedness of WetlandBird Beta Diversity in North China. Land 2022, 11, 1090. [Google Scholar] [CrossRef]
- Ehlers Smith, D.A.; Si, X.; Ehlers Smith, Y.C.; Downs, C.T. Seasonal Variation in Avian Diversity and Tolerance by Migratory Forest Specialists of the Patch-Isolation Gradient across a Fragmented Forest System. Biodivers. Conserv. 2018, 27, 3707–3727. [Google Scholar] [CrossRef]
- Cubley, E.S.; Bateman, H.L.; Riddle, S.B.; Holmquist-Johnson, C.; Merritt, D.M. Predicting Bird Guilds Using Vegetation Composition and Structure on a Wild and Scenic River in Arizona. Wetlands 2020, 40, 1829–1842. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Batalha, D.C.; Collares-Pereira, M.J. Gradients in Stream Fish Assemblages across a Mediterranean Landscape: Contributions of Environmental Factors and Spatial Structure. Freshw. Biol. 2002, 47, 1015–1031. [Google Scholar] [CrossRef]
- Tinoco, B.A.; Santillán, V.E.; Graham, C.H. Land Use Change Has Stronger Effects on Functional Diversity than Taxonomic Diversity in Tropical Andean Hummingbirds. Ecol. Evol. 2018, 8, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Delgado, L.; Iñiguez-Armijos, C.; Díaz, M.; Escudero, A.; Gosselin, E.; Waits, L.P.; Espinosa, C.I. The Good, the Bad, and the Ugly of Urbanization: Response of a Bird Community in the Neotropical Andes. Front. Ecol. Evol. 2022, 10, 844944. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban-rural gradients: How many functional groups are there? J. Ornithol. 2012, 154, 655–662. [Google Scholar] [CrossRef]
- Maure, L.A.; Rodrigues, R.C.; Alcântara, Â.V.; Adorno, B.F.C.B.; Santos, D.L.; Abreu, E.L.; Tanaka, R.; Gonçalves, R.M.; Hasui, E. Functional Redundancy in bird community decreases with riparian forest width reduction. Ecol. Evol. 2018, 8, 10395–10408. [Google Scholar] [CrossRef]




| Levels | Metrics | Abbreviation | Units | Description |
|---|---|---|---|---|
| Patch | Area of forest patches | AREA_F | Hectares | Measures the size of a patch |
| Area of urban cover patches | AREA_UC | |||
| Area of secondary vegetation patches | AREA_SV | |||
| Perimeter of forest patches | PER_F | Meters | Indicates the patch shape, with a larger perimeter indicating more irregular shapes | |
| Perimeter of urban patches | PER_UC | |||
| Perimeter of secondary vegetation patches | PER_SV | |||
| Class | Percentage of forest cover within landscape | PND_F | Percentage | Quantifies the proportional abundance of each patch type within the landscape |
| Percentage of urban cover within landscape | PND_UC | |||
| Percentage of secondary vegetation cover within landscape | PND_SV | |||
| Number of forest patches | NP_F | - | Indicates the total number of patches within a particular class | |
| Number of urban cover patches | NP_UC | |||
| Number of secondary vegetation patches | NP_SV | |||
| Shape of forest patches | SHAPE_F | - | Assesses the complexity of patch shapes within a class | |
| Shape of urban patches | SHAPE_UC | |||
| Shape of secondary vegetation patches | SHAPE_SV | |||
| Landscape | Total number of patches | NP | - | Represents the total number of patches within the landscape |
| Shannon’s diversity index | SHDI | - | Quantifies the diversity within the landscape | |
| Shannon’s evenness index | SHEI | - | Assesses the evenness of patch type distribution across the landscape | |
| Patch richness | PR | - | Refers to the number of different classes types within the landscape |
| Model | Landscape Variables | Parameter Estimated | p-Value | AIC |
|---|---|---|---|---|
| FRic | AREA_F | −0.009 | 0.032 | 0.6 |
| PND_F | 0.021 | 0.015 | ||
| FDiv | SHDI | 0.219 | 0.005 | −22.8 |
| SHEI | −0.342 | 0.024 | ||
| NP_SV | −0.005 | 0.016 | ||
| PER_F | 5.349 × 10−6 | 0.004 | ||
| AREA_SV | 2.505 × 10−5 | 0.001 | ||
| PER_SV | −0.003 | 0.003 | ||
| FEve | PND_F | −0.001 | 0.007 | −30.5 |
| FSpe | SHDI | 0.106 | 0.025 | −49.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Mosquera, V.; López-Delgado, E.; Moreno-Palacios, M. Fragmented Habitats, Fragmented Functions: Unveiling the Role of Habitat Structure in Andean Bird Communities. Ecologies 2025, 6, 52. https://doi.org/10.3390/ecologies6030052
Ramos-Mosquera V, López-Delgado E, Moreno-Palacios M. Fragmented Habitats, Fragmented Functions: Unveiling the Role of Habitat Structure in Andean Bird Communities. Ecologies. 2025; 6(3):52. https://doi.org/10.3390/ecologies6030052
Chicago/Turabian StyleRamos-Mosquera, Valentina, Edwin López-Delgado, and Miguel Moreno-Palacios. 2025. "Fragmented Habitats, Fragmented Functions: Unveiling the Role of Habitat Structure in Andean Bird Communities" Ecologies 6, no. 3: 52. https://doi.org/10.3390/ecologies6030052
APA StyleRamos-Mosquera, V., López-Delgado, E., & Moreno-Palacios, M. (2025). Fragmented Habitats, Fragmented Functions: Unveiling the Role of Habitat Structure in Andean Bird Communities. Ecologies, 6(3), 52. https://doi.org/10.3390/ecologies6030052








