Abstract
The analysis of activity patterns is a valuable tool for understanding the temporal organization of mammal communities, which is determined by biological requirements, resource availability, and competitive pressures both within and between species. Research on this ecological aspect can contribute to the development of effective conservation strategies. Cloud forest is an ecosystem of high biological relevance, as this provides habitat for a wide diversity of species in Mexico, including endemic, emblematic, and threatened taxa. Our main objectives were to analyze mammalian activity patterns and predator–prey relationships in the cloud forest of the El Cielo Biosphere Reserve, Tamaulipas, Mexico. From 2018 to 2020, twenty camera trap stations were installed, and independent photographic records were obtained, divided into 24 one-hour intervals, and subsequently classified as diurnal, nocturnal, crepuscular, or cathemeral. Temporal activity was estimated using circular statistics in RStudio v4.3.1, and activity overlap between major carnivores and their prey was assessed using the ‘overlap’ package in R. A total of 18 medium- and large-sized mammal species were recorded in this study. The activity of four species was seasonally influenced, with a predominantly nocturnal pattern observed during the dry season. The activity overlap analysis revealed potential temporal similarity between predators and their prey. For example, Panthera onca exhibited a high overlap with Mazama temama (Δ = 0.83), Puma concolor with Nasua narica (Δ = 0.91), and Ursus americanus with M. temama (Δ = 0.77). These findings suggest that the activity patterns of certain species can be influenced by seasonality and that large predators may favor specific prey whose activity overlaps with their own.
1. Introduction
The cloud forest is identified by the presence of condensation of humid air from the sea, giving rise to dense cloud formations with high humidity and rainfall [,]. This ecosystem is characterized by the convergence of species from temperate and tropical regions, resulting in a high diversity of endemic, emblematic, and endangered animal and plant species listed in the IUCN (International Union for Conservation of Nature) Red List, as in the Mexican Official Standard (NOM-059-SEMARNAT-2010). However, factors such as deforestation, changes in land use, illegal logging, and overexploitation of resources have caused a decrease in the duration of rainfall and fog days, increasing the duration and intensity of drought periods [,]. This means there has been a significant reduction in this ecosystem, which currently represents only 1% of the total area of the Mexican territory [,].
Mammals are the main taxa of this ecosystem, as they play a crucial role in the maintenance and regulation of ecosystem dynamics, mainly in seed dispersal, predation, and soil nutrient cycling [,]. Within the mammalian group, medium-sized mammals are characterized by a weight of more than one kilogram, while large mammals weigh more than 20 kg []. These organisms require extensive habitat surface areas and have low rates of reproduction and density, making them ideal for assessing ecosystem conservation status.
Mammal activity patterns are a reliable indicator of ecosystem health, enabling the monitoring of temporary variations in populations and facilitating the acquisition of knowledge regarding population dynamics [,]. In addition to the capacity to discern potential human disturbances that could modify their behavior. The daily activities of mammals depend on the satisfaction of their biological needs, which are influenced by the abundance of resources, mainly water and food; the presence of other predators; prey capture and competition; and abiotic factors such as lunar phases and daily and seasonal variations (rainy and dry) [,]. It has been demonstrated that during the rainy season, when food production and availability are at their peak, environmental conditions favor breeding and mating seasons. In contrast, during the dry season, the decrease in vegetation cover and rainfall causes mammals to migrate to areas with available water sources, avoiding encounters with their predators [,].
Research conducted in Mexican cloud forests has revealed that this ecosystem exhibits diverse activity patterns, including diurnal and nocturnal activity in medium-sized mammals and predators, as well as 24-hour activity in large mammals and generalist species []. This diversity of activity underscores the significance of ecosystem conservation, as some species display temporal flexibility to avoid predators or humans, which can be crucial for their persistence in anthropized environments but also entails risks such as increased energy expenditure or reduced trophic opportunities [,,]. Activity patterns are also influenced by interactions between coexisting species, reflecting a temporal niche partitioning that avoids direct competition and facilitates ecological stability, such as predator–prey relationships [,].
The implementation of camera traps has facilitated the observation of species that are typically difficult to observe and inhabit low-density habitats. This technology has been instrumental in detecting species with diurnal and nocturnal activity patterns. Moreover, it has enabled the identification of organisms at the taxonomic and, often, individual level []. Camera trapping represents a low-cost tool with minimal impact on wildlife and is effective for monitoring cryptic species or in hard-to-reach regions [].
In the state of Tamaulipas in Mexico, most studies on mammals have focused primarily on aspects such as species diversity [,], relative abundance [], or estimating population densities []. In contrast, relatively few studies have specifically addressed the analysis of activity patterns, despite the fact that this temporal dimension represents a key component of the ecological niche and interspecific interactions within communities. Research focused on the activity of mammals has been predominantly conducted at the El Cielo Biosphere Reserve (ECBR), where the activities of mesopredators L. wiedii, L. pardalis, and H. yagouaroundi have been studied. This research concludes that species employ complementary spatio-temporal strategies that facilitate their coexistence without direct competition [], and top predators, such as P. onca and P. concolor, where it is concluded that both felines present a nocturnal–crepuscular pattern, for which there is a high temporal overlap, and therefore, both predators are geographically distributed in a complementary way to coexist effectively [,]. However, there is a scarcity of ecological information on other mammals distributed in this reserve. This study aimed to analyze mammal activity patterns in the rainy and dry seasons to determine their response to changes in their activity. In addition, a detailed analysis of the predator–prey relationship was conducted by examining their overlapping activity overall and within each season.
2. Materials and Methods
The El Cielo Biosphere Reserve (ECBR) is located in the state of Tamaulipas. It is a protected area located in the Mexican transition zone between the Nearctic and Neotropical regions, covering part of the municipalities of Gómez Farías, Jaumave, Llera, and Ocampo (22°55′30” N, −99°26′30” W). The reserve has a rainy season that runs from April to October and a dry season that lasts from November to March []. The reserve’s vegetation is further divided into eastern and western regions. The eastern side is characterized by vegetation that is exposed to wet winds and trade winds, with a vegetation cover of semideciduous forest 27.8%, cloud forest 10.26%, oak pine forest 10.55%, and oak forest 20% (Figure 1). In contrast, the western side is dominated by xeric vegetation, typical of the northern plateau [,].
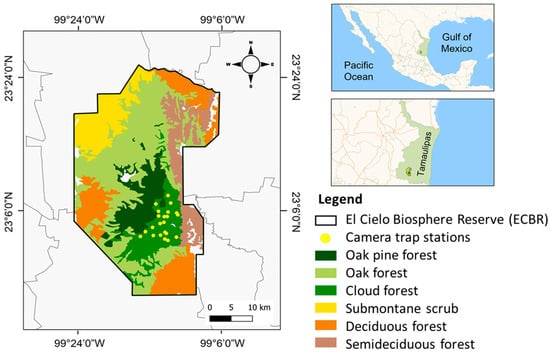
Figure 1.
State of Tamaulipas, El Cielo Biosphere Reserve, types of vegetation, and camera trap stations.
This study was conducted from 2018 to 2020 using camera traps in rainy and dry seasons. Twenty stations were established with a camera trap (Scoutguard HCO SG565) per station along roads and trails where evidence of medium and large mammal activity was observed. The distance between stations ranged from one to two km, with each camera trap placed 30 to 50 cm from the ground on tree trunks. Vegetation was cut or removed immediately in front of and to the sides of the camera to optimize visibility and avoid false triggers caused by vegetation within the camera detection radius (21 m). The cameras were programmed to be active for 24 h and to take two photographs during each capture event with a delay interval of 1.5 s; the date and hour were printed in each photographic event []. Each photograph recorded the date and time. The use of baits or lures was avoided to mitigate potential bias in the study’s results.
To avoid pseudoreplication, photocaptures were classified according to the following independence criteria: when the same camera trap took more than one photograph of the same species, (a) we counted the records separated by a minimum time lapse of 24 h; (b) consecutive photographs of different individuals of the same species that can be differentiated through unique characteristics (coat traits); (c) consecutive photographs of individuals of different species; and (d) each individual in a group photograph. Non-independent events were eliminated to avoid bias in the information [].
The activity was determined only for species with at least 11 independent photographic records with visible time, following the suggestion of [,,]. The records were grouped into 24 one-hour categories and classified into four categories: diurnal (08:01–17:59 h), nocturnal (20:01–5:59 h), and crepuscular (sunrise 6:00–08:00 h; sunset 18:00–20:00 h). While cathemeral, those species are active throughout the day [,]. Thereof, the exact date and time of each event recorded in the photo captures were used, and the sunrise and sunset hours were defined using the online platform Time and Date, which considers the latitude and the time zone [].
Temporal activity patterns were analyzed using circular statistics implemented in the RStudio statistical program [,]. Two statistical tests were applied contingent upon the data modality. The Rayleigh uniformity test (Z) was used for mammals with unimodal data sets, while the Rao spacing test (U) was used for mammals with bimodal and multimodal data sets [,,]. Both tests were performed to determine whether the independent events of each species had directionality or a uniform distribution. Thus, directionality indicated that mammals had diurnal, nocturnal, or crepuscular patterns, while a uniform distribution throughout the day suggested a cathemeral pattern [,,].
Circular histograms were used according to the Circular package to compare and describe the activity patterns of mammals in rainy and dry seasons [,]. The Watson statistical test was used to compare two samples (U2). The results indicated similarities or differences in the pattern of activity of each mammalian species by season [].
A bibliographic search was conducted to compare predator–prey interactions. The main prey of each predator within Mexico was identified, and the results were then compared with the prey of mammals distributed within the cloud forest of the ECBR. Temporal overlap values were calculated for species with more than 11 independent records from the overlap coefficient (Dhat ∆), which was determined according to the sample size of each mammal, based on the minimum number of records between the predator and prey samples. Dhat 1 was designated for samples with less than 50 records, while Dhat 4 was employed for samples with more than 75 records. Mammals with intermediate values between 50 and 75 records were assigned Dhat 1. The overlap values ranged from 0 (no overlap) to 1 (complete overlap), considering low overlap < 0.50, moderate overlap 0.50 < 0.70, and high overlap > 0.70 []. The estimate was formulated using the R-overlap package [], which subsequently enabled the development of a graph that facilitated a comparison of the overlap among major carnivores and their potential prey.
3. Results
A total of 2313 independent records were obtained. Of these, 1308 photographs were recorded during the rainy season, while 1005 photographs were obtained during the dry season. The combined analysis of these records revealed the presence of 18 species of medium and large mammals, classified into six orders, 11 families, and 17 genera. Carnivora was the most represented order, comprising six families and 11 species, followed by Artiodactyla, which included one family and two species.
The study’s results revealed the presence of three species of mammals exclusively during the dry season: Spilogale sp., Galictis vittata, and Odocoileus virginianus. Due to the limited number of independent records obtained for these four species and in accordance with the extant literature, the general or seasonal activity pattern could not be obtained.
Of the total number of mammals recorded, three species are listed on the IUCN Red List: P. onca and L. wiedii as near-threatened (NT) and Mazama temama as data-deficient (DD). The rest of the mammals in this study are classified as least concern (LC) []. Meanwhile, in NOM-059-SEMARNAT-2010, L. pardalis, L. wiedii, P. onca, Eira barbara, and U. americanus are listed as endangered (P). H. yagouaroundi and G. vittata are listed as threatened (A). The NOM-059-SEMARNAT-2010 is an official Mexican standard that aims to identify species of wild flora and fauna in a risk category [] (Table 1).

Table 1.
Species recorded by camera traps in the cloud forest of the El Cielo Biosphere Reserve (RBEC), Tamaulipas, Mexico.
3.1. Temporary Activity
Most medium-sized mammals’ activity distribution was directional towards the nocturnal hours. Dasypus novemcinctus was active during the night, with its highest activity peak between 23:00 and 24:00 h (Z = 0.66, p < 0.05) (Figure 2a). Similar to Sylvilagus floridanus (Z = 0.47, p < 0.05), it was primarily active from 20:00 to 22:00 h. While Cuniculus paca turned out to be a nocturnal mammal with a peak of activity from 2:00 to 3:00 h (U = 207.25, p < 0.05), similar to Didelphis virginiana (Z = 0.57, p < 0.05), a mammal with which it shares time slots with greater activity.
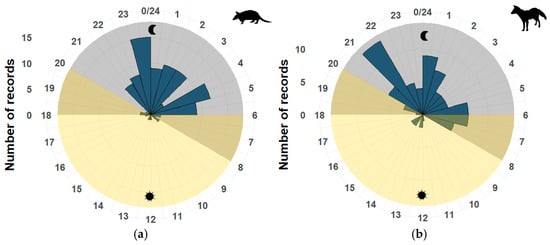
Figure 2.
Activity pattern of medium-sized mammals: (a) Dasypus novemcinctus; (b) Urocyon cinereoargenteus. The gray shaded area corresponds to the nighttime period, and the yellow area corresponds to the daytime period. The numbers around the circle correspond to the hours of the day, resembling a clock, and the number of records is in a vertical position and reflected in the graph as blue bars.
Regarding predators, those that showed nocturnal patterns were L. pardalis (Z = 0.57, p < 0.05) with an increase in activity from 21:00 to 22:00 h and from 0:00 to 3:00 h, and L. wiedii (Z = 0.47, p < 0.05) with a peak of activity from 21:00 to 23:00 h. While Urocyon cinereoargenteus (U = 158.16, p < 0.05) (Figure 2b) and Conepatus leuconotus (U = 158.16, p < 0.05) shared a peak of activity from 00:00 to 01:00 h (Supplementary Materials).
The medium-sized mammal that showed a diurnal pattern was E. barbara, which had its maximum activity peak from 11:00 to 12:00 h (Z = 0.38, p < 0.05) (Figure 3). While among the predators, U. americanus presented directional activity towards the morning hours (U = 159.66, p < 0.05), with a peak of activity from 09:00 to 10:00 h, where 25 records were obtained, as well as during the evening from 07:00 to 08:00 h with 22 records.
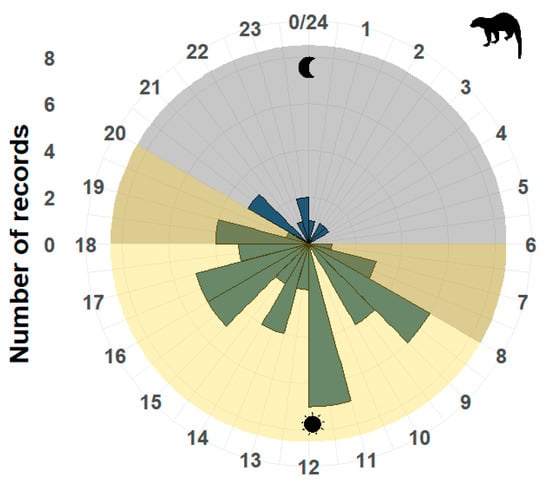
Figure 3.
Diurnal activity pattern of Eira barbara. The gray shaded area corresponds to the nighttime period, and the yellow area corresponds to the daytime period. The numbers around the circle correspond to the hours of the day, resembling a clock, and the number of records is in a vertical position and reflected in the graph as blue bars.
In addition, M. temama (U = 150.08, p > 0.05) was present during the day and twilight, with peak activity recorded during the day between 11:00 and 12:00 h and dusk between 19:00 and 20:00 h.
The species that showed a consistent pattern was Nasua narica (Z = 0.16, p > 0.05), which was recorded mainly in the afternoon. Predators such as P. onca and P. concolor show greater activity at night. These felids increase their activity at different times of the day. P. onca (Z = 0.20, p > 0.05) presents its peak activity from 20:00 to 21:00 h, while P. concolor (U = 130, p > 0.05) increases its activity from 14:00 to 15:00 h.
3.2. Seasonal Activity
In comparing seasons, medium and large mammals kept their activity pattern consistent, except for S. floridanus, D. virginiana, M. temama, and N. narica.
It was observed that S. floridanus increased its activity at dawn in the rainy season and at dusk in the dry season (U2 = 0.23, p < 0.05) (Figure 4a,b). D. virginiana increased its activity during the daytime in the dry season (U2 = 0.30, p < 0.05). At the same time, M. temama and N. narica completely changed their activity (U2 = 0.60, U2 = 0.62, p < 0.05), showing a diurnal pattern in the rainy season and a nocturnal pattern in the dry season (Figure 4c,d).
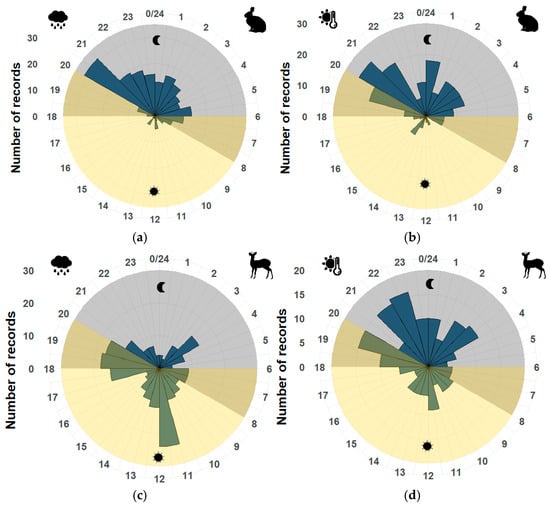
Figure 4.
Activity pattern of (a) Sylvilagus floridanus in the rainy season; (b) Sylvilagus floridanus in the dry season; (c) Mazama temama in the rainy season; and (d) Mazama temama in the dry season. The gray shaded area corresponds to the nighttime period, and the yellow area corresponds to the daytime period. The numbers around the circle correspond to the hours of the day, resembling a clock, and the number of records is in a vertical position and reflected in the graph as blue bars.
3.3. Overlap
The results of the bibliographic review indicated that the diet of P. onca and P. concolor has been the focus of extensive research. It has been documented that in northern Mexico, both species have been observed consuming prey species such as D. tajacu, N. narica, D. novemcinctus, C. paca, O. virginianus, M. temama, D. virginiana, and Mephitis macroura, in addition to certain lagomorphs and rodents []. However, P. onca has been observed to prefer prey species that exceed 10 kg in weight, while P. concolor exhibits a more generalist diet, encompassing smaller prey items [,].
The diet of U. americanus has been documented to include remains of small mammals, primarily rodents, as well as carrion, cervids such as Odocoileus hemionus and O. virginianus, and, in some cases, mephitids [,].
In other matters, the diet of mesocarnivores is varied, and they generally feed on vertebrates weighing less than one kilogram. A recurring theme in numerous studies is the predominance of rodents, including C. paca, Didelphidae, lagomorphs, Dasypus sp., birds, and select reptiles, as part of the diet of L. pardalis and L. wiedii [,,].
In this study, time overlap analysis demonstrated that predators divide their prey depending on size. P. onca exhibited a high overlap with M. temama (∆ = 0.83), similar to U. americanus (∆ = 0.77). While P. concolor exhibited a predominantly higher degree of overlap with N. narica (∆ = 0.91). On the contrary, L. pardalis and L. wiedii exhibited a higher degree of overlap in prey sharing compared to D. virginiana (∆ = 0.88).
Some potential prey modified their activity depending on the season, causing variations in their overlap with predators. Some mammals showed a greater overlap during the rainy season compared to the dry season, such as the relationship between P. onca–N. narica, P. concolor–D. virginiana, and L. wiedii–D. virginiana. Predators such as L. pardalis and U. americanus had a similar overlap with their prey in both seasons (Figure 5).
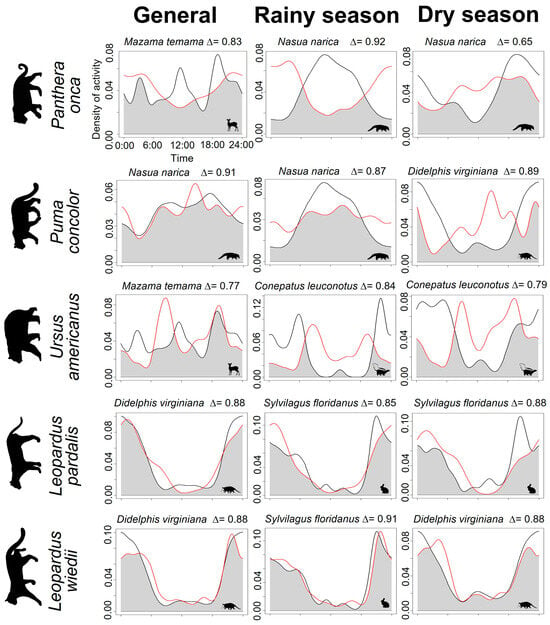
Figure 5.
Activity of carnivores and potential prey with the greatest overlap. The red line corresponds to the activity of the carnivore; the black line indicates the activity of the prey. In contrast, the gray area shows the times when the activity of the two species overlaps.
4. Discussion
The activity pattern results obtained with camera traps are essential for understanding species ecology, behavior, and adaptive strategies to environmental conditions. These patterns make it possible to identify the time periods in which species carry out essential activities such as feeding, movement, and coexistence, which are closely linked to their ecological strategies [,]. The significant number of species recorded in this study, together with the high representation of carnivores in the ecosystem, shows that the cloud forest is of great importance for the development of mammals. Furthermore, the presence of species for which little information is available, such as M. temama and the felines, which are classified as endangered or threatened, emphasizes the urgent need to implement effective conservation strategies.
In the cloud forest of the ECBR, eight nocturnal species classified as prey were recorded, consistent with the theory that smaller mammals tend to be nocturnal as a strategy to combat predation or competition [,]. Except for N. narica and E. barbara, which showed a different activity than expected, probably because they are agile mammals with terrestrial and arboreal habits, in addition to their well-developed sense of smell, which allows them to feed on fruits, small vertebrates, and carrion [,]. Other research has shown that, by showing a different pattern than other medium-sized mammals, these species avoid competition with mammals of the same size [,,].
Large mammals exhibit diurnal and cathemeral patterns, probably because they must forage for more extended periods of time to meet their energy needs []. In general, diurnal activity may be caused by vegetation, which provides protection from environmental factors, such as solar radiation, and security from predators or competitors.
In large mammals such as M. temama, a cathemeral pattern has been linked to an affinity for sites with dense, conserved herbaceous cover. Sites like the cloud forest provide protection against environmental factors while increasing the possibility of hiding and escaping predators, which gives these animals a sense of security [].
For top predators, on the other hand, the nighttime hours provide them with advantages in hunting by remaining unnoticed [,,]. This activity has been reported in other protected areas in Mexico, such as the Sierra del Abra-Tanchipa Biosphere Reserve in San Luis Potosí, the Edén Ecological Reserve in Quintana Roo, and the La Sepultura Biosphere Reserve in Chiapas [,]. P. onca showed a peak of activity at night, while P. concolor showed it during the day; this distribution of schedules could be a key factor in avoiding competition between them, since they have similar requirements [,,]. Furthermore, a cathemeral activity pattern is associated with a higher probability of encountering a high diversity of prey, which may be beneficial for generalist predators such as the P. concolor, which, according to other studies, can consume a wide variety of diurnal and nocturnal prey, which in turn allows for a constant flow of prey species, leading to the coexistence of both predators in the same habitat [,]. On the other hand, diurnal activity in felines is an indicator of the absence of human disturbances at the site, so that felines can be active during daytime hours without the risk of encountering humans [].
The response of mammals to seasonal change could be due to a reduction in the abundance of food and water []. Some studies have reported that species maintain a constant pattern because changes in photoperiod, temperature, rainfall, and food availability are factors that favor the biological development of individuals, such as mating and reproduction, which is why some mammals are more active during the rainy season []. Meanwhile, a change was observed in herbivorous species such as S. floridanus and M. temama and in arboreal species such as N. narica and D. virginiana. It is suggested that the constant pattern of species could be due to their adaptability to different conditions, utilizing additional resources in the dry season, while the increase in activity in the rainy season could be due to the mating and reproduction season.
In medium-sized mammals such as S. floridanus, their activity is related to the intensity of sunlight, such that, at high temperatures, the activity of this mammal is restricted to nocturnal and twilight hours. While, on cloudy days, diurnal activity has been observed [], which is related to the results of this research in which a greater number of daytime records were found in the rainy season, while in the dry season a greater number of records were presented at dusk, despite this, this activity is not significant enough to suggest a modification of its nocturnal pattern but only to readjust the activity schedules.
Studies of activity patterns in M. temama have indicated that this species does not exhibit an innate tendency towards a particular schedule of activity, thereby suggesting the capacity to engage in activity during both diurnal and nocturnal periods. However, the level of activity exhibited by these organisms is contingent upon various environmental factors, including temperature, humidity, and the availability of sustenance. During the rainy season, this mammal exhibited a diurnal pattern, as food and water were abundant, as was vegetation, providing protection. During the dry season, the observed pattern underwent a shift in response to rising temperatures [].
The N. narica activity is attributed to seasonal changes in the abundance of food resources. It has been observed that during times of the year when the availability of resources decreases, this mammal travels greater distances in search of food, which is why it was more active at night, in addition to avoiding predators [,].
With respect to the activity patterns exhibited by D. virginiana, the species varied across seasons, with greater diurnal activity during the dry season. This could be attributed to prolonged foraging time or the possibility that it was in the breeding season [,].
Concerning the predator–prey relationship, this is a relevant ecological topic that tells us how prey displays strategies to avoid predators or how predators can become effective hunters when they coincide with their prey. The degree of overlap reflects the behavior of the species; a high degree of overlap between the predator and prey may indicate that predators hunt when their prey is active, although the interaction may also occur opportunistically []. In this study, we found that P. onca has a greater overlap with M. temama (Δ = 0.83), P. concolor with N. narica (Δ = 0.91), and U. americanus with M. temama (Δ = 0.77). The levels of overlap can reflect the behavior of the species; a high overlap between the activity of the predator and the prey may indicate that when the prey are active, the predators hunt, although this interaction can also occur opportunistically and occur in the less active states of the prey [,]. In this way, the success that carnivores present in hunting will depend on the development of their stalking, harassment, ambush, and pursuit techniques, while the survival of the prey will be determined by the skills and strategies it develops to avoid predation [].
Some studies indicate that felid activity is primarily influenced by the activity, abundance, vulnerability, and productivity of their prey []. The study conducted in the same study area within the ECBR mentions M. temama, Didelphis sp., and Sylvilagus sp. as the most abundant mammals [], which agrees with the results obtained in this study, where M. temama was found to be one of the prey species with the highest overlap.
Multiple studies have mentioned the affinity of P. onca for larger prey, while P. concolor has a more diverse diet due to its generalist nature. In this way, a constant flow of prey is maintained, allowing both predators to coexist []. However, D. virginiana exhibited activity and habits similar to L. pardalis and L. wiedii, which could indicate that this mammal is part of its diet. Although in general, the diet of these felids consists primarily of small mammals such as mice and rabbits [,,]. Therefore, it is evident that the distribution of activities could be a key factor in avoiding competition, especially in species with similar prey, such as P. onca and P. concolor, as well as L. pardalis and L. wiedii.
The overlap that occurred in rainy and dry seasons is closely related to the activity of the prey. The activity of N. narica increased during the day in the rainy season, when resources were more abundant. During these periods, females and young have been reported to be more active during the day, while males are more active at night [], making them more likely to be hunted by predators such as P. onca and P. concolor. Meanwhile, D. virginiana increased its diurnal activity in the dry season, possibly due to increased foraging time for food and water.
In contrast, S. floridanus and C. leuconotus maintained constant activity throughout the seasons, recorded at night and crepuscular hours, which could indicate that these mammals may be a fundamental part of the diet of the carnivores.
This study focuses on ecological data concerning a vegetation type that is regarded as one of the most fragmented. However, it is notable that this vegetation type contains species that are classified as endangered or for which data are limited, including M. temama []. Moreover, northern Mexico is the sole location where all three large carnivores coexist, and it marks the extent of the species’ distribution [].
Although camera trapping has been an effective technique in the study of mammals, the potential biases of this technique must be considered. Several authors mention the importance of maximizing the detection probability of species, either through the use of multiple camera traps and/or by configuring their locations. These studies have determined that, for large mammals, the highest detection probability occurs on forest roads, while for medium-sized mammals, the probability of detection occurs in sites with less visibility, outside of wildlife trails. In turn, a separation distance of one to two kilometers between cameras for medium and large mammals has been recommended [,,]. In this study, the cameras were placed in locations to maximize detections on paths used by humans, trails used by animals, and especially in sites where more than two paths or trails coincide. Thus, it is necessary to be cautious about the results, both in species with limited records and in the placement of cameras; however, we would have missed captures of endangered animals.
5. Conclusions
The medium-sized mammals were primarily nocturnal, except for E. barbara, which had a diurnal pattern, and N. narica, which was active throughout the day. On the other hand, among large mammals, M. temama had a diurnal–crepuscular pattern, while carnivores were nocturnal, except for U. americanus, which is active during the day. In addition, rainy and dry seasons modify the activity pattern of certain species, mainly herbivores such as S. floridanus and M. temama and omnivores such as D. virginiana and N. narica.
Large carnivores demonstrated variations in their overlap with their prey. Specifically, P. onca and U. americanus exhibited a heightened degree of overlap with M. temama, while P. concolor showed a significant overlap with N. narica. In contrast, L. pardalis and L. wiedii exhibited similar prey species. The influence of climatic seasons on the overlap of specific prey species was observed, with N. narica, D. virginiana, C. leuconotus, and S. floridanus as main prey.
Supplementary Materials
The following supporting information can be downloaded at: https://github.com/Nayelimtzglez/Actividad-mamiferos.git (Uploaded at 5 May 2025).
Author Contributions
Conceptualization, N.M.-G., L.S.-D. and C.C.A.-S.; methodology, L.S.-D. and N.M.-G.; software, L.S.-D. and N.M.-G.; validation, C.C.A.-S., C.B.-V., G.R.M.-G. and Z.A.M.-d.l.R.; formal analysis, L.S.-D., N.M.-G. and V.V.-E.; investigation, N.M.-G., L.S.-D., C.C.A.-S., C.B.-V., G.R.M.-G. and Z.A.M.-d.l.R.; resources, L.S.-D. and C.B.-V.; data curation, N.M.-G., G.R.M.-G. and Z.A.M.-d.l.R.; writing—original draft preparation, N.M.-G., L.S.-D. and C.C.A.-S.; writing—review and editing, N.M.-G., L.S.-D. and C.C.A.-S.; visualization, N.M.-G., G.R.M.-G., V.V.-E. and Z.A.M.-d.l.R.; supervision, L.S.-D. and C.C.A.-S.; project administration, N.M.-G., L.S.-D. and C.B.-V.; funding acquisition, L.S.-D. All authors have read and agreed to the published version of the manuscript.
Funding
Universidad Autónoma de Tamaulipas for funding the Project UAT/PFI2015-15 and to the grant given by “Programa para el Desarrollo Profesional Docente” (PRODEP) for project UAT-PTC-221/511-6/17-8212.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at https://github.com/Nayelimtzglez/Actividad-mamiferos (accessed on 21 June 2023).
Acknowledgments
We also thank Esteban Berrones Benítez, our field guide in this research work. We thank the reviewers for their helpful comments that improved this article.
Conflicts of Interest
The authors declare no conflicts of interest. With regard to conflicts of interest with the funding bodies, we declare that there are none. Both PRODEP and the Universidad Autónoma de Tamaulipas (UAT) are institutions that support education and research on a not-for-profit basis, with no interest in obtaining financial benefit or bibliographic recognition. Their sole aim is to promote scientific outreach and to support the academic development of both staff and students.
Abbreviations
The following abbreviations are used in this manuscript:
| ECBR | El Cielo Biosphere Reserve |
| IUCN | International Union for Conservation of Nature |
| NOM | Mexican Official Standard |
References
- Gual-Díaz, M.; González-Medrano, F. Los Bosques Mesófilos de Montaña En México. In Bosques Mesófilos de Montaña de México, Diversidad, Ecología y Manejo; Gual-Díaz, M., Rendón-Correa, A., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Tlalpan, México, 2014; pp. 22–67. ISBN 978-607-8328-07-9. [Google Scholar]
- Krasilnikov, P. Montane Cloud Forests. In Encyclopedia of the World’s Biomes; Goldstein, M.I., Della-Sala, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 138–145. ISBN 9780128160961. [Google Scholar]
- Williams-Linera, G.; Toledo-Garibaldi, M.; Hernández, C.G. How Heterogeneous Are the Cloud Forest Commu-nities in the Mountains of Central Veracruz, Mexico? Plant Ecol. 2013, 214, 685–701. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ochoa-Ochoa, L.M.; Flores-Villela, O.; León-Paniagua, L.; Navarro-Sigüenza, A.G. Amenazas al Bosque Mesófilo de Montaña En México y La Importancia de Los Estudios Multitaxonómicos. Biol. Soc. 2023, 6, 42–49. [Google Scholar]
- Rzedowski, J. Análisis Preliminar de La Flora Vascular de Los Bosques Mesófilos de Montaña de México. Acta Bot. Mex. 1996, 35, 25–44. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, F.; Chávez, C.; Sánchez-Rojas, G.; Elizabeth Moreno, C.; González-Salazar, C.; Ruiz-Gutiérrez, B.O.; Torres-Bernal, R. Medium and Large Mammals of the Sierra Madre Del Sur de Guerrero, Mexico: Comprehensive Assessment of Diversity and Its Relationship with Environmental Characteristics. Rev. Mex. Biodiv. 2020, 91, e913168. [Google Scholar] [CrossRef]
- Lacher, T.E.; Davidson, A.D.; Fleming, T.H.; Gómez-Ruiz, E.P.; McCracken, G.F.; Owen-Smith, N.; Peres, C.A.; Vander Wall, S.B. The Functional Roles of Mammals in Ecosystems. J. Mammal. 2019, 100, 942–964. [Google Scholar] [CrossRef]
- Benchimol, M. Medium and Large—Sized Mammals. In Core Standard Methods for Rapid Biological Field Assessment; Larsen, T.H., Ed.; Conservation International: Arlington, VA, USA, 2016; pp. 37–50. ISBN 9781934151969. [Google Scholar]
- Lira-Torres, I.; Briones-Salas, M. Abundancia Relativa y Patrones de Actividad de Los Mamíferos de Los Chimalapas, Oaxaca, México. Acta Zool. Mex. 2012, 28, 566–585. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A. Quantifying Levels of Animal Activity Using Camera-Trap Data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Skene, D.J. Social Influences on Mammalian Circadian Rhythms: Animal and Human Studies. Biol. Rev. Camb. Philos. Soc. 2004, 79, 533–556. [Google Scholar] [CrossRef]
- McCleery, R.; Monadjem, A.L.; Conner, M.; Austin, J.D.; Taylor, P.J. (Eds.) Chapter 10. Communities and the Environment. In Methods for Ecological Research on Terrestrial Mammals, 1st ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2021; pp. 200–229. ISBN 9781421442112. [Google Scholar]
- Santiago-Moreno, J.; Gomez-Brunet, A.; Toledano-Díaz, A.; Picazo, R.; Gonzalez-Bulnes, A.; López-Sebastián, A. Seasonal Endocrine Changes and Breeding Activity in Mediterranean Wild Ruminants. Reprod. Dom. Anim. 2006, 41, 72–81. [Google Scholar] [CrossRef]
- Ikeda, T.; Uchida, K.; Matsuura, Y.; Takahashi, H.; Yoshida, T.; Kaji, K.; Koizumi, I. Seasonal and Diel Activity Patterns of Eight Sympatric Mammals in Northern Japan Revealed by an Intensive Camera-Trap Survey. PLoS ONE 2016, 11, e0163602. [Google Scholar] [CrossRef]
- Aranda, M.; Botello, F.; López-De Buen, L. Diversidad y Datos Reproductivos de Mamíferos Medianos y Grandes En El Bosque Mesófilo de Montaña de La Reserva de La Biosfera Sierra de Manantlán, Jalisco- Colima, México. Rev. Mex. Biodiv. 2012, 83, 778–784. [Google Scholar] [CrossRef]
- Frey, S.; Fisher, J.T.; Burton, A.C.; Volpe, J.P. Investigating Animal Activity Patterns and Temporal Niche Partitioning Using Camera-Trap Data: Challenges and Opportunities. Remote Sens. Ecol. Conserv. 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Caravaggi, A.; Gatta, M.; Vallely, M.C.; Hogg, K.; Freeman, M.; Fadaei, E.; Dick, J.T.A.; Montgomery, W.I.; Reid, N.; Tosh, D.G. Seasonal and Predator-Prey Effects on Circadian Activity of Free-Ranging Mammals Revealed by Camera Traps. PeerJ 2018, 6, e5827. [Google Scholar] [CrossRef]
- Yildiz, F.; Uzun, A. Daily Activity Patterns and Overlap Activity of Medium–Large Mammals in Sülüklü Lake Nature Park, Western Black Sea Region, Türkiye. Ecol. Evol. 2024, 14, e70654. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Vilchis, O.; Zarco-González, M.M.; Rodríguez-Soto, C.; Soria-Díaz, L.; Urios, V. Fototrampeo de Mamíferos En La Sierra Nanchititla, México: Abundancia Relativa y Patrón de Actividad. Rev. Biol. Trop. 2011, 59, 373–383. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F. Camera Trapping for Wildlife Research; Pelagic Publishing: London, UK, 2016; ISBN 9781784270483. [Google Scholar]
- Vargas-Contreras, J.A.; Hernández-Huerta, A. Distribución Altitudinal de La Mastofauna En La Reserva de La Biosfera “El Cielo”, Tamaulipas, México. Acta Zool. Mex. 2001, 82, 83–109. [Google Scholar] [CrossRef]
- Ortega-Huerta, M.A.; Peterson, A.T. Modelling Spatial Patterns of Biodiversity for Conservation Prioritization in North-Eastern Mexico. Diversity Distrib. 2004, 10, 39–54. [Google Scholar] [CrossRef]
- Ochoa-Espinoza, J.M.; Soria-Díaz, L.; Astudillo-Sánchez, C.C.; Treviño-Carreón, J.; Barriga-Vallejo, C.; Maldonado-Camacho, E. Diversidad y abundancia de mamíferos del bosque mesófilo de montaña del noreste de México. Acta Zool. Mex. 2023, 39, 1–18. [Google Scholar] [CrossRef]
- Carrera-Treviño, R.; Lira-Torres, I.; Martínez-García, L.; López-Hernández, M. El Jaguar Panthera onca (Carnivora: Felidae) En La Reserva de La Biosfera “El Cielo”, Tamaulipas, México. Rev. Biol. Trop. 2016, 64, 1451–1468. [Google Scholar] [CrossRef][Green Version]
- Carrera-Treviño, R.; Astudillo-Sánchez, C.C.; Garza-Torres, H.A.; Martínez-García, L.; Soria-Díaz, L. Temporal and Spatial Interactions of Sympatric Mesocarnivores at a Biosphere Reserve: Coexistence or Competition? Rev. Biol. Trop. 2018, 66, 996–1008. [Google Scholar] [CrossRef]
- Contreras-Díaz, C.A.; Soria-Díaz, L.; Gómez-Ortiz, Y.; Carrera-Treviño, R.; Astudillo-Sánchez, C.C.; Chacón-Hernández, J.C.; Martínez-García, L. Temporal and Spatial Segregation of Top Predators (Felidae) in a Mexican Tropical Biosphere Reserve. Zoologia 2021, 38, e63231. [Google Scholar] [CrossRef]
- Sanchez-Santillan, N.; Binnqüist Cervantes, G.S.; Garduño López, R. Summer Drought in the El Cielo Biosphere Reserve and its Environment, Tamaulipas, Mexico. Cuad. Geogr. Rev. Colomb. Geogr. 2018, 27, 146–163. [Google Scholar] [CrossRef]
- González-Medrano, F. La Vegetación. In Historia Natural de la Reserva de la Biosfera El Cielo, Tamaulipas, México; Sanchez-Ramos, G., Reyes-Castillo, P., Dirzo, R., Eds.; Universidad Autónoma de Tamaulipas: Tamaulipas, Mexico, 2005; pp. 88–105. ISBN 968-7662-67-0. [Google Scholar]
- Halffter, G. Historia Natural de La Reserva de La Biosfera El Cielo, Tamaulipas, México. Acta Zool. Mex. 2006, 22, 155. [Google Scholar]
- Díaz-Pulido, E.; Payán Garrido, A. Manual de Fototrampeo Una Herramienta de Investigación Para La Conservación de La Biodiversidad En Colombia; Instituto de Investigaciones de Recursos Biológicos Alexander von Humboldt y Panthera: Bogotá, Colombia, 2012; ISBN 978-958-8343-79-2. [Google Scholar]
- Linkie, M.; Ridout, M.S. Assessing Tiger-Prey Interactions in Sumatran Rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Maffei, L.; Cuéllar, E.; Noss, A. Uso de Trampas-Cámara Para La Evaluación de Mamíferos Para La Evaluación de Mamíferos En El Ecotono Chaco-Chiquitanía. Rev. Bol. Ecol. 2002, 11, 55–65. [Google Scholar]
- Maffei, L.; Cuéllar, E.; Noss, A. One Thousand Jaguars (Panthera onca) in Bolivia’s Chaco? Camera Trapping in the Kaa-Iya National Park. J. Zool. 2004, 262, 295–304. [Google Scholar] [CrossRef]
- Ávila-Nájera, D.M.; Chávez, C.; Lazcano-Barrero, M.A.; Mendoza, G.D.; Perez-Elizalde, S. Overlap in Activity Patterns between Big Cats and Their Main Prey in Northern Quintana Roo, México. Therya 2016, 7, 439–448. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.C.; Chávez, C.; List, R. Diversidad y Patrones de Actividad de Mamíferos Medianos y Grandes En La Reserva de La Biosfera La Encrucijada, Chiapas, México. Rev. Biol. Trop. 2018, 66, 634–646. [Google Scholar] [CrossRef]
- Time and Date “World Clock—Time Zone Differences.” Time and Date AS. Available online: https://www.timeanddate.com/worldclock/ (accessed on 21 June 2023).
- Sánchez, R.E.; Rivera García, P.; Marques Dos Santos, M.J. Estadística Circular: Herramienta Para Analizar Datos Angulares En Biología; Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Zaragoza: Ciudad de México, México, 2009; ISBN 970-32-3123-3. [Google Scholar]
- RStudio Team RStudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 21 June 2023).
- Rodriguez-Maturino, A.; Viggers-Carrasco, M.G.; Morales-Balderas, B.N.; López-Reyes, J.A.; Silva-Flores, R.; De León-Mata, G.D. Solapamiento En Los Patrones de Actividad de Mamíferos y Sus Presas Potenciales En Un Área de La Sierra Madre Occidental En Durango, México. Rev. Bio Cienc. 2020, 7, e962. [Google Scholar] [CrossRef]
- Buenrostro-Silva, A.; Sánchez-Núñez, O.; García-Grajales, J. Daily Activity Patterns and Relative Abundance of Medium and Large Mammals in a Communal Natural Protected Area on the Central Coast of Oaxaca, Mexico. Int. J. Biodivers. Conserv. 2020, 12, 159–168. [Google Scholar] [CrossRef]
- Nasanbat, B.; Ceacero, F.; Ravchig, S. A Small Neighborhood Well-Organized: Seasonal and Daily Activity Patterns of the Community of Large and Mid-Sized Mammals around Waterholes in the Gobi Desert, Mongolia. Front. Zool. 2021, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical-Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 0-13-100846-3. [Google Scholar]
- De Oliveira, T.G.; Tortato, M.A.; Silveira, L.; Kasper, C.B.; Mazim, F.D.; Lucherini, M.; Jácomo, A.T.; Soares, J.B.; Márquez, R.V.; Sunquist, M. Ocelot Ecology and Its Effect on the Small-Felid Guild in the Lowland Neotropics. In Biology and Conservation of Wild Felid; Macdonald, D., Loveridge, A., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 559–580. ISBN 9780191574146. [Google Scholar]
- López-Tello, E. Análisis de Actividad y Traslape: Overlap. In Fototrampeo en R. Organización y Análisis de Datos; Mandujano-Rodríguez, S., Pérez-Solano, L.A., Eds.; Instituto de Ecología A.C.: Xalapa, Veracruz, México, 2019; Volume I, pp. 155–166. ISBN 978-607-7579-89-2. [Google Scholar]
- Agostinelli, C.; Lund, U. R Package Circular: Circular Statistics (Version 0.4-93). Available online: https://cran.r-project.org/web/packages/circular/index.html (accessed on 21 June 2023).
- Meredith, M.; Ridout, M.S.; Campbell, L.A.D. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. Available online: https://cran.r-project.org/web/packages/overlap/index.html (accessed on 21 June 2023).
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. Available online: https://iucnredlist.org (accessed on 21 June 2025).
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental, Especies Nativas de Flora y Fauna Silvestres de México, Categorías de Riesgo y Especificaciones Para Su Inclusión, Exclusión o Cambio, y Lista de Especies En Riesgo. México: Diario Oficial de La Federación. Available online: https://www.dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm (accessed on 20 June 2023).
- Foster, V.C.; Sarmento, P.; Sollmann, R.; Tôrres, N.; Jácomo, A.T.A.; Negrões, N.; Fonseca, C.; Silveira, L. Jaguar and Puma Activity Patterns and Predator-Prey Interactions in Four Brazilian Biomes. Biotropica 2013, 45, 373–379. [Google Scholar] [CrossRef]
- Powell, R.A.; Zimerman, J.W.; Seaman, D.E. Ecology and Behavior of North American Black Bears: Home Ranges, Habitat and Social Organization; Chapman & Hall: London, UK, 1994; ISBN 0-412-57990. [Google Scholar]
- Hellgren, E.C.; Vaughan, M.R. Seasonal Food Habits of Black Bears in Great Dismal Swamp, Virginia-North Carolina. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1988, 42, 295–305. [Google Scholar]
- Sánchez, F.; Gómez-Valencia, B.; Álvarez, S.J.; Gómez-Laverde, M. Primeros Datos Sobre Los Hábitos Alimentarios Del Tigrillo, Leopardus pardalis, En Un Bosque Andino de Colombia. Rev. UDCA Actual. Divulg. Cient. 2008, 11, 101–107. [Google Scholar] [CrossRef]
- Bianchi, R.d.C.; Rosa, A.F.; Gatti, A.; Mendes, S.L. Diet of Margay, Leopardus wiedii, and Jaguarundi, Puma yagouaroundi, (Carnivora: Felidae) in Atlantic Rainforest, Brazil. Zoologia 2011, 28, 127–132. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Griffiths, M. Activity Periods of Indonesian Rain Forest Mammals. Biotropica 1996, 28, 105–112. [Google Scholar] [CrossRef]
- Cortés-Marcial, M.; Briones-Salas, M. Diversidad, Abundancia Relativa y Patrones de Actividad de Mamíferos Medianos y Grandes En Una Selva Seca Del Istmo de Tehuantepec, Oaxaca, México. Rev. Biol. Trop. 2014, 62, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, M.D.; Youlatos, D. Integrating Locomotion, Postures and Morphology: The Case of the Tayra, Eira barbara (Carnivora, Mustelidae). Mamm. Biol. 2016, 81, 464–476. [Google Scholar] [CrossRef]
- Pérez-Solano, L.A.; Hidalgo-Mihart, M.G.; Mandujano, S. Preliminary Study of Habitat Preferences of Red Brocket Deer (Mazama temama) in a Mountainous Region of Central Mexico. Therya 2016, 7, 197–203. [Google Scholar] [CrossRef]
- Hernández-SaintMartín, A.D.; Rosas-Rosas, O.C.; Palacio-Núñez, J.; Tarango-Arámbula, L.A.; Clemente-Sánchez, F.; Hoogesteijn, A.L. Activity Patterns of Jaguar, Puma and Their Potential Prey in San Luis Potosi, Mexico. Acta Zool. Mex. 2013, 29, 520–533. [Google Scholar] [CrossRef]
- Polisar, J.; Maxit, I.; Scognamillo, D.; Farrell, L.; Sunquist, M.E.; Eisenberg, J.F. Jaguars, Pumas, Their Prey Base, and Cattle Ranching: Ecological Interpretations of a Management Problem. Biol. Conserv. 2003, 109, 297–310. [Google Scholar] [CrossRef]
- Scognamillo, D.; Maxit, I.E.; Sunquist, M.; Polisar, J. Coexistence of Jaguar (Panthera onca) and Puma (Puma concolor) in a Mosaic Landscape in the Venezuelan Llanos. J. Zool. 2003, 259, 269–279. [Google Scholar] [CrossRef]
- Flores-Turdera, C.; Ayala, G.; Viscarra, M.; Wallace, R. Comparison of Big Cat Food Habits in the Amazon Piedmont Forest in Two Bolivian Protected Areas. Therya 2021, 12, 75–83. [Google Scholar] [CrossRef]
- De Oliveira, T. Ecología Comparativa de La Alimentación Del Jaguar y Del Puma En El Neotrópico. In El jaguar en el Nuevo Milenio; Medellín, R.A., Chetkiewicz, C., Rabinowitz, A., Redford, K.H., Robinson, J.G., Sanderson, E., Taber, A., Eds.; Fondo de 107 Cultura Económica/Universidad Nacional Autónoma de México/Wildlife Conservation Society: Ciudad de México, México, 2002; pp. 285–313. [Google Scholar]
- Paviolo, A.; Di Blanco, Y.E.; De Angelo, C.D.; Di Bitetti, M.S. Protection Affects the Abundance and Activity Patterns of Pumas in the Atlantic Forest. J. Mammal. 2009, 90, 926–934. [Google Scholar] [CrossRef]
- Marín, M.V.; Uribe-Velásquez, L.F. ¿Cómo Afecta el Estrés Calórico la Reproducción? Biosalud 2010, 9, 83–95. [Google Scholar]
- López-Tello, E.; Gallina, S.; Mandujano, S. Activity Patterns of White-Tailed Deer in the Tehuacán-Cuicatlán Biosphere Reserve, Puebla-Oaxaca, Mexico. Deer Spec. Group Newsl. 2015, 27, 32–43. [Google Scholar] [CrossRef]
- Valenzuela, D.; Ceballos, G. Habitat Selection, Home Range, and Activity of the White-Nosed Coati (Nasua narica) in a Mexican Tropical Dry Forest. J. Mammal. 2000, 81, 810–819. [Google Scholar] [CrossRef]
- Núñez-Garduño, A. Los Mamíferos Silvestres de Michoacán, Diversidad, Biología e Importancia; Universidad Michoacana de San Nicolás Hidalgo, Secretaria de Difusión Cultural y Extensión Universitaria: Michoacán, México, 2005; p. 429. [Google Scholar]
- Ceballos, G.; Oliva, G. Los Mamíferos Silvestres de México, 1st ed.; CONABIO/Fondo de Cultura Económica: Mexico City, México, 2005; p. 904. [Google Scholar]
- Romero-Muñoz, A.; Maffei, L.; Cuéllar, E.; Noss, A.J. Temporal Separation between Jaguar and Puma in the Dry Forests of Southern Bolivia. J. Trop. Ecol. 2010, 26, 303–311. [Google Scholar] [CrossRef]
- Estrada-Hernández, C.G. Dieta, Uso de Hábitat y Patrones de Actividad del Puma (Puma concolor) y el Jaguar (Panthera onca) en la Selva Maya. Rev. Mex. Mastozool. 2008, 12, 113–130. [Google Scholar] [CrossRef]
- Harmsen, B.J.; Foster, R.J.; Silver, S.C.; Ostro, L.E.T.; Doncaster, C.P. Jaguar and Puma Activity Patterns in Relation to Their Main Prey. Mamm. Biol. 2011, 76, 320–324. [Google Scholar] [CrossRef]
- Rocha-Mendes, F.; Bos-Mikich, S.; Quadros, J.; Pedro, W.A. Feeding Ecology of Carnivores (Mammalia, Carnivora) in Atlantic Forest Remnants, Southern Brazil. Biota Neotrop. 2010, 10, 21–30. [Google Scholar] [CrossRef]
- Cinta-Magallón, C.C.; Bonilla-Ruz, C.R.; Alarcón-D, I.; Arroyo-Cabrales, J. Dos Nuevos Registros de Margay (Leopardus wiedii) En Oaxaca, México, Con Datos Sobre Hábitos Alimentarios. UNED Res. J. 2012, 4, 33–40. [Google Scholar] [CrossRef]
- Gallina-Tessaro, S.; López-González, C. Manual de Técnicas para el Estudio de la Fauna, 1st ed.; Instituto de Ecología, A.C./Universidad Autónoma de Querétaro, INE-Semarnat: Querétaro, México, 2012; p. 377. [Google Scholar]
- Delfín-Alfonso, C.A.; Gallina-Tessaro, S.A.; López González, C.A. El Hábitat: Definición, Dimensiones y Escalas de Evaluación Para La Fauna Silvestre. In Manual de Técnicas Para el Studio de la Fauna; Gallina-Tessaro, S.A., López-González, C., Eds.; Instituto de Ecología A.C./Universidad Autónoma de Querétaro: Querétaro, México, 2012; pp. 285–313. [Google Scholar]
- Tobler, M.W.; Zúñiga Hartley, A.; Carrillo-Percastegui, S.E.; Powell, G.V.N. Spatiotemporal Hierarchical Modelling of Species Richness and Occupancy Using Camera Trap Data. J. Appl. Ecol. 2015, 52, 413–421. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Thorsen, N.H.; Cromsigt, J.P.G.M.; Kindberg, J.; Andrén, H.; Linnell, J.D.C.; Odden, J. Effects of Camera-Trap Placement and Number on Detection of Members of a Mammalian Assemblage. Ecosphere 2021, 12, e03662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).