Abstract
Leaf litter traits among tree species exert a direct influence on spatiotemporal nutrient turnover and an indirect influence by shifting the decomposition dynamics of leaf litter mixtures including other sympatric species. Cycas micronesica and Serianthes nelsonii are two Mariana Island tree species that are endangered, and developing a greater understanding of the influence of these trees on biogeochemistry may improve information-based conservation decisions. The objectives of this study were to quantify the influence of mixing the leaf litter of these species with 12 sympatric forest plants to determine the additive and nonadditive influences on decomposition. The C. micronesica litter was collectively antagonistic when litter mixtures were incubated in a mesocosm study and a field litterbag study, and the response was similar among the included species. The S. nelsonii litter was collectively synergistic among the same mixed species, and the response was dissimilar among the included species. The contributions of these two threatened tree species to spatiotemporal diversity in biogeochemistry are dissimilar and considerable. These findings indicate that species recovery efforts for these two species are of paramount importance for maintaining Mariana Island ecological integrity and native biodiversity by sustaining their contributions to ecosystem services.
1. Introduction
The decomposition of leaf litter in forest communities plays a central role in numerous crucial processes including regional nutrient cycling, carbon sequestration dynamics, sustaining food webs, and soil microbiology [1,2,3]. Among the drivers which control the decomposition processes is the quality of litter, indicating that the spatial and temporal aspects of litterfall need to be more fully understood [4,5,6]. Single-species litters with greater concentrations of nutrients and lower concentrations of complex molecules are expected to decompose more rapidly. These phenomena are critical to the global biodiversity crisis and for endangered species conservation science. The individual contributions of threatened tree species to ecosystem processes need to be more fully understood to develop more knowledge-based decisions for species recovery and to more fully acknowledge the contributions before they are potentially lost.
All ecological processes should be viewed with contextual integrity, and litter decomposition research must acknowledge the mixing of various plant taxa. Single-species litter decomposition studies are important for understanding species rankings in decomposition lability and recalcitrance. However, in natural forest settings, the litter from one species is never decomposed in the absence of litter from other sympatric species. Therefore, research to address the decomposition of multi-species litter mixtures provides more context-dependent outcomes [7,8]. When multi-species decomposition speed is increased or decreased relatively to the arithmetic averages of single-species incubation, the response is referred to as nonadditive. Some of the factors that lead to nonadditive litter mixture decomposition are transfers of nutrients from high-quality litters to low-quality litters during incubations, changes in water retention in the litter layer, the transfer of toxic or recalcitrant molecules from recalcitrant litters to labile litters, and changes in the microbial community due to the enhanced chemical diversity [9,10,11]. One of the issues that need to be more fully understood in relation to biodiversity loss is that the direct impact of each species represented in litter mixtures alters litter decomposition dynamics [12].
The Micronesia region of the western Pacific Ocean has not received adequate research to fully understand ecosystem function, so contributions from the region to global issues e.g., [13], remains minimal. Two endangered tree species from the Micronesian Mariana Islands associate with nitrogen-fixing root endosymbionts, and therefore are important contributors to island biogeochemical relations. The cycad Cycas micronesica K.D. Hill is the region’s only gymnosperm, and has been listed under the International Union for Conservation of Nature’s Red List since 2006 [14] and under the United States Endangered Species Act (ESA) since 2015 [15]. The legume Serianthes nelsonii Merr. is the most threatened tree species in the region, and has been listed under the Red List since 1978 [16] and under the ESA since 1987 [17]. These two tree species are valued as horticultural specimens. A greater understanding of how these important trees contribute to ecosystem function is urgently needed. Towards that goal, leaf litter nutrient relations have been determined [18] and single-species litter decomposition has been reported, indicating that low-quality C. micronesica litter is relatively recalcitrant and high-quality S. nelsonii litter is relatively labile [19,20].
No reports from Micronesia have adequately addressed multi-species litter decomposition, so knowledge is lacking to understand nutrient cycling in these oceanic islands. Therefore, the objective of this study was to determine the litter mass loss of dual-species mixtures involving these two endangered tree species. In addition to the C. micronesica and S. nelsonii litter mixture incubations, the study included 12 other sympatric species, each mixed with C. micronesica or S. nelsonii. For any nonadditive decomposition outcomes, the low-quality C. micronesica litter was predicated to exhibit antagonistic changes and the high-quality S. nelsonii litter was predicted to exhibit synergistic changes to the mixed litter decompositions.
2. Materials and Methods
Four litter decomposition experiments were conducted on the island of Guam using leaf litter from 14 forest species. The experimental approach was created to determine the influence of C. micronesica and S. nelsonii leaf litter on decomposition dynamics of the remaining 12 species in two-species mixtures. Two experiments were conducted under closed canopy forest conditions to ensure ecological relevance under natural weather conditions. In addition, two experiments were conducted in a constructed mesocosm to provide more controlled conditions that are more easily repeatable. Mesocosm research is useful for bridging the gap between laboratory experimental conditions and in situ field conditions [21]. Mesocosms provide more realism than laboratory studies, yet variables can be controlled to a greater degree than field studies.
Litter collection methods, storage conditions, and litterbag preparation protocols were previously described [18,22]. The collection locality was a biodiverse karst forest habitat in northeast Guam which developed on an uplifted coralline soil series (clayey-skeletal, gibbsitic, nonacid, isohyperthermic Lithic Ustorthents). The 14 species were sympatric taxa in this habitat, and included monocot, eudicot, and gymnosperm species (Table A1). A major canopy defoliation event during Typhoon Dolphin in May 2015 was exploited to collect the litter samples from freshly dislodged stems containing intact leaves. The litter was collected within 48 h of the defoliation event. The 2015 litter samples were stored in air-conditioned laboratory conditions until the incubations were conducted. The litterbag protocol [23] was employed in the four experiments. Litterbags for the determination of single-species incubations were 20 × 20 cm and the nylon screen contained 1.5 mm diameter holes. Single-species litterbags were constructed with 3 g of litter, and therefore contained 75 g·m−2. Litterbags for the determination of two-species incubations were constructed with 0.5 g of each species for a total of 1 g, and litterbags were 10 × 10 cm. These litterbags contained 100 g·m−2. These mixture litterbags contained 0.5 g of C. micronesica or S. nelsonii litter, and 0.5 g of each of the remaining species. To ensure that the pre-incubation litter was not altered by oven drying, air-dried litter weights were used initially. Five samples of each of the 14 species were weighed as 2 g air-dried samples, dried at 75 °C for two days, and then reweighed to obtain oven dry weight. The ratio of air-dried/oven-dried weight was used to determine the weight of the air-dried litter for each species that would provide the equivalent of 3 g or 0.5 g oven-dried litter.
2.1. Mesocosm Methods
The mesocosm experiments were initiated 6 January 2018 using the University of Guam laboratory site and methods that were previously described [20]. The mesocosm exposed 0.6 m × 8.8 m of medium surface, and the substrate was created with a 10 cm layer of perlite as the base, then a surface 10 cm layer of field soil collected in a native forest community in Dededo, Guam. Care was taken to ensure that none of the 12 experimental species were in the vicinity of the soil collection sites in order to avoid any home field advantage complications among the taxa [24,25]. The litter layer was not included, but the humus layer was harvested with the soil and homogenized. The soil series was clayey, gibbsitic, nonacid, isohyperthermic Lithic Ustorthents [26]. The litterbags were installed on the same day as the mesocosm construction to ensure that the soil microorganism community did not change prior to litterbag deployment. Nutrient contents were typical for northern Guam soils (Table A2). The scattered indirect light to which the litterbags were exposed was 30–35 µmol·m−2·s−1 (Skye SKP200 Quantum Sensor, Skye Instruments, Llandrindod Wells, Powys, UK). The plastic mesocosm cover was removed one time per week and overhead sprinklers were employed to apply 5 cm of irrigation water. This was approximately the average of historical weekly rainfall for Guam. The mean daily maximum temperature was 29–30 °C, and the mean daily minimum temperature was 27–28 °C. Environmental factors such as temperature, light, and water relations influence decomposition [27], and therefore, the previously mentioned factors are needed to compare to similar studies.
Eight replications were constructed for each single-species litterbag category. The litterbags were deployed on the surface of the mesocosm substrate in eight blocks, and each block contained a single litterbag for each 3 g replication. Four of the blocks were randomly selected for harvest on 7–8 April 2018 (three months of incubation), and the remaining four blocks were harvested 10–11 July 2018 (six months of incubation). Eight litterbags were constructed for each two-species combination, and deployed as a second mesocosm experiment. There were 10 co-occurring species for the mesocosm experiment (Table A1). This dual-species mixture experiment also included 1 g litterbags for each species. The deployment date, three-month harvest date, and six-month harvest dates were as described for the single-species experiment.
Following harvest, adhering soil and humus particles were gently washed from the surface of each litterbag, and the remaining litter was carefully removed and the litterbags were discarded. The litter was allowed to dry in ambient conditions, then adhering soil was gently brushed from the litter. The litter was placed on a filter paper, then gently washed with reverse osmosis water. The litter was dried at 75 °C for two days and then weighed.
2.2. Field Methods
The field experiments were initiated 15 December 2022 on a site of the University of Guam campus, and the soil series was the same as for the mesocosm study. The litterbags were constructed according to the methods described for the mesocosm experiments, except that there were 12 co-occurring species included (Table A1). A fragment forest comprised Leucaena leucocephala (Lam.) de Wit was selected because of the homogeneity of emergent canopy species that reduced spatial heterogeneity in litterfall history. Soil nutrients were similar to those of the mesocosm study, but nitrogen concentrations were elevated due to the L. leucocephala tree cover (Table A3). The small plants that contributed to the subcanopy were removed to enable the deployment of the litterbags. All stems that directly contributed to the emergent canopy were left intact to avoid creating large canopy gaps. Each block was 0.8 m × 1.0 m, and the litterbags for each block were installed in a contiguous manner. The blocks were not contiguous. The entire footprint of this field site was 6.4 m × 9.2 m.
The amount of incident light at the soil surface was directly quantified with a 0.75 m line quantum sensor (EMS-7, PP Systems, Amesbury, MA, USA) and was 105–140 µmol·m−2·s−1. Rainfall was allowed to impact the litterbags. A mean of 5.1 cm rainfall per week occurred during the first three months, and a mean of 7.4 cm rainfall per week occurred during the second three-month period. The mean daily maximum temperature was 28–29 °C, and the mean daily minimum temperature was 26–28 °C.
Four of the blocks were randomly selected and all of the litterbags within each were harvested 14–15 March 2023 to provide remaining litter weight after three months of incubation. The four remaining blocks were harvested 20–21 June 2023 to yield the six-month samples. Oven dry weight was determined as described above.
2.3. Statistical Methods
Several one-way and two-way analysis of variance (ANOVA) approaches were employed to determine the levels of significance in the response variables. The same tests were used for the mesocosm and field experiments. Parametric prerequisites were confirmed with Levene’s test and the Shapiro–Wilk test.
The differences in the remaining litter dry weight among the species were determined with a one-way ANOVA for 12 species in the mesocosm study and 14 species in the field study. This ANOVA included only the 3 g litterbag replications. The three- and six-month harvests were analyzed separately.
The influence of C. micronesica litter on the decomposition speed of the co-occurring species was analyzed as a two-factor ANOVA. The treatment factor contained two levels with the calculated additive weight and actual weight of each replication considered as the treatment factor and the species were treated as a second factor in a 2 × 10 factorial for the mesocosm study and a 2 × 12 factorial for the field study.
The influence of the S. nelsonii litter on the decomposition speed of the co-occurring species was analyzed with the same two-factor ANOVA approach. These two-way ANOVAs did not include the 3 g litterbag replications, but included the 1 g litterbags containing only single-species and dual-species litter.
The C. micronesica and S. nelsonii litterbag treatments were analyzed with a t-test comparing the calculated additive weight with the actual weight. The three-month and six-month data were analyzed separately.
In order to standardize each dual-species dataset with a single-response variable, the relative deviation from additivity was calculated for each replication as ((absolute weight − additive weight)/additive weight) × 100. A positive outcome indicated nonadditive antagonism with less decomposition than predicted. A negative outcome indicated nonadditive synergism with greater decomposition than predicted.
In order to more fully understand some of the underlying mechanisms of the experimental outcomes, a Pearson correlation analysis was conducted using litter chemical trait dissimilarity. Total N and C were determined by dry combustion (FLASH EA1112 CHN Analyzer, Thermo Fisher, Waltham, MA, USA). Samples were also digested by a microwave system with nitric acid and peroxide, and then Ca, K, and P were quantified by inductively coupled plasma optical emission spectroscopy (Spectro Genesis; SPECTRO Analytical Instruments, Kleve, Germany). Litter tissue lignin concentration was determined according to the acetyl-bromide method. The chemical concentrations were described in detail from previous reports [18,28]. The differences between each species pair for concentrations of lignin (∆Lig), carbon (∆C), nitrogen (∆N), phosphorus (∆P), potassium (∆K), and calcium (∆Ca) were sorted then correlated with the relative deviation from the additivity metric. Additionally, the stoichiometric variables ∆C:N, ∆C:P, and ∆C:K were correlated with the same metric.
The statistical procedures were executed with R [29]. For variables which were significant, means separation was accomplished with the Tukey–Kramer post hoc test of each pairwise comparison.
3. Results
3.1. Single-Species Incubations
The biomass loss during the incubations in the mesocosm differed among the 12 species during the first three months (f11,55 = 438.278, p < 0.001) and after six months (f11,55 = 608.378, p < 0.001). The least biomass loss occurred for B. tetrandra, C. nucifera, C. micronesica, and H. longipetiolata. More than 80% of the original litter remained after 3 months of incubation for these species (Table 1). Biomass loss was greater during the 3- to 6-month period for these recalcitrant species, and more than 40% of the original biomass remained in the litterbags at 6 months. The labile species experienced the greatest biomass loss and included M. citrifolia, P. grandis, P. serratifolia, and S. nelsonii. Less than 20% of the original biomass remained after 3 months of incubation for these species (Table 1). The speed of biomass loss during the 3- to 6-month incubation period also increased for these species such that less than 10% of the original biomass remained in the litterbags at 6 months. The remaining species in the study exhibited intermediate decomposition speed in the mesocosm (Table 1).

Table 1.
Remaining litter biomass of 12 plant species from 3 g litterbags after 3 or 6 months of incubation in a mesocosm in Guam. Mean ± SE, n = 4.
The mass loss during the incubations in the field study differed among the 14 species during the first three months (f13,55 = 89.811, p < 0.001) and after six months (f13,55 = 104.055, p < 0.001). The same four species (B. tetrandra, C. nucifera, C. micronesica, H. longipetiolata) exhibited recalcitrant decomposition, but the biomass loss was greater than in the mesocosm study for both litterbag harvest periods (Table 2). The same four species (M. citrifolia, P. grandis, P. serratifolia, S. nelsonii) exhibited labile decomposition (Table 2). The remaining species including the added F. prolixa and O. mariannensis were intermediate compared to the four recalcitrant and four labile species (Table 2).

Table 2.
The remaining litter biomass of 14 plant species from 3 g litterbags after 3 or 6 months of incubation in a field litterbag study in Guam. Mean ± SE, n = 4.
In summary, there was a 11.5-fold difference among the 6-month biomass means for the field study and a 67.5-fold difference among the 6-month biomass means for the mesocosm study. In general, the recalcitrant species exhibited greater mass loss and the labile species exhibited less mass loss in the field study when compared with the mesocosm study.
3.2. Mixing Litter with Cycas micronesica
The biomass remaining after 3 months of incubation in the mesocosm with C. micronesica litter mixtures was influenced by the main treatment factor (f1,60 = 22.386; p < 0.001) and the main species factor (f9,60 = 105.662; p < 0.001). In contrast, the interaction of treatment × species was not significant (f9,60 = 0.952; p = 0.488), indicating that the extent and direction of the nonadditive effects of the C. micronesica litter was similar among the species. The actual biomass of litter remaining after 3 months of incubation was 7% greater than the calculated additive mean (Figure 1a), and after 6 months of incubation, the actual biomass was 6% greater than the additive means (Figure 1b). These results indicated that the C. micronesica litter rendered an antagonistic influence on the decomposition of litter from the 10 forest species.
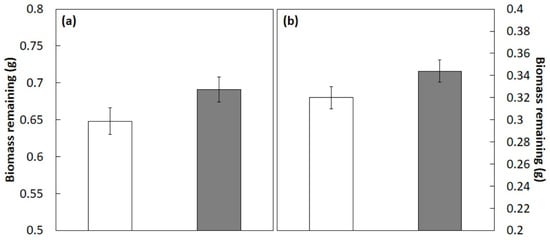
Figure 1.
Litter biomass remaining after 3 months (a) or 6 months (b) of incubation in a Guam mesocosm. The original 1 g of litter tissue was derived from 10 plant species from Guam’s northern karst forests that were mixed 50:50 with Cycas micronesica litter. Open bars represent the additive mean, and shaded bars represent the actual biomass. Mean ± SE, n = 40. Differences are significant at p < 0.001.
The leaf litter mixtures contained 50% C. micronesica litter separated into six non-overlapping means after 3 months of incubation (Table 3). The H. longipetiolata litter exhibited the least biomass loss, and the M. citrifolia and P. serratifolia litters exhibited the greatest level of biomass loss. Biomass loss was about 33% for the combined 3-month data set. These litter mixtures lost about 67% for the combined 6-month dataset, and the differences among the species mixtures were diminished (Table 3). The mixtures containing B. tetrandra and C. nucifera merged with the H. longipetiolata litter as the most recalcitrant mixtures, and the mixtures containing P. grandis merged with the M. citrifolia and P. serratifola as the most labile mixtures.

Table 3.
Remaining litter biomass of 10 plant species from the 1 g litterbags after 3 or 6 months of incubation in a mesocosm in Guam. The overall means were a combination of the predicted additive mean from single-species incubations and the actual remaining biomass of 50:50 mixtures with Cycas micronesica litter. Mean ± SE, n = 8.
The biomass remaining after 3 months of incubation in the field study with C. micronesica litter mixtures was influenced by the main treatment factor (f1,95 = 15.659; p < 0.001) and the main species factor (f11,95 = 18.678; p < 0.001). In contrast, the interaction of treatment × species was not significant (f11,95 = 0.652; p = 0.778), indicating that the extent and direction of the nonadditive effects of the C. micronesica litter was similar among the species, as for the mesocosm study. The actual biomass of litter remaining after 3 months of incubation was 9% greater than the calculated additive mean (Figure 2a), and after 6 months of incubation, the actual biomass was 8% greater than the additive means (Figure 2b). These results indicated that the C. micronesica litter rendered an antagonistic influence on the decomposition of litter from the 12 forest species.
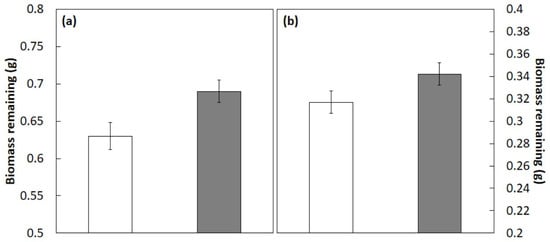
Figure 2.
Litter biomass remaining after 3 months (a) or 6 months (b) of incubation in a Guam field study. Original 1 g of litter tissue was derived from 12 plant species from Guam’s northern karst forests that were mixed 50:50 with Cycas micronesica litter. Open bars represent the additive mean, shaded bars represent the actual biomass. Mean ± SE, n = 48. Differences significant at p < 0.001.
The 12 species with litter that was mixed with C. micronesica litter exhibited a 1.6-fold difference among the 3-month biomass means and a 1.6-fold difference among the 6-month biomass means for the field study (Table 4). There were five overlapping groups of means in the 3-month dataset and four groups of non-overlapping means in the 6-month groups of means. The relatively diminished differences among the species indicated that mixing the leaf litter with the C. micronesica leaf litter reduced the differences among the individual species to a greater extent in the field study than in the mesocosm study. The litter mixtures with the M. citrifolia and P. serratifolia litter remained the most labile litter mixtures, with about half of the litter lost by 3 months of incubation and about three-fourths of the litter lost by 6 months.

Table 4.
Remaining litter biomass of 12 plant species from 1 g litterbags after 3 or 6 months of incubation in a field study in Guam. Overall means were a combination of the predicted additive mean from single-species incubations and the actual remaining biomass of 50:50 mixtures with Cycas micronesica litter. Mean ± SE, n = 8.
In summary, the influence of mixing the leaf litter of various forest species with C. micronesica leaf litter on changes in decomposition was similar among the 12 different species, as indicated by the lack of significant treatment × species interactions in the 3- and 6-month factorial ANOVAs. Therefore, the magnitude and direction of non-additivity was not influenced by the litter identity when mixed with C. micronesica litter. The species that exhibited the least amount of decomposition as non-mixed litter also exhibited the least amount of decomposition when mixed with C. micronesica. The species that exhibited the greatest levels of decomposition as non-mixed litter also exhibited the greatest levels of decomposition when mixed with C. micronesica. However, the decomposition was collectively non-additive, and as a group, the C. micronesica litter caused an antagonism that slowed down the litter decomposition of these 12 forest species. The overall means were similar for the mesocosm versus the field study for these litter treatments that were mixed with C. micronesica litter. Mixtures with C. micronesica litter exhibited a collective 7% antagonism in mesocosm and 9% antagonism in field incubations.
3.3. Mixing Litter with Serianthes nelsonii
The biomass remaining after 3 months of incubation in the mesocosm with S. nelsonii litter mixtures was influenced by the treatment main factor (f1,60 = 63.316; p < 0.001), the species main factor (f9,60 = 334.874; p < 0.001), and the interaction of treatment × species (f9,60 = 9.176; p < 0.001). Therefore, the extent and direction of non-additive effects of the S. nelsonii litter was heterogeneous among the 10 species (Table 5). The means separated into nine non-overlapping groups. Some species exhibited an additive effect as shown by a lack of significant difference between the additive mean and the actual mean. These included A. mariannensis, M. citrifolia, P. grandis, P. serratifolia, and T. rotensis. The remainder of the species exhibited a nonadditive effect of litter mixing, whereby the 50:50 mixture with S. nelsonii accelerated litter decomposition in a synergistic manner. The species with the greatest level of synergistic decomposition were the most recalcitrant species. For example, the actual biomass mean for B. tetrandra was 18% less than the predicted additive mean.

Table 5.
Remaining litter biomass of 10 plant species from 1 g litterbags after 3 or 6 months of incubation in a mesocosm in Guam. Means are the predicted additive mean from single-species incubations and the actual remaining biomass of 50:50 mixtures with Serianthes nelsonii litter. Mean ± SE, n = 4.
The biomass remaining after 6 months of incubation in the mesocosm with S. nelsonii litter mixtures was influenced by the treatment main factor (f1,60 = 46.189; p < 0.001), the main species factor (f9,60 = 815.342; p < 0.001), and the interaction of treatment × species (f9,60 = 3.97; p < 0.001). Therefore, the extent and direction of non-additive effects of the S. nelsonii litter was heterogeneous among the 10 species as with the 3-month incubations (Table 5). There was more overlap among the 6-month means than the 3-month means, indicating the means converged as the leaf litter decomposed. The same five species exhibited an additive effect as shown by a lack of significant difference between the additive mean and the actual mean. The 50:50 mixture with S. nelsonii accelerated litter decomposition for the five remaining species. The three most recalcitrant species were B. tetrandra, C. nucifera, and H. longipetiolata, and the actual remaining mixed litter was 14% less for these three species than what was predicted from the additive means.
The biomass remaining after 3 months of incubation in the field study with S. nelsonii litter mixtures was influenced by the main treatment factor (f1,95 = 69.140; p < 0.001), the species main factor (f11,95 = 90.123; p < 0.001), and the interaction of treatment × species (f11,95 = 2.785; p = 0.005). The significant interaction indicated that the extent and direction of the non-additive effects of the S. nelsonii litter was not similar among the 12 species (Table 6). The number of species exhibiting an additive response was less than it was for the mesocosm study, in that only the labile M. citrifolia, P. grandis, and P. serratifolia litter means revealed an additive decomposition response. The recalcitrant species exhibited the greatest level of synergy when mixed with S. nelsonii, with actual biomass means that were about 19% less than the predicted additive means. About 71% of the litter had decomposed by 3 months in this field study, and about 91% of the litter had decomposed by 6 months.

Table 6.
Remaining litter biomass of 12 plant species from 1 g litterbags after 3 or 6 months of incubation in a field study in Guam. Means are the predicted additive mean from single-species incubations and the actual remaining biomass of 50:50 mixtures with Serianthes nelsonii litter. Mean ± SE, n = 4.
The biomass remaining after 6 months of incubation in the field study with S. nelsonii litter mixtures was influenced by the treatment main factor (f1,95 = 38.954; p < 0.001), the species main factor (f11,95 = 76.499; p < 0.001), and the interaction of treatment × species (f11,95 = 2.205; p = 0.023). The extent and direction of nonadditive effects of the S. nelsonii litter was heterogeneous among the 12 species (Table 6). Seven of the 12 species exhibited additive litter decomposition when mixed with S. nelsonii after 6 months of incubation in the field study. The recalcitrant species like B. tetrandra and H. longipetiolata exhibited the greatest nonadditive response with actual litter weight of 18% to 19% less than that predicted by the additive mean.
In summary, the influence of mixing the leaf litter of various forest species with S. nelsonii leaf litter on changes in decomposition was not consistent among 12 different species, as indicated by the significant treatment × species interactions in the 3- and 6-month factorial ANOVAs. Three to seven species exhibited additive decomposition in this mixture, depending on the study and incubation duration. For the species which demonstrated nonadditive litter decomposition, the S. nelsonii litter caused a universal synergism that increased the litter mass loss. About 70% of the mixed litter had decomposed by 3 months in the field study, and about 86% of the litter had decomposed by 6 months. Mixtures with labile S. nelsonii litter exhibited 7% synergism in the mesocosm and 12% synergism in the field incubations.
3.4. General Trends
The mixture of C. micronesica and S. nelsonii litter exhibited a significant antagonistic effect after 3 months (Figure 3a) or 6 months (Figure 3b) of incubation in the mesocosm. The recalcitrant C. micronesica litter significantly antagonized the S. nelsonii litter, with an actual decomposition that was about 8% greater than the additive mean after 3 months and about 11% greater than the additive mean after 6 months.
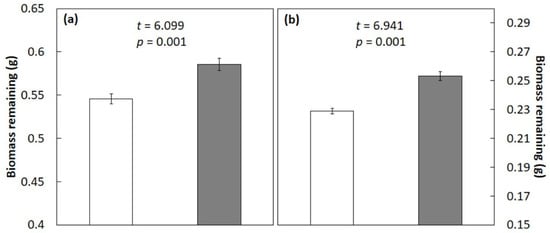
Figure 3.
Litter biomass remaining after 3 months (a) or 6 months (b) of incubation in a Guam mesocosm study. Original 1 g of litter tissue was derived from a 50:50 mixture of Cycas micronesica litter and Serianthes nelsonii litter. Open bars represent additive mean, and shaded bars represent actual biomass. Mean ± SE, n = 4.
The mixture of C. micronesica and S. nelsonii litter exhibited additive decomposition effects after 3 months (Figure 4) or 6 months (Figure 4b) of incubation in the field study. Under these field conditions, the relative influences of these two contrasting litter types on the bulk litter sample clearly cancelled the effects of each other during incubation.
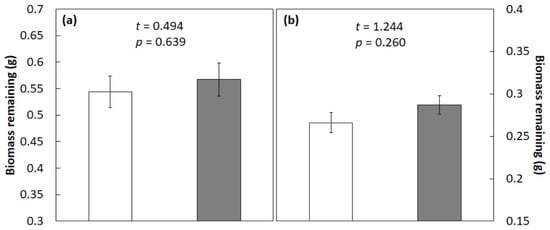
Figure 4.
Litter biomass remaining after 3 months (a) or 6 months (b) of incubation in a Guam field study. Original 1 g of litter tissue was derived from a 50:50 mixture of Cycas micronesica litter and Serianthes nelsonii litter. Open bars represent additive mean, and shaded bars represent actual biomass. Mean ± SE, n = 4.
The relative decomposition of each species combination may best be revealed graphically by plotting the relative increase or decrease in decomposition as compared with additivity. For the 3 month mesocosm data, the overall means were about 6% antagonized by C. micronesica and 7% synergized by S. nelsonii (Figure 5a). Most species that were antagonized by C. micronesica exhibited near additivity for S. nelsonii, and most species that were synergized by S. nelsonii exhibited near additivity for C. micronesica. However, H. tiliaceus and P. tectorius appeared to be influenced in opposite directions by the two species.
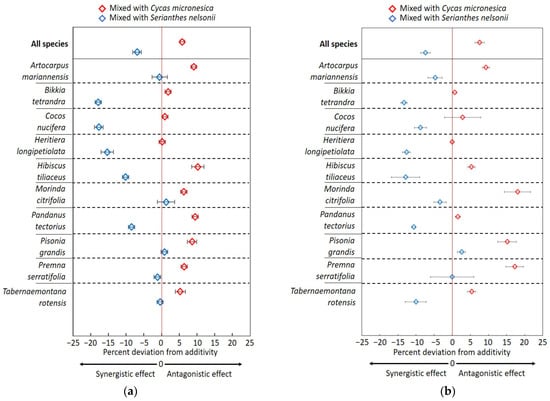
Figure 5.
Graphic comparison of mixing the litter of 10 Guam forest species with Cycas micronesica litter or Serianthes nelsonii litter after (a) 3 months of incubation and (b) 6 months in a mesocosm. Original 1 g of litter tissue was derived from a 50:50 mixture. Vertical red line is additivity.
The deviation from additivity was greater after 6 months of incubation than after 3 months of incubation in the mesocosm study (Figure 5b). The litter mixed with C. micronesica decomposed about 8% slower and the litter mixed with S. nelsonii decomposed about 7% faster than the additive means. Variation among the replications also increased, as shown by the relatively greater SE bars.
The data from the field litterbag study exhibited greater deviations from additivity than the mesocosm study (Figure 6a). After 3 months of decomposition, the litter mixed with C. micronesica decomposed about 10% slower and the litter mixed with S. nelsonii decomposed about 13% faster than the additivity.
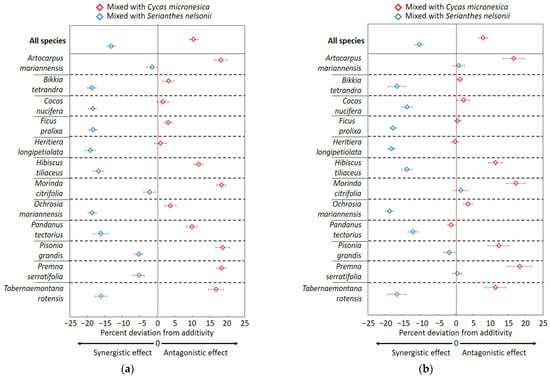
Figure 6.
Graphic comparison of the mixing litter of 12 Guam forest species with Cycas micronesica litter or Serianthes nelsonii litter after (a) 3 months of incubation or (b) 6 months in a field litterbag study. Original 1 g of litter tissue was derived from a 50:50 mixture. Vertical red line is additivity.
More of the species mixtures exhibited additivity after 6 months of incubations in the Guam field litterbag study than in the mesocosm study (Figure 6b). Overall, the mixtures with C. micronesica litter decomposed about 8% slower and the mixtures with S. nelsonii litter decomposed 10% faster than additivity.
In summary, the litterbags containing mixed C. micronesica and S. nelsonii litter revealed a significant antagonism in the mesocosm study but not in the field study. The C. micronesica litter antagonized the mixed species in litterbags and the S. nelsonii litter synergized the mixed species in litterbags. The level of antagonism caused by C. micronesica litter was similar to the level of synergism caused by the S. nelsonii litter in the mesocosm study. In contrast, the synergistic effects of the S. nelsonii litter were relatively greater than the antagonistic effects of C. micronesica litter in the field study. These dissimilarities in some of the response variables illuminated the importance of using various research protocols to improve the context dependency to more fully understand complex litter decomposition dynamics.
3.5. Pearson Correlations
The differences in various leaf litter chemicals between the pairwise comparisons of species and C. micronesica exhibited correlations with the deviation from decomposition additivity after 3 months and 6 months of incubation in the mesocosm study (Figure 7). After 3 months of incubation, the variables with the greatest correlations were ∆C:N > ∆C:K > ∆N. After 6 months of incubation, the variables with the greatest correlations were ∆N > ∆C:N > ∆lig. For these mesocosm incubations, the 3-month data were not correlated with the 6-month data.
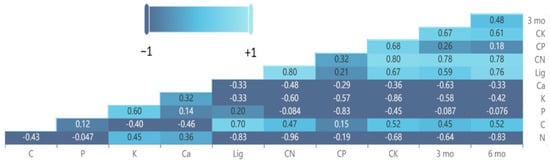
Figure 7.
Heat map and coefficients for Pearson correlations among the leaf litter of various litter species mixed with Cycas micronesica litter after 3 and 6 months of incubation in a mesocosm litterbag study. Lig = lignin.
The differences in various leaf litter chemicals between the pairwise comparisons of species and S. nelsonii exhibited correlations with the deviation from decomposition additivity after 3 months and 6 months of incubation in the mesocosm study (Figure 8). After 3 months of incubation, the variables with the greatest correlations were ∆N > ∆C:N > ∆C:K. After 6 months of incubation, none of the litter chemical traits exhibited a substantial correlation with the litter mass loss. The 3-month data were again not correlated with the 6-month data for the S. nelsonii mesocosm mixtures.
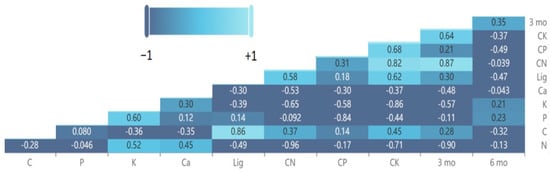
Figure 8.
Heat map and coefficients for Pearson correlations among leaf litter of various litter species mixed with Serianthes nelsonii litter after 3 and 6 months of incubation in a mesocosm litterbag study. Lig = lignin.
The differences in various leaf litter chemicals between the pairwise comparisons of species and C. micronesica exhibited correlations with the deviation from decomposition additivity after 3 months and 6 months of incubation in the field study (Figure 9). After 3 months of incubation, the variables with the greatest correlations were ∆C:N > ∆N. After 6 months of incubation, the variables with the greatest correlations were ∆N > ∆C:N > ∆lig. In contrast to the mesocosm study, the 3-month data exhibited substantial correlation with the 6-month data.
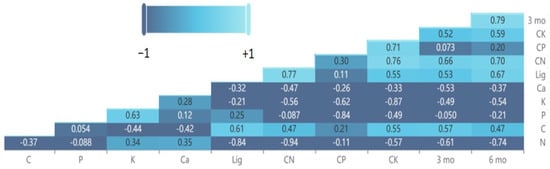
Figure 9.
Heat map and coefficients for Pearson correlations among the leaf litter of various litter species mixed with Cycas micronesica litter after 3 and 6 months of incubation in a field litterbag study. Lig = lignin.
The differences in various leaf litter chemicals between the pairwise comparisons of species and S. nelsonii exhibited correlations with the deviation from decomposition additivity after 3 months and 6 months of incubation in the field study (Figure 10). After 3 months and 6 months of incubation, the variables with the greatest correlations were ∆N > ∆C:N. As with the C. micronesica field study mixtures, the 3-month data were correlated with the 6-month data.
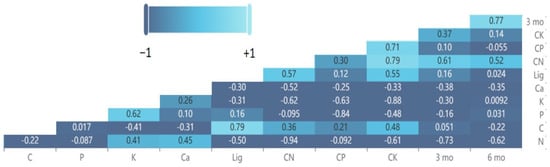
Figure 10.
Heat map and coefficients for Pearson correlations among the leaf litters of various litter species mixed with Serianthes nelsonii litter after 3 and 6 months of incubation in a field litterbag study. Lig = lignin.
4. Discussion
Litterbags incubated in two studies in Guam confirmed the prediction of the nonadditive effects of C. micronesica and S. neslonii litter on mixtures with 12 Guam forest species. Mixtures with the recalcitrant C. micronesica litter exhibited a collective antagonism, and the mixtures with labile S. nelsonii litter exhibited a collective synergism. Literature reviews reveal that the nonadditive effects of litter mixtures occur in a majority of these studies [10,30,31], and these Guam data corroborate those reports.
4.1. Recalcitrance Versus Lability
A considerable body of literature exists on the direct influence of litter quality on single-species decomposition [4,5,6]. The role of litter quality may also explain much of the extent and direction of nonadditive trends in mixed litters in relation to recalcitrance and the lability of the constituents of the bulk litter matrix [32,33,34,35]. Indeed, the low quality C. micronesica litter exhibited recalcitrant decomposition traits when decomposed alone but also antagonized the mixed litter incubations. In contrast, the high quality S. nelsonii litter exhibited labile decomposition traits when decomposed alone but also synergized mixed litter incubations.
Several mechanisms of nonadditive litter mixture decomposition have been proposed [9,10,11]. For the synergism caused by high-quality litter types, the direct transfer of nutrients from high-to-low quality litters within the mixture has been verified [36]. For antagonism caused by low-quality litter types, the role of recalcitrant components like lignin and cellulose and their transfer among the mixed leaf materials may be partly causal [37,38]. In addition to the transfer of these labile and recalcitrant chemical components among the mixed litter materials, their transfer to the soil substrate can facilitate or impede changes in the microbial community due to the enhanced chemical diversity. The litterfall zone beneath a S. nelsonii tree may therefore generate a mini-ecocline whereby the collective chemical environment facilitates litter decomposition within the ecocline in relation to the zones outside of the litterfall zone. Contrarily, the litterfall zone beneath a C. micronesica tree may generate a mini-ecocline whereby the collective chemical environment impedes litter decomposition in relation to the zones outside of the litterfall zone. These heterogeneous creations of mini-ecoclines may subsequently lead to mini-ecotones that benefit the community by improving biodiversity heterogeneity at the local level.
Species recovery plans are crucial for identifying approaches for conserving threatened plant species [39]. These plans typically explicate the need for expanded research to improve knowledge about the contributions of endangered species to ecosystem function. This Guam case study illuminates how two endangered tree species contribute to biogeochemical relations and add to the global literature on how biodiversity losses may influence litter decomposition [12]. If ongoing conservation efforts are ultimately unsuccessful, their loss will exert a direct negative impact on ecosystem function.
4.2. Mesocosm Versus Field Incubations
The overall litter mass loss for single-species litterbags was similar for the mesocosm versus field study incubations, but individual litter types behaved differently. The 6-month litter mass loss was greater in the field venue for recalcitrant species but greater in the mesocosm venue for the labile species. Moreover, nonadditive effects were greater in the field incubations than the mesocosm incubations for both C. micronesica and S. nelsonii litter mixing. The 3-month data were not correlated with the 6-month data for the mesocosm study, but were highly correlated for the field study.
The quality of the litter material is consistently considered the major driver of heterogeneous decomposition processes, but site factors also play a role. Among these are the temperature, humidity and rainfall patterns, soil substrate nutrient supply, incident light, slope aspect, altitude, and local decomposer community identities [27,40,41,42,43,44,45,46,47]. The temperature and soil nutrient regimes of the two studies were similar, so these factors were not likely causal of the differences between the mesocosm and field studies. The plastic cover of the mesocosm provided 100% humidity for the duration of the study, but diel fluctuations of ambient humidity characterized the field study. Additionally, the weekly application of a consistent amount of irrigation in the mesocosm created a homogeneous temporal pattern in relation to water inputs, but heterogeneous inputs of water occurred for the open field litterbags. For example, the field litterbags received more than 32 cm of rainfall during one week in May 2023, and these stochastic water relations may have influenced decomposer activities or leached more nutrients from the litterbag contents. Rainfall pulses per se have been identified as consequential drivers of litter decomposition [48]. Moreover, incident light was less than 2% of sunlight and homogeneous throughout every photoperiod in the mesocosm study, but was 5% to 7% of sunlight and heterogeneous in the field study. The mesocosm litter was never exposed to sunflecks, but the field litter was impacted with bursts of full sun during midday sunflecks. Specific short wavelengths of the incident light, as occurs during sunflecks, have been identified as causal of the photodegradation influences on leaf litter decomposition [49,50,51].
These various drivers may impart a portion of their influence indirectly by modifying the soil microbial community function [52]. Recalcitrant litter components such as lignin and cellulose may deter the activity of the decomposer community, thereby altering the decomposer community. The role of the decomposer community identity on decomposition may be more important in the initial decomposition of fresh litters when the bacterial community dominates the processes, and become more muted in later stages of decomposition when the fungal decomposer community becomes more influential [9]. Decomposition during these two short-term litterbag studies may have been partly influenced by an inconsistent initial decomposer community. The mesocosm substrate was obtained from a native tree forest which had a decomposer community that had developed under mixed litters, and the field study was conducted under the canopy of the non-native L. leucocephala which produced a labile leaf litter [53,54,55,56]. This legume tree increases soil fertility partly through its beneficial influence on the soil microorganism community [57].
4.3. Litter Quality Drivers
Several of the initial litter quality metrics were correlated with the differences in litter mixture effects, but ∆N and ∆C:N emerged as the most prevalent drivers for every correlation. These two metrics were highly correlated with the results of 3- and 6-month incubations for both C. micronesica and S. nelsonii mixtures. The recalcitrant lignin fraction emerged as influential in defining the C. micronesica mixtures for the 3- to 6-month incubation period, but not the initial 3-month period. Lignin differences among these litter types may be important during the later stages of decomposition when C. micronesica litter is part of the mixture.
4.4. Future Directions
The loss of global biodiversity is raising alarm bells among many research priorities, and the influence on litter decomposition is among those priorities [12]. The forest ecological communities in the oceanic islands of Micronesia have largely been ignored in this research agenda. The ongoing loss of biodiversity is increasing threats to ecosystem function in numerous ways, and this study augments recent papers [18,19,20,22] that indicate species recovery efforts for C. micronesica and S. nelsonii are of paramount importance for maintaining ecosystem function and biogeochemical spatiotemporal diversity in Guam.
Design of successful research on litter decomposition in Guam and other Micronesian islands will require more context dependency in the protocols. The direct influence of the home field advantage [24,25,58] on litter decomposition was purposefully disallowed in both of these pioneer studies by design. This design assured a level substrate playing field among all of the single- and dual-species litterbags. The home field advantage of litters that fall on soil substrates that have historically been influenced by conspecific litters predicts a universal positive influence on litter mass loss speed. The extent of this advantage is modified by how dissimilar the chemical constituents of the fresh litter are from the bulk litter matrix [59]. More litterbag incubations using home and away microsites as the experimental substrates within in situ forests settings will be required to tease apart the many context-dependent issues in this C. micronesica and S. nelsonii research agenda.
Copious resources have been spent in recent years for C. micronesica and S. nelsonii conservation and recovery [60,61,62]. Most heavily funded projects have focused on expensive endeavors that do not address the primary threats and resulted in no adaptive management research that could have added horticultural and ecological knowledge. Therefore, the history of failed terrestrial resource conservation in Guam is partly a social science issue in which the policy- and decision-makers have suppressed science to rely on anecdotes and gray literature to inform decisions. This established culture has caused international species experts to be marginalized from having a seat at the table for C. micronesica and S. nelsonii conservation decisions [60,61]. The greatest need for continued research on the role of biodiversity and specifically threatened taxa on ecosystem function is a change in culture of the decision-makers who continue to invest millions of U.S. dollars of public funds into ineffective conservation programs. These ineffective conservation expenditures have led to a federal lawsuit [61].
Human activities continue to drive global change factors which collectively threaten biodiversity, demanding greater levels of coordination among various disciplines and requiring a change in direction [63]. Global climate change includes elevated carbon dioxide concentrations, and these atmospheric changes may decrease leaf litter mass loss through feedback loops [64]. Invasive species have emerged as another major driver of global change [65], and invasive insect herbivores comprise the primary threat to C. micronesica survival [62]. These invasive species may exert direct changes to litter decomposition dynamics [20], and this phenomenon deserves further study as the interplay between climate change and invasive species is more fully researched. Climate change and invasive species will continue to be major drivers that should be studied in linkages rather than in isolation as biodiversity management policy continues to evolve [66].
5. Conclusions
Cycas micronesica and Serianthes nelsonii leaf litters influenced the decomposition of dual species litter mixtures in mesocosm and field conditions on the island of Guam. The C. micronesica litter was collectively antagonistic, whereas the S. nelsonii litter was collectively synergistic. These two tree species are endangered and these findings enable a greater understanding of how losing them from the palette of native trees may influence the biogeochemistry of the forest communities. The contributions of these two threatened tree species are dissimilar and considerable. Species recovery efforts for these two species are of paramount importance for maintaining Mariana Island ecological integrity and native biodiversity by sustaining their contributions to ecosystem services
Funding
This research was funded in part by the United States Forest Service grant numbers 13-DG-11052021-210 and 17-DG-11052021-217 and United States Department of Agriculture NIFA grant number GUA0801.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
I thank Gil Cruz and Frankie Matanane for aid in field methods. I thank Nirmala Dongol for aid in tissue collation and preparation.
Conflicts of Interest
Author Marler represented the company Philippine Native Plants Conservation Society. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A

Table A1.
List of plant species selected to decompose in northern Guam. M = mesocosm study; F = field study.
Table A1.
List of plant species selected to decompose in northern Guam. M = mesocosm study; F = field study.
| Species | Family | Study | Notes |
|---|---|---|---|
| Artocarpus mariannensis Trécul | Moraceae | M,F | Eudicot tree |
| Bikkia tetrandra (L.f.) A. Rich. | Rubiaceae | M,F | Eudicot shrub |
| Cocos nucifera L. | Arecaceae | M,F | Monocot tree |
| Cycas micronesica K.D. Hill 1,2 | Cycadaceae | M,F | Gymnosperm tree |
| Ficus prolixa G. Forst. | Moraceae | F | Eudicot tree |
| Heritiera longipetiolata Kaneh. 2 | Malvaceae | M,F | Eudicot tree |
| Hibiscus tiliaceus L. | Malvaceae | M,F | Eudicot tree |
| Morinda citrifolia L. | Rubiaceae | M,F | Eudicot tree |
| Ochrosia mariannensis A.DC. | Apocynaceae | F | Eudicot tree |
| Pandanus tectorius Parkinson ex Du Roi | Pandanaceae | M,F | Monocot tree |
| Pisonia grandis R. Br. | Nyctaginaceae | M,F | Eudicot tree |
| Premna serratifolia L. | Lamiaceae | M,F | Eudicot tree |
| Serianthes nelsonii Merr. 1,2 | Fabaceae | M,F | Eudicot tree |
| Tabernaemontana rotensis (Kaneh.) P.T. Li 2 | Apocynaceae | M,F | Eudicot tree |
1 Associated with nitrogen-fixing root endosymbionts. 2 Listed under the United States Endangered Species Act.

Table A2.
The concentration of plant nutrients in mineral soils in a Guam mesocosm. Total carbon and nitrogen content was determined by dry combustion. Available phosphorus was determined by the Olsen method [67]. Other macro- and micro-nutrients were quantified following digestion with diethylenetriaminepentaacetic acid [68]. Analysis was performed using inductively coupled plasma optical emission spectrometry. Mean ± standard error, n = 4.
Table A2.
The concentration of plant nutrients in mineral soils in a Guam mesocosm. Total carbon and nitrogen content was determined by dry combustion. Available phosphorus was determined by the Olsen method [67]. Other macro- and micro-nutrients were quantified following digestion with diethylenetriaminepentaacetic acid [68]. Analysis was performed using inductively coupled plasma optical emission spectrometry. Mean ± standard error, n = 4.
| Variable | Soil Trait |
|---|---|
| pH | 7.82 ± 0.13 |
| Calcium | 7.91 ± 0.17 mg·g−1 |
| Carbon | 90.44 ± 5.62 mg·g−1 |
| Copper | 6.09 ± 0.61 µg·g−1 |
| Iron | 34.72 ± 2.14 µg·g−1 |
| Magnesium | 880.27 ± 31.29 µg·g−1 |
| Manganese | 64.19 ± 5.11 µg·g−1 |
| Nitrogen | 8.05 ± 0.72 mg·g−1 |
| Phosphorus | 80.22 ± 7.27 µg·g−1 |
| Potassium | 577.64 ± 19.54 µg·g−1 |
| Zinc | 17.17 ± 3.29 µg·g−1 |

Table A3.
The concentration of plant nutrients in mineral soils in a Guam field study. Chemical analyses were as described in Table A2. Mean ± standard error, n = 4.
Table A3.
The concentration of plant nutrients in mineral soils in a Guam field study. Chemical analyses were as described in Table A2. Mean ± standard error, n = 4.
| Variable | Soil Trait |
|---|---|
| pH | 7.85 ± 0.17 |
| Calcium | 7.88 ± 0.15 mg·g−1 |
| Carbon | 91.44 ± 4.98 mg·g−1 |
| Copper | 5.39 ± 0.55 µg·g−1 |
| Iron | 32.75 ± 2.10 µg·g−1 |
| Magnesium | 870.22 ± 29.22 µg·g−1 |
| Manganese | 68.12 ± 5.15 µg·g−1 |
| Nitrogen | 8.92 ± 0.78 mg·g−1 |
| Phosphorus | 77.82 ± 6.32 µg·g−1 |
| Potassium | 562.52 ± 17.55 µg·g−1 |
| Zinc | 18.38 ± 2.97 µg·g−1 |
References
- Liu, Q.; Li, J.; Ye, S.; Guo, Y.; Wang, S. Characteristics and hotspots of forest litter decomposition research: A bibliometric analysis. Land Degrad. Dev. 2024, 35, 2684–2699. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Decomposition as a Process—Some Main Features. In Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2020; pp. 13–43. [Google Scholar]
- Zhang, P.; Fornara, D.; Yang, H.; Yu, R.-P.; Callaway, R.M.; Li, L. Plant litter strengthens positive biodiversity–ecosystem functioning relationships over time. Trends Ecol. Evol. 2023, 38, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- Pankaj; Bhardwaj, K.K.; Yadav, R.; Goyal, V.; Sharma, M.K. Pattern of litterfall production and nutrient addition in soil through litterfall by different tree species: A review. Environ. Conserv. J. 2023, 25, 257–266. [Google Scholar] [CrossRef]
- Mukamparirwa, V.; Maliondo, S.M.S.; Mugunga, C.P. Synergistic and antagonistic effects of mixed-leaf litter decomposition on nutrient cycling. Plants 2024, 13, 3204. [Google Scholar] [CrossRef]
- Chapman, S.K.; Newman, G.S.; Hart, S.C.; Schweitzer, J.A.; Koch, G.W. Leaf litter mixtures alter microbial community development: Mechanisms for non-Additive effects in litter decomposition. PLoS ONE 2013, 8, e62671. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Song, Q.; Compson, Z.G.; LeRoy, C.J.; Luan, F.; Wang, H.; Hu, Y.; Yang, Q. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef]
- Porre, R.J.; VanderWerf, W.; DeDeyn, G.B.; Stomph, T.J.; Hoffland, E. Is litter decomposition enhanced in species mixtures? A meta-analysis. Soil Biol. Biochem. 2020, 145, 107791. [Google Scholar] [CrossRef]
- Handa, I.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; Noon, B.R.; Masino, S.A.; Noss, R.F. Coordinating old-growth conservation across scales of space, time, and biodiversity: Lessons from the US policy debate. Front. For. Glob. Chang. 2025, 8, 1493879. [Google Scholar] [CrossRef]
- Bösenberg, J.D. Cycas micronesica. The IUCN Red List of Threatened Species 2022: e.T61316A68906033. Available online: https://www.iucnredlist.org/species/61316/68906033 (accessed on 16 June 2025).
- United States Fish & Wildlife Service. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Fed. Regist. 2015, 80, 59424–59497. [Google Scholar]
- Wiles, G.; Williams, E. Serianthes nelsonii. The IUCN Red List of Threatened Species 2017: e.T30437A98715973. Available online: https://www.iucnredlist.org/species/30437/98715973 (accessed on 16 June 2025).
- United States Fish and Wildlife Service. Determination of endangered status for Serianthes nelsonii Merr. (Hayun lagu or Tronkon guafi). Fed. Regist. 1987, 52, 4907–4910. [Google Scholar]
- Marler, T.E. Leaf elemental concentrations, stoichiometry, and resorption in Guam’s coastal karst forests. Diversity 2021, 13, 545. [Google Scholar] [CrossRef]
- Marler, T.E. Perennial trees associating with nitrogen-fixing symbionts differ in leaf after-life nitrogen and carbon release. Nitrogen 2020, 1, 111–124. [Google Scholar] [CrossRef]
- Marler, T.E. Aulacaspis yasumatsui infestations accelerate Cycas leaf litter decomposition and nutrient release. Pedosphere 2024, 34, 681–684. [Google Scholar] [CrossRef]
- Odum, E.P. The Mesocosm. BioScience 1984, 34, 558–562. [Google Scholar] [CrossRef]
- Marler, T.E.; Cruz, G.N. Temporal variation of litterfall and nutrient return of Serianthes nelsonii Merr. in a tropical karst forest. Plants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Bärlocher, F.; Gessner, M.O.; Graça, M.A.S. (Eds.) Methods to Study Litter Decomposition; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Shigyo, N.; Umeki, K.; Hirao, T. Soil microbial identity explains home-field advantage for litter decomposition. New Phytol. 2024, 243, 2146–2156. [Google Scholar] [CrossRef]
- Daumal, M.M.; Oguro, M.; Ueda, M.U.; Takayanagi, S.; Nakashizuka, T.; Kurokawa, H. What makes decomposition faster under conspecific trees? The factors controlling the magnitude of home-field advantage. Oikos 2024, 2024, e10532. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U.S. Department of Agriculture, Soil Conservation Service: Washington, DC, USA, 1988.
- Liu, Y.; Li, L.; Wang, S.; Li, X. Precipitation modulates the net effect of solar radiation on litter decomposition and CO2 emission- a meta-analysis. Front. Plant Sci. 2023, 14, 1200155. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Coconut leaf age and coconut rhinoceros beetle herbivory influence leaflet nutrients, metals, and lignin. Horticulturae 2018, 4, 9. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 10 June 2025).
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Li, Y.N.; Zhou, X.M.; Zhang, N.L.; Ma, K.P. The research of mixed litter effects on litter decomposition in terrestrial ecosystems. Acta Ecol. Sin. 2016, 36, 4977–4987. [Google Scholar]
- Kou, L.; Jiang, L.; Hättenschwiler, S.; Zhang, M.; Niu, S.; Fu, X.; Dai, X.; Yan, H.; Li, S.; Wang, H. Diversity-decomposition relationships in forests worldwide. eLife 2020, 9, e55813. [Google Scholar] [CrossRef]
- Mori, A.S.; Cornelissen, J.H.C.; Fujii, S.; Okada, K.-I.; Isbell, F. A meta-analysis on decomposition quantifies afterlife effects of plant diversity as a global change driver. Nat. Commun. 2020, 11, 4547. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.J.; Cavender-Bares, J.; Hobbie, S.E. Functional diversity of leaf litter mixtures slows decomposition of labile but not recalcitrant carbon over two years. Ecol. Monogr. 2020, 90, e01407. [Google Scholar] [CrossRef]
- Zhou, G.; Wan, J.; Gu, Z.; Ding, W.; Hu, S.; Du, Q.; Meng, S.; Yang, C. Functional diversity accelerates the decomposition of litter recalcitrant carbon but reduces the decomposition of labile carbon in subtropical forests. Forests 2023, 14, 2258. [Google Scholar] [CrossRef]
- Versini, A.; Laclau, J.P.; Mareschal, L.; Plassard, C.; Diamesso, L.A.; Ranger, J.; Zeller, B. Nitrogen dynamics within and between decomposing leaves, bark and branches in Eucalyptus planted forests. Soil Biol. Biochem. 2016, 101, 55–64. [Google Scholar] [CrossRef]
- He, W.; Ma, Z.; Pei, J.; Teng, M.; Zeng, L.; Yan, Z.; Huang, Z.; Zhou, Z.; Wang, P.; Luo, X.; et al. Effects of predominant tree species mixing on lignin and cellulose degradation during leaf litter decomposition in the Three Gorges Reservoir, China. Forests 2019, 10, 360. [Google Scholar] [CrossRef]
- Gao, J.; Kang, F.; Han, H. Effect of litter quality on leaf-litter decomposition in the context of home-field advantage and non-additive effects in temperate forests in China. Polish J. Environ. Stud. 2016, 25, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- United States Fish and Wildlife Service. Recovery Plan for Serianthes nelsonii; USFWS: Portland, OR, USA, 1994.
- Mcclaugherty, C.A.; Pastor, J.; Aber, J.D.; Melillo, J.M. Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 1985, 66, 266–275. [Google Scholar] [CrossRef]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Jasińska, J.; Sewerniak, P.; Markiewicz, M. Links between slope aspect and rate of litter decomposition on inland dunes. Catena 2019, 172, 501–508. [Google Scholar] [CrossRef]
- Esquivel, J.; Park, B.B.; Casanoves, F.; Delgado, D.; Park, G.E.; Finegan, B. Altitude and species identity drive leaf litter decomposition rates of ten species on a 2950 m altitudinal gradient in Neotropical rain forests. Biotropica 2020, 52, 11–21. [Google Scholar] [CrossRef]
- Park, B.B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Youn, W.B.; Choi, H.S.; Jung, S. Leaf litter decomposition of deciduous Quercus acutissima Carruth. and evergreen Quercus glauca Thunb. in an inter-site experiment in three contrasting temperate forest stands in South Korea. Ann. For. Sci. 2021, 78, 34. [Google Scholar] [CrossRef]
- Wang, Q.W.; Pieristè, M.; Liu, C.; Kenta, T.; Robson, T.M.; Kurokawa, H. The contribution of photodegradation to litter decomposition in a temperate forest gap and understorey. New Phytol. 2021, 229, 2625–2636. [Google Scholar] [CrossRef]
- Hussain, M.B.; Al-Hadidi, S.H.; Erfanian, M.B.; Yahia, M.N.D.; Mullungal, M.N.; Alsafran, M.; Bai, Y.; Alatalo, J.M. Photodegradation and its effect on plant litter decomposition in terrestrial ecosystems: A systematic review. Soil Syst. 2023, 7, 6. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballaré, C.L. Photodegradation in terrestrial ecosystems. New Phytol. 2024, 244, 769–785. [Google Scholar] [CrossRef]
- Anaya, C.A.; Jaramillo, V.J.; Martinez-Yrizar, A.; Garcia-Oliva, F. Large rainfall pulses control litter decomposition in a tropical dry forest: Evidence from an 8-year study. Ecosystems 2012, 15, 652–663. [Google Scholar] [CrossRef]
- Wang, Q.W.; Pieristè, M.; Kotilainen, T.K.; Forey, E.; Chauvat, M.; Kurokawa, H.; Robson, T.M.; Jones, A.G. The crucial role of blue light as a driver of litter photodegradation in terrestrial ecosystems. Plant Soil 2023, 488, 23–38. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, L.; Dai, Y.; Chen, S.; Li, C.; Yuan, T.; Ma, X.; Liu, A. Effects of enhanced UV-B radiation on decomposition and nutrient release rates of litter from Cunninghamia lanceolata (Lamb.) Hook. Forests 2024, 15, 686. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Zhang, B.; Li, L.; Lin, L.; Li, X.; Zeng, Q. The effect of litter decomposition mostly depends on seasonal variation of ultraviolet radiation rather than species in a hyper-arid desert. Front. Environ. Sci. 2024, 12, 1379442. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, in press. [CrossRef]
- Budelman, A. The decomposition of the leaf mulches of Leucaena leucocephala, Gliricidia sepium and Flemingia macrophylla under humid tropical conditions. Agrofor. Syst. 1988, 7, 33–45. [Google Scholar] [CrossRef]
- Sandhu, J.; Sinha, M.; Ambasht, R.S. Nitrogen release from decomposing litter of Leucaena leucocephala in the dry tropics. Soil Biol. Biochem. 1990, 22, 859–863. [Google Scholar] [CrossRef]
- Jama, B.A.; Nair, P.K. Decomposition and nitrogen-mineralization patterns of Leucaena leucocephala and Cassia siamea mulch under tropical semiarid conditions in Kenya. Plant Soil 1996, 179, 275–285. [Google Scholar] [CrossRef]
- Casanova-Lugo, F.; Cetzal-Ix, W.R.; Escobedo-Cabrera, A.; Estrada-Medina, H.; Aryal, D.R.; Villanueva-López, G. Decomposition and nutrient release of leaves of tree legumes with agroforestry potential in the sub-humid tropic. Agrofor. Syst. 2024, 98, 3165–3177. [Google Scholar] [CrossRef]
- Gulati, S.; Kaur, J. Potential of decomposing leaf litter of Leucaena leucocephala in influencing mycoflora of the soil and its role in increasing soil fertility. Vegetos 2024, 37, 1271–1287. [Google Scholar] [CrossRef]
- Podzikowski, L.Y.; Duell, E.B.; Burrill, H.M.; Bever, J.D. Home- field advantage, N- priming and precipitation independently govern litter decomposition in a plant diversity manipulation. Funct. Ecol. 2024, 38, 820–831. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. Multiple mechanisms for trait effects on litter decomposition: Moving beyond home-field advantage with a new hypothesis. J. Ecol. 2012, 100, 619–630. [Google Scholar] [CrossRef]
- Marler, T.E.; Musser, C.; Cascasan, A.N.J.; Cruz, G.N.; Deloso, B.E. Adaptive management lessons for Serianthes nelsonii conservation. Horticulturae 2021, 7, 43. [Google Scholar] [CrossRef]
- Center for Biological Diversity; Center for Biological Diversity and Prutehi Litekyan versus United States Department of Defense; Carlos del Toro; United States Fish and Wildlife Service; Haaland, D. CIV 23-00019. Complaint for Declaratory and Injunctive Relief Under the Endangered Species Act, Administrative Procedure Act, and Freedom of Information Act. United States District Court of Guam. 2023. Available online: https://www.biologicaldiversity.org/programs/biodiversity/pdfs/Camp-Blaz-Complaint.pdf (accessed on 16 June 2025).
- Marler, T.E.; Lindström, A.J.; Terry, L.I.; Deloso, B.E. Decades of IUCN recommendations for biocontrol of invasive pest on the Guam cycad: You can lead policy-makers to conservation proposals but you cannot make them follow. J. Threat. Taxa 2024, 16, 26150–26162. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondizio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, Y.; Ai, L.; Wu, F.; Wu, Q.; Zhang, X.; Zhu, J.; Ni, X. Declines in carbon and nitrogen release from decomposing litter under elevated CO2 in terrestrial ecosystems. J. Plant Ecol. 2025, 18, rtaf002. [Google Scholar] [CrossRef]
- Mainka, S.A.; Howard, G.W. Climate change and invasive species: Double jeopardy. Integr. Zool. 2010, 5, 102–111. [Google Scholar] [CrossRef]
- Colberg, E.; Bradley, B.; Morelli, T.; Brown-Lima, C. Climate-smart invasive species management for 21st century global change challenges. Glob. Chang. Biol. 2024, 30, e17531. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954.
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media extractant, identification and evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).