Some Ecological Characteristics of a Neophyte of the Canary Islands: Pluchea ovalis (Asteraceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Species
2.2. Study Area
2.3. Germination Chamber Tests
2.4. Greenhouse Germination Experiment and Root Functional Trait Analysis
2.5. Field Study: Effect of the Presence of Pluchea ovalis on Native Plant Communities and Physicochemical Soil Properties
2.6. Statistical Analysis
3. Results
3.1. Effect of Light and Temperature on Germination Rate
3.2. Effect of Environmental Conditions on Germination and Plant Development
3.3. Effect of Pluchea ovalis on Soil Properties and Native Plant Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, R.; Simberloff, D.; Lonsdale, M.W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Jaksic, F.M.; Castro, S.A. Biological invasions in the Anthropocene. In Biological Invasions in the South American Anthropocene: Global Causes and Local Impacts; Springer Nature: Cham, Switzerland, 2021; pp. 19–47. [Google Scholar]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar]
- Baker, H.G. Patterns of plant invasion in North America. In Ecology of Biological Invasions of North America and Hawaii; Springer: New York, NY, USA, 1986; pp. 44–57. [Google Scholar]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The Population Biology of Invasive Species. Annu. Rev. Ecol. Evo. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Ruiz, G.M.; Rawlings, T.K.; Dobbs, F.C.; Drake, L.A.; Mullady, T.; Huq, A.; Colwell, R.R. Global spread of microorganisms by ships. Nature 2000, 408, 49–50. [Google Scholar] [PubMed]
- Kier, G.; Kreft, H.; Ming-Lee, T.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Biol. Sci. 2009, 106, 9322–9327. [Google Scholar] [CrossRef] [PubMed]
- Pfadenhauer, W.G.; Bradley, B.A. Quantifying vulnerability to plant invasion across global ecosystems. Ecol. Appl. 2024, 34, e3031. [Google Scholar]
- Moser, D.; Lenzner, B.; Weigelt, P.; Dawson, W.; Kreft, H.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Winter, M.; Capinha, C.; et al. Remoteness promotes biological invasions on islands worldwide. Biol. Sci. 2018, 115, 9270–9275. [Google Scholar]
- Blackburn, T.M.; Bellard, C.; Ricciardi, A. Alien versus native species as drivers of recent extinctions. Front. Ecol. Environ. 2019, 17, 203–207. [Google Scholar]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar]
- De Nascimento, L.; Willis, K.J.; Fernández-Palacios, J.M.; Criado, C.; Whittaker, R.J. The long–term ecology of the lost forests of La Laguna, Tenerife (Canary Islands). J. Biogeogr. 2009, 36, 499–514. [Google Scholar]
- De Nascimento, L.; Nogué, S.; Naranjo-Cigala, A.; Criado, C.; McGlone, M.; Fernández-Palacios, E.; Fernández-Palacios, J.M. Human impact and ecological changes during prehistoric settlement on the Canary Islands. Quat. Sci. Rev. 2020, 239, 106332. [Google Scholar] [CrossRef]
- Banco de Datos de Biodiversidad de Canarias (BIOTA). Available online: https://www.biodiversidadcanarias.es/biota/ (accessed on 11 October 2024).
- Red Canaria de Alerta Temprana de Especies Exóticas Invasoras (REDEXOS). Available online: https://www3.gobiernodecanarias.org/cptss/sostenibilidad/biodiversidad/redexos/ (accessed on 24 October 2024).
- Rodríguez-Navarro, M.L.; Fariña-Trujillo, B.; Rivero-Quintero, A. La expansion de las especies de Pluchea Cass. (Asteraceae) y su potencial comportamiento invasor en Tenerife (Islas Canarias). Rev. Sci. Insul. 2021, 4, 11–25. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystems function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Q.; Wang, S.; Yang, G.; Xue, S. A global meta-analysis of the impacts of exotic plant species invasion on plant diversity and soil properties. Sci. Total Environ. 2022, 810, 152286. [Google Scholar] [CrossRef]
- Slesak, R.A.; Harrington, T.B.; D’Amato, A.W.; Peter, D.H. Removal of invasive scoth broom increases its negative effects on soil chemistry and plant communities. Oecologia 2022, 198, 243–254. [Google Scholar] [CrossRef]
- Zarafshar, M.; Bazot, S.; Matinizadeh, M.; Bordbar, S.K.; Rousta, M.J.; Kooch, Y.; Enayati, K.; Abbasi, A.; Negahdarsaber, M. Do tree plantations or cultivated fields have the same ability to maintain soil quality as natural forests? Appl. Soil Ecol. 2020, 151, 103536. [Google Scholar] [CrossRef]
- Stanek, M.; Piechnik, L.; Stefanowicz, A.M. Invasive red oak (Quercus rubra L.) modifies soil physicochemical properties and forest understory vegetation. For. Ecol. Manag. 2020, 472, 118253. [Google Scholar] [CrossRef]
- Wen, B. Effects of high temperature and water stress on seed germination of the invasive species Mexican sunflower. PLoS ONE 2015, 10, e0141567. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Udo, N.; Tarayre, M.; Atlan, A. Evolution of germination strategy in the invasive species Ulex europaeus. J. Plant Ecol. 2016, 10, 375–385. [Google Scholar]
- Hahn, M.A.; Lanz, T.; Fasel, D.; Müller-Schärer, H. Increased seed survival and seedling emergence in a polyploidy plant invader. Am. J. Bot. 2013, 100, 1555–1561. [Google Scholar] [CrossRef]
- Henery, M.L.; Bowman, G.; Mráz, P.; Treier, U.A.; Gex-Fabry, E.; Schaffner, U.; Müller-Schärer, H. Evidence for a combination of pre-adapted traits and rapid adaptive change in the invasive plant Centaurea stoebe. J. Ecol. 2010, 98, 800–813. [Google Scholar] [CrossRef]
- Hierro, J.L.; Eren, Ö.; Khetsuriani, L.; Diaconu, A.; Török, K.; Montesinos, D.; Andonian, K.; Kikodze, D.; Janoian, L.; Estanga-Mollica, M.E.; et al. Germination responses of an invasive species in native and non-native ranges. Oikos 2009, 118, 529–538. [Google Scholar]
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org/ (accessed on 24 October 2024).
- Sauerbier, H.; Cabrera-Calixto, F.; Muer, T. Flora Vascular de Canarias, 1st. ed.; Turquesa Ediciones: San Cristóbal de La Laguna, Spain, 2023; p. 1192. [Google Scholar]
- King-Jones, S. Revision of Pluchea Cass (Compositae, Plucheae) in the Old World. Englera 2001, 23, 3–136. [Google Scholar]
- Padrón-Mederos, M.A.; Reyes-Betancort, J.A. Apuntes florísticos y taxonómicos para la flora de las Islas Canarias. Acta Botánica Malacit. 2009, 34, 242–251. [Google Scholar]
- Verloove, F.; Reyes-Betancort, J.A. Additions to the flora of Tenerife (Canary Islands, Spain). Collect. Bot. 2011, 30, 63–78. [Google Scholar]
- Barone-Tosco, R.; Hernández-Romero, F. La planta Pluchea ovalis invade el sur de Tenerife. Quercus 2014, 399, 42–43. [Google Scholar]
- Martín-Osorio, V.E.; Wildpret, W. Pluchea dioscoridis (L.) DC (Asteraceae) new introduced and naturalized species in Tenerife, Canary Islands. Vieraea 2014, 42, 323–326. [Google Scholar]
- Verloove, F. New xenophytes from Gran Canaria (Canary Islands, Spain), with emphasis on naturalized and (potentially) invasive species. Coll. Bot. 2013, 33, 59–82. [Google Scholar]
- Verloove, F. New xenophytes from the Canary Islands (Gran Canaria and Tenerife; Spain). Acta Bot. Croat. 2010, 76, 120–131. [Google Scholar]
- Del Arco-Aguilar, M.J.; González-González, R.; Garzón-Machado, V.; Pizarro-Hernández, B. Actual and potential natural vegetation on the Canary Islands and its conservation status. Biodivers. Conserv. 2010, 19, 3089–3140. [Google Scholar]
- Del Arco-Aguilar, M.J.; Wildpret-de la Torre, W.; Pérez-de Paz, P.L.; Rodríguez-Delgado, O.; Acebes-Ginovés, J.R.; García-Gallo, A.; Martín-Osorio, V.E.; Reyes-Betancort, A.; Salas-Pascual, M.; Díaz, M.A.; et al. Mapa de Vegetación de Canarias, 1st ed.; Grafcan Ediciones: Santa Cruz de Tenerife, Spain, 2006; p. 294. [Google Scholar]
- Del Arco-Aguilar, M.J.; Rodríguez-Delgado, O. Vegetation of the Canary Islands, 1st ed.; Springer: Cham, Switzerland, 2018; pp. 92–293. [Google Scholar]
- Agencia Estatal de Meteorología (AEMET). Available online: https://www.aemet.es/es/portada (accessed on 7 December 2024).
- Côme, D. Obstacles to germination. Obs. Germ. 1970, 6, 162. [Google Scholar]
- Aravind, J.; Vimala, D.; Radharani, J.; Jacob, S.R.; Srinivasa, K. The Germinationmetrics Package: A Brief Introduction; ICAR-National Bureau of Plant Genetic Resources: New Delhi, India, 2019. [Google Scholar]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of data analysis for seed germination. Crop Sci. 1984, 24, 1192–1199. [Google Scholar]
- Orchard, T. Estimating the parameters of plant seedling emergence. Seed Sci. Technol. 1977, 5, 61–69. [Google Scholar]
- Benech Arnold, R.; Fenner, M.; Edwards, P. Changes in germinability, ABA content and ABA embryonic sensitivity in developing seeds of Sorghum bicolor (L.) Moench. induced by water stress during grain filling. New Phytol. 1991, 118, 339–347. [Google Scholar] [PubMed]
- Al-Mudaris, M. Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt-J. Agric. Trop. Subtrop. 1998, 99, 147–154. [Google Scholar]
- Goloff, A.A., Jr.; Bazzaz, F.A. A germination model for natural seed populations. J. Theor. Biol. 1975, 52, 259–283. [Google Scholar]
- Seethepalli, A.; York, L.M. RhizoVision Explorer—Interactive software for generalized root image analysis designed for everyone (2.0.3). Zenodo 2020. [Google Scholar] [CrossRef]
- Ryser, P. The importance of tissue density for growth and life span of leaves and roots: A comparisson of five ecologically contrasting grasses. Funct. Ecol. 1996, 10, 717–723. [Google Scholar]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2020, 232, 1123–1158. [Google Scholar]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Anon. Métodos Oficiales de Análisis, 1st ed.; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1986. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemist, 15th ed.; The Association: Arlington, TX, USA, 1990. [Google Scholar]
- Fernández-Lugo, S.; de Nascimento, L.; Mellado, M.; Bermejo, L.A.; Arévalo, J.R. Vegetation change and chemical soil composition after 4 years of goat grazing exclusion in a Canary Islands pasture. Agric. Ecosyst. Environ. 2009, 132, 276–282. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-69440-8. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 1st ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Palmer, M.W. Ordination Methods-an Overview; Oklahoma State University: Stillwater, OK, USA, 2004. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Luo, J.; Cardina, J. Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Res. 2010, 52, 112–121. [Google Scholar] [CrossRef]
- Westman, W.E.; Panetta, F.D.; Stanely, T.D. Ecological studies on reproduction and establishment of the woody weed, groundsel bush (Baccharis halimifolia L.: Asteraceae). Aust. J. Agr. Res. 1975, 26, 855–870. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Montejo, L.; Gamboa, A.; Albert-Puentes, D.; Hernández, F. Germinación y dormancia de arbustos y trepadoras del bosque siempreverde de la Sierra del Rosario, Cuba. Pastos Forrajes 2015, 38, 11–28. [Google Scholar]
- Leger, E.A.; Rice, K.J. Assessing the speed and predictability of local adaptation in invasive California poppies (Eschscholzia californica). J. Evol. Biol. 2007, 20, 1090–1103. [Google Scholar] [CrossRef]
- Beckmann, M.; Bruelheide, H.; Erfmeier, A. Germination responses of three grassland species differ between native and invasive origins. Ecol. Res. 2011, 26, 763–771. [Google Scholar] [CrossRef]
- Kudoh, H.; Nakayama, M.; Lihová, J.; Marhold, K. Does invasion involve alternation of germination requirements? A comparative study between native and introduced strains of an annual Brassicaceae, Cardamine hirsuta. Ecol. Res. 2007, 22, 869–875. [Google Scholar] [CrossRef]
- Kurt, S.; Erdağ, B. In vitro germination and axillary shoot propagation of Centaurea zeybekii. Biologia 2009, 64, 97–101. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Di Gristina, E.; Barone, G.; Sottlie, F.; Domina, G. Seed germination and vegetative and in vitro propagation of Hieracium lucidum sbsp. lucidum (Asteraceae), a critically endangered endemic taxon of the sicilian flora. PeerJ 2024, 12, e16839. [Google Scholar]
- Falkner, M.B.; Laven, R.D.; Aplet, G.H. Experiments on germination and early growth of three rare and endemic species of Hawaiian Tetramolopium (Asteraceae). Biol. Cons. 1997, 80, 39–47. [Google Scholar]
- Rodrigues de Queiroz, A.; Maricle, B.R. Effects of light levels on germination of five Asteraceae species native to the tallgrass prairie. Trans. Kans. Acad. Sci. 2019, 122, 267–273. [Google Scholar]
- Shahda, R.H.; El-Bana, M.; El-Bous, M.M.; Zaghloul, M.S. Variations in achene traits, germination, and seedling growth of Pluchea dioscoridis (L.) DC. as affected by maternal climatic aridity. Egypt. J. Bot. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Inderjit. Influence of Pluchea lanceolata (Asteraceae) on selected soil properties. Am. J. Bot. 1998, 85, 64–69. [Google Scholar]
- Koné, A.W.; Edoukou, E.F.; Gonnety, J.T.; N’Dri, A.N.A.; Assémien, L.F.E.; Angui, P.K.T.; Tondoh, J.E. Can the shrub Chromolaena odorata (Asteraceae) be considered as improving soil biology and plant nutrient availability? Agrofor. Syst. 2012, 85, 233–245. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O.; Ogeh, J.S. Response of soil properties and yam yield to Chromolaena odorata (Asteraceae) and Tithonia diversifolia (Asteraceae) mulches. Arch. Agron. Soil Sci. 2014, 60, 209–224. [Google Scholar]
- Inderjit; Dakshini, K.M.M. Allelopathic potential of the phenolics from the roots of Pluchea lanceolata. Physiol. Plant. 1994, 92, 571–576. [Google Scholar]
- Fahmy, G.M.; Al-Sawaf, N.A.; Turki, H.; Ali, H.I. Allelopathic potential of Pluchea dioscoridis (L.) DC. J. Appl. Sci. Res. 2012, 8, 3129–3142. [Google Scholar]
- Youssef, N.; Diatta, J. Potential efficiency of wild plant species (Pluchea dioscoridis (L.) DC.) for phytoremediation of trace elements on contaminated locations. Sustainability 2023, 15, 119. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Mac-Nally, R. Invasional meltdown: Invader—Invader mutualism facilitates a secondary invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [PubMed]
- Real Decreto 630/2013, de 2 de Agosto, por el que se regula el Catálogo español de especies exóticas invasoras. Available online: https://www.boe.es/eli/es/rd/2013/08/02/630/con (accessed on 27 November 2024).

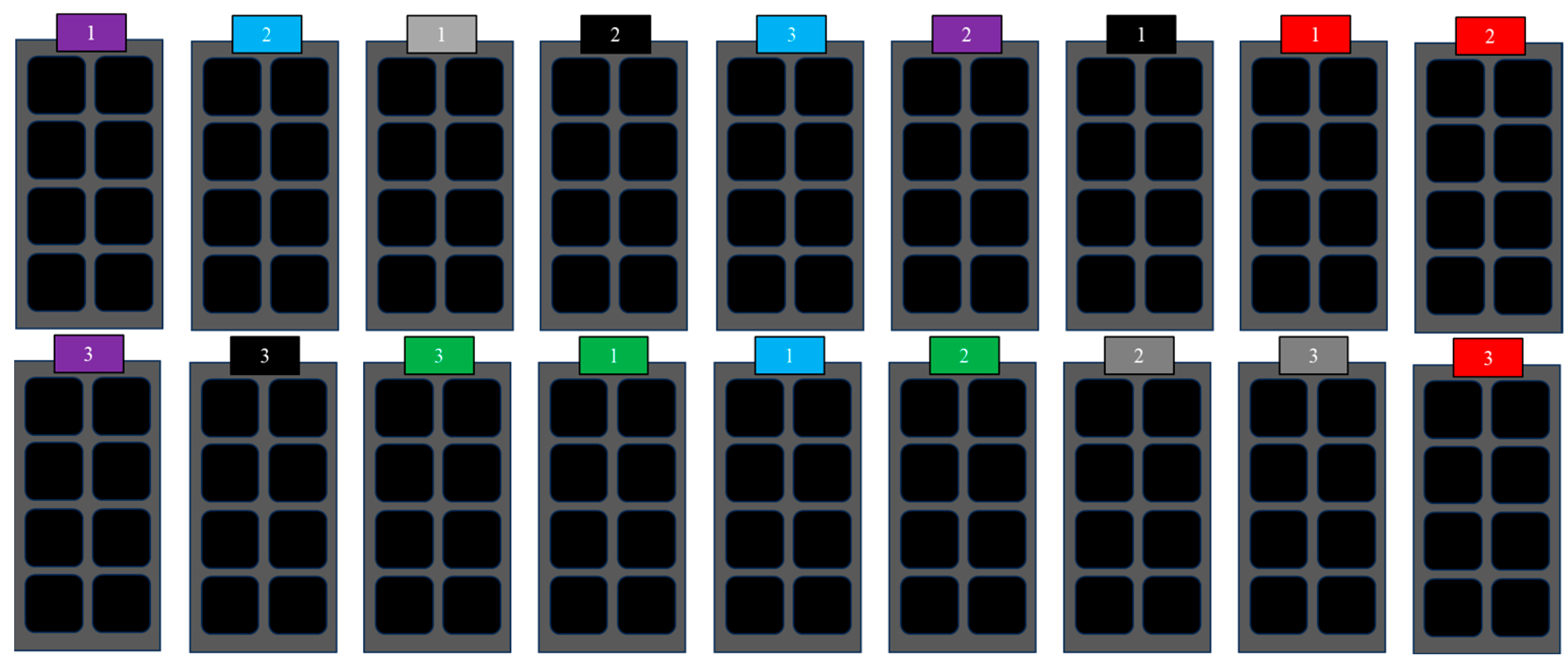
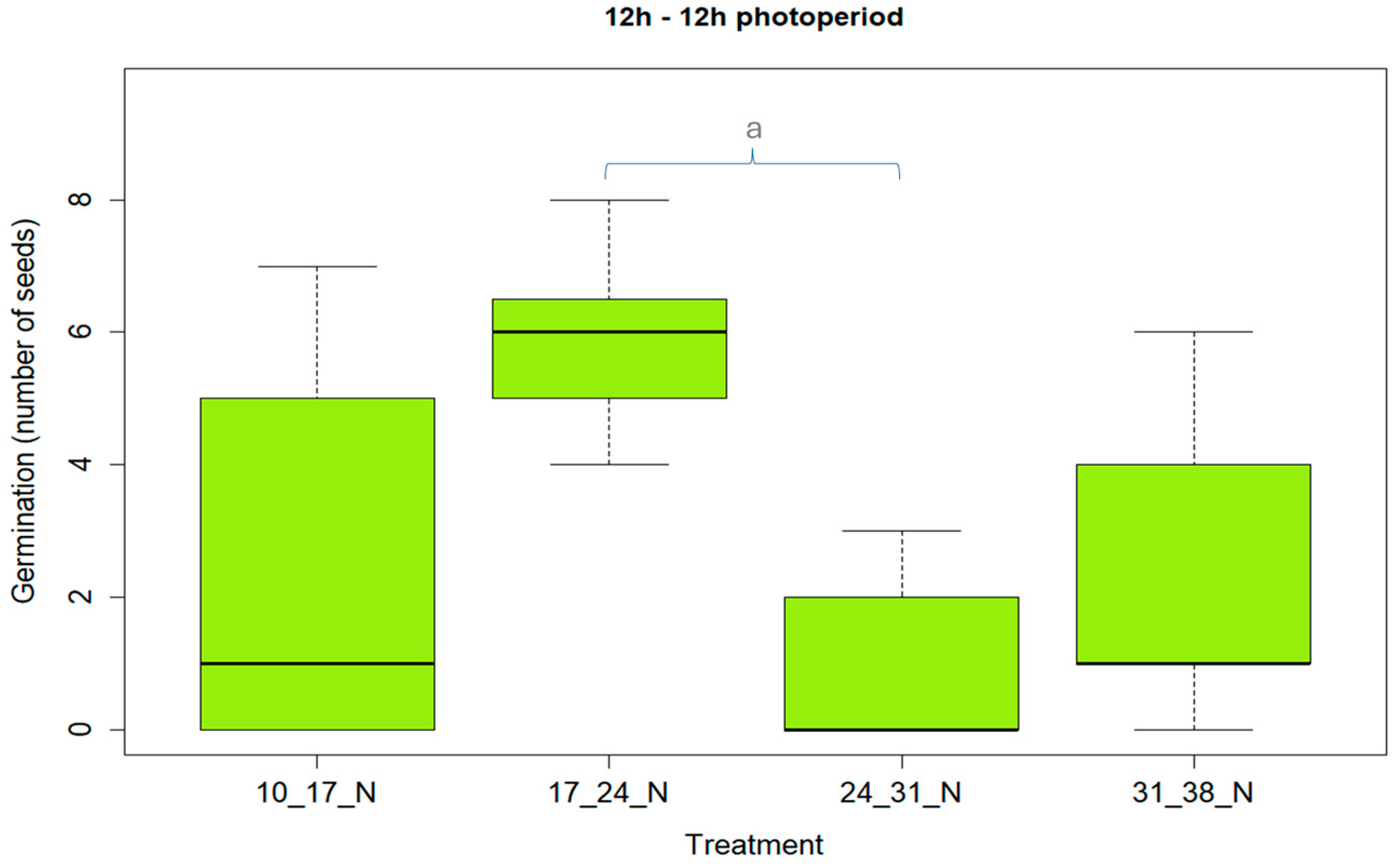

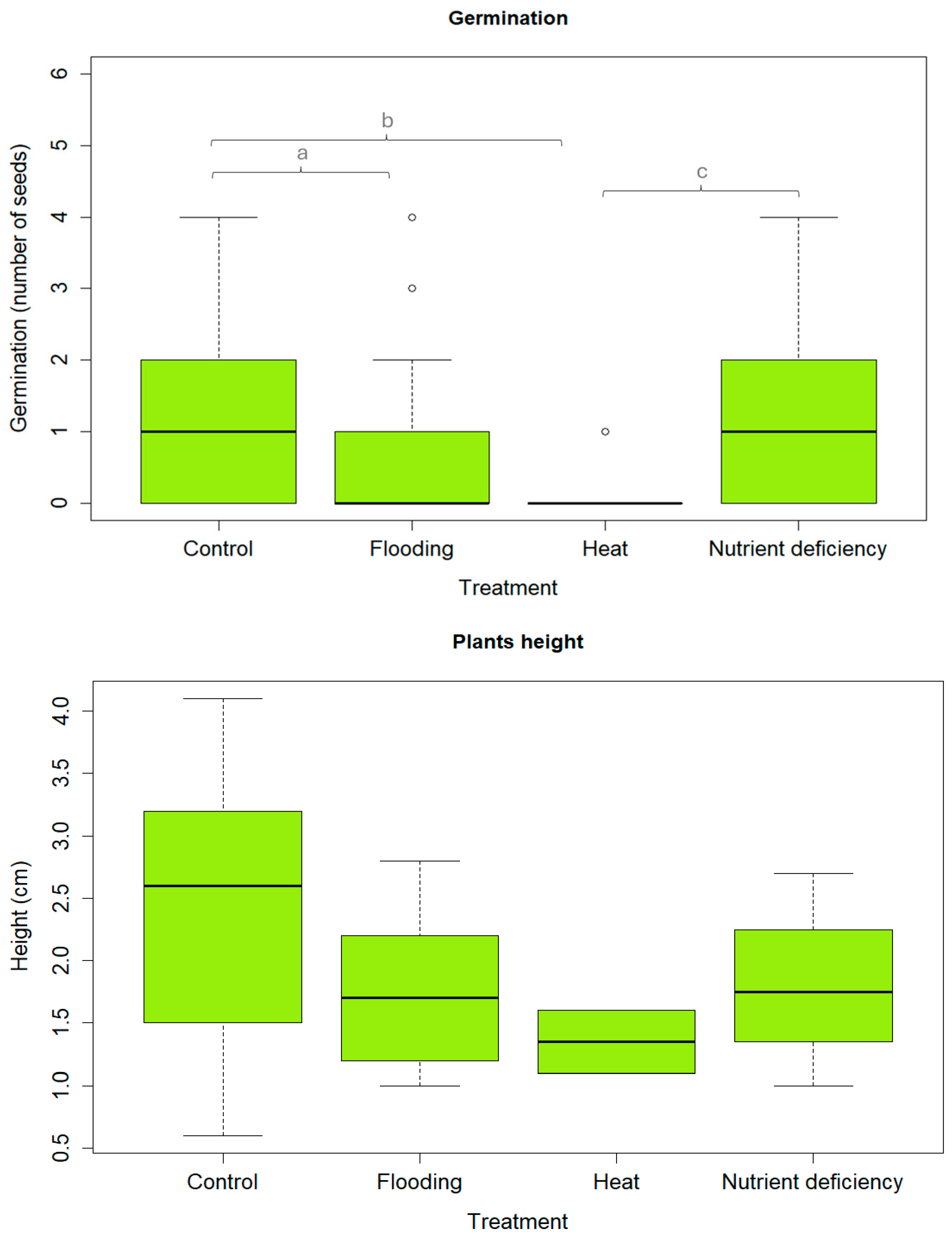

| Treatment | FGP (%) | MGT (Days) | GI | TSG (Days) | FGT (Days) |
|---|---|---|---|---|---|
| 10–17 °C N | 24 | 8 ± 6.48 | 2.88 | 14 | 5 |
| 10–17 °C D | 12 | 14.33 ± 6.57 | 0.68 | 5 | 13 |
| 17–24 °C N | 58.8 | 4.11 ± 8.78 | 9.34 | 1 | 4 |
| 17–24 °C D | 22.5 | 8.89 ± 6.46 | 2.5 | 12 | 4 |
| 24–31 °C N | 8.89 | 3.63 ± 6.61 | 1.46 | 3 | 2 |
| 24–31 °C D | 3.33 | 8.67 ± 6.68 | 0.38 | 5 | 7 |
| 31–38 °C N | 24.44 | 3.68 ± 6.54 | 3.98 | 4 | 2 |
| 31–38 °C D | 14.44 | 12.15 ± 6.52 | 1.13 | 11 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestano, M.; Suárez, I.; González-Montelongo, C.; Sierra Cornejo, N.; Arévalo, J.R. Some Ecological Characteristics of a Neophyte of the Canary Islands: Pluchea ovalis (Asteraceae). Ecologies 2025, 6, 28. https://doi.org/10.3390/ecologies6020028
Pestano M, Suárez I, González-Montelongo C, Sierra Cornejo N, Arévalo JR. Some Ecological Characteristics of a Neophyte of the Canary Islands: Pluchea ovalis (Asteraceae). Ecologies. 2025; 6(2):28. https://doi.org/10.3390/ecologies6020028
Chicago/Turabian StylePestano, Miguel, Isabel Suárez, Cristina González-Montelongo, Natalia Sierra Cornejo, and José Ramón Arévalo. 2025. "Some Ecological Characteristics of a Neophyte of the Canary Islands: Pluchea ovalis (Asteraceae)" Ecologies 6, no. 2: 28. https://doi.org/10.3390/ecologies6020028
APA StylePestano, M., Suárez, I., González-Montelongo, C., Sierra Cornejo, N., & Arévalo, J. R. (2025). Some Ecological Characteristics of a Neophyte of the Canary Islands: Pluchea ovalis (Asteraceae). Ecologies, 6(2), 28. https://doi.org/10.3390/ecologies6020028








