Abstract
Parancistrocerus fulvipes (Saussure) (Hymenoptera: Vespidae), a predatory potter wasp, plays a crucial role in ecosystem services by preying on microlepidopteran larvae. This study investigated the effect of the temperature on the spring emergence and survivorship of P. fulvipes. We used seven different temperature regimes ranging from 5 to 38 °C to determine the developmental rate and thermal requirement for the emergence of P. fulvipes at each temperature. The development rates were determined using linear regression and a biophysical model (i.e., the Lactin model). Adult emergence occurred between 22 and 33 °C, and the lower developmental thresholds were 18.5, 17.1, and 17.8 °C for males, females, and both genders combined, respectively. The optimal temperature and upper-temperature threshold for spring emergence were 33 and 38 °C, respectively. The degree-day requirements for adult emergence were 201, 218, and 208 for males, females, and both genders combined. The lowest mortality was observed at 22 °C, while no P. fulvipes emerged at ≤13 °C and ≥38 °C. These findings provide a predictive model for the spring emergence of P. fulvipes, which can optimize ecosystem service programs in various agricultural systems, particularly in the context of climate change and pest management.
1. Introduction
Parancistrocerus fulvipes (Saussure) (Hymenoptera: Vespidae) is commonly known as the potter wasp and belongs to the subfamily Eumeninae, which includes solitary wasps that construct their brood nests individually [1,2]. P. fulvipes overwinters as fully grown larvae, and pupation and adult emergence occur in spring. P. fulvipes males emerge first in May or June in the mid-Atlantic region of the USA, followed by females 10 to 14 days later. This emergence timing can vary depending on the local climate conditions. After spring emergence, P. fulvipes mates, and the females start constructing nests by using pre-existing cavities such as holes in wood or trees, bamboo, reeds, or the old nests of bees and other wasps. If no pre-existing cavities are available, P. fulvipes individuals may construct their burrows in the ground using mud or chewed leaves [1]. P. fulvipes females lay a single egg and then provision it with paralyzed larvae from various families, including Tortricidae, Nolidae, Crambidae, and Gelechiidae [2,3,4]. Because these lepidopteran larvae feed on foliage in various agroecosystems, P. fulvipes plays a significant role in controlling the key pests of fruit trees and field crops. Each nest consists of multiple brood cells, and the females seal a brood cell with mud after provisioning it with prey [2,3].
The temperature is a key factor influencing the growth and development of arthropods, including insects [5]. Warmer temperatures can accelerate the development of insects, leading to short developmental periods [6,7] and earlier emergence [8,9]. In addition, the temperature can affect the longevity, fecundity, and mortality of insects [10]. Therefore, the shifts in temperature due to global warming can significantly affect prey–predator interactions or pollinator-blooming synchrony if spring emergence does not align with the occurrence and abundance of prey species and blooming, respectively. Previous studies showed that temperature increases could significantly affect hymenopteran insects’ phenology, impacting their activity patterns and interactions with their food sources. One of the hymenopteran insect species that has been studied in detail regarding the effect of the temperature on spring emergence is Osmia cornifrons (Hymenoptera: Megachilidae), a solitary mason bee species commonly used for orchard pollination. Adams [11], White, Son, and Park [8], and Ahn et al. [12] explored how temperature variations could alter the spring emergence timing of the bee, potentially leading to mismatches with flowering periods. Similarly, Lee, He, and Park [9] investigated climate change’s impacts on O. cornifrons in the eastern USA, highlighting that rising temperatures in the future could disrupt the synchrony between bees and floral resources due to accelerated spring emergence. These studies emphasize the potential for climate-induced phenological shifts to impact population interactions. Therefore, understanding how the temperature affects the spring emergence of P. fulvipes is essential in predicting potential disruptions in ecological relationships under a warming climate.
To understand the effect of the temperature on the growth, survival, and reproduction of poikilothermic organisms such as insects, temperature-dependent development models have been used as a crucial tool across taxa and environmental conditions. These models describe the relationship between the temperature and insect development rate [13]. Insects can develop between two temperature thresholds: the upper developmental threshold (UDT) and lower developmental threshold (LDT). Development between the UDT and LDT typically shows a skewed, unimodal nonlinear pattern. The temperature at which the insect develops at the fastest rate is called TOpt [14,15], and the temperature between TOpt and the LDT is known as the thermal window [14]. Using these parameters, the thermal ecology of insects (i.e., development, spring emergence, and phenology) can be described as their adaptation to seasonal temperature regimes [14,16]. Both linear and nonlinear models are generally used to determine temperature-dependent development; linear models are used to determine the LDT from mid-range temperatures, while nonlinear models provide more accurate descriptions across a wide temperature range [13,17]. Nonlinear models can also be used to estimate operative temperature ranges, the UDT, and TOpt [16].
Temperature-dependent development models not only provide parameters to describe the thermal biology of insects but also provide tools for diverse applications in insect ecology, pest management, and climate change research. These models can be used further to predict the phenology of insects, optimize the mass rearing of beneficial insects, assess the establishment potential of natural enemies or pollinator insects, and simulate climate change’s impacts on insect distributions [13,18,19]. For instance, these models help to forecast the emergence of insects or their peak population periods, which is crucial for pest management strategies [20]. In addition, the parameters derived from temperature-dependent development models (e.g., LDT and degree-day requirement) provide significant insights into insect ecology and pest management. Threshold temperatures (i.e., LDT and UDT) define the temperature ranges within which insects can develop, offering valuable information about their potential geographic distribution [21]. The concept of degree days also allows for the prediction of developmental events based on heat accumulation over time, which is particularly useful in pest management and phenology forecasting [17]. These studies have provided valuable insights for research on the thermal biology of P. fulvipes. Specifically, the timing of the spring emergence of P. fulvipes is critical for successful reproduction and thus it should be synchronized with the presence of its prey species.
This study was conducted to determine the effect of the temperature on the spring emergence of P. fulvipes. The objectives of this study were to determine the thermal window for development, the temperature thresholds (i.e., LDT, TOpt, and UDT), and the survivorship of P. fulvipes at seven different temperature regimes. This study used both linear and nonlinear models to address potential challenges for the population management of P. fulvipes as a beneficial wasp species that preys on lepidopteran pests under changing temperature regimes. By examining these effects, we determined how the temperature influences the emergence of P. fulvipes and its reliance on temperature changes.
2. Materials and Methods
2.1. Study Sites and Insects
We used nest blocks to collect overwintering P. fulvipes larvae (Figure S1). The nest blocks were originally developed for mason bee propagation [16], but P. fulvipes commonly utilize them for their nesting throughout the growing season. Each nest was created from white pine timber (14 cm in length × 9 cm in width × 2 cm in height) by carving them with a router. Transparency film (PP2500, 3M, Inc., St. Paul, MN, USA) was attached to the top of the block, allowing us to examine the nests without destroying them.
More than 100 nest blocks were placed in March 2023 on the Organic Research Farm of West Virginia University in Morgantown, West Virginia, USA. Naturally occurring P. fulvipes populations were allowed to nest until the end of the year, before being transferred to the Entomology Laboratory at West Virginia University.
During the nesting season of P. fulvipes, starting in May, we examined ten nests, focusing on individual cells to determine the quantity and identity of provisioned lepidopteran larvae in each nest. We carefully counted the larvae in each nest cell and identified them to the lowest possible taxonomic level.
2.2. Experimental Design and Conditions
In the Entomology Laboratory, fully grown larvae of P. fulvipes undergoing the overwintering phase were gently transferred to 24-well plates. Then, 42 P. fulvipes larvae were placed in each of seven incubators maintained at 5.0 ± 0.10, 13.0 ± 0.20, 22.0 ± 0.50, 27.5 ± 0.11, 30.0 ± 0.13, 33.0 ± 0.5, and 38.0 ± 0.35 °C (i.e., 294 P. fulvipes in total). The temperature in each incubator was monitored with a HOBO data logger (H08-007-02, OnSet Computer Corporation, Pocasset, MA, USA), and the photoperiod was kept constant at 9:15 h (L:D).
Each P. fulvipes individual was checked daily, and the developmental stage and mortality were recorded. Pupal development was noted when noticeable movement began to occur, and the date of adult emergence was recorded. The developmental periods of P. fulvipes at different temperatures were compared by using PROC GLM in SAS 9.4 [22] and Tukey’s studentized range test (HSD) for mean separation at a 5% error rate.
2.3. Emergence Rate Model
The rate of spring emergence of P. fulvipes at each temperature was calculated as the reciprocal of the median development time (1/median) in days. We used the median developmental time rather than mean values because median values are less sensitive to extreme values or outliers in the data compared to the mean. This makes it a more reliable measure of the central tendency, especially when dealing with biological data that may contain atypical observations [23,24]. Over the entire thermal range, the relationship between the temperature and the spring emergence rate was described by the nonlinear Lactin model, which was modified from the Logan type I model [25]:
where R(T) is the rate of development at temperature T; ρ can be interpreted as a composite Q10 value for enzyme-catalyzed biochemical reactions; TL is the lethal maximum temperature; and Δ is the width of the decline phase in the development rate above the optimum temperature [26].
R(T) = exp (ρT) − exp [ρTL − (TL − T)/Δ],
The parameter values were estimated by the least squares method using nonlinear regression analysis (PROC NLIN in SAS 9.4) [22]. The goodness of fit for nonlinear equations was assessed by the adjusted coefficient of determination.
2.4. Thermal Window, Optimal Temperature, and Degree Days Required for Adult Emergence
The thermal window was defined as the range of temperatures between the minimum (LDT) and maximum (TOpt) rates of spring emergence [14]. A narrow thermal window indicates that P. fulvipes is sensitive to temperature changes. TOpt was calculated when the model predicted the temperature at the highest developmental rate or shortest development time.
The linear portion of the data was (i.e., 22–33 °C) used to develop a linear model [6,12,16]: y = a+ bx, where y is the rate of development at temperature x, a is the y-intercept, and b is the slope of the regression model. The lower developmental threshold (LDT) was obtained by calculating −(a/b), and the thermal constant in degree days (DD) was obtained by calculating 1/b [27]. All linear regression analyses were conducted using PROC REG in SAS 9.4 [22].
3. Results
3.1. Nesting and Provisioning of P. fulvipes
We found that P. fulvipes could be propagated with artificial nests that were originally developed for mason bees (Figure 1), and the number of brood cells ranged from two to eight per nest. P. fulvipes females secured the nest by constructing vestibular cells at the entrance after the completion of each nest; the vestibular cells are known to function as barriers against predators or parasitic wasps (Figure S1).
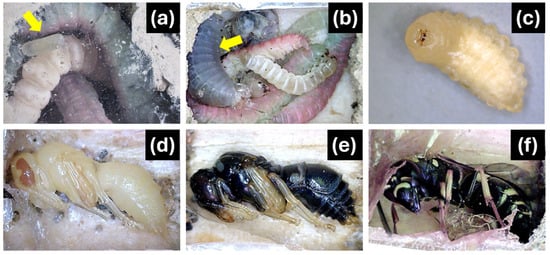
Figure 1.
Different stages of P. fulvipes in the nest. (a) One egg (yellow arrow) per cell is laid by the female P. fulvipes with provisioned lepidopteran larvae in the cell; (b) a hatched larva (yellow arrow) consumes provisioned prey; (c) a fully grown larva in the overwintering stage; (d,e) the larva becomes a pupa in the spring; (f) the adult P. fulvipes emerges.
We observed that each female P. fulvipes provisioned its larva with an average of nine lepidopteran larvae per cell, mainly in the families of Tortricidae, Gelechiidae, and Crambidae. These lepidopterans are key pests for fruit trees and various vegetables, forage crops, and field crops.
The identification of microlepidopteran larvae belonging to the families Gelechiidae and Tortricidae presents significant challenges, often necessitating supplementary information such as host plant associations or the rearing of larvae to the adult stage for accurate species determination. In our study, efforts were made to rear the larvae found in the P. fulvipes nests; however, only a single larva developed to the pupal stage, and no individuals successfully completed development to the adult stage. This lack of successful development can be attributed to the fact that the larvae were paralyzed by the mother P. fulvipes before their transportation to the nest, thereby hindering the normal growth and metamorphosis of prey species when we attempted to rear them to the adult stage.
3.2. Development and Spring Emergence of P. fulvipes
The adult emergence of P. fulvipes occurred at 22–33 °C (Table 1). At 13 °C, only one larva successfully developed into a pupa, but it failed to reach the adult stage. At 5 and 38 °C, no P. fulvipes individuals developed to a pupa. The emergence pattern of P. fulvipes adults depended upon the temperature to which they were exposed (Table 1). The slowest adult emergence was observed at 22 °C, where the first adult male and female emerged 48.9 and 79.8 days after they were placed in the incubator. In contrast, the earliest adult male and female emergence occurred at 33 °C after 13.5 and 15.5 days, respectively.

Table 1.
Developmental times (days), survivorship, and sex ratios of P. fulvipes at different constant temperatures.
Although adult emergence occurred more rapidly as the temperature was increased to 33 °C, the survivorship of P. fulvipes decreased with increasing temperatures. The survivorship rates of P. fulvipes from the fully grown larval stage to adults were 0, 0, 91.0, 88.1, 85.7, 81.0, and 0% at 5.0, 13.0, 22.0, 27.5, 30.0, 33.0, and 38.0 °C, respectively. At 33 °C, the fastest emergence of P. fulvipes was observed, while the lowest survival was recorded.
3.3. Spring Emergence Model
The nonlinear Lactin 1 model was used to fit the developmental rate data over the entire thermal range (Table 2).

Table 2.
Parameter estimates of the Lactin 1 model for the spring emergence of P. fulvipes males, females, and both males and females combined.
The developmental time was significantly influenced by the temperature (Figure 2) for males (F = 65.8; df = 2, 5; p = 0.003; r2 = 0.98), females (F = 33.2; df = 2, 5; p = 0.009; r2 = 0.96), and both genders combined (F = 26.3; df = 2, 5; p = 0.017; r2 = 0.95).
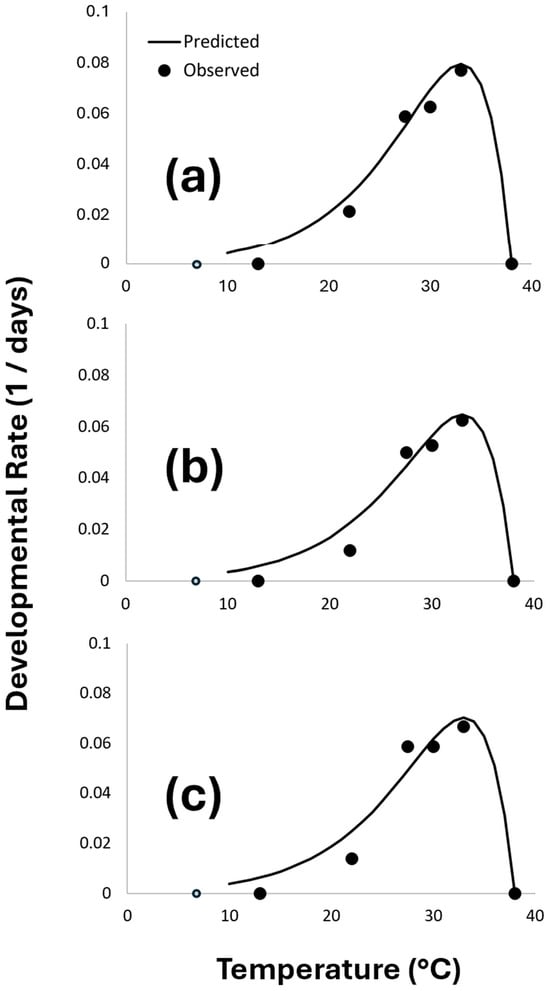
Figure 2.
Nonlinear temperature-dependent development model (Lactin model) of P. fulvipes: (a) males, (b) females, and (c) both genders combined. The open circle indicates a data point that was excluded from the regression analysis because no adult emerged at ≤13 °C.
3.4. Thermal Window and Degree Days Required for Adult Emergence
The developmental rate data in the mid-range temperatures (22–33 °C) or the linear portion of the data were fit to the linear model for males (F = 48.1; df = 1, 3; p = 0.020; r2 = 0.96), females (F = 25.1; df = 1, 3; p = 0.037; r2 = 0.93), and both genders combined (F = 13.1; df = 1, 3; p = 0.068; r2 = 0.87) (Figure 3).
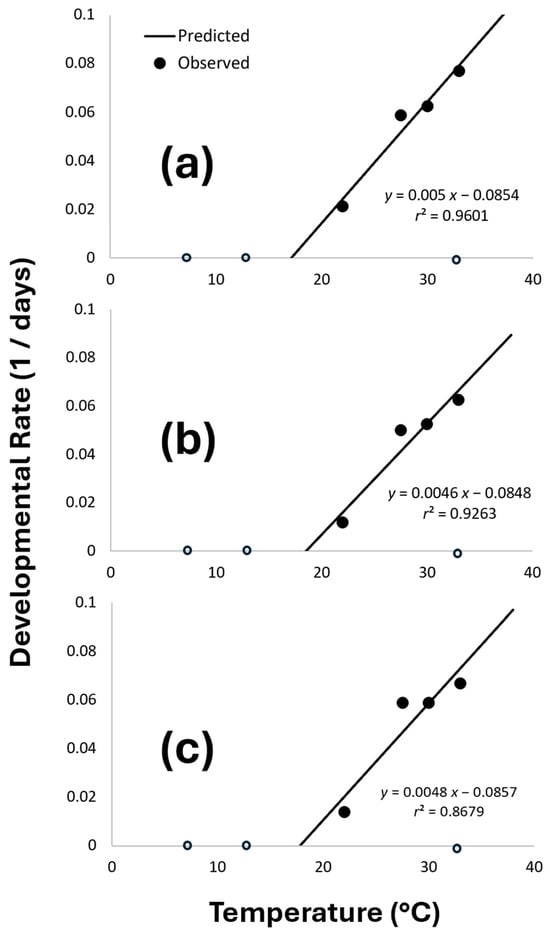
Figure 3.
Linear regression of temperature-dependent development of P. fulvipes: (a) males, (b) females, and (c) both genders combined. Only the linear portion of the data was used for regression analyses, and open circles indicate that no adult emerged and thus the data points were excluded from the regression analysis.
From the linear regression analysis, the LDTs (i.e., x-intercepts in Figure 3) for the males, females, and both genders combined were 18.5 ± 1.67, 17.1 ± 2.05, and 17.8 ± 3.01 °C, respectively, indicating that P. fulvipes could not develop and emerge at the temperature of ≤17.1 °C.
From the Lactin model, the optimal temperatures (TOpt) for males, females, and both genders combined were 33.0, 33.1, and 33.0 °C, respectively. The thermal windows between the LDT and TOpt for the males, females, and both genders combined were 14.5, 16.0, and 15.2 °C, respectively. The UDT was very similar between the male and female P. fulvipes (Table 2).
The degree-day requirements for adult emergence were 201 ± 28.9, 218 ± 43.4, and 208 ± 57.3 for males, females, and both genders combined, respectively.
4. Discussion
This research utilized development models that incorporated both high and low temperature ranges to determine the temperature-dependent conditions necessary for P. fulvipes to emerge in spring and survive. The findings revealed that the temperature played a crucial role in the spring emergence patterns of P. fulvipes. Even minor temperature elevations resulted in significant shifts in both the emergence rates and mortality. The implications of these results are that, in the context of global warming, even a slight increase in temperature could have significant consequences for P. fulvipes. P. fulvipes would appear sooner in spring, with higher mortality due to the temperature changes, and the life cycle of P. fulvipes could be accelerated. These potential changes could have far-reaching effects. Firstly, they may impact the population dynamics of P. fulvipes in various agricultural ecosystems, where these insects serve as important natural predators. Secondly, and perhaps more critically, there is a risk of creating temporal asynchrony between P. fulvipes and its prey species. This misalignment could occur if P. fulvipes emerges and becomes active earlier in spring, potentially before its prey populations have reached sufficient levels.
Higher temperatures accelerated the development and emergence rates but also led to increased mortality. We observed 100% failure in the development of P. fulvipes to either pupae or adults at the lowest temperatures (i.e., 5 and 13 °C) and the highest temperatures (i.e., 38 °C). With the exception of these temperatures, the highest mortality was observed at 33 °C, suggesting that even a small temperature increase due to climate change could have a substantial impact on the population of P. fulvipes. High and low temperatures also seem to affect the sex-biased mortality of P. fulvipes. The male to female sex ratios of surviving P. fulvipes individuals were 0.52, 0.95, 0.67, and 0.44 at 22.0, 27.5, 30.0, and 33.0 °C, respectively. The observed sex ratio of P. fulvipes was 0.95 at 27 °C, which was the closest to the sex ratio of 1.0 seen in nature. However, it is important to note that our study was conducted at seven constant temperatures, but the temperature fluctuates diurnally and seasonally, causing potential discrepancies between laboratory observations and field conditions. Consequently, the parameters derived from our constant-temperature experiments may introduce a degree of error when extrapolating them to estimate developmental timelines and spring emergence dates in natural settings. This methodological limitation should be considered when interpreting and applying our results to real-world scenarios.
The timing of the spring emergence of insects is critical for survival and successful reproduction. The prediction of the female population is particularly important because female P. fulvipes individuals engage in major predatory activities by hunting and provisioning prey larvae for the next generation. The results of our study indicate the thermal requirements for the spring emergence of P. fulvipes males and females, obtained using linear regression analysis. Our model predicted the emergence of P. fulvipes adults at 200–208 degree days. The degree-day requirement and LDT obtained by the linear model, coupled with weather data and monitoring data for field populations, are useful in predicting insects’ phenology in the field [8,10]. In addition to the LDT, the UDT is commonly used to accurately calculate the degree days from weather data using high-temperature cut-off techniques [28]. Based on the results of our study, thermal accumulation ≥ 38 °C needs to be excluded from degree-day accumulation.
P. fulvipes is important to agroecosystems as it controls small lepidopteran herbivores. Although P. fulvipes can occupy the artificial nests of solitary bees for orchard pollination (e.g., Osmia bees), we observed in the field that the main nesting activities of P. fulvipes occurred considerably later (i.e., June–October) than those of Osmia bees (April–early June). This study found that P. fulvipes males emerged earlier than females, which explains why male brood cells were located closer to the nest entrance, so that males could emerge first. Additional field observations in this research indicated that females emerged 10 to 14 days later than males in Morgantown, WV, USA (unpublished data). Based on our results, females require 17 more degree days than males for spring emergence and have a 1.4 °C lower LDT than males. If the average daily mid-May temperature increases by 2–3 °C in the future due to global warming, P. fulvipes males will emerge only 5–8 days earlier than females in our research site.
Temperature increases also affect the synchrony of P. fulvipes and its prey. For example, tortricid larvae, one of the major prey of P. fulvipes, have a lower LDT (9–10 °C), with a thermal requirement of 80–392 degree days to be in the larval stage [29], indicating that the spring emergence of P. fulvipes (LDT of 18 °C and 208 degree days) and the presence of tortricid larvae can be asynchronous when the spring temperature is 10–18 °C, because no degree-day accumulation would occur for P. fulvipes, while tortricid larvae still gain degree days. In addition, at >33 °C, the developmental rate and survivorship of P. fulvipes decrease considerably, but the tortricid larvae can still develop, with high survivorship at >33 °C [27], further indicating that a temperature increase would shift these predator–prey interactions.
Many previous studies have predicted that future climate changes will have severe impacts on various ecosystems. Specifically, many entomological studies have reported that climate change affected the growth, development, survivorship, and population dynamics of insects, including the spatial and temporal asynchrony of interacting populations (see [30,31,32] for recent reviews). Among the various events caused by climate change, a temperature increase could be the most influential driver of ecosystem function [33]. While some eusocial bees and parasocial wasps can control the temperatures of their hives, solitary wasps such as P. fulvipes cannot manipulate the temperature within their nests. Our study showed that P. fulvipes was unable to complete development at ≤13 °C and ≥38 °C, indicating that the development of P. fulvipes is sensitive to temperature changes, especially in the case of pupation and development to adults. The narrow thermal window found for P. fulvipes indicates that there are important considerations and complications when managing bees for agricultural production and annual population increases.
Although P. fulvipes has the potential to control key orchard and field crop pests, the lack of literature on the ecology of P. fulvipes is obvious. Since early studies describing the life cycle were performed in the 1950s and 1960s [34,35], no detailed life history studies have been published. Even recent research on P. fulvipes has mainly focused on the taxonomy [36] and its interaction with parasitic mites [35], further indicating the lack of ecological studies on P. fulvipes. Our study is the first to report the thermal ecology of P. fulvipes, but future studies would need to investigate the propagation, mass production, economic value, ecosystem services, and pest control capabilities of P. fulvipes. The results of our study can be used as the foundation for such future studies.
5. Conclusions
Our study demonstrates the effect of the temperature on the development, survivorship, and spring emergence of P. fulvipes. Its potential mortality and mismatch with its prey’s phenology due to increased temperatures underscores the need for adaptive crop and farm management practices. The results of our study have three implications for the population management of P. fulvipes. Firstly, the spring emergence of male and female P. fulvipes can be predictable. This study provides major parameters to precisely predict its development and spring emergence; these parameters include the LDT, UDT, TOpt, number of degree days to spring emergence, and thermal window. Secondly, the LDT and TOpt found in this study can be used to determine temperatures to delay the spring emergence of P. fulvipes by placing overwintering larvae below the LDT or accelerating their spring emergence by placing them at the optimum temperature. Storage temperatures exceeding the UDT should be avoided due to 100% mortality. Lastly, our study provides fundamental knowledge of how the temperature affects the development and spring emergence of P. fulvipes and a management tool for population management. P. fulvipes is a key predator for microlepidopteran pests in various agroecosystems; thus, the mass production of P. fulvipes for the biological control of pests can be achieved by utilizing the model and results presented in this study. In addition, the model can be further used for ecological studies of P. fulvipes and assessments of climate change impacts on P. fulvipes populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies6010020/s1, Figure S1. An observation nest block with five nests (rows) was used in this study. The transparent film on the top of the nest block allowed us to locate and observe P. fulvipes. The numbers in the picture indicate cells with immature stages of P. fulvipes.
Author Contributions
Conceptualization, R.K. and Y.-L.P.; methodology, R.K.; software, R.K. and Y.-L.P.; validation, R.K. and Y.-L.P.; formal analysis, R.K. and Y.-L.P.; investigation, R.K.; resources, Y.-L.P.; data curation, R.K. and Y.-L.P.; writing—original draft preparation, R.K. and Y.-L.P.; writing—review and editing, R.K. and Y.-L.P.; visualization, R.K. and Y.-L.P.; supervision, Y.-L.P.; project administration, Y.-L.P.; funding acquisition, Y.-L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the West Virginia University Agriculture and Forestry Experiment Station (WVA00785).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We thank Erica Edinger, Nellie Heitzman, and Kushal Naharki for their help in constructing the wasp nests. We also thank Vicki Kondo for helping to identify the lepidopteran larvae found in the nests.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Iwata, K. Evolution of Instinct. Comparative Ethology of Hymenoptera; Smithsonian Institution and the National Science Foundation: St. Paul, MN, USA, 1976; 535p. [Google Scholar]
- Krombein, K.V. Biosystematic studies of Ceylonese wasps III. Life history, nest and associates of Paraleptomenes mephitis (Cameron) (Hymenoptera: Eumenidae). J. Kans. Entomol. Soc. 1978, 51, 721–734. [Google Scholar]
- Cowan, D.P. The Social Biology of Wasps. The Solitary and Presocial Vespidae; Comstock Publishing Associates: Ithaca, NY, USA, 1991; 678p. [Google Scholar]
- Boesi, R.; Polidori, C.; Tormos, J.; Bevacqua, S.; Asís, J.D.; Andrietti, F. Trap-nesting Ancistrocerus sikhimensis (Hymenoptera: Eumenidae) in Nepal: Nest structure and associates (Hymenoptera: Chrysididae; Acarina: Saproglyphidae). Fla. Entomol. 2005, 88, 135–140. [Google Scholar] [CrossRef]
- Shi, P.; Ge, F.; Sun, Y.; Chen, C. A simple model for describing the effect of temperature on insect developmental rate. J. Asia-Pac. Entomol. 2011, 14, 15–20. [Google Scholar] [CrossRef]
- Gilbert, N.; Raworth, D.A. Insects and temperature—A general theory. Can. Entomol. 1996, 128, 1–13. [Google Scholar] [CrossRef]
- Ahn, J.J.; Son, Y.; He, Y.; Lee, E.; Park, Y.-L. Effects of temperature on development and voltinism of Chaetodactylus krombeini (Acari: Chaetodactylidae): Implications for climate change impacts. PLoS ONE 2016, 11, e0161319. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Son, Y.; Park, Y.-L. Temperature-dependent emergence of Osmia cornifrons (Hymenoptera: Megachilidae) adults. J. Econ. Entomol. 2009, 102, 2026–2032. [Google Scholar] [CrossRef]
- Lee, E.; He, Y.; Park, Y.-L. Effects of climate change on the phenology of Osmia cornifrons: Implications for population management. Climatic Change 2018, 150, 305–317. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, D.-S. Effect of temperature on the fecundity and longevity of Ascotis selenaria (Lepidoptera: Geometridae): Developing an oviposition model. J. Econ. 2016, 109, 1267–1272. [Google Scholar]
- Adams, L.R. Determination of Accumulation of Degree Days Required for the Emergence of Osmia cornifrons (Megachilidae) in Pennsylvania. Master’s Thesis, Pennsylvania State University, College Park, PA, USA, 2001. [Google Scholar]
- Ahn, J.J.; Park, Y.-L.; Jung, C. Modeling Spring emergence of Osmia cornifrons Radoszkowski (Hymenoptera: Megachilidae) females in Korea. J. Asia-Pac. Entomol. 2014, 17, 901–905. [Google Scholar] [CrossRef]
- Malekera, M.J.; Acharya, R.; Mostafiz, M.M.; Hwang, H.-S.; Bhusal, N.; Lee, K.-Y. Temperature-dependent development models describing the effects of temperature on the development of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Insects 2022, 13, 1084. [Google Scholar] [CrossRef]
- Dixon, A.F.; Honěk, A.; Keil, P.; Kotela, M.A.A.; Šizling, A.L.; Jarošík, V. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 2009, 23, 257–264. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.; Johnson, D.; Craigen, R. Improved rate model of temperature-dependent development by arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- McKinney, M.; Ahn, J.J.; Park, Y.-L. Thermal biology of Osmia cornifrons (Hymenoptera: Megachilidae) eggs and larvae. J. Apic. Res. 2017, 56, 421–429. [Google Scholar] [CrossRef]
- Régnier, B.; Legrand, J.; Rebaudo, F. Modeling temperature-dependent development rate in insects and implications of experimental design. Environ. Entomol. 2022, 51, 132–144. [Google Scholar] [CrossRef]
- Bhagarathi, L.K.; Maharaj, G. Impact of climate change on insect biology, ecology, population dynamics, and pest management: A critical review. World J. Adv. Res. Rev. 2023, 19, 541–568. [Google Scholar] [CrossRef]
- Prasad, K.H. Insect Ecology: Concepts to Management; Climate Change and Insect Ecology; Springer: Singapore, 2022; pp. 223–228. [Google Scholar]
- Mohamed, S.A.; Azrag, A.G.; Obala, F.; Ndlela, S. Estimating the demographic parameters of Tuta absoluta (Lepidoptera: Gelechiidae) using temperature-dependent development models and their validation under fluctuating temperature. Biology 2022, 11, 181. [Google Scholar] [CrossRef]
- Taylor, F. Ecology and evolution of physiological time in insects. Am. Nat. 1981, 117, 1–23. [Google Scholar] [CrossRef]
- SAS Institute. Using JMP Student Edition for Windows and Macintosh: The User’s Guide to Statistics with JMP Student Edition; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Padmavathi, C.; Katti, G.; Sailaja, V.; Padmakumari, A.P.; Jhansilakshmi, V.; Prabhakar, M.; Prasad, Y.G. Temperature thresholds and thermal requirements for the development of the rice leaf folder, Cnaphalocrocis medinalis. J. Insect Sci. 2013, 13, 96. [Google Scholar] [CrossRef]
- Ma, Z.S.; Bechinski, E.J. A new modelling approach to insect reproduction with same-shape reproduction distribution and rate summation: With particular reference to Russian wheat aphid. Bull. Entomol. Res. 2009, 99, 445–455. [Google Scholar] [CrossRef]
- Logan, J.; Wollkind, D.; Hoyt, S.; Tanigoshi, L. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: History and critique. Can. J. Zool. 1997, 75, 1561–1574. [Google Scholar] [CrossRef]
- Campbell, A.; Frazer, B.D.; Gilbert, N.G.A.P.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Aghdam, H.R.; Fathipour, Y.; Radjabi, G.; Rezapanah, M. Temperature-dependent development and temperature thresholds of codling moth (Lepidoptera: Tortricidae) in Iran. Environ. Entomol. 2009, 38, 885–895. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Schweiger, O.; Settele, J.; Kudrna, O.; Klotz, S.; Kühn, I. Climate change can cause spatial mismatch of trophically interacting species. Ecology 2008, 89, 3472–3479. [Google Scholar] [CrossRef]
- Krombein, K.V. Some symbiotic relations between saproglyphid mites and solitary vespid wasps (Acarina, Saproglyphidae and Hymenoptera, Vespidae). J. Wash. Acad. Sci. 1961, 51, 89–93. [Google Scholar]
- Cooper, K.W. Biology of Eumenine wasps. V. Digital communication in wasps. J. Exp. Zool. 1957, 134, 469–513. [Google Scholar] [CrossRef]
- Li, T.; Carpenter, J.M. Descriptions of eight new species of the genus Parancistrocerus Bequaert (Hymenoptera: Vespidae: Eumeninae), with a key to the Oriental species. Zootaxa 2019, 4551, 251–274. [Google Scholar] [CrossRef]
- Graham, J.R.; Campbell, J.W.; Ellis, J.D. Uninvited Guests: Identifying Parasites and Other Nest Associates of Solitary Bees and Wasps Using Artificial Nest Sites in North Central Florida. Southeast. Nat. 2023, 22, 192–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).