Abstract
Diplodia pinea is a fungal pathogen that causes Diplodia shoot blight in pines and is widely spread in red pine (Pinus resinosa) and jack pine (Pinus banksiana) forests in Michigan. The objective of this study was to examine whether infection with D. pinea compromises wood quality in pine stands. Acoustic data was collected using an acoustic tomographer from the stem region at breast height (1.3 m) of red pine and jack pine trees across two categories of forest health condition (control vs. Diplodia-affected), in two latitudinal regions (Lower Peninsula vs. Upper Peninsula), and two levels of initial stand density (low vs. high). The acoustic data was used to infer the wood quality (i.e., density) in these two tree species since material of higher density generally has higher sound velocity rates. Red pine had significantly higher wood quality (i.e., higher sound velocities) in the Upper Peninsula region compared to the Lower Peninsula region. Within each latitudinal region, red pine sound velocities did not show significant differences between forest health condition or initial stand density levels. Jack pine showed no significant differences in sound velocities across the treatment categories. The results indicate that latitudinal region appears to have more impact on red pine wood quality than the influence of forest health condition (presence of Diplodia shoot blight) or initial stand density. All analyzed factors (latitudinal region, forest health condition, and stand density) did not have a significant impact on the wood quality of jack pine.
1. Introduction
Diplodia pinea (Desm.) is one of the most common fungal pathogens to infect more than 20 pine species [1]. D. pinea is widespread geographically in the United States, and is found to occur in 30 eastern and central states plus California in the west and Hawaii [1]. Globally, D. pinea has been observed to cause crown wilt and infection of woody stems of Pinus radiata (D. Don) in Chile [2]. Furthermore, D. pinea was newly detected as a pathogen of Austrian pine (Pinus nigra Arnold) in Estonia [3] and urban trees in the United States [4]. Another species of Diplodia (i.e., Diplodia mutila) was determined to cause blue stain in wood samples of Caribbean pine (Pinus caribaea Morelet) in Venezuela [5]. Symptoms of infection of D. pinea include shoot blight, branch dieback, dead tops, death, stem cankers, and blue staining on dead wood [6].
Diplodia shoot blight preferentially affects young pine stands [7]. However, young jack pine (Pinus banksiana Lamb.) stands in the northern lower peninsula of Michigan is the only summer nesting habitat for Kirkland’s warbler (Dendroica kirtlandii), a federally protected and endangered songbird [8]. An improved understanding of how abiotic and biotic factors may predispose jack pine stands to Diplodia will be instrumental for sustainable management of this habitat for the continued survival of the Kirtland’s warbler. Jack pine is a shade-intolerant species [9]. In North America, the southern portion of the range of Jack pine is situated partly in Michigan in the northern portion of the Lower Peninsula and in the Upper Peninsula region. Jack pine stands typically regenerate via stand-replacing fires.
Red pine (Pinus resinosa Ait.) is a shade-intolerant native conifer that covers approximately 769,000 ha of forest land in Michigan [10]. In Michigan, the southern distributional range of red pine occurs in the northern Lower Peninsula region while the central range of red pine occurs in the Upper Peninsula region based on the range map of Rudolf [11]. Red pine is economically important in the Great Lakes region for a variety of uses including pulpwood and in particular utility poles where wood quality is a critical issue. Red pine stands are routinely managed using different initial levels of stand density, which in turn can improve resilience to climatic stress [12].
Climate can predispose trees to being susceptible to secondary factors (i.e., insects and fungal diseases), which in turn can lead to forest decline [13]. Drought and intraspecific competition in pine stands have been implicated as predisposing factors for increased shoot blight risk caused by D. pinea [10]. Crown damage caused by harsh winters (via increased mechanical load of snow on crowns) may have predisposed red pine stands to D. pinea affection through providing an easier entry point for the fungal pathogen [14]. Negative relationships between radial growth with precipitation in D. pinea-affected stands may promote increased dispersal of spores in D. pinea-affected stands that could occur with significant rain and any increased storm and wind activity [14].
Acoustic tomography is based on the principle that material of higher density will propagate sound at faster velocities than material of lower density [15,16]. In forestry-related applications, acoustic sensors have been used for wood quality assessment [15,16,17]. In arboriculture and urban forestry settings, acoustic tomography is used primarily to measure the mechanical properties of wood [18,19] and to evaluate internal stem decay during tree risk assessments [20,21,22]. In particular, acoustic sensors are used to detect for internal decay and defects in tree stems to proactively determine if a tree needs to be cut down to limit the risk to human injury or death [15]. Prior acoustic-based studies of red pine [23] and jack pine [24] have focused solely on assessing wood properties and their connection to wood quality. No prior studies, though, have used an acoustic based assessment of the impact of D. pinea on wood properties of red pine and jack pine.
The main objective of the present study was to examine whether infection with D. pinea compromises wood quality in red pine and jack pine stands in Michigan. Furthermore, the study also examined whether differences in acoustic stem velocities existed across two latitudinal regions (Lower Peninsula vs. Upper Peninsula), and two levels of initial stand density (low vs. high).
2. Materials and Methods
Plantation stands of red pine and jack pine (pole sized) were selected in two different latitudinal regions: i.e., the northern Lower Peninsula (LP) to the Upper Peninsula (UP) region of Michigan (Figure 1). Specifically, the location of the study area was in Hiawatha National Forest situated in the UP, and Huron National Forest situated in the LP.
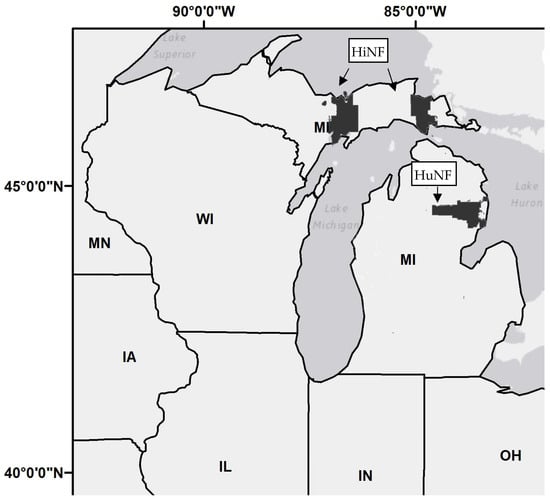
Figure 1.
Location of the study area in Hiawatha National Forest (HiNF) situated in the Upper Peninsula of Michigan (MI), and Huron National Forest (HuNF) situated in the Lower Peninsula. National forests are shaded in black.
In each region, sites were selected for two categories of forest health condition: healthy-looking reference vs. affected by D. pinea. Selected healthy-looking reference stands had <5% of visual crown dieback while Diplodia-affected stands had at least 15% crown dieback. Infection with Diplodia was confirmed based on visual symptoms of crown dieback (based on estimation of crown area with brown needles) and by culturing pine needles in agar media [6]. Furthermore, black fruiting bodies (pycnidia) found on pine needles were placed on microscope slides and were then examined under a compound microscope to identify the presence of D. pinea spores, which look like brown jelly beans [1]. Half of the total number of sites for red pine (20 sites) and jack pine (20 sites) that were selected in this study were originally planted at higher density (>1977 trees per ha) while the other half of the study sites were planted at low density (<988 trees per ha) in order to examine the influence of intraspecific tree competition. During site selection, stands were not selected if they showed signs of other disturbance agents besides D. pinea. Furthermore, given the persistence of stumps in the stand, stands were avoided that had a high degree of natural tree mortality or high degree of past thinning.
In the Upper Peninsula region of Michigan, field sampling was conducted in the Hiawatha National Forest in a total of eight sites for red pine (Table 1) and eight sites for jack pine (Table 2). In the Lower Peninsula region of Michigan, field sampling was conducted in the Huron National Forest in a total of 12 sites for red pine (Table 1) and 12 sites for jack pine (Table 2). Furthermore, for both regions, half of the number of either healthy-looking reference or Diplodia-affected stands were selected to have a low initial stand density while the other stands had a high initial stand density.

Table 1.
List of red pine sites sampled in the Upper Peninsula (UP) and Lower Peninsula (LP) of Michigan. Sites were further categorized by different levels of forest health condition (healthy-looking reference vs. Diplodia-affected) and level of initial stand density (low vs. high level).

Table 2.
List of jack pine sites sampled in the Upper Peninsula (UP) and Lower Peninsula (LP) of Michigan. Sites were further categorized by different levels of forest health condition (healthy-looking reference vs. affected by Diplodia) and level of initial stand density (low vs. high level).
At each site, the field data collection was conducted by first establishing a circular plot with a randomly selected focal tree representing the plot center. Stands with low initial density had a radius of 9.78 m (0.03 ha) while stands with high initial density stands consisted of a circular plot radius of 7.99 m (0.02 ha). It was required that each plot was a minimum of 30 m from the stand’s boundary. Along the compass bearings of NE, SE, SW and NW, trees that were closest to the halfway point of the radius of the plot were selected for sampling. While the selection process for trees near the mid-point of each cardinal radius did mean they were selected close to each other in the stand, which in turn could lead to spatial autocorrelation issues, the trees still experienced a unique competition environment based on the different categories of their initial stand densities. Consequently, 4 trees were measured from each of the 40 sites. All trees sampled in the D. pinea-affected sites all had dieback. For each selected tree at a measurement height of 1.3 m on the stem aboveground line, an acoustic tomographer (microsecond timer manufactured by FAKOPP: Figure 2) was used to determine the transit time in units of microseconds to travel between the start sensor and end sensor [25]. This microsecond timer requires that the sound is initiated with a small tap of a provided hammer. Each tree was measured in two orientations: (a) North to South direction, and (b) West to East direction. For each direction, 3 measurements were taken. Before an acoustic measurement was taken, each sensor was hammered in so that the tip of the sensor was seated in the xylem tissue and not in the inner or outer bark region. A bark gauge (Suunto) was used to verify the depth of the bark. The diameter outside bark in combination with the depth of the sensor was used to calculate the distance between the start and end sensor of the acoustic tomographer.

Figure 2.
Demonstration of the use of a microsecond timer developed by FAKOPP. The hammer is used to initiate the sound pulse on the start sensor and the unit is able to determine how long it takes for the sound to travel through the stem to end sensor. In combination with the distance between the sensors, the sound velocity can be calculated. Photo taken by Sophan Chhin.
Sound velocity (V) was calculated using the following formula:
where V = correct velocity (m/s); distance = distance between the start and stop transducer placed on opposite sides of the stem (m); transit time (s) = the time required for sound to travel from the start sensor to the stop sensor (Wang et al., 2004); and correction = delay time associated with measuring equipment (which was seven microseconds for unit used in this study).
V = distance/(transit time − correction)
The three sound velocity values for each direction were averaged, and then the two directions were averaged so that each tree was represented by a single sound-velocity value. The sound velocity of each tree in a total of eight categories consisting of all possible combinations of two latitudinal regions (UP = Upper Peninsula; LP = Lower Peninsula), two forest health conditions (C = control; D = Diplodia-affected), and two levels of original stand density (L = low; H = high) were analyzed using one-way analysis of variance with Systat statistical software (version 13).
3. Results
Red pine had significantly higher wood quality (i.e., higher sound velocities) in the Upper Peninsula region compared to the Lower Peninsula region (Figure 3). Within each latitudinal region, red pine did not show significant differences between the forest health condition or stand density levels. In the Upper Peninsula, high-density red pine stands affected by D. pinea (category UP.D.H) had the lowest sound velocities in that latitudinal region but this was not statistically significant (p = 0.067) (Figure 3).
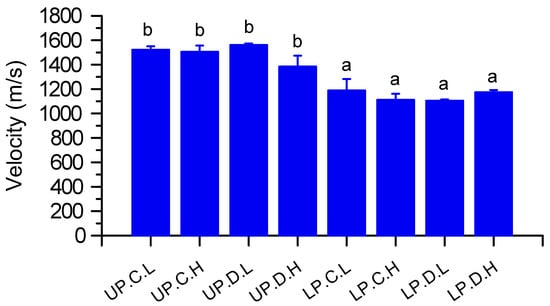
Figure 3.
Acoustic velocity through the stems of red pine (Pinus resinosa) sample from two regions (UP = Upper Peninsula; LP = Lower Peninsula of Michigan state, USA), forest health condition (C = control; D = affected by Diplodia), and level of initial stand density (L = low; H = high). Bars are means ± 1 standard error. Bars labelled with different lowercase letters are statistically significant from each other (p < 0.05).
Jack pine showed no significant differences across the eight treatment categories (Figure 4). In the Lower Peninsula, high density stands of Jack pine affected by Diplodia did have the lowest sound velocities but this was not statistically significant (p = 0.087).
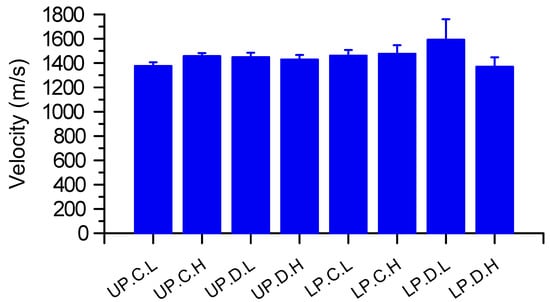
Figure 4.
Acoustic velocity through the stems of jack pine (Pinus banksiana) sample from two latitudinal regions (UP = Upper Peninsula; LP = Lower Peninsula of Michigan State, USA), forest health condition (C = control; D = affected by Diplodia), and level of initial stand density (L = low; H = high). Bars are means ± 1 standard error. There were no significant differences between any of the categories of jack pine (all p ≥ 0.05).
4. Discussion
Most acoustic tomography-based studies have focused primarily on either wood-quality concerns from a forest product standpoint (e.g., [26]) or tree risk assessments in urban areas [20,21,22]. For instance, Zhang et al. [27] examined acoustic propagation through red pine stems sampled from a plantation in Wisconsin, USA, but only from a forest product perspective. Consequently, the present study represents the first attempt at utilizing this technology for understanding the potential impact of a forest pathogen on the structural integrity of the stemwood of pine species. At the 5% significance level, there was no statistically significant impact of the pathogen on the sound velocity of both investigated pine species. This could be due to the low rate of presence of D. pinea even in the Diplodia-affected stands. However, at the 10% significance level, some patterns were noticeable. Namely, in the Upper Peninsula, high-density red pine stands affected by D. pinea did have the lowest sound velocities and therefore showed some level of degraded wood quality in this category (p = 0.067). Furthermore, in the Lower Peninsula, high-density stands of jack pine affected by D. pinea did have the lowest sound velocities and, therefore, lower wood quality (p = 0.087). However, these patterns observed at the 10% level of significance may be due as much to the high-density conditions as the potential impact of the pathogen.
Initial stand density did not have a significant (5% level) impact on wood quality in this study. It could be argued that a higher initial stand density alone could lead to slower growth rates and higher wood density and, therefore, higher stem velocities. However, D. pinea is known to preferentially impact trees with a past history of stand-related stress such as high competition and/or a past history of climatic stress [10]. At the 10% level of significance, the results of the study seemed to suggest that higher-stand-density stands succumbed more to the impact of D. pinea, which, in turn, negatively impacted wood quality (i.e., for red pine in the Upper Peninsula and jack pine in the Lower Peninsula).
The results of the present study showed a clear impact of latitudinal region on the acoustic velocities in red pine wood. We speculate that given the more northerly location of the Upper Peninsula and the colder climate associated with it, the slower diameter growth rates led to a higher wood density and, therefore, correspondingly higher stem velocities. This would suggest a tradeoff between reduced growth and productivity and better wood quality in colder climates. This finding suggests that acoustic tomography may have potential applications in other ecological studies.
The sound velocities observed for red pine and jack pine reported in this study are within the range or higher than that reported by Mattheck and Bethge [28] for pine species sampled in Germany: i.e., 1066–1146 m/s. While Mattheck and Bethge [28] do not list the specific species of pine they tested, our slightly higher velocities are not surprising as red pine is reported to have moderately high material properties for many commercial pine species in the United States [29]. This would suggest that even stands affected by D. pinea did not have their wood quality affected below the reference velocity. This in turn may mean that stands with a minor degree of fungal infestation may still yield usable forest products. Zhang et al. [27] only reported on the transit times of acoustic waves but unfortunately did not report values of acoustic velocities, which therefore limits the opportunity for comparisons.
Overall, the effectiveness of acoustic tomography appears likely connected to the stage of decay. At one end of the spectrum, Li et al. [30] noted that acoustic tomography had difficulties detecting early decay in black cherry (Prunus serotina). Furthermore, Wang et al. [31] found that acoustic tomography typically underestimated the degree of heartwood decay in black cherry. At the other end of the detection spectrum, Burcham et al. [22] and Nicoletti et al. [20] found that different types of acoustic tomography was affective in detecting extensive internal decay in urban trees. The current study likely falls under the lower end of the decay spectrum, which limited the capacity for detection of D. pinea-induced stem decay (at the 5% level of statistical significance). Consequently, future studies should examine stands of red pine and jack pine that are affected more severely by D. pinea to further examine the utility of using acoustic-based detection systems for forest health applications.
Author Contributions
Formal analysis, S.C.; funding acquisition, S.C.; methodology, S.C.; writing—original draft, S.C.; writing—review and editing, S.C. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the USDA Forest Service (Forest Health Protection, Grant No. 11-DG-11420004-148). This work was also supported by the United States Department of Agriculture (USDA), National Institute of Food and Agriculture (NIFA), McIntire Stennis Project #1017946 and WVU West Virginia Agriculture and Forestry Experiment Station (Scientific Article #3464).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available until full completion of all project components.
Acknowledgments
We thank K. Lazda for his logistical support and GIS data for sampling Huron National Forest. We thank D. Berry, E. David, A. Djoko, K. Finley, M. Magruder, K. Minnix, A. Monks, and M. Rooney for their assistance in field and laboratory data collection. We also thank two anonymous reviewers for providing constructive comments on a prior version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peterson, G.W. Diplodia Blight of Pines; Forest Insect & Disease Leaflet 161; US Forest Service: Lincoln, NE, USA, 1997. Available online: http://www.na.fs.fed.us/spfo/pubs/fidls/diplodia/diplodiafidl.htm (accessed on 5 April 2017).
- Chou, C.K.S. Crown wilt of Pinus radiata associated with Diplodia pinea infection of woody stems. Eur. J. For. Pathol. 1987, 17, 398–411. [Google Scholar]
- Hanso, M.; Drenkhan, R. Diplodia pinea is a new pathogen on Austrian pine (Pinus nigra) in Estonia. Plant Pathol. 2009, 58, 797. [Google Scholar] [CrossRef]
- Wallis, C.; Eyles, A.; Chorbadjian, R.A.; Riedl, K.; Schwartz, S.; Hansen, R.; Cipollini, D.; Herms, D.A.; Bonello, P. Differential effects of nutrient availability on the secondary metabolism of Austrian pine (Pinus nigra) phloem and resistance to Diplodia pinea. For. Pathol. 2011, 45, 52–58. [Google Scholar] [CrossRef]
- Mohali, S.; Encinas, O. Association of Diplodia mutila with blue stain of Caribbean pine in Venezuela. For. Pathol. 2001, 31, 187–189. [Google Scholar] [CrossRef]
- Munck, I.A.; Smith, D.R.; Sickley, T.; Stanosz, G.R. Site-related influences on cone-borne inoculum and asymptomatic persistence of Diplodia shoot blight fungi on or in mature red pines. For. Ecol. Manag. 2009, 257, 812–819. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Leisso, R. Diplodia shoot blight and asymptomatic persistence of Diplodia pinea on or in stems of jack pine nursery seedlings. For. Pathol. 2007, 37, 145–154. [Google Scholar] [CrossRef]
- Kashian, D.M.; Barnes, B.V.; Walker, W.S. Landscape ecosystems of northern lower Michigan and the occurrence and management of the Kirtland’s warbler. For. Sci. 2003, 49, 140–159. [Google Scholar]
- Rudolf, P.O.; Laidly, P.R. Pinus banksiana Lamb. (Jack Pine). In Silvics of North America: 1. Softwoods; Burns, R.M., Honkala, B.H., Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Gilmore, D.W.; Palik, B. A Revised Managers Handbook for Red Pine in the North Central Region; General Technical Report NC-264; North Central Research Station, US Forest Service: St. Paul, MI, USA, 2005.
- Rudolf, P.O. Pinus resinosa Ait. (Red Pine). In Silvics of North America: 1. Softwoods; Burns, R.M., Honkala, B.H., Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Magruder, M.; Chhin, S.; Monks, D.; O’Brien, J. Effects of initial stand density and climate on red pine productivity within Huron National Forest, Michigan. Forests 2012, 3, 1086–1103. [Google Scholar] [CrossRef]
- Manion, P.D. Tree Disease Concepts, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1991. [Google Scholar]
- Chhin, S.; O’Brien, J. Dendroclimatic analysis of red pine affected by Diplodia shoot blight in different latitudinal regions in Michigan. Can. J. For. Res. 2015, 45, 1757–1767. [Google Scholar] [CrossRef]
- Bucur, V. Techniques for high resolution imaging of wood structure: A review. Meas. Sci. Technol. 2003, 14, R91–R98. [Google Scholar] [CrossRef]
- Wang, X. Acoustic measurements on trees and logs: A review and analysis. Wood Sci. Technol. 2013, 47, 965–975. [Google Scholar] [CrossRef]
- Wang, X.; Ross, R.J.; Carter, P. Acoustic evaluation of wood quality in standing trees. Part I. Acoustic wave behavior. Wood Fiber Sci. 2007, 39, 28–38. [Google Scholar]
- Dahle, G.A.; Carpenter, A.; DeVallance, D. Non-destructive Measurement of the Flexural Modulus of Elasticity of Wood Using Acoustical Stress Waves. Arboric. Urban For. 2016, 42, 227–233. [Google Scholar]
- Persad, A.; Rocha, O.; Dahle, G.A.; Grabosky, J.; DeVallance, D. Optical, acoustical and fine root analyses of emerald ash borer infested ash trees. Arboric. Urban For. 2019, 45, 211–220. [Google Scholar] [CrossRef]
- Nicolotti, G.; Socco, L.V.; Martinis, R.; Godio, A.; Sambuelli, L. Application and comparison of three tomographic techniques for detection of decay in trees. J. Arboric. 2003, 29, 66–78. [Google Scholar] [CrossRef]
- Gilbert, E.A.; Smiley, E.T. Picus sonic tomography for the quantification of decay in white oak (Quercus alba) and Hickory (Carya spp.). J. Arboric. 2004, 30, 277–281. [Google Scholar] [CrossRef]
- Burcham, D.C.; Brazee, N.J.; Marra, R.E.; Kane, B. Geometry matters for sonic tomography of trees. Trees 2023, 34, 837–848. [Google Scholar] [CrossRef]
- Newton, P.F. Acoustic-based non-destructive estimation of wood quality attributes within standing red pine trees. Forests 2017, 8, 380. [Google Scholar] [CrossRef]
- Newton, P.F. Acoustic-based prediction of end-product-based fibre determinates within standing jack pine trees. Forests 2019, 10, 605. [Google Scholar] [CrossRef]
- Wang, X.; Divos, F.; Pilon, C.; Brashaw, B.K.; Ross, R.J.; Pellerin, R.F. Assessment of Decay in Standing Timber Using Stress Wave Timing Nondestructive Evaluation Tools; General Technical Report FPL-GTR-147; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 2004.
- Carter, P.; Briggs, D.; Ross, R.J.; Wang, X. Acoustic testing to enhance western forest values and meet customer wood quality needs. In Productivity of Western Forests: A Forest Products Focus; Gen. Tech. Rep., P.NW-GTR-642; Harrington, C.A., Schoenholtz, S.H., Eds.; Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2005; pp. 121–129. [Google Scholar]
- Zhang, H.; Wang, X.; Su, J. Experimental investigation of stress wave propagation in standing trees. Holzforschung 2011, 65, 743–748. [Google Scholar] [CrossRef]
- Mattheck, C.G.; Bethge, K.A. Detection of decay in trees with the Metriguard Stress Wave Timer. J. Abroriculture 1993, 19, 374–378. [Google Scholar] [CrossRef]
- Kretschmann, D.E. Mechanical properties of wood. In Wood Handbook: Wood as An Engineering Material; General Technical Report FPL-GTR-190; USDA, Forest Products Laboratory: Madison, WI, USA, 2010. [Google Scholar]
- Li, L.; Wang, X.P.; Wang, L.; Allison, R.B. Acoustic tomograph in relation to 2D ultrasonic velocity and hardness mappings. Wood Sci. Technol. 2012, 46, 551–561. [Google Scholar] [CrossRef]
- Wang, X.; Wiedenbeck, J.; Lian, S. Acoustic tomography for decay detection in black cherry trees. Wood Fiber Sci. 2009, 41, 127–137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
