Abstract
In this study, the long-term (i.e., over a 27-year period) dynamics of 137Cs content are presented for seven species of fish in both the cooling pond (CP) of the Chornobyl Nuclear Power Plant and the Kaniv Reservoir (KR). The decline of 137Cs specific activity in fish exhibits various patterns. For certain years in the KR, fish belonging to different ecological groups experienced an increase rather than a decrease in specific activity levels of 137Cs. From 2012 to 2014, the concentration of 137Cs in all studied species in the KR ranged from 4 to 23 Bq/kg. In the CP during 2012–2013, fish still showed high contamination levels, ranging from 770 to 8300 Bq/kg. The ecological half-life (Teco) was determined for all the studied fish species. For most fish species (i.e., P. fluviatilis, B. bjoerkna, A. brama, S. lucioperca, A. aspius), the shortest 137Cs Teco values were obtained in the CP, being a highly radiocaesium-contaminated waterbody. In contrast, two fish species (R. rutilus and S. glanis) in the CP exhibited a considerably slower rate of 137Cs removal from their bodies compared to even the relatively cleaner KR. Moreover, the 137Cs Teco in R. rutilus and S. glanis was nearly twice as long as that observed in other species within the CP. We assume that the redistribution of 137Cs in the body of fish is affected by multidirectional mechanisms: accumulation, retention, and/or excretion. The functioning of these mechanisms can vary among different fish species. The observed level of 137Cs content in a particular fish species at a given time point results from the combined effects of these mechanisms. Fish likely have the ability to absorb and accumulate radiocaesium in their bodies selectively, and this demand appears to be species-specific.
1. Introduction
The total release of radioactive substances from the damaged Unit 4 of the Chornobyl Nuclear Power Plant (ChNPP) accident in 1986 was about 14 × 1018 Bq. 137Cs and other cesium radioisotopes were released at approximately 0.085 × 1018 Bq [1].
1.1. 137Cs in Fish of the Cooling Pond (CP) of ChNPP
The cooling pond of the Chornobyl Nuclear Power Plant serves as a vital component of the plant’s cooling system, playing a crucial role in regulating the temperature of the reactor cores and auxiliary equipment. Investigations after the ChNPP accident revealed that up to 7.4 × 1015 Bq of various fission products were initially released into the CP [2]. During the Chornobyl accident and the elimination of its consequences, radioactive substances penetrated the CP in different ways. The primary sources of contamination at the CP were solid and liquid aerosols generated during the reactor explosion and subsequent burning, which were then deposited onto the reservoir’s water surface. The liquid effluents from the nuclear power plant itself, which contained fuel and irradiated materials resulting from the accident, also contributed to the contamination of CP. During the elimination of the accident, fire suppression and cleanup of the industrial site irradiated building structures, pieces of graphite and fuel (and heterogeneous contaminated “debris”) fell into the water. Dust formation and waste from the work at the Chornobyl nuclear power plant during the sarcophagus (shelter structure) construction contributed to the additional ingress of radioactive substances into the CP. The location’s proximity (approx. 2–3 km) to the destroyed power unit contributed to significant radioactive contamination of the CP [2,3,4]. In addition, the significant accumulation of radioactive substances by various environmental objects was facilitated by the relative isolation of the CP, which made it one of the most contaminated reservoirs in the Chornobyl Exclusion Zone [5].
Immediately after the onset of 137Cs influx into open waterbodies, a commonly observed trend is the rapid accumulation of this radionuclide by aquatic organisms. In the initial minutes and hours, bacteria and filamentous algae become saturated with this radionuclide, followed by higher aquatic plants over a period ranging from several days to several weeks. The peak concentration of 137Cs in nonpredatory fish species was observed within 1–3 months, while in predatory fish species, it was recorded within 2–15 months after the entry of this radionuclide into the waterbody [6,7,8].
Absorption of radionuclides in fish occurs through the gill apparatus and digestive tract [9,10]. Over the past decades, research on the mechanisms of 137Cs uptake in fish organisms has identified the predominant pathway of this radionuclide entering their bodies as food ingestion [11,12,13,14,15,16].
Before the Chornobyl accident, the 137Cs specific activity in fish living in CP was: Blicca bjoerkna: 2–14, Carassius Carassius: 2–25, Cyprinus carpio: 1–12, Abramis brama: 0.3–9, Rutilus rutilus: 0.4–9, Sander lucioperca: 3–30 Bq/kg. After the accident, the amount of 137Cs in fish in CP significantly increased. The benthophagous fishes reached the maximum specific activity of 137Cs (up to 276,000 Bq/kg in B. bjoerkna) during the years 1986–1987. By the time the water level began to decrease in 2013, the content in benthophage fish had decreased by about 100 times, and in 2012–2013 was at the level of 800–3400 Bq/kg [17].
The specific activity dynamics of 137Cs in fish of higher trophic levels exhibit distinct patterns. In the case of Perca fluviatilis and Silurus glanis, unlike fish of lower trophic levels, the highest specific activity of 137Cs was observed two years after the accident (1987–1988). From 1986 until now, fish with higher trophic levels consistently exhibited higher radiocaesium content levels than nonpredatory fish species [17]. Changes in the specific activity of 137Cs in fish largely depend on the trophic level effect [18].
The studies on the distribution of radiocaesium in various organs of fish in the water reservoir have indicated that stable relationships in the radionuclide content in fish organisms were established within 1–4 years of the accident. Specifically, the highest levels of specific activity of 137Cs were found in the muscles, while the fat exhibited the lowest [19].
1.2. 137Cs in Fish of the Kaniv Reservoir (KR)
Before the Chornobyl Nuclear Power Plant accident, the contamination of the KR with artificial radionuclides was solely attributed to global fallout stemming from nuclear weapons testing. After the ChNPP accident, the contamination of the KR occurred in three stages. The first stage (starting on 30 April 1986) was the short-term acute period caused by the deposition of radioactive aerosols onto the reservoir water’s surface. The second phase of increased radionuclide influx occurred from May 16 to May 22, 1986, resulting from the transportation of contaminated radionuclides from the northern catchment areas to the water masses. Following the completion of this period of intense influx, the third stage began, characterized by the chronic influx of 137Cs and 90Sr into the KR, originating from the Kyiv Reservoir catchment areas and the Desna River. Since 1987, 137Cs and 90Sr have contributed to water contamination with artificial radionuclides [20,21].
As a result of the transfer of 137Cs through the food chain, the content of this radionuclide in the muscle tissue of most nonpredatory fish species in the KR reached its peak within 2–6 months of the accident, reaching levels of 100–200 Bq/kg. Due to an unavoidable delay in the passage of 137Cs through the food chain in ichthyophages, the highest levels were registered 5–12 months after the accident. In the muscles of Esox lucius, the 137Cs content reached 200 Bq/kg; they reached 250 Bq/kg in S. glanis and S. lucioperca, 300 Bq/kg in P. fluviatilis, and 600 Bq/kg in Aspius aspius [20]. Following the initial peak, the concentration of 137Cs in fish from the KR gradually decreased over time. The specific activity levels of radiocaesium in fish of KR were 2.5–40.0 Bq/kg (2012–2014), which is below the regulatory food safety norm [22].
1.3. 137Cs in Fish after an Accident at Fukushima Dai-ichi Nuclear Power Plant
Subsequent investigations conducted after the Fukushima Dai-ichi nuclear accident in 2011 have provided further evidence supporting the previously observed general trends in the 137Cs contamination of various fish species following the Chornobyl accident. The determinant factor of 137Cs content in fish is the level of environmental contamination, whereby higher levels of radioactive contamination in waterbodies and their catchment areas result in an increased contamination of the fish. Studies have shown that freshwater fish exhibit significantly higher levels of 137Cs accumulation than their marine counterparts, with concentrations exceeding approximately a hundredfold. Additionally, several factors, such as the route of entry into the fish’s organism (mainly through food consumption), as well as species-specific characteristics, have been identified as influencing the concentration levels of radiocaesium in fish [23].
One study [24] reported that the highest radiocaesium concentration was detected in the muscles of predatory fish species. This distribution pattern of radiocaesium among fish organs is likely characteristic of when this radionuclide enters fish organisms during any accident involving the release of radiocaesium into waterbodies. After the release of cesium radioisotopes into freshwater waterbodies in Japan, there was a delay in reaching peak levels in predatory fish compared to herbivorous and planktivorous species [25]. This peak-level delay was also observed in the CP and the KR after the Chornobyl accident [17,20]. Furthermore, according to [25], predatory salmonid fish exhibited longer ecological half-lives for radiocaesium (Teco: 1.2–2.6 years) compared to phyto- and planktivorous fish (0.99 and 0.69 years, respectively). The ecological half-life (Teco) of 137Cs refers to the time it takes for the specific activity of radiocaesium in an ecosystem or environmental object to decrease by half.
The main pathway of radiocaesium uptake in fish is through their food consumption. This applies not only to freshwater fish but also to marine species. Studies conducted on marine fish species off the coast of Japan after 2011 indicate that the primary source of contamination in olive flounder was the composition of their diet. This species’ estimated Teco of radiocaesium is approximately 5–6 months [26]. Subsequent investigations have revealed that the Teco is influenced by the sampling location, with a maximum Teco of 0.49 years observed at the Fukushima Dai-ichi Nuclear Power Plant port, compared to 0.38 years in other coastal areas of Japan [27].
According to [28], the duration of the radiocaesium half-life in fish is influenced by the sampling location and the specific fish species. Among three fish species—fat greenling, marbled flounder, and red stingray—a more rapid decline in 137Cs content was observed at the Fukushima Dai-ichi Nuclear Power Plant port compared to the surrounding area, resulting in shorter Teco values (ranging from 0.24 years to 0.53 years) compared to fish from the 20 km radius zone (which ranged from 0.70 years to 1.16 years). In contrast, the Japanese flounder exhibited a notably longer period of ecological half-life of 137Cs at the Fukushima Dai-ichi Nuclear Power Plant port (0.88 years) compared to the 29 km radius area (0.66 years).
In the case of Plaice, a species of marine flatfish, the ecological half-life of 137Cs can exceed 0.22 years, indicating the persistence of risks for humans for an extended period after the accidental release of radiocaesium into waterbodies [29].
Similarly, comparable half-life periods for 137Cs were observed in other marine organisms following the Fukushima Dai-ichi Nuclear Power Plant accident. The mollusks showed half-life periods of 0.52 years for two-shelled mollusks and 0.28 years for gastropods. However, in higher crustaceans (Malacostraca) and polychete worms (Polychaeta), the decrease in 137Cs concentration was more slight, resulting in more extended half-life periods of 0.57 years and 1.33 years, respectively [30].
1.4. Ecological Half-Life of 137Cs in Fish
The duration of the ecological half-life of 137Cs in different fish species was estimated in lakes across Europe after the Chornobyl accident [31]. For the same fish species, Perch (P. fluviatilis), the Teco varied in different waterbodies: 2 years in Lake Hillesjön (Sweden) and only 1 year in IJsselmeer (The Netherlands). In Lake Hillesjön, Pike (E. lucius) exhibited a Teco of 2.4 years, while in the Finnish Lake Iso Valkjärvi, it was 4.6 years. The authors attributed the differences in Teco to temperature influences. The time elapsed since water contamination, lake characteristics, and fish species can also affect this parameter.
In a study [32], Pike in Lake Vorsee (Germany) showed a 137Cs Teco of 2.1 years, which is comparable to the results presented in [31]. For Small Cyprinidae from the same waterbody, the authors calculated two half-life periods: an initial short-term period (immediately after contamination) lasting 0.64 ± 0.1 years, and a significantly more extended period of 6.7 ± 2.2 years.
In the Savannah River (USA), the effective half-life (Teff) of 137Cs in largemouth bass, sunfishes, and catfishes was determined to be 7.6–8.1 years, which was significantly shorter than the Teff of 137Cs in soil and vegetation [33]. The authors also noted that Teff is lower in waterbodies with high water turnover compared to relatively stagnant reservoirs, where Teff for bullheads (Ameiurus spp.) may exceed the physical half-life. For largemouth bass and sunfishes, it may be 13.4 to 16.7 years.
Based on the analysis of reported studies and scientific publications, it can be concluded that the period of the ecological half-life of 137Cs in fish organisms depends on several factors, including the level of contamination of the waterbody with this radionuclide and the ecological group to which the fish belongs. The primary objective of this study is to determine the ecological half-life of 137Cs in freshwater fish species inhabiting two waterbodies contaminated with radiocaesium following the Chornobyl nuclear accident. In this work, we included fish with different feeding habits found in the cooling pond of the Chornobyl nuclear power plant and the Kaniv Reservoir of the Dnieper River cascade.
2. Materials and Methods
2.1. Sampling Sites
Fish sampling was conducted in the cooling pond of the Chornobyl Nuclear Power Plant and the Kaniv reservoir (Figure 1).
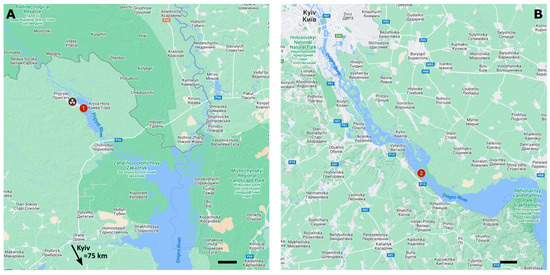
Figure 1.
Fish sampling sites: (A) CP (No. 1) within the Chornobyl exclusion zone and (B) KR (No. 2) in the Kyiv south region, both located in Ukraine. Scale bars represent a distance of 5 km. Maps were created using Google My Maps (Google LLC).
Coordinates of the sampling sites:
- The Cooling Pond of ChNPP: 30.1429120 E, 51.3732884 N, (point No. 1);
- Kaniv Reservoir: 30.9694639 E, 50.0493128 N, (point No. 2).
The Chornobyl Nuclear Power Plant’s cooling pond is an artificial reservoir located southeast of the nuclear power plant site. It was created by separating a section of the Pripyat River floodplain using an enclosing dam. The shape of the CP is close to oval, surrounded by a dam along its perimeter, with a stream-dividing dam running along its longitudinal axis. The coastal pumping station is northwest of the CP, replenishing water losses by drawing from the river. Before the commissioning of the first phase of the nuclear power plant in 1982, the area of the CP was 12.7 km2, with a volume of 59 million m3. In 1982, with the launch of the third unit of the second phase of the nuclear power plant, the area of the CP expanded to approximately 22.9 km2, with a water volume of about 150 million m3. The average width is 2 km, the length is 11.4 km, and the average depth is 6.6 m, reaching a maximum of 20 m. The length of the enclosing dam is 21.6 km. Almost 40% of the water volume is located below the average depth of the reservoir, and depths exceeding 10 m account for 28% of the total water mass. These areas of the CP are typically closed basins where the deposition of radionuclides mainly occurs [34]. A drainage channel is constructed along the downstream slope of the dam, consisting of two main sections: the northern section (water discharge of 1–1.2 m3/s) and the southern section (water discharge of 0.9–1 m3/s). Drainage water from the northern drainage channel is released through stone-filled filtering prisms directly into the Pripyat River. In contrast, drainage water from the southern drainage channel flows into the Hlynitsa Stream and the Pripyat River. Filtration losses from the CP amount to 70–100 million m3/year and ultimately flow into the Pripyat River [35]. Fish catching was conducted in the northern part of the CP (Figure 1A).
The Kaniv reservoir (KR) is the youngest among the cascade of reservoirs created on the Dnieper River. It was filled with water from 1974 to 1976. The KR covers an area of 675 km2, with a length of approximately 123 km and a maximum width of 8 km (Figure 1B). Its maximum depth is 21 m. Shallow waters characterize the shoreline of the KR. The major rivers that flow into it are the Desna, Stuhna, and Trubizh. The largest cities along the coast of the KR include Kyiv, Ukrayinka, Pereyaslav, and Kaniv. The reservoir dam is located east of Kaniv and comprises the Kaniv Hydroelectric Power Plant and a lock. The dam was constructed between 1972 and 1978 and stretches 16 km [36]. The water level in the reservoir fluctuates within a range of 0.5 m, and complete water exchange occurs 17–18 times per year. The primary water sources for the KR are the Desna River and the Kyiv Reservoir, fed by the waters of the Dnieper, Pripyat, and Teteriv rivers.
2.2. Preparation of the Fish Samples
For the study, seven fish species commonly found in large reservoirs in Ukraine [37] were selected (Table 1).

Table 1.
Fish species and range of samples periods.
Fish catching was primarily conducted during the summer and autumn using recreational fishing gear, such as spinning rods, fishing rods, and fyke nets (with mesh sizes ranging from 14 to 120 mm). In 1986, the sampling took place from late August to November, while in 2013 and 2014, it occurred in May and June. Not all fish species were sampled in 1986 in the CP of the ChNPP.
Cessation of water supply from the Pripyat River and subsequent large-scale transformations of the cooling pond could have led to unpredictable changes in the specific activity levels of 137Cs in fish. Therefore, this study utilized data on the accumulation of this radionuclide in fish only until 2013, which marked the beginning of the gradual transformation of the CP.
In this study, we determined the 137Cs content for fish of various feeding types:
- Benthophages: B. bjoerkna (Silver bream), A. brama (Bream), and R. rutilus (Roach) are characterized as benthic feeders. Their diet primarily comprises benthic invertebrates, such as mollusks, small crustaceans, mosquito larvae, worms, and organic detritus that settle on the bottom.
- Mixed type of nutrition (Omnivores): P. fluviatilis (Perch) and S. glanis (European Catfish) exhibit versatile dietary behavior. As they mature, these species have the capacity to adapt their feeding preferences, gradually incorporating other fishes into their diet. Their feeding habits can also be influenced by seasonal variations and the availability of food resources, allowing them to adjust and expand their feeding spectrum dynamically.
- Ichthyophages: S. lucioperca (Pikeperch) and A. aspius (Asp) are prominent piscivorous species, exhibiting a predatory feeding strategy focused on consuming fish.
For the determination of 137Cs content, fish specimens of similar size and mass were used for each species. Primary sample preparation at the capture site involved species identification, weighing, and length measurement. Data were recorded in a field diary. Whenever feasible, each captured fish was placed entirely in a polyethylene bag. In cases where packaging the entire sample was not possible due to its size, a portion of the carcass was separated at the capture site. Fish samples were then transported to the laboratory for further processing.
In the laboratory, the fish were additionally cleaned of debris and scales. For fish weighing up to 1 kg, the head and fins were removed, the resulting carcass was cut open along the belly, and all internal organs, spine, ribs, and skin were removed. If the fish weighed more than 1 kg, muscles from the near head, middle, and posterior parts were excised. When multiple fish of the same species were selected, an average sample was prepared.
Subsequent sample preparation involved homogenization using a blender. The obtained mass was thoroughly mixed, and a required amount (100–250 g) was stored for gamma spectrometric analysis. The selected sample was placed in calibrated plastic containers and stored in a freezer at a temperature of −18 °C until the quantitative determination of radionuclides was performed. Prior to measurements, the samples were taken out of the freezer and left to thaw at room temperature in the laboratory for approximately 12 h. Each fish sample consisted of 1–7 individuals.
2.3. Radiometry
To determine the ecological half-life (Teco) of 137Cs in fish species, specific activity data of this radionuclide were obtained through gamma spectrometric measurements. From 1986 to 2002, the quantification of 137Cs was performed using gamma spectrometry methods with germanium coaxial drift detectors (type DGDК-40 or DGDК-180) with an energy resolution of 3.5–4.5 keV for 60Co (1332 keV line) and multichannel analyzers ICA-70, AFORA, NOKIA, NOKIA LP4900B. To allow 137Cs quantification in relatively “clean” fish samples from the KR, the spectrometer was protected with a metal shield with a thickness of 150 mm, made from the hull materials of ships built before 1945, i.e., before the release of radionuclides into the environment due to nuclear explosions. From 2001 to 2003 and from 2009, measurements were conducted using a “CANBERRA” gamma spectrometer based on a germanium coaxial detector GC-6020 with a resolution of 1.8 keV for 60Co (1332 keV line) and an efficiency of 60%. The digital processor DSP-9660 by “CANBERRA” was used. The detection unit was shielded with a 100 mm lead shield to prevent the influence of background gamma radiation.
The duration of specific activity measurements for fish samples varied depending on the sampling site. For CP samples, the range was 600 to 14,400 s, and for KR samples, 7200 to 86,400 s. The measurement errors did not exceed 10%. The fish samples were not subjected to any thermal treatment (heating) or drying. The measurements of 137Cs specific activity were conducted for raw mass, and the specific activity data of the fish samples were also calculated for the fresh weight (Bq/kg fresh weight).
2.4. Calculation of the Ecological Half-Life of 137Cs
To describe the process of radionuclide extraction from the environmental object, the time required for the radionuclide activity in the object to decrease by half has been considered. For this purpose, two calculated parameters are used: the ecological half-life (Teco) and the effective half-life (Teff).
The sum of the reciprocals of T1/2 (the physical half-life of the radioactive element) and Teco (the ecological half-life of the same isotope) determines the reciprocal value of the effective half-life, i.e., 1/Teff = 1/Teco + 1/T1/2. The ecological half-life (Teco) represents an integral value that sums up all processes contributing to the reduction of radioactivity in the environment (object) beyond physical decay [32]. The physical half-life (T1/2) for 137Cs is 30.05 years [38]. Teff, Teco, and T1/2 have the dimension of time (years).
The activity of the radionuclide at a given time t is determined using the formula: A(t) = A(0) 2−μt, where A(0) represents the specific activity of the radionuclide at time zero, and μ is the coefficient that characterizes the rate of activity decay over time. A higher coefficient indicates a faster decay of the radionuclide. When t = 1/μ, 2−μt becomes equal to 1/2, and A(t) = A(0)/2. If 1/μ is denoted as Teff, then A(t) = A(0) 2−t/Teff. The equation describes the logarithmic representation of the power law: L(t) = ln(A(0)) − ln 2(t/Teff) = b − at, where b represents ln(A(0)) and a represents (ln 2)/Teff (the tangent of the slope angle of the line). Hence, Teff = (ln 2)/a. Thus, we arrive at the formula mentioned above: 1/Teff = 1/T1/2 + 1/Teco [39].
For a better overview, we also provide the Teff and β values. β is defined as the reciprocal of the ecological half-life, i.e., 1/Teco. β parameter represents the rate at which the specific activity of the investigated radionuclide (in our case, 137Cs) decreases in the studied environmental object. The dimension of β is expressed as 1/year. The starting (zero) point for the calculations was the year in which the maximum levels of radiocaesium content were recorded for each fish species and each sampling site.
3. Results
3.1. Dynamics of 137Cs Content in Fish from the Cooling Pond of ChNPP and Kaniv Reservoir
Figure 2 depicts the dynamics of 137Cs content in the studied fish species. Significant decreases in specific activity levels of this radionuclide were observed in all fish species during the observation period. In the CP, the decrease in 137Cs content occurred more intensively, ranging from 27 to 108 times. Among all fish species, S. glanis showed the slightest decrease in 137Cs content in the CP, reducing by only 9.5 times between 1988 and 2012. In the KR, the reduction in specific activity of 137Cs in fish was not as intense, ranging from 5 to 14 times. B. bjoerkna stood out among the investigated species, exhibiting the highest intensity of concentration decrease in the CP and KR, with reductions of 143 and 27 times, respectively. By 2013–2014, the specific activity levels of 137Cs in all fish species in the KR had decreased significantly below the permissible limits for fish consumption established in Ukraine, which is 150 Bq/kg [22]. The lowest radiocaesium content was found in benthophagous fish, ranging from 4 to 7 Bq/kg. Ichthyophages and fish of mixed type of nutrition had higher concentrations of 137Cs, ranging from 12 to 23 Bq/kg.
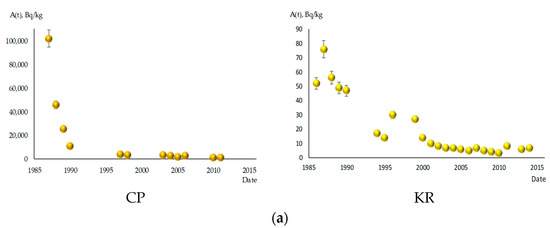
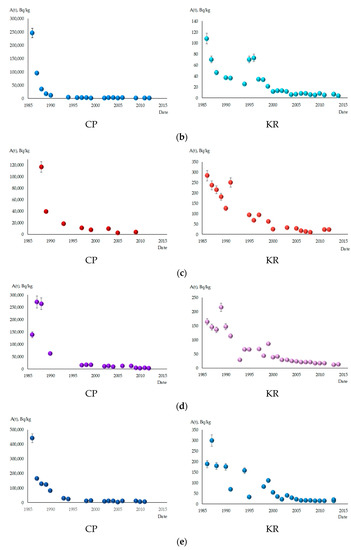
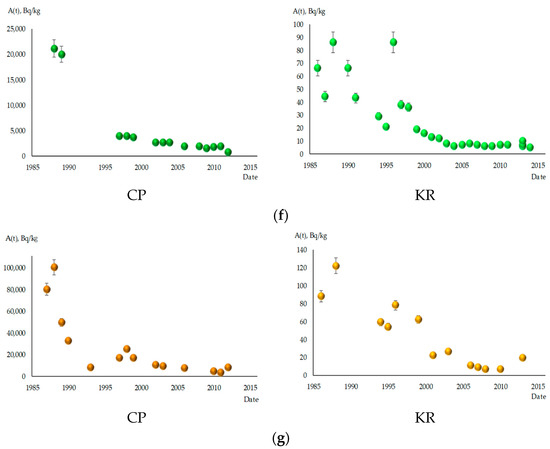
Figure 2.
The content of 137Cs in studied fish species in the cooling pond of ChNPP (CP) and Kaniv Reservoir (KR), A(t) in Bq/kg fresh weight. (a) A. brama; (b) B. bjoerkna; (c) A. aspius; (d) P. fluviatilis; (e) S. lucioperca; (f) R. rutilus; (g) S. glanis.
Even 26 years after the accident (in 2012), the content of 137Cs in fish remains very high in the CP. This is particularly evident in ichthyophages and fish of mixed type of nutrition, where the specific activity of 137Cs reaches values of 3060–4900 Bq/kg. The highest radiocaesium concentration was recorded in S. glanis, with 8300 Bq/kg. Benthophagous fish exhibited lower specific activity levels of this radionuclide, ranging from 770 in R. rutilus to 1700 in B. bjoerkna, while A. brama occupied an intermediate position with 930 Bq/kg. The decrease in 137Cs content in fish follows an exponential pattern. This is particularly evident in the CP for all species without exception. For benthophage fish in the CP, the most pronounced, 10–25-fold decrease in specific activity of 137Cs occurred in the first 5 years after the accident, from 1986–1987 to 1990–1991. Subsequently, the decrease in specific activity of 137Cs slowed down, and over approximately 20 years, from 1990–1991 to 2011–2013, it decreased by no more than 10 times.
In the KR, the dynamics of 137Cs content in some fish species belonging to different ecological groups showed a saltatory pattern, meaning that the decrease in the 137Cs activity was not smooth but rather occurred in sudden jumps or leaps, especially in the first 10–15 years after the accident. Relative stabilization of radiocaesium content levels in this reservoir occurred later than in the CP. In the KR, it took place around the year 2000, while in the CP, it occurred on average by 1997. For fish species at higher trophic levels (S. glanis, S. lucioperca, and P. fluviatilis), maximum levels of 137Cs content were recorded in 1987–1989, in contrast to benthophagous fish, where the maximum specific activity of this radionuclide was recorded in 1986–1987.
3.2. Calculation of Teco of 137Cs
The results of calculating the ecological half-life (Teco), β, and effective half-life (Teff) of 137Cs in fish species in the cooling pond of Chornobyl Nuclear Power Plant and Kaniv Reservoir are presented in Table 2.

Table 2.
Teco, β, and Teff values in fish species in CP and KR.
The minimum values of Teco for most (five out of seven) investigated species were determined in CP. This means that in the highly contaminated reservoir, the rate of decrease in 137Cs specific activity (β) in fish of different trophic levels was greater, compared to the relatively “clean” KR (Figure 2, Table 2).
In KR, the duration of the Teco is generally longer. In this reservoir, the maximum duration of the ecological half-life was observed in fish of mixed type of nutrition, P. fluviatilis (9.92 years) and the benthophage fish A. brama (9.11 years). In contrast, in CP, these same species have a minimum ecological half-life of 5.58 and 5.84 years, respectively.
An exception to this trend is the ecological half-life of 137Cs in R. rutilus (benthophages) and S. glanis (fish of mixed type of nutrition). In these species, the decrease rate of the 137Cs specific activity β in the CP is considerably slower than in the KR. It should be noted that the ecological half-life of radiocaesium in R. rutilus and S. glanis in the Kaniv Reservoir was practically the same as the Teco for other species.
Among the fish species examined, it was found that in five species, there was an inverse relationship between the ecological half-life of 137Cs and its content in the fish’s body. This means that higher levels of specific activity of radiocaesium corresponded to a faster removal rate (β) from the body. However, the relationship is direct for two species (R. rutilus and S. glanis), where higher concentrations of 137Cs in the fish resulted in longer ecological half-life periods.
4. Discussion
The accumulation of 137Cs by fish following the Chornobyl accident can be divided into several stages. In most species, the accumulation followed a two-stage pattern. A sharp decrease in radiocaesium concentrations characterized the first stage, while the second stage exhibited a slower decline in the levels of this radionuclide. For ichthyophages and fish of a mixed type of nutrition, three stages can be distinguished: the first stage is characterized by an increase in 137Cs content in the first few years following the accident, the second stage exhibits a rapid decrease in the concentrations of this radionuclide, and the third stage shows stabilization of radiocaesium levels. These dynamics of 137Cs accumulation are likely influenced by the trophic levels effect, which implies a certain delay in the uptake and elimination of 137Cs by fish occupying higher trophic levels compared to those at lower trophic levels [18]. This effect can be attributed to the fact that, in the case of ichthyophages, 137Cs enter the fish body in a form processed by organisms in the previous trophic chain, making it more biologically accessible.
Another reason for the higher concentrations of 137Cs in predatory fish, compared to nonpredatory fish species, can be attributed to the increased acidity within their gastrointestinal tracts. Higher acidity promotes the dissolution of 137Cs and, consequently, increases its availability for absorption in the gastrointestinal tract [40].
The decrease in 137Cs specific activity in fish of CP occurs at a faster rate compared to fish in the KR. This difference is particularly notable among benthophages fish in the years following the accident. In certain years, an increase is observed instead of a decrease in the 137Cs specific activity in fish from the KR (B. bjoerkna, R. rutilus) (see Figure 2). In one study [41], possible explanations for these observations were examined, including specific radioactivity of 137Cs in the water, different levels of radionuclide water contamination at different spots of the reservoirs, the size effect, seasonality, water temperature, trophic level, species-specific characteristics and dietary spectrum, and key indicators of water quality. However, the influence of these factors on the recorded faster excretion of radiocaesium from the body of fish in the CP, in comparison with the rate of the excretion of this radionuclide from fish in KR, was not established. An exception could be the more rapid decrease in 137Cs specific activity in the CP, which, mediated through food chains, may determine the levels of 137Cs specific activity in aquatic organisms, including fish. The study also hypothesized the existence of other factors or a combination of factors that affect the dynamics of specific activity of 137Cs in fish.
The different duration of the ecological half-life of 137Cs in fish of the same species but in waterbodies with different pollution levels (see Table 2) can be interpreted as evidence of the manifestation of the phenomenon of radiation hormesis [42]. Hormesis is associated with the positive effects of low doses of toxic substances on organisms. It is consistent with the Arndt–Schulz law, which states: “For every substance, small doses stimulate, moderate doses inhibit, large doses kill” [43]. Radiation hormesis has been studied in various organisms, ranging from viruses and bacteria to primates and humans [43,44,45]. Its effect involves the beneficial influence of ionizing radiation within a specific range of doses, which can manifest as accelerated growth and development in irradiated biological entities and increased fertility [43]. Radiation hormesis can occur both in the case of external exposure and internal irradiation caused by incorporating radioactive isotopes into biological tissues [46]. Animal experiments have demonstrated that pre-exposure to low doses promotes the occurrence of a radioadaptive effect [47]. Studies have been conducted on the combined effects of low doses of ionizing radiation and heavy metals on the Danio rerio (Zebrafish). Positive effects of low doses of ionizing radiation on the adaptive response of fish to the action of heavy metals have been observed [48]. Another study [49] demonstrated the radiation stimulation of embryonic development in fish using low doses of radiation.
Today, there is no unified conceptual framework for the phenomenon of radiation hormesis. One of the explanations for the manifestation of radiation stimulation is that it is a secondary response to damage. It can be considered as the recovery mechanism at different levels of biological organization (repair, cell repopulation, regeneration, organism-level repopulation) [43]. Our experimental results are difficult to explain with this concept of radiation hormesis. Here, we observe an effect when small amounts of 137Cs are retained by the body of fish of the same species for a longer time compared to large amounts of radiocaesium in a more polluted reservoir. This is true for five out of the seven studied fish species that belong to different ecological groups (P. fluviatilis is a fish of mixed type of nutrition, B. bjoerkna and A. brama are benthophages, S. lucioperca and A. aspius are ichthyophages). In these species, the rate of decrease in 137Cs concentrations (β) is lower in KR than in the CP.
Experiments on plants indicate that the phenomenon of hormesis is more frequently observed in certain species and varieties [50]. It can be postulated that such a conclusion extends to other biotic entities, particularly fish, indicating the manifestation of species-specific responses of organisms to the effects of ionizing radiation. In our research, this species-specific manifestation has been represented using the Teco values for two fish species belonging to different ecological groups: the benthophagous R. rutilus, and fish of a mixed type of nutrition, S. glanis. These two species exhibit a duration of Teco in the CP that is almost twice as long as in other species (Table 2). Additionally, the value of Teco of 137Cs for these species in the KR does not significantly differ in duration from the Teco of other species. Since these two species belong to different ecological groups, the differences in Teco of 137Cs cannot be explained using the trophic level effect or the high amount of radioactive cesium in their nutrition, as these factors also influence other fish species. The observed phenomenon may likely be attributed to an increased demand for 137Cs in R. rutilus and S. glanis, indicating the species-specific responses to the level of 137Cs in their food and, indirectly, in their habitat.
Another manifestation of species-specific responses to ionizing radiation can be observed in P. fluviatilis (a fish of mixed type of nutrition) and the benthophagous A. brama. These two species demonstrated the shortest Teco of 137Cs in the CP (5.58 and 5.84) and the longest Teco in the “clean” KR (9.98 and 9.11). This indicates that these two species also exhibit a specific reaction to the content of 137Cs in their food: the rates of decrease in the concentration of this radionuclide are the lowest in the “clean” waterbody and highest in heavily contaminated environments.
The existence of two distinct mechanisms responsible for the interaction between fish and 137Cs can explain the parameters of radiocaesium accumulation and removal from the fish body. The first mechanism regulates the absorption of 137Cs from food, while the second mechanism is responsible for the release of this radionuclide by fish. Both mechanisms operate continuously, and their relative influence likely depends on the degree of environmental contamination and the fish species involved. The dominance of one mechanism at a given time leads to a decrease, increase, or stabilization of the 137Cs concentration in fish at a consistent level.
The values of Teco in fish, indicating the prolonged retention of small quantities of 137Cs, can be attributed to the favorable effects of this radionuclide on the fish organism (the manifestation of the radiation hormesis effect). At the organism level, there is a selective accumulation of this radionuclide from food. The relatively extended retention of 137Cs in small quantities by fish can be considered a rule, with exceptions observed for species that require significant amounts of this radionuclide.
5. Conclusions
Towards the end of the fish sampling for this study and prior to the water level reduction in the CP (2013) and the KR, there was a notable decrease in the detected levels of 137Cs in fish across various ecological groups compared to the early postaccident years. The reduction of 137Cs concentrations in fish was carried out according to the exponential law in the CP and, to a lesser extent, for KR. The relative decrease in radiocaesium content is more pronounced in fish of different species of CP than in KR.
After the onset of a period of relative stabilization, the levels of 137Cs specific activity in predatory fish and fish of a mixed type of nutrition (S. lucioperca, A. aspius, R. rutilus, S. glanis) had higher levels of radiocaesium in the CP and KR, compared to with nonpredatory species (B. bjoerkna, A. brama, and R. rutilus).
The duration of Teco varies among different fish species in the CP and KR. P. fluviatilis, B. bjoerkna, A. brama, S. lucioperca, and A. aspius belong to different ecological groups and have a much greater rate of decrease in the specific activity levels of 137Cs (β) in the CP compared to KR. Among the two studied species, R. rutilus (benthophagous) and S. glanis (a fish of mixed type of nutrition), the duration of 137Cs Teco was nearly twice as long as that observed in all fish species in the CP. Furthermore, Teco in R. rutilus and S. glanis is higher in the CP compared to the KR. For the other two species, P. fluviatilis (a fish of a mixed type of nutrition) and benthophagous A. brama, the shortest 137Cs Teco values were observed in the KR, while the longest Teco values were found in the CP. This indicates the manifestation of species-specific absorption/excretion of 137Cs from the body of fish, as well as the species-specific manifestation of radiation hormesis; therefore, different amounts of absorbed radiocaesium have a positive effect on fish.
Author Contributions
N.Z. conceptualized the study; O.S.B. developed the methodology; L.P.P. conducted validation; O.S.B. and V.S. performed formal analysis; N.Z. carried out the investigation; O.S.B. and L.P.P. curated the data; N.Z. drafted the original manuscript; V.S. provided critical review and editing; and V.S. visualized the findings. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not required for this study.
Informed Consent Statement
This study does not involve human subjects and is exempt from informed consent requirements.
Data Availability Statement
All data supporting the reported results have been comprehensively included in the article.
Acknowledgments
The authors would like to express their gratitude to the late Oleg Zarubin for his extensive contributions to this study. Oleg Zarubin, who dedicated his research efforts from 1986 until his passing in 2017, conducted pioneering investigations on the impact of the Chornobyl Nuclear Power Plant accident on the accumulation of various artificial radionuclides in fish from the Cooling-pond of Chornobyl Nuclear Power Plant and Kaniv Reservoir of the Dnieper River. Oleg Zarubin’s profound expertise and valuable research materials greatly influenced the outcome of this manuscript. While deeply missed, his legacy inspires scientific endeavors in the field.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- International Atomic Energy Agency. Environmental Consequences of the Chernobyl Accident and Their Remediation: Twenty Years of Experience; STI/PUB/1239; Report of the Chernobyl Forum Expert Group “Environment”; Radiological Assessment Reports Series; International Atomic Energy Agency: Vienna, Austria, 2006; ISBN 92-0-114705-8. [Google Scholar]
- Voitsekhovich, O.V.; Kanivets, V.V.; Laptev, G.V. The Current State of Radioactive Contamination of Water Objects in the Zone of Influence of the Accident. In Radioecology of Water Objects in the Zone of Influence of the Accident at the Chernobyl Nuclear Power Plant; Chernobyltekhinform: Kyiv, Ukraine, 1997; pp. 60–96. (In Ukrainian) [Google Scholar]
- Kuzmenko, M.I. Radioecological Problems of Water Bodies of Ukraine. Hydrobiol. J. 1998, 34, 95–119. (In Ukrainian) [Google Scholar] [CrossRef]
- Kononovich, A.L.; Oskolkov, B.Y.; Korotkov, V.T. Radiation State of the Cooling-Pond of the Chernobyl Nuclear Power Plant and Its Radioecological Status. In Proceedings of the 4th International Scientific and Technical Conference “Results of 8 Years of Work to Eliminate the Consequences of the Chernobyl Accident”. 160–164. (In Ukrainian)
- Zarubin, O.L. Dynamics of the Content of Cesium-137 in Some Fish Species of the Cooling-pond of the Chernobyl Nuclear Power Plant. In Proceedings of the Scientific Conference of the Institute for Nuclear Research, Kyiv, Ukraine, 22–26 January 1996; pp. 286.2–290.2. (In Ukrainian). [Google Scholar]
- Zarubin, O.L.; Vishnevsky, I.N.; Trishin, V.V.; Zalissky, A.A.; Laktionov, V.A. Optimization of the Selection of Biological Objects in Radioecological Monitoring of Freshwater Reservoirs. In Proceedings of the International Conference BIORAD-2001 “Biological Effects of Low Doses of Ionizing Radiation and Radioactive Contamination Environment”, Syktyvkar, Russia, 20–24 March 2001; pp. 129–130. [Google Scholar]
- Zarubin, O.L.; Laktionov, V.A.; Lukashev, D.V.; Zalissky, A.A.; Golovach, A.I. Application of Biological Objects in Radiation Monitoring of Water Bodies. In Proceedings of the International Conference “Fifteen Years of the Chernobyl Disaster. Experience of Overcoming”, Kyiv, Ukraine, 18–20 April 2001. (In Ukrainian). [Google Scholar]
- Zarubin, O.L. Optimization of the Choice of Biotic Objects for a Comprehensive Assessment of Radionuclide Pollution of Water Bodies. In Proceedings of the Russian Scientific Conference “Medico-Biological Problems of Anti-Radiation and Anti-Chemical Protection”, Saint-Petersburg, Russia, 20–21 May 2004; pp. 453–454. (In Russian). [Google Scholar]
- Polikarpov, G.G. Radioecology of Marine Plants and Animals. In Modern Problems of Radiobiology; Atomizdat: Moscow, Russia, 1971; pp. 354–367. (In Russian) [Google Scholar]
- Polikarpov, G.G. Radioecology of Marine Organisms; Atomizdat: Moscow, Russia, 1964. (In Russian) [Google Scholar]
- Ilyin, D.I.; Moskalev, Y.I. On the Distribution, Excretion and Accumulation Coefficients of Strontium-90, Cesium-137 and Phosphorus-32 in Fish. In Distribution, Biological Action and Migration of Radioactive Isotopes; Medgiz: Moscow, Russia, 1961. (In Russian) [Google Scholar]
- Kanevsky, Y.P. Dynamics of K, Rb, and Cs Metabolism in Anadromous Cyclostomes during the Freshwater (pre-spawning) Period of Life. In Radioecology of Aquatic Organisms; Zinatne: Riga, Latvia, 1973; p. 129. (In Russian) [Google Scholar]
- Fleishman, D.G. Accumulation of Artificial Radionuclides by Freshwater Fish. In Modern Problems of Radiobiology; Atomizdat: Moscow, Russia, 1971; p. 395. (In Russian) [Google Scholar]
- Gustafson, P.F. Comments on Radionuclides in Aquatic Ecosystems. In Radioecological Concentration Processes; Pergamon Press: Oxford, UK, 1967; p. 853. [Google Scholar]
- Häsänen, E. Biological Half–Time of Caesium-137 in Three Species of Fresh-Water Fish: Perch, Roach and Rainbow Trout. In Radioecological Concentration Processes; Pergamon Press: Oxford, UK, 1967; p. 921. [Google Scholar]
- King, S.F. Uptake and Transfer of Caesium-137 by Chlamydomonads, Daphnie and Bluegill Fingerlings. Ecology 1964, 45, 852–859. [Google Scholar] [CrossRef]
- Zarubin, O.L.; Zarubina, N.E.; Zalissky, A.A.; Malyuk, I.A.; Kostyuk, V.A. Dynamics of Specific Activity of 137Cs in Fish of Different Types of Food in the Cooling-Pond of the Chernobyl Nuclear Power Plant. Hydrobiol. J. 2014, 50, 107–119. (In Ukrainian) [Google Scholar] [CrossRef]
- Kryshev, I.I.; Ryabov, I.N. About the Efficiency of Trophic Levels in the Accumulation of Cs-137 in Fish of the Chernobyl NPP’ Cooling Pond. In Proceedings of the First International Conference “Biological and Radioecological Aspects of the Consequences of the Chernobyl NPP accident”, Zeleny Mys, Ukraine, 10–18 September 1990. (In Russian). [Google Scholar]
- Zarubin, O.L.; Kostyuk, V.A.; Zalissky, A.A.; Babenko, V.V.; Litvinskaya, T.A.; Malyuk, I.A.; Laktionov, V.A.; Moshna, B.A. Dynamics of Distribution of 137Cs in Organs and Tissues of Fish from Different Ecological Groups of the Cooling Pond. Hydrobiol. J. 2012, 48, 109–115. (In Ukrainian) [Google Scholar] [CrossRef]
- Zarubin, O.L.; Zarubina, N.E. Radionuclide Contamination of the Kaniv Reservoir and Coastal Terrestrial Ecosystems. In Scientific notes of Ternopil National Pedagogical University named after Volodymyr Hnatyuk. Series: Biology. Special Issue “Hydroecology”. 2010; Volume 2, 201–203. (In Ukrainian) [Google Scholar]
- Zarubin, O.L.; Kanivets, V.V. The Content of Radionuclides in the Water of the Kanevsky Reservoir after the 1986 Chernobyl NPP Accident. Collect. Sci. Work Inst. Nucl. Res. 2005, 3, 110–130. (In Ukrainian) [Google Scholar]
- GN 6.6.1.1-130-2006; Permissible Levels of Radionuclides 137Cs and 90Sr in Food and Drinking Water. State Hygienic Standards; Approved. Order of the Ministry of Health of Ukraine from 19.08.97 No. 255. Ministry of Health of Ukraine: Kyiv, Ukraine, 2006. (In Ukrainian)
- Arai, T. Radioactive Cesium Accumulation in Freshwater Fishes after the Fukushima Nuclear Accident. SpringerPlus 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Furota, T.; Kagami, M.; Tagami, K.; Uchida, S. Inequality in the Distribution of 137Cs Contamination within Freshwater Fish Bodies and its Affecting Factors. Nat. Sci. Rep. 2021, 11, 5769. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Tomiya, A.; Enomoto, M.; Sato, T.; Morishita, D.; Izumi, S.; Niizeki, K.; Suzuki, S.; Morita, T.; Kawata, G. Radiological Impact of the Nuclear Power Plant Accident on Freshwater Fish in Fukushima: An Overview of Monitoring Results. J. Environ. Radioact. 2016, 151, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Tateda, Y.; Tsumune, D.; Tsubono, T.; Aono, T.; Kanda, J.; Ishimaru, T. Radiocesium Biokinetics in Olive Flounder Inhabiting the Fukushima Accident-Affected Pacific Coastal Waters of Eastern Japan. J. Environ. Radioact. 2015, 147, 130–141. [Google Scholar] [CrossRef]
- Tateda, Y.; Tsumune, D.; Misumi, K.; Aono, T.; Kanda, J.; Ishimaru, T. Biokinetics of Radiocesium Depuration in Marine Fish Inhabiting the Vicinity of the Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2017, 166, 67–73. [Google Scholar] [CrossRef]
- Wada, T.; Fujita, T.; Nemoto, Y.; Shimamura, S.; Mizuno, T.; Sohtome, T.; Kamiyama, K.; Narita, K.; Watanabe, M.; Hatta, N.; et al. Effects of the Nuclear Disaster on Marine Products in Fukushima: An Update after Five Years. J. Environ. Radioact. 2016, 164, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Pouila, S.; Oberhänslia, F.; Swarzenskia, P.W.; Bustamanteb, P.; Metian, M. The Role of Salinity in the Trophic Transfer of 137Cs in Euryhaline Fish. J. Environ. Radioact. 2018, 189, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sohtome, T.; Wada, T.; Mizuno, T.; Nemoto, Y.; Igarashi, S.; Nishimune, A.; Aono, A.; Ito, Y.; Kanda, J.; Ishimaru, T. Radiological impact of TEPCO’s Fukushima Dai-ichi Nuclear Power Plant Accident on Invertebrates in the Coastal Benthic Food Web. J. Environ. Radioact. 2014, 138, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.; Bergström, U.; Håkanson, L.; Heling, R.; Monte, L.; Suolanen, V. Estimation of Ecological Half-lives of Caesium-137 in Lakes Contaminated by Chernobyl Fallout. In Proceedings of the International Symposium on Environmental Impact of Radioactive Releases, Vienna, Austria, 8–12 May 1995; IAEA-SM-339/58. pp. 291–298. [Google Scholar]
- Zibold, G.; Klemt, E. Ecological Half-Times of 137Cs and 90Sr in Forest and Freshwater Ecosystems. Radioprotection 2005, 40, S497–S502. [Google Scholar] [CrossRef]
- Paller, M.H.; Jannik, G.T.; Baker, R.A. Effective Half-Life of Caesium-137 in Various Environmental Media at the Savannah River Site. J. Environ. Radioact. 2014, 131, 81–88. [Google Scholar] [CrossRef]
- Available online: https://uk.wikipedia.org/wiki/KanivReservoir (accessed on 5 July 2023).
- Kryshev, I.I.; Ryabov, I.N.; Chumak, V.K.; Zarubin, O.L.; Blynova, L.D.; Nikityn, A.I. Radioecological Processes in the Cooling Reservoir of the Chernobyl NPP. In Radioecological Consequences of the Chernobyl Accident; Nuclear Society of the USSR: Moscow, Russia, 1991; pp. 54–70. (In Russian) [Google Scholar]
- Dzepo, S.P.; Skalsky, A.S.; Bugai, A.D. Results of Monitoring and Special Studies of Radioactive Contamination of Groundwater in the Exclusion Zone. In Radioecology of Water Objects in the Zone of Influence of the Accident at the Chernobyl Nuclear Power Plant; Chernobyltechinform: Kyiv, Ukraine, 1997; pp. 166–196. (In Ukrainian) [Google Scholar]
- Freyhof, J.; Kottelat, M. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland; Berlin, Germany, 2007; ISBN 978-2-8399-0298-4. [Google Scholar]
- Bé, M.-M.; Chisté, V.; Dulieu, C.; Browne, E.; Baglin, C.; Chechev, V.; Kuzmenko, N.; Helmer, R.; Kondev, F.; MacMahon, D.; et al. Table of Radionuclides, Cs-137; Monographie BIPM-5; Bureau International des Poids et Mesures: Sèvres, France, 2006; Volume 3, ISBN 92-822-2218-7. [Google Scholar]
- Zarubina, N.E.; Semak, V.; Burdo, O.S.; Ponomarenko, L.P. Ecological Half-Life of 137Cs in Fungi. Ecologies 2023, 4, 11–19. [Google Scholar] [CrossRef]
- Zarubin, O.L. 137Сs in Fish of Kaniv Reservoir after 20 Years after Accident on ChNPP. Nucl. Phys. At. Energy 2006, 2, 106–114. (In Ukrainian) [Google Scholar]
- Zarubin, O.L.; Zarubina, N.E.; Kostyuk, V.A.; Malyuk, I.A.; Osadchaya, N.N.; Kanivets, V.V.; Zalissky, A.A. On the dynamics of the specific activity of 137Cs in fish from the Kaniv reservoir and the cooling pond of the Chornobyl NPP. In Proceedings of the IX International Scientific and Practical Conference “Ecological Safety: Problems and Ways of Excellence”, Alushta, Ukraine, 9–13 September 2013; V. 1. Rider: Kharkiv, Ukraine, 2013; pp. 115–119. [Google Scholar]
- Luckey, T.D. Radiation Hormesis: The Good, the Bad, and the Ugly. Dose-Response 2006, 4, 169–190. [Google Scholar] [CrossRef]
- Grodzinsky, D.M.; Shilina, Y.V.; Мikhyeyev, O.N.; Guscha, M.I. Radiation Hormesis—Retrospectivity and Modernity. Saf. Probl. Nucl. Pow. Plan. Chorn. 2005, 3, 17–30. (In Ukrainian) [Google Scholar]
- Moffett, J.R. Miasmas, Germs, Homeopathy and Hormesis: Commentary on the Relationship Between Homeopathy and Hormesis. Hum. Exp. Toxicol. 2010, 29, 539–543. [Google Scholar] [CrossRef]
- Tang, S.; Liang, J.; Xiang, C.; Xiao, Y.; Wang, X.; Wu, J.; Li, G.; Cheke, R.A. A General Model of Hormesis in Biological Systems and Its Application to Pest Management. J. R. Soc. Interface 2019, 16, 20190468. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, D.M.; Kolomiets, K.D.; Kutlahmedov, Y.O. Anthropogenic Radionuclide Anomaly and Plants; Grodzinsky, D.M., Ed.; Lybid: Kyiv, Ukraine, 1991. (In Ukrainian) [Google Scholar]
- Luckey, T.D. A Rosetta Stone for Ionizing Radiation. Radiat. Prot. Manag. 1994, 11, 73–79. [Google Scholar]
- Ostrovskaya, S.S.; Krizhanovsky, D.G.; Trushenko, O.S.; Shevchenko, I.F.; Gerasimchuk, P.G.; Konovalova, O.S. Influence of Ionizing Radiation and Heavy Metals on Organisms with the Impact of Modeling Effects and Radiation Hormesis. Bull. Probl. Biol. Med. 2022, 4, 84–91. [Google Scholar] [CrossRef]
- Hudkov, I.M. Basics of General and Agricultural Radiobiology; Publishing House of the Ukrainian Academy of Sciences: Kyiv, Ukraine, 1991; ISBN 7987-0005-4. (In Ukrainian) [Google Scholar]
- Grodzinsky, D.M. Radiobiology, 2nd ed.; Lybid: Kyiv, Ukraine, 2000; ISBN 966-06-0143-3. (In Ukrainian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
