Abstract
Micro-pollutants especially estrogens, progesterone, androgens, glucocorticoids, and growth hormones, are biological and chemical impurities that find their way into natural aquatic environments in trace quantities (ng/L), and possess a significant disturbance by impacting human and aquatic life. Due to the significant progress in in the analysis and detection techniques, these trace elements have been observed and quantified in several studies. However, as a result of limited methods and management technology, the adverse effects by these micro-pollutants in surface and coastal water is largely unknown. For this study, the compounds of estrogens, progesterone, androgens, glucocorticoids, and growth hormones have been selected according to their high frequent detection value in environmental waters. The concentration of the selected steroid and hormones ranges from 0.1–196 ng/L (estrogens), less than 0.1 to 439 ng/L (progesterone), 0.06–86 ± 2 (androgens), less than 0.1 to 433 ng/L (glucocorticoids), and 26.6 ng/g to 100 ng/L (growth hormones), and their percentage of removal efficiency varies from less than 10% to 99%, as the measurement of compounds concentration was found to be very low. Here, we report that future studies are necessary to detect the entry routes of these compounds into the environmental water, as well as to explore the technological approaches which are able to resolve this issue permanently.
1. Introduction
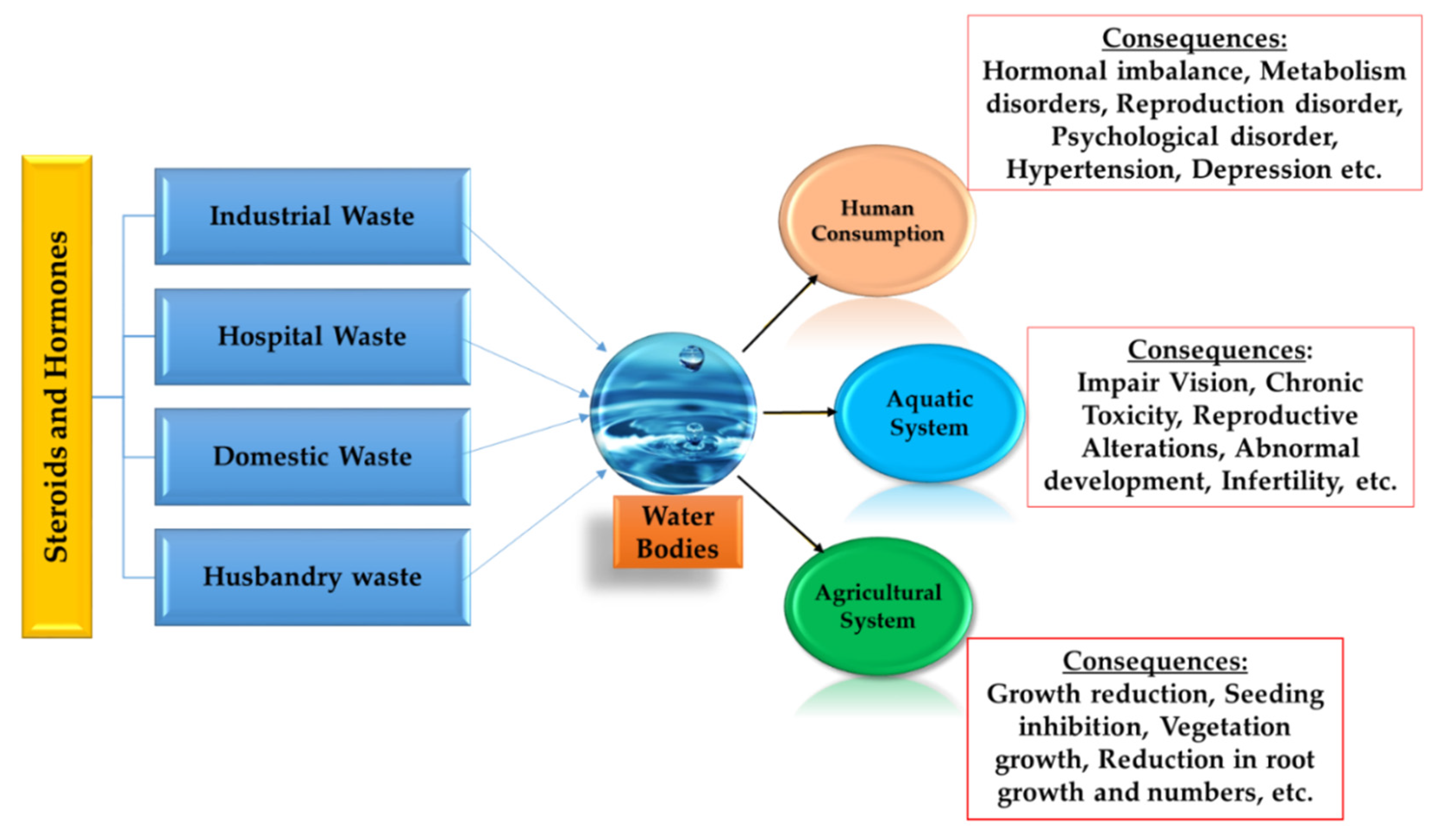
As the global population grows exponentially, it has constituted a parallel increase in the need for the world’s limited availability of freshwater. Hence, preserving the virtue of accessible water resources is one of the vital environmental concerns of the 21st century [1,2,3,4,5,6]. The potential adverse effect on human and ecological health due to the production, use, and disposal of numerous chemicals that offer improvements in industry, agriculture, medical treatment, and even common household conveniences have brought immense concern nowadays [7,8,9,10,11]. The usage of steroid hormones in pharmaceutical as well as personal care products (PPCPs), livestock, and husbandry have become a burning issue, as their presence pollutes the water resources in a significant way [12,13,14]. Over the past few years, due to the presence in the environment and the risks associated with steroid hormones, this has brought immense concern. Furthermore, both estrogen and androgen are highlighted in stressing the environment in several studies [5,8,13,14,15,16,17,18]. Existing wastewater treatment plants (WWTPs) are struggling to remove these compounds effectively and completely [1,2,3,4,16,17,18]. Figure 1 shows a summary of the sources, as well as the impacts, of steroids and hormones.
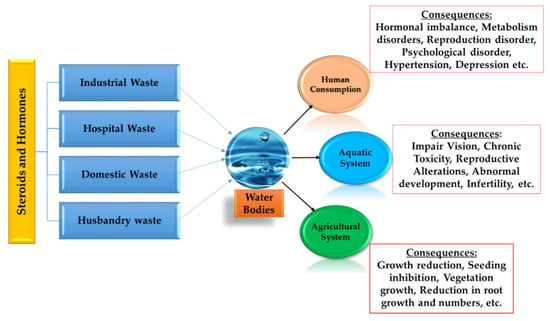
Figure 1.
Schematic diagram of main sources and fates of steroids and hormones in human world, plants, and the aquatic environment [19,20,21].
The existence of these steroid hormones acting as endocrine-disrupting compounds (EDCs) in the water resources has become a prominent topic in the field of environmental research and policy nowadays, although it was first discovered in the 1990s [13,21]. EDCs have attracted the attention of scientists, as they play a major role in altering the regular reproduction of humans and wildlife, as well as their development and growth, and interference in the aging or longevity of aquatic organisms [19,20,21,22,23,24,25]. Pharmaceutical and personal care products (PPCPs) which contain natural and synthetic steroid hormones are referred to as contaminants of emerging concern (CECs), due to their multiple forms of operation, noxious actions, and adverse effects on different species [16,24,25]. Steroid and hormones, which are considered as EDCs, and are also frequently found in WWTP, include androgens, estrogens, glucocorticoids, progestogens, and growth hormones at trace concentration (ng/L) levels [13,14]. These compounds were selected on the basis of usage density and the percentage of their existence in effluent concentrations, as well as their impact as endocrine-disrupting chemicals.
The existing technology to remove hormones and steroids can be categorized into three different methods (physical removal technology, biodegradation of the compounds, and using advanced chemical oxidation processes (AOP)). Many researchers have shown that only 27% of micro pollutants can be removed from most of the WWTP, which is below the limit that can be detected; meanwhile, of the remaining 64% of compounds, less than 50% of them can be eliminated, and the remainder cannot be removed at all. [12,13,14,15,16,17,22,23,24,25]. Different types of steroids and hormones have been detected in the effluents of WWTP in different countries at different concentrations. Concentrations of detected compounds vary from μg/L to ng/L, and some research has shown that the detected pollutants are found in concentrations that are even higher than their toxicity limits [12,15,24,25,26,27,28,29,30].
The primary purpose of this study was to collect the data of occurrence and the fate of the steroid and hormonal compounds, measured from the intake sources to WWTP discharges. Specific objectives included: (1) summarizing the presence and concentrations of targeted compounds; (2) compiling the health and environmental impacts of steroids and hormones; and (3) addressing the current removable technologies and their efficiency.
2. Motivation
As the targeted steroid and hormonal compounds do not yet have specific limits established for drinking water in most countries, their frequent existence in raw water used for regular drinking water production engages the attention of environmentalists and the general public [31,32,33,34,35,36]). According to the perspective of pure water consumption, the question is: up to what concentration should the water system allow the presence of these substances before they impact the final product [37,38]. The potential long-term negative impact of steroids and hormones on human health, plants, and aquatic environments at currently found concentrations is yet to be proved [39,40,41,42]. As a result, scientists are addressing this scenario as unquantified risk [41]. As reported by The World Health Organization (WHO), due to the lower concentrations, these compounds are potentially less harmful to human health [39,40]. In addition to being aware of their long-term consequences on the world’s vulnerable populations, WHO also addressed the need of some preventive approaches to limit the concentrations of these compounds in the water [40]. Until specific limit values are established for the endocrine-disrupting steroids and hormones, thorough research and risk assessments should be the first priority.
Most steroids and hormones have three cyclohexane and cyclopentane rings, referred to as the tetra cyclic structure [43,44]. However, different classes (natural and synthetic) have different cyclic structures, based on their physical properties (Table 1) [45]. Physical and chemical characteristics of different steroid and hormone compounds are critical for understanding and predicting their presence and fate into the global environment. Steroids and hormones do not completely dissolve in the water. The octanol–water partition coefficient (Kow) measures the hydrophobicity of steroids and hormones, and the organic partition coefficient (Koc) can explain their sorption coefficient value. From Table 1, the log of Kow for frequently found steroid and hormone compounds is in the range of 1.4–5. [37,46]. GCs have very little ability to adsorb onto sludge than the other classes of steroids of steroids and hormones. However, while sorption had little role to play in the removal of GCs, it played an important role in the removal of other steroids and hormones in WWTPs.

Table 1.
Physiochemical properties of targeted steroid and hormones.
Overall, four of the most frequently found natural and synthetic estrogens (E1, E2, E3, and EE2) are produced from human and animal excretions, PPCPs, and the cattle (dairy and meat) and poultry industries [41,42]. Sadly, these estrogenic compounds are being detected in feces, both liquid and solid waste collected from husbandry, bayou effluents, and human fecal matter, all of which directly applied to agricultural land [47,48]. The presence of these steroid estrogens has been globally confirmed by researchers, not only in the groundwater, but also in freshwater. It is reported that husbandry industries discharge around 58% estradiol (E2), which is excreted through urine; on the other hand, the percentage of 17α-ethinylestradiol (EE2) and estriol (E3) discharge is about 96% and 69%, respectively [42,47,48]. The maximum concentrations of estrogen products found in groundwater in North America were E1 (79 ng/L), E2 (147 ng/L), E3 (1745 ng/L), and EE2 (230 ng/L). On the other hand, in Europe, recorded numbers varied between 0.02–100 ng/L [49,50]. Some European countries, such as France, Italy, the Netherlands, and Spain, as well as American countries such as Brazil and the USA, had it confirmed by many researchers that there were no traces of estrogen found in drinking water [51,52,53,54,55,56]. However, in China, the presence of trace estrogens were found and evaluated (E1 (9.9 ng/L), E2 (0.1 ng/L), and EE2 (0.3 ng/L)) [57,58,59,60,61], whereas E1 and E2 were not detected in European countries (France and Spain) [61,62,63,64,65].
As mentioned earlier, limited fruitful studies have been published so far about the consequences of various steroids being present in surface water and effluents of WWTPs compared to estrogen [66,67,68,69]. However, the levels of concentrations of progesterone found in the wastewaters is much higher than estrogen, as its discharge through human urine has been reported 100–1000 times more than estrogen [70,71].The recorded concentrations of progesterone in surface water and wastewater effluents has ranged from 0.95 to 66 ng/L, 0.8–2.3 ng/L, respectively [67,69]. On the other hand, 96% of the total steroids and hormones found in WWTP are natural and synthetic androgens. Natural androgens, such as androsterone, epiandrosterone, and androstenedione, have frequently been found with a maximum concentration of 2977 ng/L, 640 ng/L, 270 ng/L, respectively [70,71]. Both China and Brazil have encountered higher concentrations of testosterone in their groundwater and drinking water. In China, the maximum testosterone concentration found in surface water was 480 ng/L, whereas in Brazil, it was slightly lower than 330 ng/L [72,73,74,75,76,77]. Glucocorticoids (GCs) can pollute natural aquatic environments, and are a cause of great concern due to their impact on human and ecosystem health. Furthermore, the excretion of natural GCs has been recorded to be 10 times higher than the previously mentioned estrogen and androgen [78,79]. The concentration of selected glucocorticoids in surface water and wastewater effluents was found in ranges from 0.05 ng/L to 433 ng/L. A total of 14 states in the USA have reported GCs in their wastewater effluents [80,81,82].
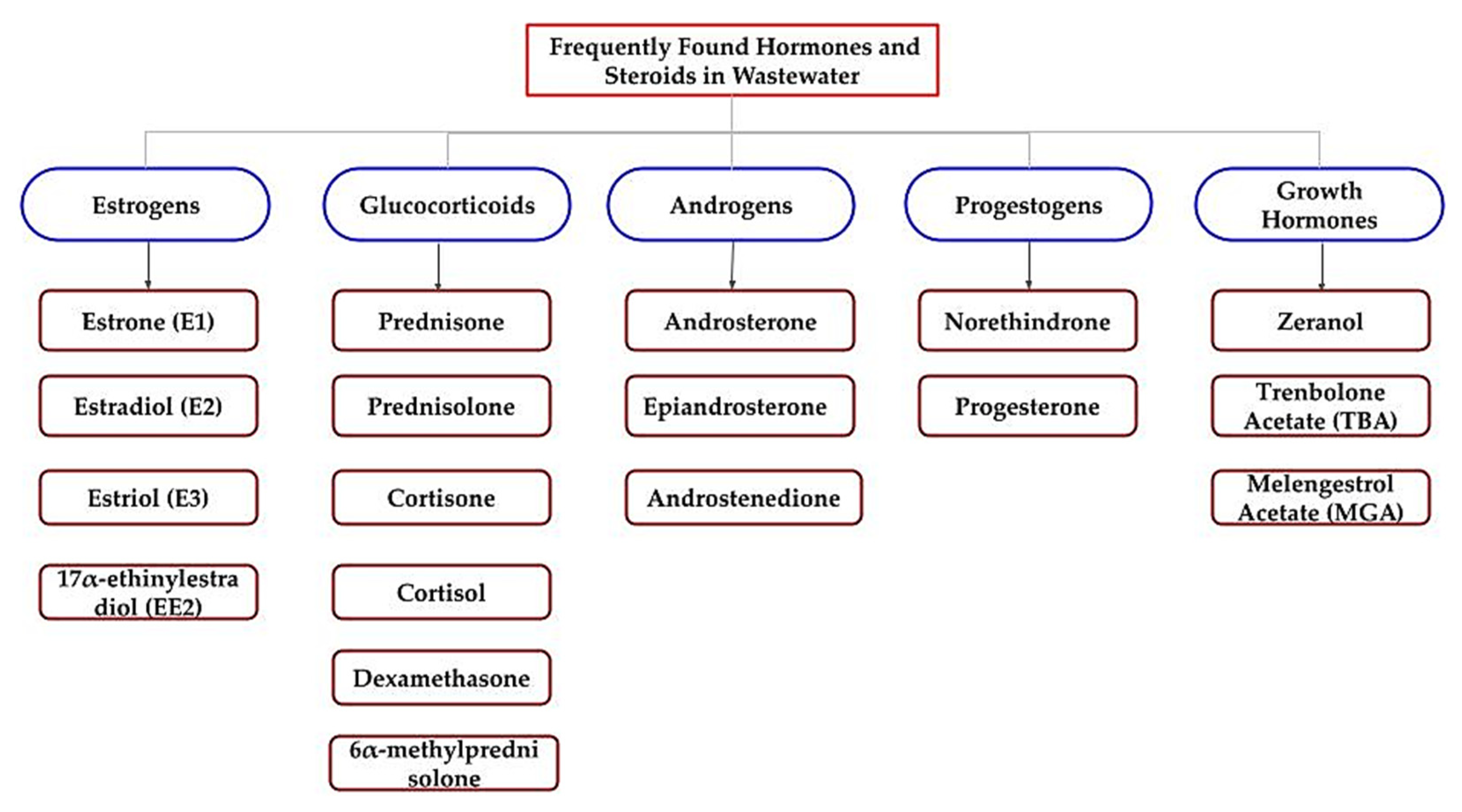
Overall, three synthetic hormones, which act as growth promotion chemicals in the husbandry industry, have been approved by the U.S. Food and Drug Administration (USFDA) [83,84]. Zeranol (commercially available in Zeraplix), trenbolone acetate (TBA), and melengestrol acetate (MGA) are the three growth hormones which have been detected in surface waters, as well as in soil near a husbandry and agricultural industry land, at concentration levels of 10–100 ng/L and 1–100 ng/g, respectively [85,86,87,88]. These hormones can easily enter the surface and groundwater through runoff from husbandry waste, as well as through animal manure and urine into soils as fertilizers [89,90,91]. Figure 2 summarizes all of the targeted steroids and hormones for this study, as mentioned above.
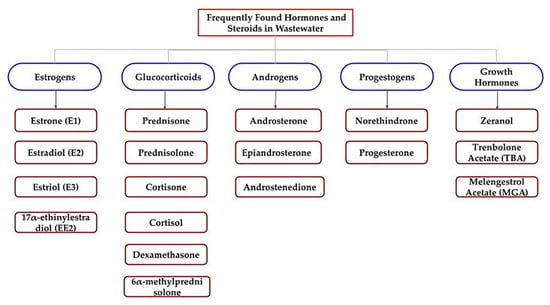
Figure 2.
Scheme of the potential endocrine disruption steroids and hormones.
3. Sources of Steroid Hormones in WWTPs
Table 2 and Table 3 represent a comprehensive scenario of the current incidence of steroids and hormones found in the world. The tables were selected based on different hormone compounds, year, sources such as country or location, environmental health impact, and the level of impact. This paper was effectuated according to the studies accomplished until 2021, as well as those published in databases such as Google Scholar, Microsoft Academic, Elsevier, Science.gov, Scopus, Researchgate, Science direct, Refseek, Pubmed Central, etc., using hormone and steroid removal, micro pollutant removal, sex hormones removal, steroidal hormones environmental and health impact, hormone removal from different sources of wastewater, and removal efficiency of micro pollutants mainly hormone and steroids as the keywords.

Table 2.
Significant incidences of steroid and hormone compounds.

Table 3.
Occurrence and health impact of hormones.
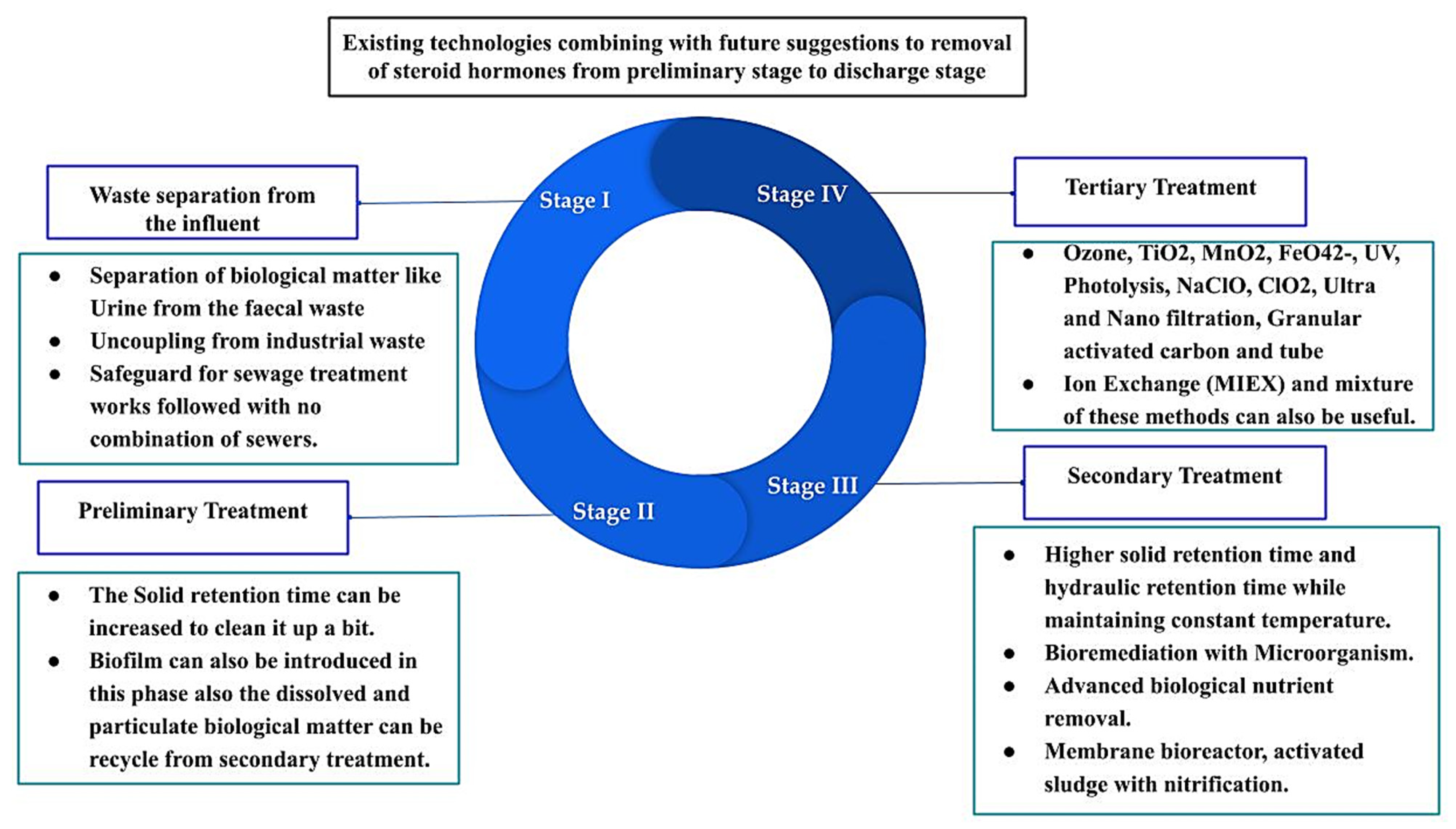
Existing wastewater treatment plants are designed for the removal of nitrogen, carbon, and mostly phosphorus, and also the partial removal of these compounds has been detected in different cases [132,133,134,135]. Nevertheless, large differences in the efficiency of steroid hormone removal have been highlighted by many researchers. The removal efficiency varies between 0% to 99% in different countries, based on location and concentrations [134,135,136,137]. Different removal technologies accentuate the relevance of geographical location parameters. Over the last two decades, technical analysis progress, along with industrial development, have empowered scientists to think again about the occurrence and fate of natural and synthetic steroids and hormones in wastewater treatment plants, even at concentrations at the ng/L level [137,138,139]. Conventional treatment plants have three major facilities, namely preliminary, primary, and secondary. Tertiary treatment is needed when discharging the effluent to the groundwater or surface waters. Figure 3 shows the current approaches and some potential future directions of removing these steroids and hormones from wastewater treatment plants.
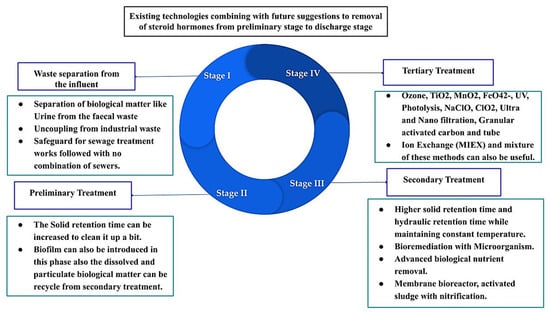
Figure 3.
Existing technologies combined with future suggestions for removing steroids and hormones from the preliminary stage to the discharge stage of a wastewater treatment plant.
In spite of the fact that existing WWTPs are designed with the aim of removing nutrients and suspended solids as much as possible, partial removal of steroids and hormones has been detected in many cases [140,141,142,143,144]. The efficiency of steroid and hormone removal systems have been varied. Based on the specific topographic locations and levels of concentrations, the removal efficiency ranges between less than 10% to 98% [145]. Over the last few decades, improvements in the technical sector have forced scientists to reconsider the occurrence and fate of natural and synthetic steroids and hormones in wastewater treatment plants, even at the ng/L level [141,145]. The removal efficiency of treatment plants regarding the targeted steroids and hormones are listed in Table 4. Possible removal methods of steroids and hormones from wastewater treatment units include volatility, photocatalysis, nanotechnology, chlorination, adsorption, biological degradation, and Fe (VI) treatment as a tertiary treatment technology etc. [143,146,147,148,149,150,151,152,153,154]. Table 4 shows the removal efficiency of each recorded method in various studies. The regeneration or disposal of adsorbents must be performed after the treatment. Additional treatment technology would need to be investigated and considered before disposal, although regeneration can be expensive and energy comprehensive. Sometime the regeneration phases are not only somewhat difficult, but they also have the disadvantage of the loss of the adsorbent. In spite of the higher adsorption capacity of activated carbons, this technology demonstrates several weaknesses. Activated carbons are very expensive, and the expense will surely increase with the quantity needed for any experiment. Therefore, there is an urgent need to investigate or develop new materials which have the same effective performance as activated carbon, but without the disadvantages. Over the last couple of decades, scientists and researchers have published several articles regarding this challenge. There are two ways in which this challenge can be addressed. These are the adaptation of abundant and natural materials, which are very cheap, as well as conventional and easily available in either raw or modified forms, and the development and usage of new synthetic materials.

Table 4.
Engineering processes and their efficiency for steroid and hormone removal.
4. Conclusions
Water quality concerns have achieved significant importance, both in the USA and internationally. The objective of WWTP is to remove steroid and hormone compounds, which may have negative effects on both health of both the environment and humans, completely. Studies have shown that the steroid and hormone removal technology currently used in most of the wastewater treatment plants are, unfortunately, insufficient. Therefore, these compounds, which are potentially unsafe, may enter surface water and, sometimes, groundwater. Current regulations aimed at reducing emissions to the environment are confined to a limited number of these compounds. As a result, the majority of steroids and hormones remain a problem for the existing legal norms. Among all of the treatment processes, adsorption, removing with nanoparticles, and oxidation methods have shown greater efficiency in hormone removal, relative to other technologies. Over time, wastewater effluents has been considered as the leading source of steroids and hormones in aquatic environments; however, surface runoff, as well as livestock sewage, has been identified as another source of these compounds. These removal technologies and their efficiency varies according to the geography of the country and the type of process. This is why the accuracy of implementation and methods is dissimilar. There is always disagreement and inconsistency in inspecting the occurrence and fate of steroids and hormones over the course of the sludge stabilizing. Scientists are considering this as one of the main problems related to the quantification of very low concentrations of steroid and hormone compounds in sludge.
Although physical and chemical treatment processes, prior to biological treatment, can further decrease concentrations of hormones and steroids in wastewater effluents (ultra low levels), their economical aspect and feasibility require further escalation. Without lowering the regulatory levels of estrogens, the treatment process which has been previously used to treat drinking water, in order to eradicate the endocrine disrupting steroids and hormones, may not be needed in wastewater sewage. Researchers, thus, should focus on finding the benefits of STWs, and emphasize the activated sludge process as the most economical wastewater treatment process that does not cause further side streams, which would involve additional treatment and disposal. In addition to this, further research and scientific analysis in this field is required to identify the effects of pre-treatments, mainly thermal, mechanical, and chemical, which can be modified before application in aerobic and anaerobic digestion.
Author Contributions
Conceptualization, M.M.S.Y. and S.W.L.; methodology, M.M.S.Y.; software, M.M.S.Y.; validation, M.M.S.Y. and S.W.L.; formal analysis, M.M.S.Y. and R.K.; investigation, S.W.L.; resources, M.M.S.Y.; data curation, M.M.S.Y. and R.K.; writing—original draft preparation, M.M.S.Y. and R.K.; writing—review and editing, M.M.S.Y. and R.K.; visualization, S.W.L.; supervision, S.W.L.; project administration, S.W.L.; funding acquisition, S.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Grdulska, A.; Kowalik, R. Pharmaceuticals in water and wastewater—Overview (Farmaceutyki w wodach i ściekach). Struct. Environ. 2020, 12, 79–84. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Herrera-Melián, J.A.; Rodríguez-Rodríguez, R.; Ojeda-González, Z.; Landívar-Andrade, V.; Santana-Rodríguez, J.J. Pharmaceutical and personal care product residues in a macrophyte pond-constructed wetland treating wastewater from a university campus: Presence, removal and ecological risk assessment. Sci. Total Environ. 2020, 703, 135596. [Google Scholar] [CrossRef]
- Zwart, N.; Jonker, W.; Ten Broek, R.; de Boer, J.; Somsen, G.; Kool, J.; Lamoree, M.H. Identification of mutagenic and endocrine disrupting compounds in surface water and wastewater treatment plant effluents using high-resolution effect-directed analysis. Water Res. 2020, 168, 115204. [Google Scholar] [CrossRef]
- Lei, K.; Lin, C.Y.; Zhu, Y.; Chen, W.; Pan, H.Y.; Sun, Z.; He, M.C. Estrogens in municipal wastewater and receiving waters in the Beijing-Tianjin-Hebei region, China: Occurrence and risk assessment of mixtures. J. Hazard. Mater. 2020, 389, 121891. [Google Scholar] [CrossRef] [PubMed]
- Shehab, Z.N.; Jamil, N.R.; Aris, A.Z. Occurrence, environmental implications and risk assessment of Bisphenol A in association with colloidal particles in an urban tropical river in Malaysia. Sci. Rep. 2020, 10, 20360. [Google Scholar] [CrossRef] [PubMed]
- Pratush, A.; Ye, X.; Yang, Q.; Kan, J.; Peng, T.; Wang, H.; Hu, Z. Biotransformation strategies for steroid estrogen and androgen pollution. Appl. Microbiol. Biotechnol. 2020, 104, 2385–2409. [Google Scholar] [CrossRef] [PubMed]
- Čelić, M.; Škrbić, B.D.; Insa, S.; Živančev, J.; Gros, M.; Petrović, M. Occurrence and assessment of environmental risks of endocrine disrupting compounds in drinking, surface and wastewaters in Serbia. Environ. Pollut. 2020, 262, 114344. [Google Scholar] [CrossRef]
- Vulliet, E.; Baugros, J.B.; Flament-Waton, M.M.; Grenier-Loustalot, M.F. Analytical methods for the determination of selected steroid sex hormones and corticosteriods in wastewater. Anal. Bioanal. Chem. 2007, 387, 2143–2151. [Google Scholar] [CrossRef]
- Kolodziej, E.P.; Gray, J.L.; Sedlak, D.L. Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ. Toxicol. Chem. Int. J. 2003, 22, 2622–2629. [Google Scholar] [CrossRef]
- Cartinella, J.L.; Cath, T.Y.; Flynn, M.T.; Miller, G.C.; Hunter, K.W.; Childress, A.E. Removal of natural steroid hormones from wastewater using membrane contactor processes. Environ. Sci. Technol. 2006, 40, 7381–7386. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.T.; Churchley, J.; Huddersman, K.D. Pilot-scale removal of trace steroid hormones and pharmaceuticals and personal care products from municipal wastewater using a heterogeneous fenton’s catalytic process. Int. J. Chem. Eng. 2013, 2013, 760915. [Google Scholar] [CrossRef]
- Combalbert, S.; Hernandez-Raquet, G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Appl. Microbiol. Biotechnol. 2010, 86, 1671–1692. [Google Scholar] [CrossRef]
- Williams, R.J.; Johnson, A.C.; Smith, J.J.; Kanda, R. Steroid estrogens profiles along river stretches arising from sewage treatment works discharges. Environ. Sci. Technol. 2003, 37, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Persistence and impact of steroidal estrogens on the environment and their laccase-assisted removal. Sci. Total Environ. 2019, 690, 447–459. [Google Scholar] [CrossRef]
- Samavat, H.; Kurzer, M.S. Estrogen metabolism and breast cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Q.; Lin, L.; Wang, X.; Chen, B.; Luan, T.; Tam, N.F. Partitions and vertical profiles of 9 endocrine disrupting chemicals in an estuarine environment: Effect of tide, particle size and salinity. Environ. Pollut. 2016, 211, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Ying, G.G.; Liu, S.; Lai, H.J.; Chen, Z.F.; Pan, C.G.; Chen, J. Analysis of 21 progestagens in various matrices by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) with diverse sample pretreatment. Anal. Bioanal. Chem. 2014, 406, 7299–7311. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Ying, G.G.; Liu, Y.S.; Yang, Y.Y.; He, L.Y.; Chen, J.; Zhao, J.L. Occurrence and removal of progestagens in two representative swine farms: Effectiveness of lagoon and digester treatment. Water Res. 2015, 77, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.A.; Shappell, N.W.; Billey, L.O.; Bermudez, D.S.; Wilson, V.S.; Kolpin, D.W.; Meyer, M.T. Bioassay of estrogenicity and chemical analyses of estrogens in streams across the United States associated with livestock operations. Water Res. 2013, 47, 3347–3363. [Google Scholar] [CrossRef]
- Zhi, E.; Song, Y.; Duan, L.; Yu, H.; Peng, J. Spatial distribution and diversity of microbial community in large-scale constructed wetland of the Liao River Conservation Area. Environ. Earth Sci. 2015, 73, 5085–5094. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Tang, Z.; Huang, J.; Zhou, Q.; Vymazal, J. Effects of plant biomass on bacterial community structure in constructed wetlands used for tertiary wastewater treatment. Ecol. Eng. 2015, 84, 38–45. [Google Scholar] [CrossRef]
- Weizel, A.; Schlüsener, M.P.; Dierkes, G.; Ternes, T.A. Occurrence of glucocorticoids, mineralocorticoids, and progestogens in various treated wastewater, rivers, and streams. Environ. Sci. Technol. 2018, 52, 5296–5307. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Oller, I.; Klamerth, N.; Agüera, A.; Rodríguez, E.M.; Malato, S. Application of solar AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. 2013, 47, 1521–1528. [Google Scholar] [CrossRef]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Nakrst, J.; Bistan, M.; Tišler, T.; Zagorc-Končan, J.; Derco, J.; Gotvajn, A.Ž. Comparison of Fenton’s oxidation and ozonation for removal of estrogens. Water Sci. Technol. 2011, 63, 2131–2137. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1259, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee. 2019. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/?qid=1582731822045&uri=CELEX:52019DC0128 (accessed on 25 February 2020). European Union Strategic Approach to Pharmaceuticals in the Environment.
- Datel, J.V.; Hrabankova, A. Pharmaceuticals load in the svihov water reservoir (Czech Republic) and impacts on quality of treated drinking water. Water 2020, 12, 1387. [Google Scholar] [CrossRef]
- Eckhardt, P.; Hrabánková, A.; Novotná, E.; Hrkal, Z.; Rozman, D. PPCP Monitoring in Drinking Water Supply Systems: The Example of Káraný Waterworks in Central Bohemia. Water 2018, 10, 1852. [Google Scholar] [CrossRef]
- Snyder, S.A. Occurrence of pharmaceuticals in US drinking water. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; American Chemical Society: Washington, DC, USA, 2010; pp. 69–80. [Google Scholar] [CrossRef]
- World Health Organization. WHO/HSE/WSH/11.05, Pharmaceuticals in Drinking Water. 2011. Available online: http://www.who.int/water_sanitation_health/publications/2011/pharmaceuticals/en (accessed on 25 February 2020).
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/water_sanitation_health/publications/2012/pharmaceuticals/en/ (accessed on 24 February 2020).
- Chiang, Y.R.; Wei, S.T.S.; Wang, P.H.; Wu, P.H.; Yu, C.P. Microbial degradation of steroid sex hormones: Implications for environmental and ecological studies. Microb. Biotechnol. 2020, 13, 926–949. [Google Scholar] [CrossRef]
- Jari, Y.; Roche, N.; Necibi, M.C.; El Hajjaji, S.; Dhiba, D.; Chehbouni, A. Emerging Pollutants in Moroccan Wastewater: Occurrence, Impact, and Removal Technologies. J. Chem. 2022. [Google Scholar] [CrossRef]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? TRENDS Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment—A review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Caldas, S.S.; Arias, J.L.O.; Rombaldi, C.; Mello, L.L.; Cerqueira, M.B.; Martins, A.F.; Primel, E.G. Occurrence of pesticides and PPCPs in surface and drinking water in southern Brazil: Data on 4-year monitoring. J. Braz. Chem. Soc. 2019, 30, 71–80. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways—A critical review. J. Environ. Chem. Eng. 2021, 9, 104558. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Yazdan, M.M.; Ahad, M.T.; Mallick, Z.; Mallick, S.P.; Jahan, I.; Mazumder, M. An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems. Pollutants 2021, 1, 141–155. [Google Scholar] [CrossRef]
- Biswas, S.; Shapiro, C.A.; Kranz, W.L.; Mader, T.L.; Shelton, D.P.; Snow, D.D.; Ensley, S. Current knowledge on the environmental fate, potential impact, and management of growth-promoting steroids used in the US beef cattle industry. J. Soil Water Conserv. 2013, 68, 325–336. [Google Scholar] [CrossRef]
- Kim, M.K.; Zoh, K.D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Zheng, W.; Yates, S.R.; Bradford, S.A. Analysis of steroid hormones in a typical dairy waste disposal system. Environ. Sci. Technol. 2008, 42, 530–535. [Google Scholar] [CrossRef]
- Karnjanapiboonwong, A.; Suski, J.G.; Shah, A.A.; Cai, Q.; Morse, A.N.; Anderson, T.A. Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water Air Soil Pollut. 2011, 216, 257–273. [Google Scholar] [CrossRef]
- Dévier, M.H.; Le Menach, K.; Viglino, L.; Di Gioia, L.; Lachassagne, P.; Budzinski, H. Ultra-trace analysis of hormones, pharmaceutical substances, alkylphenols and phthalates in two French natural mineral waters. Sci. Total Environ. 2013, 443, 621–632. [Google Scholar] [CrossRef]
- Maggioni, S.; Balaguer, P.; Chiozzotto, C.; Benfenati, E. Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ. Sci. Pollut. Res. 2013, 20, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Gorga, M.; González-Alonso, S.; Petrovic, M.; Barceló, D.; Valcárcel, Y. Monitoring endocrine disrupting compounds and estrogenic activity in tap water from Central Spain. Environ. Sci. Pollut. Res. 2014, 21, 9297–9310. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water Res. 2011, 45, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Yazdan, M.M.; Ahad, M.T.; Jahan, I.; Mazumder, M. Review on the evaluation of the impacts of wastewater disposal in hydraulic fracturing industry in the United States. Technologies 2020, 8, 67. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Ruan, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Falconer, I.R.; Chapman, H.F.; Moore, M.R.; Ranmuthugala, G. Endocrine—disrupting compounds: A review of their challenge to sustainable and safe water supply and water reuse. Environ. Toxicol. Int. J. 2006, 21, 181–191. [Google Scholar] [CrossRef]
- Zhang, T.; Xiong, G.; Maser, E. Characterization of the steroid degrading bacterium S19-1 from the Baltic Sea at Kiel, Germany. Chem. Biol. Interact. 2011, 191, 83–88. [Google Scholar] [CrossRef]
- Zhang, H.C.; Xu, T.; Hu, X.L.; Pang, W.H.; Yin, D.Q. The distributions, removals and estrogenic effects of selected endocrine disrupting chemicals in two drinking water factories in China. J. Water Health 2013, 11, 41–50. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Zhang, H.; Li, Z.; Ma, Y.; Wu, M.; Liu, X. Spatial and seasonal variations of occurrences and concentrations of endocrine disrupting chemicals in unconfined and confined aquifers recharged by reclaimed water: A field study along the Chaobai River, Beijing. Sci. Total Environ. 2013, 450, 162–168. [Google Scholar] [CrossRef]
- Chen, F.; Ying, G.G.; Kong, L.X.; Wang, L.; Zhao, J.L.; Zhou, L.J.; Zhang, L.J. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ. Pollut. 2011, 159, 1490–1498. [Google Scholar] [CrossRef]
- Vulliet, E.; Wiest, L.; Baudot, R.; Grenier-Loustalot, M.F. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2008, 1210, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Félix–Cañedo, T.E.; Durán–Álvarez, J.C.; Jiménez–Cisneros, B. The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Sci. Total Environ. 2013, 454, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jardim, W.F.; Montagner, C.C.; Pescara, I.C.; Umbuzeiro, G.A.; Bergamasco, A.M.D.D.; Eldridge, M.L.; Sodré, F.F. An integrated approach to evaluate emerging contaminants in drinking water. Sep. Purif. Technol. 2012, 84, 3–8. [Google Scholar] [CrossRef]
- Khalil, A.M.; Hashem, T.; Gopalakrishnan, A.; Schafer, A.I. Cyclodextrin composite nanofiber membrane: Impact of the crosslinker type on steroid hormone micropollutant removal from water. ACS Appl. Polym. Mater. 2021, 3, 2646–2656. [Google Scholar] [CrossRef]
- Baştürk, E.; Karataş, M. Removal of pharmaceuticals by advanced treatment methods. J. Environ. Management 2021, 300, 113808. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Verebey, Z.; Sharma, V.K.; Kovacsics, L.; Fekete, J. Simultaneous determination of corticosteroids, androgens, and progesterone in river water by liquid chromatography–tandem mass spectrometry. Chemosphere 2010, 78, 972–979. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.G.; Zhao, J.L.; Zhou, L.J.; Yang, B.; Chen, Z.F.; Lai, H.J. Occurrence and fate of androgens, estrogens, glucocorticoids and progestagens in two different types of municipal wastewater treatment plants. J. Environ. Monit. 2012, 14, 482–491. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Trinh, P.B.; Burkhardt, C.J.; Schäfer, A.I. Incorporation of single-walled carbon nanotubes in ultrafiltration support structure for the removal of steroid hormone micropollutants. Sep. Purif. Technol. 2021, 264, 118405. [Google Scholar] [CrossRef]
- Shore, L.S.; Shemesh, M. Naturally produced steroid hormones and their release into the environment. Pure Appl. Chem. 2003, 75, 1859–1871. [Google Scholar] [CrossRef]
- Yang, G.; Fan, M.; Zhang, G. Emerging contaminants in surface waters in China—A short review. Environ. Res. Lett. 2014, 9, 074018. [Google Scholar] [CrossRef]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Jardim, W.F. Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Praveena, S.M.; Lui, T.S.; Hamin, N.A.; Razak, S.Q.N.A.; Aris, A.Z. Occurrence of selected estrogenic compounds and estrogenic activity in surface water and sediment of Langat River (Malaysia). Environ. Monit. Assess. 2016, 188, 442. [Google Scholar] [CrossRef] [PubMed]
- Kuster, M.; de Alda, M.J.L.; Hernando, M.D.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Analysis and occurrence of pharmaceuticals, estrogens, progestogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). J. Hydrol. 2008, 358, 112–123. [Google Scholar] [CrossRef]
- Kuster, M.; Díaz-Cruz, S.; Rosell, M.; de Alda, M.L.; Barceló, D. Fate of selected pesticides, estrogens, progestogens and volatile organic compounds during artificial aquifer recharge using surface waters. Chemosphere 2010, 79, 880–886. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, J.L.; Wilding, A. Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1022, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Delgado-González, C.R.; Madariaga-Navarrete, A.; Fernández-Cortés, J.M.; Islas-Pelcastre, M.; Oza, G.; Iqbal, H.; Sharma, A. Advances and Applications of Water Phytoremediation: A Potential Biotechnological Approach for the Treatment of Heavy Metals from Contaminated Water. Int. J. Environ. Res. Public Health 2021, 18, 5215. [Google Scholar] [CrossRef] [PubMed]
- Runnalls, T.J.; Margiotta-Casaluci, L.; Kugathas, S.; Sumpter, J.P. Pharmaceuticals in the aquatic environment: Steroids and anti-steroids as high priorities for research. Hum. Ecol. Risk Assess. 2010, 16, 1318–1338. [Google Scholar] [CrossRef]
- Torres, M.J.; Canto, G. Hypersensitivity reactions to corticosteroids. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 273–279. [Google Scholar] [CrossRef]
- Ammann, A.A.; Macikova, P.; Groh, K.J.; Schirmer, K.; Suter, M.J. LC-MS/MS determination of potential endocrine disruptors of cortico signalling in rivers and wastewaters. Anal. Bioanal. Chem. 2014, 406, 7653–7665. [Google Scholar] [CrossRef]
- Stavreva, D.A.; George, A.A.; Klausmeyer, P.; Varticovski, L.; Sack, D.; Voss, T.C.; NHager, G.L. Prevalent glucocorticoid and androgen activity in US water sources. Sci. Rep. 2012, 2, 937. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.L.; Gaughan, J.B.; Kreikemeier, W.M.; Parkhurst, A.M. Behavioural effects of yearling grain-finished heifers exposed to differing environmental conditions and growth-promoting agents. Aust. J. Exp. Agric. 2008, 48, 1155–1160. [Google Scholar] [CrossRef]
- Sinnett-Smith, P.A.; Dumelow, N.W.; Buttery, P.J. Effects of trenbolone acetate and zeranol on protein metabolism in male castrate andfemale lambs. Br. J. Nutr. 1983, 50, 225–234. [Google Scholar] [CrossRef]
- Schiffer, B.; Daxenberger, A.; Meyer, K.; Meyer, H.H. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: Environmental studies. Environ. Health Perspect. 2001, 109, 1145–1151. [Google Scholar] [CrossRef]
- Lange, I.G.; Daxenberger, A.; Schiffer, B.; Witters, H.; Ibarreta, D.; Meyer, H.H. Sex hormones originating from different livestock production systems: Fate and potential disrupting activity in the environment. Anal. Chim. Acta 2002, 473, 27–37. [Google Scholar] [CrossRef]
- Kolok, A.S.; Snow, D.D.; Kohno, S.; Sellin, M.K.; Guillette, L.J., Jr. Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. Sci. Total Environ. 2007, 388, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Hunt, S.L.; Snow, D.D.; Kranz, W.L.; Mader, T.L.; Shapiro, C.A.; Donk, S.J.V.; Zhang, T.C. Effect of growth promotants on the occurrence of endogenous and synthetic steroid hormones on feedlot soils and in runoff from beef cattle feeding operations. Environ. Sci. Technol. 2012, 46, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Durhan, E.J.; Lambright, C.S.; Makynen, E.A.; Lazorchak, J.; Hartig, P.C.; Wilson, V.S.; Ankley, G.T. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ. Health Perspect. 2006, 114 (Suppl. 1), 65–68. [Google Scholar] [CrossRef]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Lee, L.S.; Carmosini, N.; Sassman, S.A.; Dion, H.M.; Sepulveda, M.S. Agricultural contributions of antimicrobials and hormones on soil and water quality. Adv. Agron. 2007, 93, 1–68. [Google Scholar] [CrossRef]
- Koh, Y.K.K.; Chiu, T.Y.; Boobis, A.; Cartmell, E.; Scrimshaw, M.D.; Lester, J.N. Treatment and removal strategies for estrogens from wastewater. Environ. Technol. 2008, 29, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiao, R.; Sun, H.; Xu, H.; He, Y.; Wang, D. Removal of microorganic pollutants in aquatic environment: The utilization of Fe (VI). J. Environ. Manag. 2022, 316, 115328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, H.; van der Hoek, J.P.; Yu, K.; Cao, Y. Recent applications of biological technologies for decontaminating hormones in livestock waste and wastewater. Curr. Opin. Environ. Sci. Health 2021, 24, 100307. [Google Scholar] [CrossRef]
- Necibi, M.C.; Dhiba, D.; El Hajjaji, S. Contaminants of emerging concern in African wastewater effluents: Occurrence, impact and removal technologies. Sustainability 2021, 13, 1125. [Google Scholar] [CrossRef]
- Okoye, C.O.; Okeke, E.S.; Okoye, K.C.; Echude, D.; Andong, F.A.; Chukwudozie, K.I.; Okoye, H.U.; Ezeonyejiaku, C.D. Occurrence and fate of pharmaceuticals, personal care products (PPCPs) and pesticides in African water systems: A need for timely intervention. Heliyon 2022, e09143. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, Z.X.; Liu, Y.Y.; Wu, Q.Y.; Sun, Y.X. Relative developmental toxicities of reclaimed water to zebrafish embryos and the relationship with relevant water quality parameters. Water Cycle 2021, 2, 85–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.B.; Kumar, V.; Necibi, M.C.; Mu, Y.J.; Shi, C.Z.; Chaurasia, D.; Chauhan, S.; Chaturvedi, P.; Sillanpää, M.; et al. Synthetic organic antibiotics residues as emerging contaminants waste-to-resources processing for a circular economy in China: Challenges and perspective. Environ. Res. 2022, 211, 113075. [Google Scholar] [CrossRef]
- Furuichi, T.; Kannan, K.; Giesy, J.P.; Masunaga, S. Contribution of known endocrine disrupting substances to the estrogenic activity in Tama River water samples from Japan using instrumental analysis and in vitro reporter gene assay. Water Res. 2004, 38, 4491–4501. [Google Scholar] [CrossRef]
- Bednárek, J.; Matějová, L.; Jankovská, Z.; Vaštyl, M.; Sokolová, B.; Peikertová, P.; Šiler, P.; Verner, A.; Tokarský, J.; Koutník, I.; et al. The Influence of Structural Properties on the Adsorption Capacities of Microwave-assisted Biochars for Metazachlor Removal from Aqueous Solutions. J. Environ. Chem. Eng. 2022, 108003. [Google Scholar] [CrossRef]
- Fernandez, M.P.; Ikonomou, M.G.; Buchanan, I. An assessment of estrogenic organic contaminants in Canadian wastewaters. Sci. Total Environ. 2007, 373, 250–269. [Google Scholar] [CrossRef]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.M.; Evans, N.; Cardon, M.C.; Rosenblum, L.; Iwanowicz, L.R.; Hartig, P.C.; Wilson, V.S. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ. Sci. Technol. 2017, 51, 4781–4791. [Google Scholar] [CrossRef]
- Yarahmadi, H.; Duy, S.V.; Hachad, M.; Dorner, S.; Sauvé, S.; Prévost, M. Seasonal variations of steroid hormones released by wastewater treatment plants to river water and sediments: Distribution between particulate and dissolved phases. Sci. Total Environ. 2018, 635, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Šauer, P.; Stará, A.; Golovko, O.; Valentová, O.; Bořík, A.; Grabic, R.; Kroupová, H.K. Two synthetic progestins and natural progesterone are responsible for most of the progestagenic activities in municipal wastewater treatment plant effluents in the Czech and Slovak republics. Water Res. 2018, 137, 64–71. [Google Scholar] [CrossRef]
- Karpińska, J.; Kotowska, U. New Aspects of Occurrence and Removal of Emerging Pollutants. Water 2021, 13, 2418. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Wu, J. Removal of steroid hormones from mariculture system using seaweed Caulerpa lentillifera. Front. Environ. Sci. Eng. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Mourão, L.C.; Souza, G.B.; Dias, I.M.; Andrade, L.A.; Souza, P.L.; Cardozo-Filho, L.; Oliveira, G.R.; Oliveira, S.B.; Alonso, C.G. Treatment of hormones in wastewater from the pharmaceutical industry by continuous flow supercritical water technology. J. Environ. Chem. Eng. 2021, 9, 106095. [Google Scholar] [CrossRef]
- Kolok, A.S.; Ali, J.M.; Rogan, E.G.; Bartelt-Hunt, S.L. The fate of synthetic and endogenous hormones used in the US beef and dairy industries and the potential for human exposure. Curr. Environ. Health Rep. 2018, 5, 225–232. [Google Scholar] [CrossRef]
- Hatt, J.W.; Germain, E.; Judd, S.J. Powdered Activated Carbon-Microfiltration for Waste-Water Reuse. Sep. Sci. Technol. 2013, 48, 690–698. [Google Scholar] [CrossRef]
- Nakada, N.; Yasojima, M.; Okayasu, Y.; Komori, K.; Tanaka, H.; Suzuki, Y. Fate of oestrogenic compounds and identification of oestrogenicity in a wastewater treatment process. Water Sci. Technol. 2006, 53, 51–63. [Google Scholar] [CrossRef]
- Desbrow, C.E.J.R.; Routledge, E.J.; Brighty, G.C.; Sumpter, J.P.; Waldock, M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 1998, 32, 1549–1558. [Google Scholar] [CrossRef]
- Johnson, A.C.; Belfroid, A.; Di Corcia, A. Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci. Total Environ. 2000, 256, 163–173. [Google Scholar] [CrossRef]
- Körner, W.; Spengler, P.; Bolz, U.; Schuller, W.; Hanf, V.; Metzger, J.W. Substances with estrogenic activity in effluents of sewage treatment plants in southwestern Germany. 2. Biological analysis. Environ. Toxicol. Chem. Int. J. 2001, 20, 2142–2151. [Google Scholar] [CrossRef]
- Belfroid, A.C.; Van der Horst, A.; Vethaak, A.D.; Schäfer, A.J.; Rijs, G.B.J.; Wegener, J.; Cofino, W.P. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci. Total Environ. 1999, 225, 101–108. [Google Scholar] [CrossRef]
- Servos, M.R.; Bennie, D.T.; Burnison, B.K.; Jurkovic, A.; McInnis, R.; Neheli, T.; Ternes, T.A. Distribution of estrogens, 17β-estradiol and estrone, in Canadian municipal wastewater treatment plants. Sci. Total Environ. 2005, 336, 155–170. [Google Scholar] [CrossRef]
- Jenkins, R.L.; Wilson, E.M.; Angus, R.A.; Howell, W.M.; Kirk, M. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol. Sci. 2003, 73, 53–59. [Google Scholar] [CrossRef]
- Hansen, P.D.; Dizer, H.H.B.; Marx, A.; Sherry, J.; McMaster, M.; Blaise, C. Vitellogenin: A Biomarker for Endocrine Disruptors. Trends Anal. Chem. 1998, 17, 448–451. [Google Scholar] [CrossRef]
- Leffers, H.; Næsby, M.; Vendelbo, B.; Skakkebæk, N.E.; Jørgensen, M. Oestrogenic potencies of Zeranol, oestradiol, diethylstilboestrol, Bisphenol-A and genistein: Implications for exposure assessment of potential endocrine disrupters. Hum. Reprod. 2001, 16, 1037–1045. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Y.C. Transformation of MCF-10A Human Breast Epithelial Cells by Zeranol and Estradiol-17β. Breast J. 2004, 10, 514–521. [Google Scholar] [CrossRef]
- Xu, P.; Ye, W.; Jen, R.; Lin, S.H.; Kuo, C.T.; Lin, Y.C. Mitogenic activity of zeranol in human breast cancer cells is enhanced by leptin and suppressed by gossypol. Anticancer Res. 2009, 29, 4621–4628. [Google Scholar]
- Chang, H.; Hu, J.; Shao, B. Occurrence of natural and synthetic glucocorticoids in sewage treatment plants and receiving river waters. Environ. Sci. Technol. 2007, 41, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, J.P.; Johnson, A.C. Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 2005, 39, 4321–4332. [Google Scholar] [CrossRef]
- Länge, R.; Hutchinson, T.H.; Croudace, C.P.; Siegmund, F.; Schweinfurth, H.; Hampe, P.; Sumpter, J.P. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. Int. J. 2001, 20, 1216–1227. [Google Scholar] [CrossRef]
- Pawlowski, S.; Van Aerle, R.; Tyler, C.R.; Braunbeck, T. Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol. Environ. Saf. 2004, 57, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.J.; Arao, Y.; Korach, K.S. Estrogen hormone physiology: Reproductive findings from estrogen receptor mutant mice. Reprod. Biol. 2014, 14, 3–8. [Google Scholar] [CrossRef]
- Petersen, L.H.; Hala, D.; Carty, D.; Cantu, M.; Martinović, D.; Huggett, D.B. Effects of progesterone and norethindrone on female fathead minnow (Pimephales promelas) steroidogenesis. Environ. Toxicol. Chem. 2015, 34, 379–390. [Google Scholar] [CrossRef]
- Orlando, E.F.; Kolok, A.S.; Binzcik, G.A.; Gates, J.L.; Horton, M.K.; Lambright, C.S.; Guillette, L.J., Jr. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 2004, 112, 353–358. [Google Scholar] [CrossRef]
- Morthorst, J.E.; Holbech, H.; Bjerregaard, P. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat. Toxicol. 2010, 98, 336–343. [Google Scholar] [CrossRef]
- Sone, K.; Hinago, M.; Itamoto, M.; Katsu, Y.; Watanabe, H.; Urushitani, H.; Iguchi, T. Effects of an androgenic growth promoter 17β-trenbolone on masculinization of Mosquitofish (Gambusia affinis affinis). Gen. Comp. Endocrinol. 2005, 143, 151–160. [Google Scholar] [CrossRef]
- Ankley, G.T.; Jensen, K.M.; Makynen, E.A.; Kahl, M.D.; Korte, J.J.; Hornung, M.W.; Gray, L.E. Effects of the androgenic growth promoter 17-β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ. Toxicol. Chem. Int. J. 2003, 22, 1350–1360. [Google Scholar] [CrossRef]
- Nuzzo, J.B. The biological threat to US water supplies: Toward a national water security policy. Biosecur. Bioterror. Biodef. Strategy Pract. Sci. 2006, 4, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Wojnarowicz, P.; Yang, W.; Zhou, H.; Parker, W.J.; Helbing, C.C. Changes in hormone and stress-inducing activities of municipal wastewater in a conventional activated sludge wastewater treatment plant. Water Res. 2014, 66, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Curini, R.; D’Ascenzo, G.; Di Corcia, A.; Gentili, A.; Samperi, R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ. Sci. Technol. 2000, 34, 5059–5066. [Google Scholar] [CrossRef]
- D’ascenzo, G.; Di Corcia, A.; Gentili, A.; Mancini, R.; Mastropasqua, R.; Nazzari, M.; Samperi, R. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 2003, 302, 199–209. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Gas chromatography–mass spectrometry determination of pharmacologically active substances in urine and blood samples by use of a continuous solid-phase extraction system and microwave-assisted derivatization. J. Chromatogr. B 2012, 891, 12–19. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography–mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci. Total Environ. 2012, 419, 208–215. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Liu, H.; Ru, J.; Qu, J.; Dai, R.; Wang, Z.; Hu, C. Removal of persistent organic pollutants from micro-polluted drinking water by triolein embedded absorbent. Bioresour. Technol. 2009, 100, 2995–3002. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Removal of natural hormones by nanofiltration membranes: Measurement, modeling, and mechanisms, Environ. Sci. Technol. 2004, 38, 1888–1896. [Google Scholar] [CrossRef]
- Lopez, J. Endocrine-Disrupting Chemical Pollution: Why the EPA Should Regulate These Chemicals under the Clean Water Act. Sustain. Dev. Law Policy 2009, 10, 19. [Google Scholar]
- Ohko, Y.; Iuchi, K.-I.; Niwa, C.; Tatsuma, T.; Nakashima, T.; Iguchi, T.; Kubota, Y.; Fujishima, A. 17β-Estradiol Degradation by TiO2 Photocatalysis as a Means of Reducing Estrogenic Activity. Environ. Sci. Technol. 2002, 36, 4175–4181. [Google Scholar] [CrossRef]
- Rose, L.J.; Rice, E.W. Inactivation of bacterial biothreat agents in water, a review. J. Water Health 2014, 12, 618–633. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, M.T.M.; Nygaard, J.M.; Ghosh, A.K.; Hoek, E.M. Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 2010, 261, 255–263. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ. Sci. Technol. 2005, 39, 7656–7661. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Humplik, T.; Lee, J.; O’hern, S.C.; Fellman, B.A.; Baig, M.A.; Hassan, S.F.; Wang, E.N. Nanostructured materials for water desalination. Nanotechnology 2011, 22, 292001. [Google Scholar] [CrossRef]
- World Health Organization. Economic and Health Effects of Increasing Coverage of Low Cost Household Drinking-Water Supply and Sanitation Interventions to Countries Off-Track to Meet MDG Target 10: Background Document to the “Human Development Report 2006”; No. WHO/SDE/WSH/07.05; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- WHO/UNICEF Joint Water Supply, and Sanitation Monitoring Programme. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Thomas, A.G.; Syres, K.L. Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem. Soc. Rev. 2012, 41, 4207–4217. [Google Scholar] [CrossRef]
- Díez, A.M.; Ribeiro, A.S.; Sanromán, M.A.; Pazos, M. Optimization of photo-Fenton process for the treatment of prednisolone. Environ. Sci. Pollut. Res. 2018, 25, 27768–27782. [Google Scholar] [CrossRef]
- Yin, K.; He, Q.; Liu, C.; Deng, Y.; Wei, Y.; Chen, S.; Luo, S. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism. Chemosphere 2018, 212, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Romão, J.S.; Hamdy, M.S.; Mul, G.; Baltrusaitis, J. Photocatalytic decomposition of cortisone acetate in aqueous solution. J. Hazard. Mater. 2015, 282, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X.; Sun, W.; Ren, D.; Li, X.; Li, X.; Pan, X. Occurrence, removal, and fate of progestogens, androgens, estrogens, and phenols in six sewage treatment plants around Dianchi Lake in China. Environ. Sci. Pollut. Res. 2014, 21, 12898–12908. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Tache, Y. Stress-related alterations of visceral sensation: Animal models for irritable bowel syndrome study. J. Neurogastroenterol. Motil. 2011, 17, 213–234. [Google Scholar] [CrossRef]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Jia, A.; Wu, S.; Daniels, K.D.; Snyder, S.A. Balancing the budget: Accounting for glucocorticoid bioactivity and fate during water treatment. Environ. Sci. Technol. 2016, 50, 2870–2880. [Google Scholar] [CrossRef]
- Saha, B.; Karounou, E.; Streat, M. Removal of 17β-oestradiol and 17α-ethinyl oestradiol from water by activated carbons and hypercrosslinked polymeric phases. React. Funct. Polym. 2010, 70, 531–544. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.G.; Zhao, J.L.; Liu, S.; Zhou, L.J.; Chen, F. Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate (VI) treatment of secondary wastewater effluents. Water Res. 2012, 46, 2194–2204. [Google Scholar] [CrossRef]
- Wang, F.; Sun, W.; Pan, W.; Xu, N. Adsorption of sulfamethoxazole and 17β-estradiol by carbon nanotubes/CoFe2O4 composites. Chem. Eng. J 2015, 274, 17–29. [Google Scholar] [CrossRef]
- Patel, S.; Han, J.; Gao, W. Sorption of 17β-estradiol from aqueous solutions on to bone char derived from waste cattle bones: Kinetics and isotherms. J. Environ. Chem. Eng. 2015, 3, 1562–1569. [Google Scholar] [CrossRef]
- Teixeira, A.P.C.; Purceno, A.D.; De Paula, C.C.A.; Da Silva, J.C.C.; Ardisson, J.D.; Lago, R.M. Efficient and versatile fibrous adsorbent based on magnetic amphiphilic composites of chrysotile/carbon nanostructures for the removal of ethynilestradiol. J. Hazard. Mater. 2013, 248–249, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Fang, L.F.; Shen, Y.J.; Yu, W.H.; Zhu, B.K.; Hélix-Nielsen, C.; Zhang, W. Ionic dendrimer based polyamide membranes for ion separation. ACS Nano 2021, 15, 7522–7535. [Google Scholar] [CrossRef]
- Tagliavini, M.; Engel, F.; Weidler, P.G.; Scherer, T.; Schäfer, A.I. Adsorption of steroid micropollutants on polymer-based spherical activated carbon (PBSAC). J. Hazard. Mater. 2017, 337, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Pretali, L.; Albini, A.; Cantalupi, A.; Maraschi, F.; Nicolis, S.; Sturini, M. TiO2-Photocatalyzed Water Depollution, a Strong, yet Selective Depollution Method: New Evidence from the Solar Light Induced Degradation of Glucocorticoids in Freshwaters. Appl. Sci. 2021, 11, 2486. [Google Scholar] [CrossRef]
- Oseph, L.; Zaib, Q.; Khan, I.A.; Berge, N.D.; Park, Y.G.; Saleh, N.B.; Yoon, Y. Removal of bisphenol A and 17α-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res. 2011, 45, 4056–4068. [Google Scholar] [CrossRef]
- Klauson, D.; Pilnik-Sudareva, J.; Pronina, N.; Budarnaja, O.; Krichevskaya, M.; Käkinen, A.; Preis, S. Aqueous photocatalytic oxidation of prednisolone. Open Chem. 2013, 11, 1620–1633. [Google Scholar] [CrossRef]
- Ellerie, J.R.; Apul, O.G.; Karanfil, T.; Ladner, D.A. Comparing graphene, carbon nanotubes, and superfine powdered activated carbon as adsorptive coating materials for microfiltration membranes. J. Hazard. Mater. 2013, 26, 91–98. [Google Scholar] [CrossRef]
- Yasir, M.; Masar, M.; Sopik, T.; Ali, H.; Urbanek, M.; Antos, J.; Machovsky, M.; Kuritka, I. ZnO nanowires and nanorods based ZnO/WO3/Pt heterojunction for efficient photocatalytic degradation of estriol (E3) hormone. Mater. Lett. 2022, 319, 132291. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).