Abstract
Microencapsulation is a fundamental technology for protecting active compounds from environmental degradation by factors such as light, heat, and oxygen. This process significantly improves their stability, bioavailability, and shelf life by entrapping an active core within a protective matrix. Therefore, a thorough understanding of the physicochemical interactions between these components is essential for developing stable and efficient delivery systems. The composition of the microcapsule and the encapsulation method are key determinants of system stability and the retention of encapsulated materials. Recently, the application of computational tools to predict and optimize microencapsulation processes has emerged as a promising area of research. In this context, molecular dynamics (MD) simulation has become an indispensable computational technique. By solving Newton’s equations of motion, MD simulations enable a detailed study of the dynamic behavior of atoms and molecules in a simulated environment. For example, MD-based analyses have quantitatively demonstrated that optimizing polymer–core interaction energies can enhance encapsulation efficiency by over 20% and improve the thermal stability of active compounds. This approach provides invaluable insights into the molecular interactions between the core material and the matrix, ultimately facilitating the rational design of optimized microstructures for diverse applications, including pharmaceuticals, thereby opening new avenues for innovation in the field. Ultimately, the integration of computational modeling into microencapsulation research not only represents a methodological advancement but also pivotal opportunity to accelerate innovation, optimize processes, and develop more effective and sustainable therapeutic systems.
1. Introduction
One of the main purposes of encapsulating an active ingredient is to protect it from external factors such as oxygen, pressure, heat, light, and water. In some cases, encapsulation is also required to prevent changes in the physical state of the compound.
Microencapsulation is the process of entrapping a small amount of an active or functional ingredient, commonly referred to as the core, within a surrounding matrix or coating made of materials known as encapsulants [1]. This approach has gained widespread use because it not only enhances the stability of active compounds but also improves their bioavailability. Furthermore, it has been extensively applied in the development of food products and pharmaceuticals [2,3].
Over the years, microencapsulation techniques have been refined, employing different encapsulating agents depending on the nature of the core material and the specific requirements of the process. This technology helps shield active compounds from environmental stresses, ensuring physicochemical stability, enabling controlled release, and extending shelf life [2]. A typical microcapsule consists of a core (the active substance) and a surrounding coating or matrix. Understanding the molecular and physicochemical interactions between these two components is essential, as they determine the stability and performance of the encapsulated system [3].
Several factors and mechanisms influence the stability of microcapsules; therefore, their composition must be carefully selected based on the compatibility between the core and the encapsulating matrix. Different encapsulation methods and processing conditions can generate diverse microstructures, which directly affect encapsulation efficiency and the retention of active compounds [4]. Microencapsulation applied in the food industry is used to protect active ingredients during processing and storage, helping to preserve their quality and shelf life—for example, in the encapsulation of flavors, polyunsaturated oils, vitamins, probiotics, among others [5,6]. Meanwhile, in the administration of active pharmaceutical ingredients, microencapsulation is employed to enhance bioavailability, stability, controlled release, and targeted delivery, and in some cases, to mask unpleasant tastes, increase efficacy, and minimize side effects [7]. Recent studies have also utilized microencapsulation to improve the bioactive stability of fruit and vegetable by-products, revalorizing bioactive compounds such as polyphenols, carotenoids, and flavonoids [8].
The integration of computational tools to predict and optimize microencapsulation processes has recently emerged as a promising research area. Methodologies such as molecular dynamics simulations and finite element modeling are increasingly being applied. In particular, computer-aided drug design has proven to be highly effective, as it allows researchers to evaluate binding affinities and pharmacokinetic properties of compounds with greater precision [9]. Molecular dynamics simulations, for example, are used to investigate the physical movements of atoms over time by solving Newton’s equations of motion. These simulations provide detailed information on atomic trajectories, offering valuable insights into the dynamic behavior of molecules within a simulated environment [10]. The application of computational simulation tools has proven to be essential for understanding and optimizing microencapsulation processes. Computational fluid dynamics (CFD) enables precise modeling of droplet formation and size distribution in multiphase systems, providing quantitative correlation with experimental results [11]. Likewise, at the molecular level, molecular dynamics (MD) offers detailed insights into interfacial interactions between encapsulating materials and active cores. Its use reveals how the composition and bonding energy determine the cohesion and mechanical strength of the composite [12].
2. Microencapsulation Techniques
Over the past decades, numerous microencapsulation techniques have been developed, each with distinct advantages and limitations. Widely applied methods include atomization, co-extrusion, spray coating, spray drying, coacervation, and emulsion-based systems, spanning a broad range of particle sizes (from hundreds of nanometers to several micrometers), encapsulation efficiencies (typically 60–95%), morphologies, material requirements, and costs [3,13]. The choice of method depends on the properties of the core and encapsulant, desired release profile, stability, and economic feasibility; for example, optimization of lipid and terpene composition in invasomal delivery systems improved entrapment efficiency to ~74% [14].
Despite methodological differences, all microencapsulation processes share four fundamental stages: core formation, shell formation, incorporation, and solidification, with process conditions at each stage determining microstructure and performance (Figure 1) [4,13,15]. Spray drying converts emulsions of core and polymer into solid microcapsules through rapid dehydration, with efficiency influenced by solution viscosity and surface tension [16,17,18,19]. Coacervation relies on phase separation of colloidal systems to achieve high encapsulation efficiency and controlled release, particularly for food applications [20,21,22,23,24]. Co-extrusion enables precise core–shell architectures, especially for viscous or concentrated materials [4,13,25], while emulsion-based methods use stabilized dispersed droplets for flexible and scalable encapsulation [4,13,26].
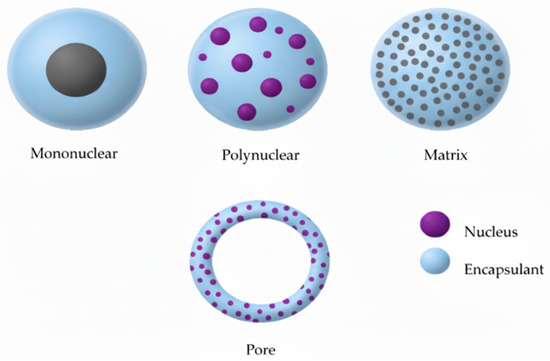
Figure 1.
Diagrams of different microstructures.
In addition, computational approaches such as molecular dynamics (MD) and continuum modeling can complement these experimental methods by predicting particle formation, stability, and encapsulation efficiency at the molecular and mesoscale levels, guiding formulation optimization and reducing reliance on trial-and-error experimentation [27,28,29]. By integrating these computational insights, researchers can better anticipate the performance of different encapsulation strategies and streamline the development of efficient and reproducible delivery systems (Figure 1).
Atomization serves as a foundational approach, generating fine droplets under controlled conditions, often integrated with other techniques for optimized particle formation [13,19]. While these experimental methods provide essential insight into microcapsule formation, the complexity of multiphase systems and the interplay of fluid dynamics, heat and mass transfer, and interfacial phenomena make complete experimental optimization challenging. Computational approaches, such as computational fluid dynamics (CFD), finite element modeling (FEM), and molecular dynamics (MD) simulations, have therefore become invaluable tools for predicting and refining microencapsulation processes [11,30,31,32,33,34,35,36,37,38,39,40,41]. By simulating the behavior of core–shell systems and microstructural dynamics, these methods can identify optimal process parameters, reduce trial-and-error experimentation, and improve product performance across applications ranging from pharmaceuticals to food and personal care products [22,30,40,42,43,44].
CFD modeling, for example, enables detailed investigation of fluid flow, heat and mass transfer, and interfacial interactions, offering insights that are difficult to obtain experimentally [11,30,31]. FEM complements this by providing numerical solutions for stress, deformation, and transport phenomena within heterogeneous systems, often using representative volume elements (RVEs) to approximate material behavior [32,33,34,35] MD simulations. Meanwhile, it allows atomic-level exploration of dynamic molecular interactions, conformational flexibility, and thermal properties, providing critical information for drug design, biomaterials, and functional food development [36,37,38,39,40,41]. Together, these approaches form a complementary multi-scale framework: CFD captures macroscopic flow behavior and transport processes that govern droplet formation and mixing; FEM bridges this mesoscopic level by modeling mechanical deformation, stress distribution, and local structural responses; and MD provides atomic-scale resolution of molecular interactions at the core–shell interface. By linking insights from each scale, this integrated approach enables a more complete representation of encapsulation mechanisms, improving the predictive accuracy of simulations and guiding experimental design.
In the context of MD simulations, typical time scales range from 1 to 100 nanoseconds, with system sizes extending from tens to several hundred nanometers, depending on the complexity of the polymeric matrix [45]. Commonly used force fields such as Chemistry at HARvard Macromolecular Mechanics (CHARMM) [46], GROningen Molecular Simulation (GROMOS) [47], and Condensed-phase Optimized Molecular Potentials for Atomistic Simulation Studies (COMPASS) [48] have been widely employed to model biopolymers, surfactants, and encapsulant materials with high reliability. Software packages including GROningen Machine for Chemical Simulations (GROMACS), Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS), and Materials Studio are among the most frequently used platforms due to their flexibility and accuracy in reproducing molecular interactions [49]. Several studies have demonstrated the successful integration of MD results with experimental validation, confirming the predictive capability of these models in encapsulation performance [50,51]. A steady increase in related publications over the past decade further reflects the growing relevance of MD simulation in understanding and optimizing polymer-based delivery systems.
The predictive power of these computational tools is increasingly corroborated through experimental validation. For instance, Berger et al. (2024) [52] used MD simulations to guide the encapsulation of polar molecules in synthetic oleosomes, while Zhang et al. (2023) [53] optimized protein–sucrose ester microcapsules based on molecular docking predictions. Similarly, microfluidic production of alginate microspheres for cephalexin encapsulation benefited from simulation-driven parameter optimization, enhancing experimental efficiency [54].
Collectively, these studies demonstrate how computational modeling, coupled with experimental validation, enables the rational design and optimization of microencapsulation processes, ensuring reproducible, high-performance outcomes [40,42,43,44].
To aid non-specialist readers, we include here a brief summary of scale, force fields, validation examples, and software commonly used in MD studies of polymeric matrices. Atomistic MD is typically applied at length scales of ~1–10 nm and time scales from picoseconds to hundreds of nanoseconds (and, in special cases, microseconds), sufficient to resolve local polymer conformations, small-molecule diffusion, and binding events at interfaces; coarse-grained (CG) MD reduces degrees of freedom to access larger length scales (tens to hundreds of nm) and longer timescales (microseconds to milliseconds), enabling study of mesoscale aggregation, phase separation, and drying behavior. Common force fields for biomolecules and soft matter include CHARMM, AMBER and GROMOS families (widely used for proteins and biopolymers), OPLS variants, and condensed-phase/condensed-matter force fields such as COMPASS (often used for synthetic polymers and polymer blends); choice of force field depends on system composition and the property of interest, and care must be taken when parametrizing novel monomers or small molecules. Representative software platforms include GROMACS, NAMD, AMBER, LAMMPS, Desmond, and commercial suites such as Materials Studio; GROMACS and LAMMPS are particularly widespread for large-scale MD and CG simulations. Several studies illustrate close integration of MD with experiments: for example, Gosecki et al. (2023) [51] combined drying-protocol MD with synthesis and encapsulation assays to rationalize and achieve high clotrimazole encapsulation efficiency (~83%), and other recent reviews and case studies demonstrate the use of MD predictions to guide formulation and to interpret spectroscopic or thermal measurements.
3. Simulation and Modeling Approaches
The experimental techniques described above provide essential insights into microcapsule formation, yet the complexity of multiphase systems—including fluid dynamics, heat and mass transfer, and interfacial interactions—makes complete experimental optimization challenging. Computational modeling offers a complementary approach, allowing researchers to predict system behavior, evaluate design parameters, and optimize processes before experimental trials. By building on knowledge gained from experimental methods, these tools enhance efficiency, reduce costs, and improve reproducibility, ultimately supporting the development of high-performance microcapsules for a wide range of applications.
Computational fluid dynamics (CFD) modeling has emerged as a valuable tool for studying complex processes such as microencapsulation [11]. These processes involve coupled phenomena, including fluid dynamics, heat and mass transfer, and interfacial interactions, which are often difficult to fully capture or optimize through experiments alone [11,30,31]. The inherent heterogeneity of multiphase systems further increases the complexity, requiring a detailed understanding of interactions among components. Computational modeling addresses these challenges by enabling systematic evaluation and optimization, enhancing the efficiency and quality of microencapsulation processes. Ultimately, these improvements translate into superior product performance and broader applications, spanning drug delivery, food processing, cosmetics, personal care products, and even the petroleum industry [22,30].
The finite element method (FEM) is a numerical technique that approximates solutions to differential and integral equations by subdividing complex domains into smaller, simpler elements. Each element is solved individually, and the results are combined to approximate the behavior of the complete system [32].
FEM is widely applied across engineering and materials science, including stress and deformation analysis in vehicles, aircraft, buildings, and bridges, as well as modeling heat transfer, fluid flow, magnetic fields, and other transport phenomena [33,34]. More recently, it has proven valuable in simulation and design for textiles, pharmaceuticals, and food processing.
A key principle of FEM is the use of a representative volume element (RVE) or unit cell to characterize the behavior of a heterogeneous material. This approach is essential because capturing the full three-dimensional arrangement of all phases in a volume comparable to experimental samples is often unfeasible and computationally prohibitive. Using RVEs enables both experimental and computational studies to predict material behavior with sufficient accuracy while maintaining practicality [32,35].
Molecular dynamics (MD) simulations have become indispensable for investigating the microstructure and properties of substances at the atomic level [36,37]. By simulating the time-dependent movements of atoms according to Newton’s laws of motion, MD provides dynamic insights into molecular interactions, complementing static structural data [36,38,39].
Over the past decade, MD has significantly expanded its influence in biomolecular research, enabling the visualization of molecular structures, estimation of thermal properties, and exploration of conformational landscapes. Beyond fundamental science, MD has been applied to materials science, food technology, drug design, and therapeutic development [40,41].
In pharmaceutical research, MD simulations are particularly valuable for exploring molecular flexibility, binding mechanisms, and dynamic stability. They also allow estimation of free energy, which is critical for accurately ranking binding affinities and optimizing drug discovery and development [10]. Molecular dynamics (MD) simulations provide not only structural and dynamic insights but also enable the estimation of free energies of binding or interaction, which is essential for understanding the stability and performance of polymer–drug and polymer–encapsulant systems. Common approaches include MM/GBSA (Molecular Mechanics/Generalized Born Surface Area) and MM/PBSA (Molecular Mechanics/Poisson–Boltzmann Surface Area), which combine molecular mechanics energies with continuum solvation models to evaluate interaction energetics [55,56,57,58,59]. These methods allow the quantification of binding affinities, identification of favorable interaction conformations, and assessment of energetic stability, providing a rational basis for formulation optimization. Several studies have integrated these computational predictions with experimental validation, demonstrating that MD-based free energy calculations can correlate accurately with encapsulation efficiency and functional performance, thereby reducing trial-and-error experimentation and guiding the design of more efficient polymeric delivery systems.
The predictive power of MD and other computational simulations is increasingly validated through experimental studies. For example, Berger et al. (2024) [52] demonstrated that synthetic oleosomes could encapsulate polar molecules by modulating core polarity with mixtures of sunflower and castor oils. MD simulations accurately predicted the interactions that guided the experimental design, confirming the approach’s potential to overcome limitations in specificity.
Similarly, Zhang et al. (2023) [53] fabricated protein–sucrose ester microcapsules via spray drying, guided by molecular docking simulations that provided insights into structural changes. These predictions were subsequently validated through experiments, confirming the reliability of the simulation approach.
In another study, the development of a microfluidic chip to produce alginate microspheres for cephalexin encapsulation relied on simulation-based tests to evaluate droplet formation and optimize critical parameters, which enhanced the efficiency of experimental trials [54].
Overall, computational simulations have proven highly effective in predicting process feasibility, evaluating material interactions, and guiding experimental design in microencapsulation [40,42,43,44]. This combination of in silico modeling and in vitro validation represents a powerful strategy for advancing encapsulation technologies and ensuring reproducible, high-performance outcomes (Figure 2).
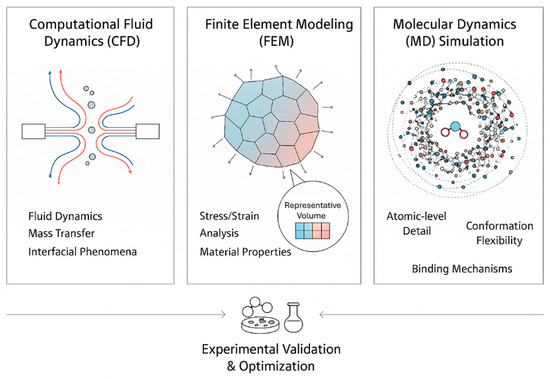
Figure 2.
Overview of computational modeling approaches in microencapsulation, including CFD, FEM, and molecular dynamics, with experimental validation.
4. Comparative Studies of Microencapsulation Processes in Pharmaceutical Industry
The continuous development of advanced drug delivery systems is driven by the need to maximize therapeutic efficacy while reducing adverse effects. Recent progress in pharmaceutical technology has highlighted the value of modified delivery strategies, in which dosage forms are specifically engineered to achieve and maintain therapeutic drug concentrations at targeted sites throughout the treatment period [60,61]. These approaches are increasingly displacing conventional administration methods, with microencapsulation standing out as one of the most versatile and widely adopted delivery platforms.
Microparticulate carriers are designed to transport active compounds directly to their intended site of action [62]. Within the pharmaceutical field, this innovation represents a significant advancement, especially for drug classes where localized and controlled release is essential.
Non-steroidal anti-inflammatory drugs (NSAIDs) exemplify such a case. They remain among the most widely prescribed agents owing to their anti-inflammatory, analgesic, and antipyretic properties. However, their therapeutic action is closely linked to a considerable risk of toxicity, resulting in a broad spectrum of adverse effects. Gastrointestinal complications are common, affecting both the upper tract (e.g., peptic ulcers) and the lower tract (NSAID-induced enteropathy), while cardiovascular events are often considered the most serious consequences [60,63,64].
Table 1 summarizes the principal microencapsulation processes applied in the pharmaceutical industry, highlighting their use across different drug classes. Beyond these applications, the versatility of microencapsulation has also extended into other scientific and technological fields, where it plays a key role in structuring, protecting, and controlling the release of active compounds.

Table 1.
Comparison between methods of microencapsulation for NSAIDs.
Given these challenges, strategies that improve drug stability and modulate release profiles are of particular importance. In this context, microencapsulation has gained significant relevance, not only in pharmaceuticals but also in fields such as cell engineering, food technology, and textiles [73]. Within drug delivery, this technique offers distinct advantages: it structures materials, protects the encapsulated product, and enables precise control over the release of active compounds. It also facilitates the use of substances that are otherwise difficult to formulate due to properties such as insolubility, volatility, reactivity, hygroscopicity, or unstable physical states [73,74].
Encapsulation of active pharmaceutical ingredients provides an additional protective barrier against degradation caused by oxygen, light, heat, or moisture, while simultaneously enhancing bioavailability and supporting prolonged release. Evidence from in vivo studies consistently highlights these benefits, demonstrating improved drug stability, sustained therapeutic levels, and greater overall bioavailability [60,73,75].
5. Application of Microencapsulation in Clinical Settings
Recent advances in microencapsulation technology with clinical applications highlight its growing integration into nanomedicine. For example, micro- and nanoparticles have been investigated for their potential to modulate mitochondrial permeability transition pores in the treatment of idiopathic pulmonary fibrosis, demonstrating superior stability and therapeutic efficiency compared to conventional approaches [76].
Beyond pulmonary applications, microencapsulation has also proven highly effective in preserving bioactive compounds derived from plants, essential oils, and vegetables for use across the food, pharmaceutical, and textile industries. By enhancing shelf life, improving oxidative stability, protecting sensitive compounds, and enabling controlled release, this technology extends the functional utility of otherwise fragile biomolecules [77].
Over the past decade, pharmaceutical research has increasingly applied microencapsulation to optimize drug loading and release profiles [78], thereby mitigating adverse effects commonly associated with various active agents. Porous biomaterials have emerged as particularly attractive carriers in this context due to their high surface area [79]. Plant spores have been identified as natural porous vehicles for nutraceutical delivery and have been used for drugs such as ibuprofen and diclofenac [80]. Polymeric microcapsules have also been explored for corticosteroid and indomethacin delivery, with techniques like coaxial atomization showing promise for intra-articular administration [81]. Despite these advances, translating microencapsulation systems to clinical use remains challenging due to unpredictable biocompatibility and immunotoxicity, variability between animal models and human responses, and technical and production-related hurdles. These factors necessitate additional time-consuming and costly validation studies [82].
Table 2 summarizes the principal pharmaceutical applications of microencapsulation, underscoring its advantages in enhancing bioavailability, enabling controlled release, and protecting active ingredients.

Table 2.
Application of microencapsulation in the pharmaceutical industry.
Scaling up microencapsulation processes from laboratory to industrial production is rarely straightforward. Achieving consistent particle homogeneity and precise control over physicochemical properties presents significant difficulties. During this transition, issues such as reproducibility, microparticle toxicity, and quality assurance become increasingly prominent [64]. Moreover, scale-up can adversely affect product stability, therapeutic efficacy, and safety, while complicating compliance with regulatory quality specifications and storage requirements [65].
Further complicating the laboratory-to-industry transition, standard analytical techniques can characterize particle size and morphology but remain limited in their ability to predict in vivo performance. Consequently, many microencapsulated products struggle to meet regulatory expectations, often requiring extended studies that substantially increase development costs [83].
6. Future Directions and Research Opportunities
Microencapsulation is a biomedical technology with considerable therapeutic potential across a wide spectrum of diseases. Beyond traditional approaches, it can also be integrated with emerging techniques such as 3D printing. The development of novel bio-inks tailored for specific applications allows the creation of microspheres that incorporate drugs or bioactive compounds while enabling precise, controlled release [84,85].
Since the FDA approval of the first 3D-printed tablet, interest in leveraging this technology for drug delivery and broader biomedical applications has grown substantially [69]. This is largely due to 3D printing’s ability to generate complex matrices and, consequently, microcapsules with highly defined architectures, which enhance the stability, survival, and functionality of the encapsulated core [84].
Optimizing each microcapsule formulation requires careful consideration of the capsule’s composition and physicochemical properties. This process involves variables such as the encapsulation technique, the choice of matrix material, and the therapeutic loading capacity [53]. In recent years, the repertoire of wall materials has expanded to include lipids, which are particularly effective for encapsulating hydrophobic compounds due to their favorable affinity and adaptable interactions [86,87].
7. Conclusions
The field of microencapsulation has progressed into a versatile technology for protecting, stabilizing, and controlling the release of active compounds across industries such as food, textiles, and pharmaceuticals. While experimental approaches have enabled the development of numerous techniques, they often struggle to capture molecular-level interactions between the active core and encapsulating matrix, which strongly influence stability and encapsulation efficiency. Computational modeling, particularly molecular dynamics (MD) simulation, provides predictive insights into atomic and molecular behavior, enabling evaluation and optimization of key parameters in silico before experimental testing. This reduces reliance on trial-and-error experiments, shortens development time, and lowers costs. By guiding the design of microcapsules with improved stability, biocompatibility, and therapeutic performance, computational approaches accelerate the rational development of safer, more efficient, and highly specific delivery systems, bridging the gap from laboratory research to clinical application.
Author Contributions
Conceptualization, C.P.C.-A. and D.R.-J.; Funding acquisition, E.D.; Investigation, K.I.V.-R. and C.I.G.-G.; Methodology, K.I.V.-R. and C.I.G.-G.; Project administration, D.R.-J.; Resources, E.D.; Software, K.I.V.-R.; Supervision, D.R.-J.; Visualization, C.P.C.-A.; Writing—original draft, K.I.V.-R.; Writing—review and editing, D.R.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MD | Molecular dynamics |
| CFD | Computational fluid dynamics |
| FEM | Finite element method |
| NSAIDs | Non-steroidal anti-inflammatory and anti-rheumatic agents |
| mPTP | Modulation of mitochondrial permeability transition pore |
References
- Das, E.D.; Sakibul, I.; Rahman, M.A.; Mohammad, G.A.; Saydar, R. Recent applications of microencapsulation techniques for delivery of functional ingredient in food products: A comprehensive review. Food Chem. Adv. 2025, 6, 100923. [Google Scholar] [CrossRef]
- Hay, T.O.; Nastasi, J.R.; Prakash, S.; Fitzgerald, M.A. Comparison of Gidyea gum, gum Arabic, and maltodextrin in the microencapsulation and colour stabilisation of anthocyanin-rich powders using freeze-drying and spray-drying techniques. Food Hydrocoll. 2025, 163, 111023. [Google Scholar] [CrossRef]
- Vasisht, N. Selection of Materials for Microencapsulation; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of encapsulates and their relations with encapsulation efficiency and controlled release of bioactive constituents: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of Polyphenols and Their Application in Food Technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- Yan, C.; Kim, S.-R.; Ruiz, D.R.; Farmer, J.R. Microencapsulation for Food Applications: A Review. ACS Appl. Bio Mater. 2022, 5, 5497–5512. [Google Scholar] [CrossRef]
- Yan, C.; Kim, S.-R. Microencapsulation for Pharmaceutical Applications: A Review. ACS Appl. Bio Mater. 2024, 7, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Ingale, O.S.; Pravin, B.P.; Pawase, P.A.; Shams, R.; Dash, K.K.; Bashir, O.; Roy, S. Enhancing Bioactive Stability and Applications: Microencapsulation in Fruit and Vegetable Waste Valorization; Springer Nature: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Jibrin, A.; Uzairu, A.; Mishra, V.K.; Shallangwa, G.A.; Abechi, S.E.; Srivastava, R.; Umar, A.B. In-silico design of potential mycobacterial membrane protein large 3 (MmpL3) inhibitors via 2D-QSAR, molecular docking, drug-likeness evaluation, and molecular dynamic simulation. Microbe 2025, 8, 100531. [Google Scholar] [CrossRef]
- AlRawashdeh, S.; Barakat, K.H. Applications of Molecular Dynamics Simulations in Drug Discovery. In Computational Drug Discovery and Design; Humana: New York, NY, USA, 2024; Volume 2714, pp. 127–141. [Google Scholar]
- Ji, J.; Wang, H.; Huang, S.F.; Li, Y.Y.; Tokumasu, T. Combining experimental and computational fluid dynamics simulation approaches to optimize cross-flow velocity conditions in anaerobic membrane bioreactors. J. Water Process Eng. 2025, 78, 108790. [Google Scholar] [CrossRef]
- Wang, X.; Xie, W.; Li, L.Y.; Zhu, J.; Xing, F. Molecular Simulation Study on Mechanical Properties of Microcapsule-Based Self-Healing Cementitious Materials. Polymers 2022, 14, 611. [Google Scholar] [CrossRef]
- Oxley, J. Overview of Microencapsulation Process Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Patil, S.; Bhargav, S. Advanced invasomal delivery system for enhanced topical application of bifonazole in fungal infections. Prospect. Pharm. Sci. 2025, 23, 102–110. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Kurrey, N.K.; Anandharamakrishnan, C. Nanoencapsulation of green tea catechins by electrospraying technique and its effect on controlled release and in-vitro permeability. J. Food Eng. 2017, 199, 82–92. [Google Scholar] [CrossRef]
- Damerau, A.; Ogrodowska, D.; Banaszczyk, P.; Dajnowiec, F.; Tańska, M.; Linderborg, K.M. Baltic herring (Clupea harengus membras) oil encapsulation by spray drying using a rice and whey protein blend as a coating material. J. Food Eng. 2022, 314, 110769. [Google Scholar] [CrossRef]
- Thao, V.T.; Truong, V. Microencapsulation of agarwood (Aquilaria crassna) essential oil by spray drying. IOP Conf. Ser. Earth Environ. Sci. 2023, 1155, 012018. [Google Scholar] [CrossRef]
- Benelli, L.; Oliveira, W.P. Fluidized bed coating of inert cores with a lipid-based system loaded with a polyphenol-rich Rosmarinus officinalis extract. Food Bioprod. Process. 2019, 114, 216–226. [Google Scholar] [CrossRef]
- Jacobs, I.C. Atomization and Spray-Drying Processe; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Amiri, S.; Khaneghah, A.M. The application of the coacervation technique for microencapsulation bioactive ingredients: A critical review. J. Agric. Food Res. 2024, 18, 101431. [Google Scholar] [CrossRef]
- Movahed, M.D.; Sahari, M.A.; Barzegar, M. Microencapsulation of flaxseed oil by complex coacervation of with apricot tree (Prunus armeniaca L.) gum and low molecular chitosan: Formation and structural characterization. Appl. Food Res. 2024, 4, 100519. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Microencapsulation of hemp seed oil by pea protein isolate−sugar beet pectin complex coacervation: Influence of coacervation pH and wall/core ratio. Food Hydrocoll. 2021, 113, 106423. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Zhang, W.; Lan, D.; Wang, Y. Co-encapsulation of probiotics with acylglycerols in gelatin-gum arabic complex coacervates: Stability evaluation under adverse conditions. Int. J. Biol. Macromol. 2023, 242, 124913. [Google Scholar] [CrossRef]
- Schäfer, C.; Meyer, S.P.; Osswald, T.A. A novel extrusion process for the production of polymer micropellets. Polym. Eng. Sci. 2018, 58, 2264–2275. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle- and Microparticle-based Delivery Systems Encapsulation, Protection and Release of Active Compounds, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Qizilbash, M.; del Valle, L.J.; Zabaleta, A.G. Modeling Polymer Microencapsulation Processes Using CFD and Population Balance Models. Appl. Sci. 2024, 14, 7807. [Google Scholar] [CrossRef]
- Mackenzie, R.; Booth, J.; Alexander, C.; Garnett, M.C.; Laughton, C.A. Multiscale Modeling of Drug–Polymer Nanoparticle Assembly Identifies Parameters Influencing Drug Encapsulation Efficiency. J. Chem. Theory Comput. 2015, 11, 2705–2713. [Google Scholar] [CrossRef]
- Gooneie, A.; Schuschnigg, S.; Holzer, C. A Review of Multiscale Computational Methods in Polymeric Materials. Polymers 2017, 9, 16. [Google Scholar] [CrossRef]
- Ricardo, F.; Pradilla, D.; Luiz, R.; Solano, O.A.A. A multi-scale approach to microencapsulation by interfacial polymerization. Polymers 2021, 13, 644. [Google Scholar] [CrossRef]
- Thakur, N.; Murthy, H. Simulation study of droplet formation in inkjet printing using ANSYS FLUENT. J. Phys. Conf. Ser. 2022, 2161, 012026. [Google Scholar] [CrossRef]
- Withers, P.J. Elastic and Thermoelastic Properties of Brittle Matrix Composites. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Behera, B.K.; Hari, P.K.; Labanieh, A.R. Modelling the structure of woven fabrics. In Woven Textiles: Principles, Technologies and Applications; Woodhead Publishing Series in Textiles: Cambridge, UK, 2020; pp. 291–328. [Google Scholar] [CrossRef]
- Raju, B.; Hiremath, S.R.; Mahapatra, D.R. A review of micromechanics based models for effective elastic properties of reinforced polymer matrix composites. Compos. Struct. 2018, 204, 607–619. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Duarte, C.A.; Aragón, A.M. A Discontinuity-Enriched Finite Element Method (DE-FEM) for modeling quasi-static fracture growth in brittle solids. Comput. Methods Appl. Mech. Eng. 2025, 435, 117585. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Li, S.; Deng, L.; Liang, B.; Kong, X.; Chen, X.; Liu, D.; Yang, B. Review of molecular dynamics simulation in extraction metallurgy. Comput. Mater. Sci. 2025, 258, 114111. [Google Scholar] [CrossRef]
- Gao, T.; Nan, Y.; Chen, S.; Li, C.; Mu, B.; Wang, J.; Li, H.; Piao, C.; Li, G. Identification of antioxidant peptides in low-salt dry-cured ham skin using molecular docking and molecular dynamics simulation. LWT 2025, 231, 118220. [Google Scholar] [CrossRef]
- Lindahl, E. Molecular dynamics simulations. In Molecular Modeling of Proteins, 2nd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2014; pp. 3–26. [Google Scholar] [CrossRef]
- Vargas-Rubio, K.I.; Medrano-Roldán, H.; Reyes-Jáquez, D. Molecular dynamics simulation of a nanocluster obtained from the mining industry. Acta Univ. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Ge, W.; Wang, H.; Ma, C. UPLC-Q-TOF-MSdriven network pharmacology coupled with molecular dynamics simulation to reveal the molecular mechanism of Lycium barbarum, L. flavonoids in regulating type 2 diabetes mellitus. Food Nutr. 2025, 1, 100038. [Google Scholar] [CrossRef]
- Wang, F.; Yang, W.; Peng, S.; Zhou, B. Quinoxaline derivatives in identifying novel VEGFR-2 inhibitors: A combined 3D-QSAR, molecular docking and molecular dynamics simulation. Comput. Biol. Med. 2025, 197, 110949. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Chen, W.; Ruan, Z.; Lou, H.; Yang, D.; Jiang, B. In silico prediction of bioequivalence of atorvastatin tablets based on GastroPlusTM software. BMC Pharmacol. Toxicol. 2023, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Okumu, A.; DiMaso, M.; Löbenberg, R. Computer simulations using GastroPlusTM to justify a biowaiver for etoricoxib solid oral drug products. Eur. J. Pharm. Biopharm. 2009, 72, 91–98. [Google Scholar] [CrossRef]
- Sun, F.; Lee, L.; Zhang, Z.; Wang, X.; Yu, Q.; Duan, X.; Chan, E. Preclinical pharmacokinetic studies of 3-deazaneplanocin A, a potent epigenetic anticancer agent, and its human pharmacokinetic prediction using GastroPlusTM. Eur. J. Pharm. Sci. 2015, 77, 290–302. [Google Scholar] [CrossRef]
- Gartner, T.E.; Jayaraman, A. Modeling and Simulations of Polymers: A Roadmap. Macromolecules 2019, 52, 755–786. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Rahmani, A.; Rezaei, A.; Khosravani, M.R. Molecular Dynamics Simulation in Tissue Engineering: Concepts, Tools, and Emerging Applications. Front. Bioeng. Biotechnol. 2024, 12, 15202223. [Google Scholar] [CrossRef]
- Singh, N.; Li, W.; Noid, W.G. Recent Advances in Coarse-Grained Models for Biomolecular Simulations. Phys. Chem. 2019, 20, 3774. [Google Scholar] [CrossRef]
- Gosecki, M.; Urbaniak, M.; Martinho, N.; Gosecka, M.; Zloh, M. Evaluation of Encapsulation Potential of Selected Star-Hyperbranched Polyglycidol Architectures: Predictive Molecular Dynamics Simulations and Experimental Validation. Molecules 2023, 28, 7308. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.A.; Vietor, H.M.; Scott, D.W.; Lee, H.; Hashemipour, S.; Im, W.; Wittenberg, N.J.; Glover, K.J. Physicochemical Properties of Seed Oil Blends and Their Potential for the Creation of Synthetic Oleosomes with Modulated Polarities. ACS Omega 2024, 9, 43193–43202. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, W.; Shi, Y.; Tu, Z.; Hu, Y.; Zhang, J. Interaction between soy protein isolate/whey protein isolate and sucrose ester during microencapsulation: Multi-spectroscopy and molecular docking. LWT 2023, 188, 115363. [Google Scholar] [CrossRef]
- Valizadeh, Z.; Shahrouzi, J.R. Microencapsulation of cephalexin in alginate microspheres in a microfluidic system: Experimental and simulation study. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 717, 136738. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson−Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Wang, E.; Weng, G.; Sun, H.; Du, H.; Zhu, F.; Chen, F.; Wang, Z.; Hou, T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 10. Impacts of enhanced sampling and variable dielectric model on protein–protein Interactions. Phys. Chem. Chem. Phys. 2019, 21, 18958–18969. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Nazari, Q.A.; Greige-Gerges, H.; Fessi, H.; Elaissari, A. Encapsulation of NSAIDs for Inflammation Management: Overview Progress Challenges Prospects; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Hijos-Mallada, G.; Sostres, C.; Gomollón, F. AINE, toxicidad gastrointestinal y enfermedad inflamatoria intestinal. Gastroenterol. Hepatol. 2021, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Marcén, B.; Sostres, C.; Lanas, A. AINE y riesgo digestivo-NSAID and gastrointestinal risk. Aten Primaria 2016, 48, 73–76. [Google Scholar] [CrossRef]
- Zhang, X.; Chau, L.Y.; Chan, H.W.; Weng, J.; Wong, K.W.; Chow, S.F.; Chow, A.H.L. Physical stability and in vivo brain delivery of polymeric ibuprofen nanoparticles fabricated by flash nanoprecipitation. Int. J. Pharm. 2021, 598, 120224. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Garrigues, O.; Fessi, H.; Elaissari, A. Nanocapsules prepared via nanoprecipitation and emulsification-diffusion methods: Comparative study. Eur. J. Pharm. Biopharm. 2012, 80, 235–239. [Google Scholar] [CrossRef]
- Fayez, S.M.; Elnahas, O.S.; Fayez, A.M.; El-Mancy, S.S. Coconut oil based self-nano emulsifying delivery systems mitigate ulcerogenic NSAIDs side effect and enhance drug dissolution: Formula optimization, in-vitro, and in-vivo assessments. Int. J. Pharm. 2023, 634, 122666. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Choudhary, C.; Ajazuddin; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef]
- Moretti, M.D.L.; Gavini, E.; Juliano, C.; Pirisino, G.; Giunchedi, P. Spray-Dried Microspheres Containing Ketoprofen Formulated into Capsules and Tablets. Available online: http://www.tandf.co.uk/journals (accessed on 4 May 2025).
- Pomázi, A.; Ambrus, R.; Sipos, P.; Szabó-Révész, P. Analysis of co-spray-dried meloxicam–mannitol systems containing crystalline microcomposites. J. Pharm. Biomed. Anal. 2011, 56, 183–190. [Google Scholar] [CrossRef]
- Castro, M.A.A.; Alric, I.; Brouillet, F.; Peydecastaing, J.; Fullana, S.G.; Durrieu, V. Spray-Dried Succinylated Soy Protein Microparticles for Oral Ibuprofen Delivery. AAPS PharmSciTech 2019, 20, 79. [Google Scholar] [CrossRef]
- del Arco, M.; Fernández, A.; Martín, C.; Rives, V. Release studies of different NSAIDs encapsulated in Mg, Al, Fe-hydrotalcites. Appl. Clay Sci. 2009, 42, 538–544. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Kahouli, I.; Prakash, S. Microencapsulation for the Therapeutic Delivery of Drugs, Live Mammalian and Bacterial Cells, and Other Biopharmaceutics: Current Status and Future Directions. J. Pharm. 2013, 2013, 103527. [Google Scholar] [CrossRef]
- Rattes, A.L.R.; Oliveira, W.P. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technol. 2007, 171, 7–14. [Google Scholar] [CrossRef]
- Chen, S.; Dai, H.; Zhou, H. Research on microencapsulated aquatic feed preparation based on micro-jet spraying technology. Adv. Mater. Res. 2011, 143–144, 1071–1074. [Google Scholar] [CrossRef]
- Lu, A.; Xu, Z.; Zhao, Z.; Yan, Y.; Jiang, L.; Geng, J.; Jin, H.; Wang, X.; Liu, X.; Zhu, Y.; et al. Double Braking Effects of Nanomedicine on Mitochondrial Permeability Transition Pore for Treating Idiopathic Pulmonary Fibrosis. Adv. Sci. 2024, 11, 2405406. [Google Scholar] [CrossRef]
- Hafizah, F.; Yusop, M.; Fairuz, S.; Manaf, A.; Hamzah, F. Preservation of Bioactive Compound via Microencapsulation. Chem. Eng. Res. Bull. 2017, 19, 50–56. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Ourani-Pourdashti, S.; Azadi, A. Pollens in therapeutic/diagnostic systems and immune system targeting. J. Control. Release 2021, 340, 308–317. [Google Scholar] [CrossRef]
- Taha, N.F.; Dyab, A.K.F.; Emara, L.H.; Meligi, N.M. Microencapsulation of Diclofenac Sodium into natural Lycopodium clavatum spores: In vitro release and gastro-ulcerogenic evaluations. J. Drug Deliv. Sci. Technol. 2022, 71, 103278. [Google Scholar] [CrossRef]
- Lamela-Gómez, I.; Luzardo-Álvarez, A. Indomethacin microencapsulation by coaxial ultrasonic atomization intended for intraarticular administration. J. Drug Deliv. Sci. Technol. 2025, 109, 107002. [Google Scholar] [CrossRef]
- Zheng, C.; Li, M.; Ding, J. Challenges and Opportunities of Nanomedicines in Clinical Translation. BIO Integr. 2021, 2, 57–60. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.; Williams, K.; Phelps, J.; La Boucan, F.; Lewandowski, G.; O’gRady, K.; Yu, B.; Willerth, S.M. Microspheres for 3D bioprinting: A review of fabrication methods and applications. Front. Bioeng. Biotechnol. 2025, 13, 1551199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, M.; Chen, W.; Liu, H.; Tan, D.; Shen, S.; Lei, Y.; Xue, L. 3D printing of bioinspired compartmentalized capsular structure for controlled drug release. J. Zhejiang Univ. B 2021, 22, 1022–1033. [Google Scholar] [CrossRef]
- Ma, Z.; Bitter, J.H.; Boom, R.M.; Nikiforidis, C.V. Encapsulation of cannabidiol in hemp seed oleosomes. Food Res. Int. 2024, 195, 114948. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).