Role of Lipid Composition on the Mechanical and Biochemical Vulnerability of Myelin and Its Implications for Demyelinating Disorders
Abstract
1. Introduction
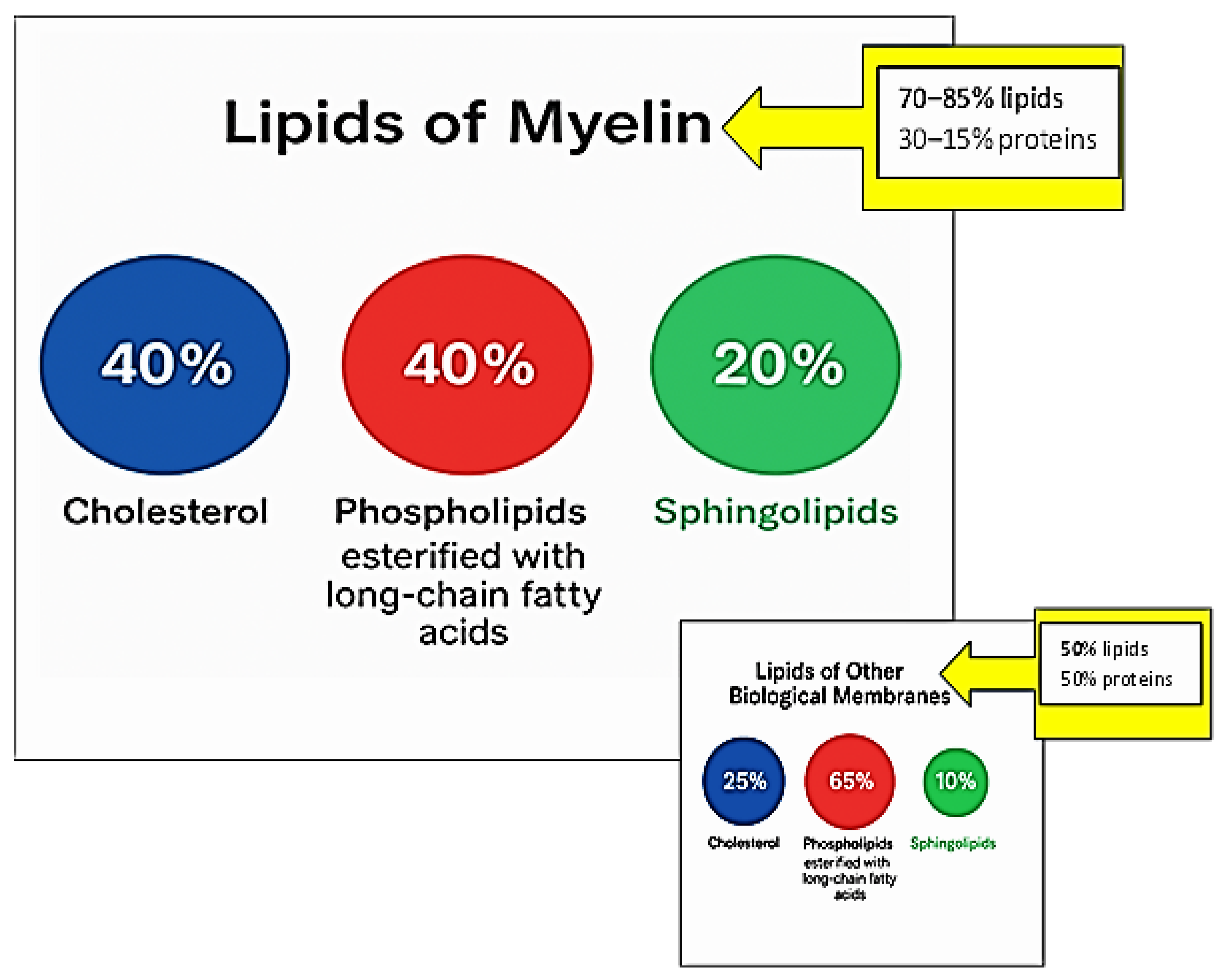
2. Distinctive Myelin Lipids
2.1. Cholesterol
2.2. Sphingolipids
2.3. Phospholipids (PC, PE, PS) Esterified with Long-Chain Fatty Acids
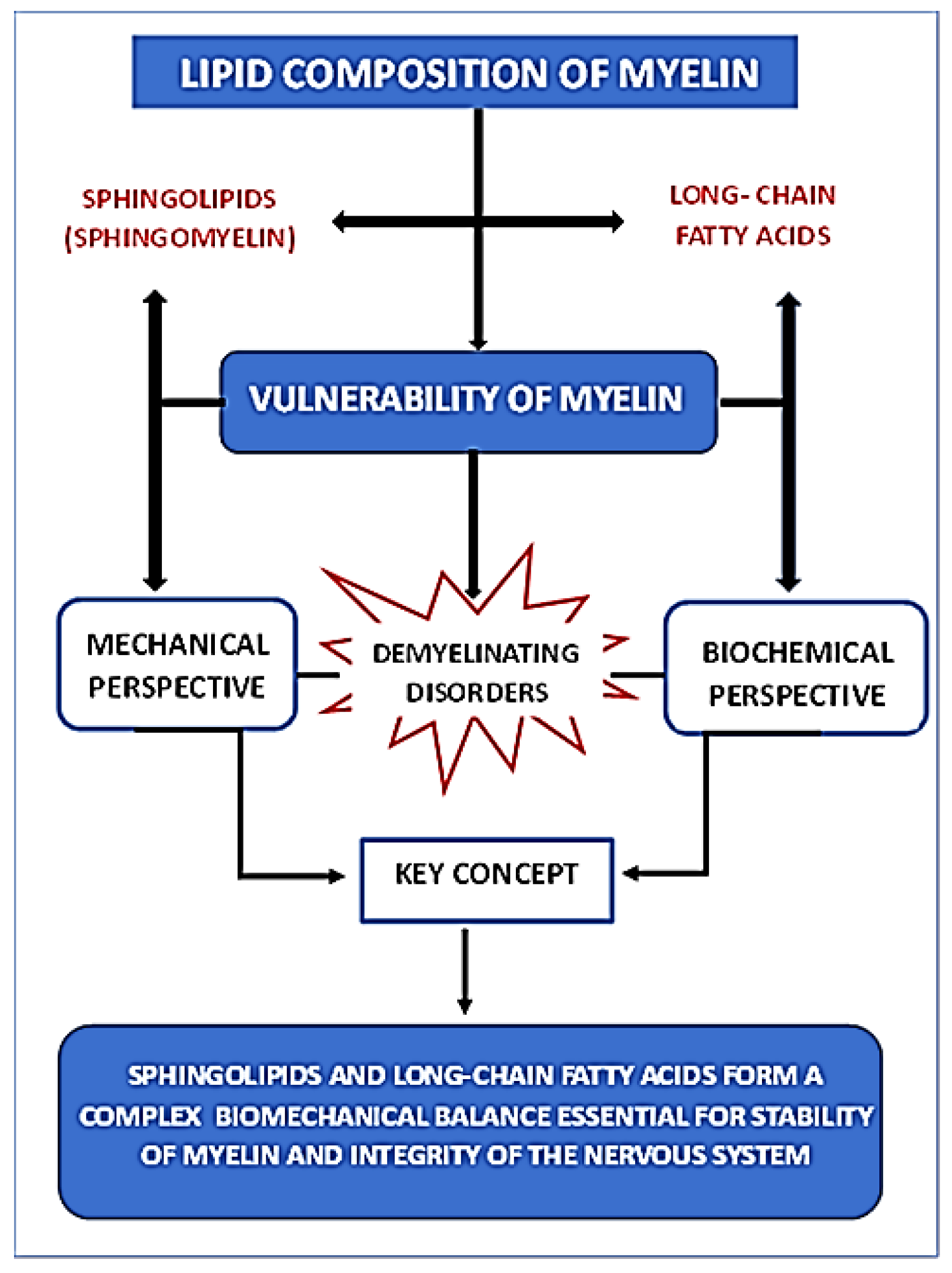
3. Mechanical Vulnerability of the Myelin Lipid Membrane
3.1. Lipid Composition, Rigidity, and Demyelination
3.2. Damage to Myelin Architecture Due to Traumatic Injury and Demyelination Mechanisms
4. Biochemical Vulnerability of Myelin: Protective Role of PUFAs
5. Myelin Vulnerability in the Context of Metabolic Disorders and Nutritional Deprivation: Myelin Lipids as an Energy Reserve
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 715281–715296. [Google Scholar] [CrossRef]
- Bonetto, G.; Belin, D.; Káradóttir, R.T. Myelin: A gatekeeper of activity dependent circuit plasticity? Science 2021, 374, eaba6905. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocytes and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Min, Y.; Kristiansen, K.; Boggs, J.M.; Husted, C.; Zasadzinski, J.A.; Israelachvili, J. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc. Natl. Acad. Sci. USA 2009, 106, 3154–3159. [Google Scholar] [CrossRef]
- Schmitt, S.; Castelvetri, L.C.; Simons, M. Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 2015, 1851, 999–1005. [Google Scholar] [CrossRef]
- O’Brien, J.S. Stability of the myelin membrane. Science 1965, 147, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Chrast, R.; Saher, G.; Nave, K.A.; Verheijen, M.H. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders andmouse models. J. Lipid Res. 2011, 52, 419–434. [Google Scholar] [CrossRef]
- Lamari, F.; Mochel, F.; Sedel, F.; Saudubray, J.M. Disorders of phospholipids, sphingolipids and fatty acids biosynthesis: Toward a new category of inherited metabolic diseases. J. Inherit. Metab. Dis. 2013, 36, 411–425. [Google Scholar] [CrossRef]
- Kister, A.; Kister, I. Overview of myelin, major myelin lipids, and myelin associated proteins. Front. Chem. 2023, 10, 1041961–1041970. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Stumpf, S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta 2015, 1851, 1083–1094. [Google Scholar] [CrossRef]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Saher, G.; Brügger, B.; Lappe-Siefke, C.; Möbius, W.; Tozawa, R.I.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef]
- Russell, D.W.; Halford, R.W.; Ramirez, D.M.; Shah, R.; Kotti, T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef]
- Norton, W.T.; Cammer, W. Isolation and characterization of myelin. In Myelin; Morell, P., Ed.; Springer: Boston, MA, USA, 1984; pp. 147–195. [Google Scholar] [CrossRef]
- Marcus, J.; Popko, B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim. Biophys. Acta 2002, 1573, 406–413. [Google Scholar] [CrossRef]
- Bakhti, M.; Aggarwal, S.; Simons, M. Myelin architecture: Zippering membranes tightly together. Cell. Mol. Life Sci. 2014, 71, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Yurlova, L.; Simons, M. Central nervous system myelin: Structure, synthesis and assembly. Trends Cell Biol. 2011, 21, 585–593. [Google Scholar] [CrossRef]
- Wattenberg, B.W. Intra- and intercellular tracking in sphingolipid metabolism in myelination. Adv. Biol. Regul. 2019, 71, 97–103. [Google Scholar] [CrossRef]
- Ozgen, H.; Baron, W.; Hoekstra, D.; Kahya, N. Oligodendroglial membrane dynamics in relation to myelin biogenesis. Cell. Mol. Life Sci. 2016, 73, 3291–3310. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 2015, 32, 117–179. [Google Scholar] [CrossRef]
- Cotter, L.; Özçelik, M.; Jacob, C.; Pereira, J.A.; Locher, V.; Baumann, R.; Relvas, J.B.; Suter, U.; Tricaud, N. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science 2010, 328, 1415–1418. [Google Scholar] [CrossRef]
- Boggs, J.M.; Rangaraj, G.; Dicko, A. Effect of phosphorylation of phosphatidylinositol on myelin basic protein-mediated binding of actin filaments to lipid bilayers in vitro. Biochim. Biophys. Acta 2012, 1818, 2217–2227. [Google Scholar] [CrossRef][Green Version]
- Musse, A.A.; Gao, W.; Homchaudhuri, L.; Boggs, J.M.; Harauz, G. Myelin basic protein as a “PI(4,5)P2-modulin”: A new biological function for a major central nervous system protein. Biochemistry 2008, 47, 10372–10382. [Google Scholar] [CrossRef]
- Nawaz, S.; Kippert, A.; Saab, A.S.; Werner, H.B.; Lang, T.; Nave, K.A.; Simons, M. Phosphatidylinosito 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J. Neurosci. 2009, 29, 4794–4807. [Google Scholar] [CrossRef]
- Noseda, R.; Belin, S.; Piguet, F.; Vaccari, I.; Scarlino, S.; Brambilla, P.; Boneschi, F.M.; Feltri, M.L.; Wrabetz, L.; Quattrini, A.; et al. DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J. Neurosci. 2013, 33, 15295–15305. [Google Scholar] [CrossRef][Green Version]
- Bolis, A.; Coviello, S.; Bussini, S.; Dina, G.; Pardini, C.; Previtali, S.C.; Malaguti, M.; Morana, P.; Del Carro, U.; Feltri, M.L.; et al. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J. Neurosci. 2005, 25, 8567–8577. [Google Scholar] [CrossRef]
- Vaccari, I.; Carbone, A.; Previtali, S.C.; Mironova, Y.A.; Alberizzi, V.; Noseda, R.; Rivellini, C.; Bianchi, F.; Del Carro, U.; D’Antonio, M.; et al. Loss of Figure 4 in both Schwann cells and motor neurons contributes to CMT4J neuropathy. Hum. Mol. Genet. 2015, 24, 383–396. [Google Scholar] [CrossRef]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Imgrund, S.; Hartmann, D.; Farwanah, H.; Eckhardt, M.; Sandho, R.; Degen, J.; Gieselmann, V.; Sandho, K.; Willecke, K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 2009, 284, 33549–33560. [Google Scholar] [CrossRef]
- Brites, P.; Mooyer, P.A.; el Mrabet, L.; Waterham, H.R.; Wanders, R.J. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain 2009, 132 Pt 2, 482–492. [Google Scholar] [CrossRef]
- Khan, M.; Singh, J.; Gilg, A.G.; Uto, T.; Singh, I. Very long-chain fatty acid accumulation causes lipotoxic response via 5-lipoxygenase in cerebral adrenoleukodystrophy. J. Lipid Res. 2010, 51, 1685–1695. [Google Scholar] [CrossRef]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Frahm, J.; et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Saeedimasine, M.; Montanino, A.; Kleiven, S.; Villa, A. Role of lipid composition on the structural and mechanical features of axonal membranes: A molecular simulation study. Sci. Rep. 2019, 9, 8000. [Google Scholar] [CrossRef]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef]
- Stollery, J.G.; Vail, W.J. Interactions of divalent cations or basic proteins with phosphatidylethanolamine vesicles. Biochim. Biophys. Acta 1977, 471, 372–390. [Google Scholar] [CrossRef]
- Lampe, P.D.; Nelsestuen, G.L. Myelin basic protein-enhanced fusion of membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 693, 320–325. [Google Scholar] [CrossRef]
- Lampe, P.D.; Wei, G.J.; Nelsestuen, G.L. Stopped-flow studies of myelin basic protein association with phospholipid vesicles and subsequent vesicle aggregation. Biochemistry 1983, 22, 1594–1599. [Google Scholar] [CrossRef]
- Maggio, B.; Yu, R.K. Interaction and fusion of unilamellar vesicles containing cerebrosides and sulfatides induced by myelin basic protein. Chem. Phys. Lipids 1989, 51, 127–136. [Google Scholar] [CrossRef]
- Haas, H.; Torrielli, M.; Steitz, R.; Cavatorta, P.; Sorbi, R.; Fasano, A.; Riccio, P.; Gliozzi, A. Myelin model membranes on solid substrates. Thin Solid Film. 1998, 327–329, 627–631. [Google Scholar] [CrossRef]
- Polverini, E.; Arisi, S.; Cavatorta, P.; Berzina, T.; Cristofolini, L.; Fasano, A.; Riccio, P.; Fontana, M.P. Interaction of myelin basic protein with phospholipid monolayers: Mechanism of protein penetration. Langmuir 2003, 19, 872–877. [Google Scholar] [CrossRef]
- Haas, H.; Steitz, R.; Fasano, A.; Liuzzi, G.M.; Polverini, E.; Cavatorta, P.; Riccio, P. Laminar order within langmuir-blodgett multilayers from phospholipid and myelin basic protein: A neutron reflectivity study. Langmuir 2007, 23, 8491–8496. [Google Scholar] [CrossRef]
- Shen, Y.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric lipid membranes: Towards more realistic model systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef]
- Heberle, F.A.; Marquardt, D.; Doktorova, M.; Geier, B.; Standaert, R.F.; Heftberger, P.; Kollmitzer, B.; Nickels, J.D.; Dick, R.A.; Feigenson, G.W.; et al. Subnanometer structure of an asymmetric model membrane: Interleaflet coupling influences domain properties. Langmuir 2016, 32, 5195–5200. [Google Scholar] [CrossRef]
- Rona, S.; Maor, R.O.; Ram, A.; Rina, A.; Ruth, A.; Yeshayahu, T.; Roy, B. Structural transition in myelin membrane as initiator of multiple sclerosis. J. Am. Chem. Soc. 2016, 138, 12159–12165. [Google Scholar] [CrossRef]
- Eicher, B.; Heberle, F.A.; Marquardt, D.; Rechberger, G.N.; Katsaras, J.; Pabst, G. Joint small-angle X-ray and neutron scattering data analysis of asymmetric lipid vesicles. J. Appl. Crystallogr. 2017, 50, 419–429. [Google Scholar] [CrossRef]
- Eicher, B.; Marquardt, D.; Heberle, F.A.; Letofsky-Papst, I.; Rechberger, G.N.; Appavou, M.-S.; Katsaras, J.; Pabst, G. Intrinsic curvature-mediated transbilayer coupling in asymmetric lipid vesicles. Biophys. J. 2018, 114, 146–157. [Google Scholar] [CrossRef]
- Shaharabani, R.; Ram-On, M.; Talmon, Y.; Beck, R. Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 11156–11161. [Google Scholar] [CrossRef]
- Min, Y.; Alig, T.; Lee, D.; Boggs, J.; Israelachvili, J.; Zasadzinski, J. Critical and off-critical miscibility transitions in model extracellular and cytoplasmic myelin lipid monolayers. Biophys. J. 2011, 100, 1490–1498. [Google Scholar] [CrossRef]
- Raasakka, A.; Ruskamo, S.; Kowal, J.; Barker, R.; Baumann, A.; Martel, A.; Tuusa, J.; Myllykoski, M.; Bürck, J.; Ulrich, A.S.; et al. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Sci. Rep. 2017, 7, 4974. [Google Scholar] [CrossRef]
- Krugmann, B.; Radulescu, A.; Appavou, M.-S.; Koutsioubas, A.; Stingaciu, L.R.; Dulle, M.; Förster, S.; Stadler, A.M. Membrane stiffness and myelin basic protein binding strength as molecular origin of multiple sclerosis. Sci. Rep. 2020, 10, 16691. [Google Scholar] [CrossRef]
- Weil, M.T.; Ruhwedel, T.; Meschkat, M.; Sadowski, B.; Möbius, W. Transmission electron microscopy of oligodendrocytes and myelin. Methods Mol. Biol. 2019, 1936, 343–375. [Google Scholar] [CrossRef]
- Simons, M.; Lyons, D.A. Axonal selection and myelin sheath generation in the central nervous system. Curr. Opin. Cell Biol. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Taib, T.; Leconte, C.; Van Steenwinckel, J.; Cho, A.H.; Palmier, B.; Torsello, E.; Lai Kuen, R.; Onyeomah, S.; Ecomard, K.; Benedetto, C.; et al. Neuroinflammation, myelin and behavior: Temporal patterns following mild traumatic brain injury in mice. PLoS ONE 2017, 12, e0184811. [Google Scholar] [CrossRef]
- Olbrich, K.; Rawicz, W.; Needham, D. Evans EWater permeability mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef]
- Burke, K.A.; Yates, E.A.; Legleiter, J. Amyloid-forming proteins alter the local mechanical properties of lipid membranes. Biochemistry 2013, 52, 808–817. [Google Scholar] [CrossRef]
- Picas, L.; Rico, F.; Scheuring, S. Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys. J. 2012, 102, L01–L03. [Google Scholar] [CrossRef]
- Brannigan, G.; Brown, F.L. Composition dependence of bilayer elasticity. J. Chem. Phys. 2005, 122, 74905. [Google Scholar] [CrossRef]
- Kelley, E.G.; Butler, P.D.; Ashkar, R.; Bradbury, R.; Nagao, M. Scaling relationships for the elastic moduli and viscosity of mixed lipid membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 23365–23373. [Google Scholar] [CrossRef]
- Eid, J.; Razmazma, H.; Jraij, A.; Ebrahimi, A.; Monticelli, L. On calculating the bending modulus of lipid bilayer membranes from buckling simulations. J. Phys. Chem. B 2020, 124, 6299–6311. [Google Scholar] [CrossRef]
- Nademi, Y.; Tang, T.; Uludağ, H. Nature of bilayer lipids affects membranes deformation and pore resealing during nanoparticle penetration. Mater. Sci. Eng. C 2022, 132, 112530. [Google Scholar] [CrossRef] [PubMed]
- Ayton, G.; Smondyrev, A.M.; Bardenhagen, S.G.; McMurtry, P.; Voth, G.A. Calculating the bulk modulus for a lipid bilayer with nonequilibrium molecular dynamics simulation. Biophys. J. 2022, 82, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Drücker, P.; Iacovache, I.; Bachler, S.; Zuber, B.; Babiychuk, E.B.; Dittrich, P.S.; Draeger, A. Membrane deformation and layer-bylayer peeling of giant vesicles induced by the pore-forming toxin pneumolysin. Biomater. Sci. 2019, 7, 3693–3705. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Akimov, S.A. The Possibility of Pore Formation in Lipid Membranes by Several Molecules of Amphipathic Peptides. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 338–350. [Google Scholar] [CrossRef]
- Soubias, O.; Sodt, A.J.; Teague, W.E.; Hines, K.G.; Gawrisch, K. Physiological changes in bilayer thickness induced by cholesterol control GPCR rhodopsin function. Biophys. J. 2023, 122, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Faizi, H.A.; Dimova, R.; Vlahovska, P.M. A vesicle microrheometer for high-throughput viscosity measurements of lipid and polymer membranes. Biophys. J. 2022, 121, 910–918. [Google Scholar] [CrossRef]
- Galimzyanov, T.R.; Bashkirov, P.V.; Blank, P.S.; Zimmerberg, J.; Batishchev, O.V.; Akimov, S.A. Monolayerwise application of linear elasticity theory well describes strongly deformed lipid membranes and the effect of solvent. Soft Matter 2020, 16, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Pinigin, K.V.; Kondrashov, O.V.; Jiménez-Munguía, I.; Alexandrova, V.V.; Batishchev, O.V.; Galimzyanov, T.R.; Akimov, S.A. Elastic deformations mediate interaction of the raft boundary with membrane inclusions leading to their effective lateral sorting. Sci. Rep. 2020, 10, 4087. [Google Scholar] [CrossRef]
- Montanino, A.; Saeedimasine, M.; Villa, A.; Kleiven, S. Localized axolemma deformations suggest mechanoporation as axonal injury trigger. Front. Neurol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Kant, A.; Johnson, V.E.; Arena, J.D.; Dollé, J.P.; Smith, D.H.; Shenoy, V.B. Modeling links softening of myelin and spectrin scaffolds of axons after a concussion to increased vulnerability to repeated injuries. Proc. Natl. Acad. Sci. USA 2021, 118, e2024961118. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Wang, C. In Vitro Application of Langmuir Monolayer Model: The interfacial Behavior of Myelin Basic Protein with a Plasma Membrane Model. J. Membr. Biol. 2022, 255, 71–78. [Google Scholar] [CrossRef]
- Gupta, M.; Weaver, D.F. Microsecond molecular dynamics studies of cholesterol-mediated myelin sheath degeneration in early Alzheimer’s disease. Phys. Chem. Chem. Phys. 2020, 24, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Eberle, D.; Fodelianaki, G.; Kurth, T.; Jagielska, A.; Möllmert, S.; Ulbricht, E.; Wagner, K.; Taubenberger, A.V.; Träber, N.; Escolano, J.C.; et al. Acquired demyelination but not genetic developmental defects in myelination leads to brain tissue stiffness changes. Brain Multiphys. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Tiwari, A.; Pradhan, S.; Sannigrahi, A.; Jha, S.; Chattopadhyay, K.; Biswas, M.; Saleem, M. In vitro reconstitution demonstrates the amyloid-beta mediated myelin membrane deformation. BioRxiv 2021. [Google Scholar] [CrossRef]
- Bianchi, F.; Hofmann, F.; Smith, A.J.; Ye, H.; Thompson, M.S. Probing multi-scale mechanics of peripheral nerve collagen and myelin by X-ray diffraction. J. Mech. Behav. Biomed. Mater. 2018, 87, 205–212. [Google Scholar] [CrossRef]
- Saeedimasine, M.; Montanino, A.; Kleiven, S.; Villa, A. Elucidating axonal injuries through molecular modelling of myelin sheaths and nodes of Ranvier. Front. Mol. Biosci. 2021, 8, 669897. [Google Scholar] [CrossRef]
- Maliha, F.; Adnan, A. Mechanical Responses of a Single Myelin Layer: A Molecular Simulation Study. Biomolecules 2023, 13, 1525. [Google Scholar] [CrossRef]
- Gaublomme, J.T.; Yosef, N.; Lee, Y.; Gertner, R.S.; Yang, L.V.; Wu Ch Pandolfi, P.P.; Mak, T.; Satija, R.; Shalek, A.K.; Kuchroo, V.K.; et al. Aviv Regev. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 2015, 163, 1400–1412. [Google Scholar] [CrossRef]
- Nguyen, C.; Lei, H.-T.; Lai, L.T.F.; Gallenito, M.J.; Mu, X.; Matthies, D.; Gonen, T. Lipid flipping in the omega-3 fatty-acid transporter. Nat. Commun. 2023, 14, 2571. [Google Scholar] [CrossRef]
- Orr, S.K.; Trépanier, M.O.; Bazinet, R.P. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 97–103. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Leyrolle, Q.; Decoeur, F.; Dejean, C.; Brière, G.; Leon, S.; Bakoyiannis, I.; Baroux, E.; Sterley, T.; Bosch-Bouju, C.; Morel, L.; et al. N-3 PUFA deficiency disrupts oligodendrocyte maturation and myelin integrity during brain development. Glia 2022, 70, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Fan, Y.Y.; Monk, J.M.; Hou, T.Y.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4+ T cells. J. Nutr. 2014, 144, 1306–1313. [Google Scholar] [CrossRef]
- Adkins, Y.; Soulika, A.M.; Mackey, B.; Kelley, D.S. Docosahexaenoic acid (22:6n-3) Ameliorated the Onset and Severity of Experimental Autoimmune Encephalomyelitis in Mice. Lipids 2019, 54, 13–23. [Google Scholar] [CrossRef]
- Unoda, K.; Doi, Y.; Nakajima, H.; Yamane, K.; Hosokawa, T.; Ishida, S.; Kimura, F.; Hanafusa, T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013, 256, 7–12. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050, Erratum in Sci. Rep. 2019, 9, 19816. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolving D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Bernardo, A.; Giammarco, M.L.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; De Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signalling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1013–1023. [Google Scholar] [CrossRef]
- Siegert, E.; Paul, F.; Rothe, M.; Weylandt, K.H. The effect of omega-3 fatty acids on central nervous system remyelination in fat-1 mice. BMC Neurosci. 2017, 18, 19. [Google Scholar] [CrossRef]
- Torkildsen, Ø.; Brunborg, L.A.; Thorsen, F.; Mørk, S.J.; Stangel, M.; Myhr, K.M.; Bø, L. Effects of dietary intervention on MRI activity, de- and remyelination in the cuprizone model for demyelination. Exp. Neurol. 2009, 215, 160–166. [Google Scholar] [CrossRef]
- Luo, C.; Ren, H.; Wan, J.B.; Yao, X.; Zhang, X.; He, C.; So, K.F.; Kang, J.X.; Pei, Z.; Su, H. Enriched endogenous omega-3 fatty acids in mice protect against global ischemia injury. J. Lipid Res. 2014, 55, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Omura, T.; Masaki, N.; Arima, H.; Banno, T.; Okamoto, A.; Hanada, M.; Takei, S.; Matsushita, S.; Sugiyama, E.; et al. Increased arachidonic acid-containing phosphatidylcholine is associated with reactive microglia and astrocytes in the spinal cord after peripheral nerve injury. Sci. Rep. 2016, 6, 26427. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, O.; Bogie, J.F.J.; Hendriks, J.J.A.; Linker, R.A.; Haghikia, A.; Kleinewietfeld, M. Western lifestyle and immunopathology of multiple sclerosis. Ann. N. Y. Acad. Sci. 2018, 1417, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmawla, M.A.; Ghaiad, H.R.; Nooh, M.M.; Amer, M.A.; El-Ghoneimy, L.T.; Mehana, N.A. Role of Vitamin D and Omega 3 Fatty Acids in Improving HDL Biogenesis among Multiple Sclerosis Patients via Orchestrating CHROME/APOA1-AS/ABCA1/APOA1 Milieu. J. Nutr. Biochem. 2025, 145, 110007. [Google Scholar] [CrossRef]
- Bjørnevik, K.; Chitnis, T.; Ascherio, A.; Munger, K.L. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult. Scler. 2017, 23, 1830–1838. [Google Scholar] [CrossRef]
- AlAmmar, W.A.; Albeesh, F.H.; Ibrahim, L.M.; Algindan, Y.Y.; Yamani, L.Z.; Khattab, R.Y. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: A systematic review. Nutr. Neurosci. 2021, 24, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, L.; Rankovic, S.; Dincic, E.; Boskovic, M.; Kolakovic, A.; Seke, M.; Taki, M.; Zivkovic, M. The Erythrocyte Fatty Acid Profile in Multiple Sclerosis Is Linked to the Disease Course, Lipid Peroxidation, and Dietary Influence. Nutrients 2025, 17, 974. [Google Scholar] [CrossRef]
- Di Sarno, A.; Romano, F.; Arianna, R.; Serpico, D.; Lavorgna, M.; Savastano, S.; Colao, A.; Di Somma, C. Lipid Metabolism and Statin Therapy in Neurodegenerative Diseases: An Endocrine View. Metabolites 2025, 15, 282. [Google Scholar] [CrossRef]
- Makris, A.; Palli, N.; Liontos, A.; Rizos, E.C.; Tsioutis, C.; Papadopoulos, D.; Agouridis, A.P. High-density lipoprotein cholesterol and multiple sclerosis: A systematic review and meta-analysis. Arch. Med. Sci.-Atheroscler. Dis. 2025, 10, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Tsai, H.H.; Yu, K.; Bronson, P.; Samuel, K.; Trinh, K.; Wei, R.; Tsai, E.; Briggs, F.B.S.; Bhargava, P.; et al. Metabolic and lipid alterations in multiple sclerosis linked to disease severity. Mult. Scler. J. 2025, 31, 433–443. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef]
- Van Meeteren, M.E.; Teunissen, C.E.; Dijkstra, C.D.; Van Tol, E.A.F. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur. J. Clin. Nutr. 2005, 59, 1347–1361. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040. [Google Scholar] [CrossRef]
- Pompura, S.L.; Hafler, D.A.; Dominguez-Villar, M. Fatty acid metabolism and T cells in multiple sclerosis. Front. Immunol. 2022, 13, 869197. [Google Scholar] [CrossRef]
- Sakoda, A.; Matsushita, T.; Nakamura, Y.; Watanabe, M.; Shinoda, K.; Masaki, K.; Isobe, N.; Yamasaki, R.; Kira, J.-i. Environmental risk factors for multiple sclerosis in Japanese people. Mult. Scler. Relat. Disord. 2020, 38, 101872. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Sultan, S.; Rytz, A.; Steiner, P.; Schneider, N. A blend containing docosahexaenoic acid, arachidonic acid, vitamin B12, vitamin B9, iron and sphingomyelin promotes myelination in an in vitro model. Nutr. Neurosci. 2020, 23, 931–945. [Google Scholar] [CrossRef]
- Rezaei Ali, M.D.; Hamidi Majid, M.D.; Seyedmirzaei Homa, M.D.; Moghadasi Abdorreza Naser, M.D. Can supplementation with antioxidants improve cognitive functions in patients with multiple sclerosis? A literature review. Ann. Med. Surg. 2025, 87, 2736–2748. [Google Scholar] [CrossRef]
- Namiecinska, M.; Piatek, P.; Lewkowicz, P. Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. Int. J. Mol. Sci. 2024, 25, 3792. [Google Scholar] [CrossRef]
- Piatek, P.; Lewkowicz, N.; Michlewska, S.; Wieczorek, M.; Bonikowski, R.; Parchem, K.; Lewkowicz, P.; Namiecinska, M. Natural fish oil improves the differentiation and maturation of oligodendrocyte precursor cells to oligodendrocytes in vitro after interaction with the blood–brain barrier. Front. Immunol. 2022, 13, 17. [Google Scholar] [CrossRef]
- Macaron, T.; Giudici, K.V.; Bowman, G.L.; Sinclair, A.; Stephan, E.; Vellas, B.; de Souto, B.P. Associations of Omega-3 Fatty Acids with Brain Morphology and Volume in Cognitively Healthy Older Adults: A narrative review. Ageing Res. Rev. 2021, 67, 101300. [Google Scholar] [CrossRef]
- Orywal, K.; Socha, K.; Iwaniuk, P.; Kaczyński, P.; Farhan, J.A.; Zoń, W.; Łozowicka, B.; Perkowski, M.; Mroczko, B. Vitamins in the Prevention and Support Therapy of Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 1333. [Google Scholar] [CrossRef]
- Shahabi, B.; Hernández-Martínez, C.; Jardí, C.; Aparicio, E.; Arija, V. Maternal Omega-6/Omega-3 Concentration Ratio During Pregnancy and Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2025, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.-T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, P.S.; Andreou, E.; Aphamis, G.; Pantzaris, M.; Ioannou, M.; Patrikios, I.S.; Giannaki, C.D. The Effects of a 6-Month High Dose Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Antioxidant Vitamins Supplementation on Cognitive Function and Functional Capacity in Older Adults with Mild Cognitive Impairment. Nutrients 2020, 12, 325. [Google Scholar] [CrossRef]
- Sedighiyan, M.; Djafarian, K.; Dabiri, S.; Abdolahi, M.; Shab-Bidar, S. The Effects of Omega-3 Supplementation on the Expanded Disability Status Scale and Inflammatory Cytokines in Multiple Sclerosis Patients: A Systematic Review and Meta-Analysis. CNS Neurol. Disord. Drug Targets 2019, 18, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, Ø.; Wergeland, S.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Lilleås, F.; Pedersen, T.; Bjørnarå, B.; et al. ω-3 Fatty Acid Treatment in Multiple Sclerosis (OFAMS Study): A Randomized, Double-Blind, Placebo-Controlled Trial. Arch. Neurol. 2012, 69, 1044–1051. [Google Scholar] [CrossRef]
- Chapman, T.W.; Hill, R.A. Myelin plasticity in adulthood and aging. Neurosci. Lett. 2020, 715, 134645. [Google Scholar] [CrossRef]
- Graciani, A.L.; Usberti Gutierre, M.; Coppi, A.A.; Arida, R.M.; Campos Gutierre, R. Myelin, aging, and physical exercise. Neurobiol. Aging 2023, 127, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolontà, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; Baes, M.; et al. Myelin lipids as nervous system energy reserves. bioRxiv 2022. bioRxiv:2022.02.24.481621. [Google Scholar] [CrossRef]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Ebrahimi, R.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 2024, 27, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.-A.; Asadollahi, E.; Sasmita, A. Expanding the function of oligodendrocytes to brain energy metabolism. Curr. Opin. Neurobiol. 2023, 83, 102782. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Cabrera-Zubizarreta, A.; Padró, D.; Matute-González, M.; Rodríguez-Antigüedad, A.; Matute, C. Widespread drastic reduction of brain myelin content upon prolonged endurance exercise. bioRxiv 2023, 10, 561303. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Cabrera-Zubizarreta, A.; Padró, D.; Matute-González, M.; Rodríguez-Antigüedad, A.; Matute, C. Reversible reduction in brain myelin content upon marathon running. Nat. Metab. 2025, 7, 697–703. [Google Scholar] [CrossRef]
- Franklyn, N.; Kesavelu, D.; Joji, P.; Verma, R.; Wadhwa, A.; Ray, C. Impact of Key Nutrients on Brain and Executive Function Development in Infants and Toddlers: A Narrative Review. J. Food Nutr. Sci. 2022, 10, 19–26. [Google Scholar] [CrossRef]
- Burzynska, A.Z.; Anderson Ch Arciniegas, D.B.; Calhoun, V.; Choi, I.-Y.; Mendez Colmenares, A.; Hiner, G.; Kramer, A.F.; Li, K.; Lee, J.; Lee, P.; et al. Metabolic syndrome and adiposity: Risk factors for decreased myelin in cognitively healthy adults. Cereb. Circ. Cogn. Behav. 2023, 5, 100180–100190. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Buttari, F.; Gilio, L.; Simonelli, I.; Carbone, F.; Micillo, T.; De Rosa, V.; Sica, F.; Furlan, R.; et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult. Scler. J. 2020, 26, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Noori, H.; Gheini, M.R.; Rezaeimanesh, N.; Saeedi, R.; Rezaei Aliabadi, H.; Sahraian, M.A.; Naser Moghadasi, A. The correlation between dyslipidemia and cognitive impairment in multiple sclerosis patients. Mult. Scler. Relat. Disord. 2019, 36, 101415. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Neto, A.; Fernandes, A.; Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef]
- Hong, Y.; Yu, J.; Su, Y.; Mei, F.; Li, M.; Zhao, K.; Zhao, L.; Deng, W.H.; Chen, C.; Wang, W.X. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxidative Med. Cell. Longev. 2020, 2020, 8172714–8172724. [Google Scholar] [CrossRef]
- Feng, Z.; Fang, C.; Ma, Y.; Chang, J. Obesity-induced blood-brain barrier dysfunction: Phenotypes and mechanisms. J. Neuroinflamm. 2024, 21, 110–132. [Google Scholar] [CrossRef]

| Lipid Type | Xnat (%) | XEAE (%) |
|---|---|---|
Phosphatidylcholine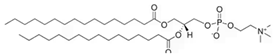 | 25.9 | 20.1 |
Phosphatidylethnolamine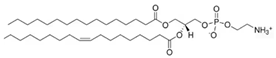 | 29.0 | 32.9 |
Phosphatidylserine  | 7.0 | 7.4 |
Sphingomyelin | 6.2 | 2.2 |
Cholesterol | 31.6 | 37.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morini, M.A.; Pedroni, V.I. Role of Lipid Composition on the Mechanical and Biochemical Vulnerability of Myelin and Its Implications for Demyelinating Disorders. Biophysica 2025, 5, 44. https://doi.org/10.3390/biophysica5040044
Morini MA, Pedroni VI. Role of Lipid Composition on the Mechanical and Biochemical Vulnerability of Myelin and Its Implications for Demyelinating Disorders. Biophysica. 2025; 5(4):44. https://doi.org/10.3390/biophysica5040044
Chicago/Turabian StyleMorini, Marcela Ana, and Viviana Isabel Pedroni. 2025. "Role of Lipid Composition on the Mechanical and Biochemical Vulnerability of Myelin and Its Implications for Demyelinating Disorders" Biophysica 5, no. 4: 44. https://doi.org/10.3390/biophysica5040044
APA StyleMorini, M. A., & Pedroni, V. I. (2025). Role of Lipid Composition on the Mechanical and Biochemical Vulnerability of Myelin and Its Implications for Demyelinating Disorders. Biophysica, 5(4), 44. https://doi.org/10.3390/biophysica5040044





