Differential Scanning Calorimetry of Proteins and the Two-State Model: Comparison of Two Formulas
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSC | Differential Scanning Calorimetry |
| BPTI | bovine pancreatic trypsin inhibitor |
| RMSD | Root Mean Square Deviation |
| NRMSD | Normalized Root Mean Square Deviation |
Appendix A. Fitting Formulas
References
- O’Neill, M.J. The Analysis of a Temperature-Controlled Scanning Calorimeter. Anal. Chem. 1964, 36, 1238–1245. [Google Scholar] [CrossRef]
- Privalov, P.L.; Monaselidze, D.R.; Mrevlishvili, G.M.; Magaldadze, V.A. Intramolecular heat of fusion of macromolecules. J. Exp. Theor. Phys. 1964, 47, 2073–2076. [Google Scholar]
- Privalov, P.L.; Griko, Y.V.; Venyaminov, S.Y.; Kutyshenko, V.P. Cold denaturation of myoglobin. J. Mol. Biol. 1986, 190, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.H.; Murphy, E.C.; Privalov, P.L. Thermodynamic effects of mutations on the denaturation of T4 lysozyme. Biophys. J. 1996, 71, 1994–2001. [Google Scholar] [CrossRef][Green Version]
- Privalov, P.L.; Dragan, A.I. Microcalorimetry of biological macromolecules. Biophys. Chem. 2007, 126, 16–24. [Google Scholar] [CrossRef]
- Hemminger, W.F.; Sarge, S.M. The baseline construction and its influence on the measurement of heat with differential scanning calorimeters. J. Therm. Anal. 1991, 37, 1455–1477. [Google Scholar] [CrossRef]
- Dupuy, J.; Leroy, E.; Maazouz, A. Determination of activation energy and preexponential factor of thermoset reaction kinetics using differential scanning calorimetry in scanning mode: Influence of baseline shape on different calculation methods. J. Appl. Polym. Sci. 2000, 78, 2262–2271. [Google Scholar] [CrossRef]
- Consalvi, V.; Chiaraluce, R.; Giangiacomo, L.; Scandurra, R.; Christova, P.; Karshikoff, A.; Knapp, S.; Ladenstein, R. Thermal unfolding and conformational stability of the recombinant domain II of glutamate dehydrogenase from the hyperthermophile Thermotoga maritima. Protein Eng. 2000, 13, 501–507. [Google Scholar] [CrossRef]
- Choma, C.T. Characterizing Protein Stability by DSC; Calorimetry Sciences Corporation: Lindon, UT, USA, 2006. [Google Scholar]
- Gibson, R.L.; Simmons, M.J.; Stitt, E.H.; Horsburgh, L.; Gallen, R.W. Selection of Formal Baseline Correction Methods in Thermal Analysis. Chem. Eng. Technol. 2022, 45, 238–248. [Google Scholar] [CrossRef]
- Privalov, P.L. Thermodynamic problems in structural molecular biology. Pure Appl. Chem. 2007, 79, 1445–1462. [Google Scholar] [CrossRef]
- Seelig, J.; Schonfeld, H.-J. Thermal protein unfolding by differential scanning calorimetry and circular dichroism spectroscopy. Two-state model versus sequential unfolding. Q. Rev. Biophys. 2016, 49, e9. [Google Scholar] [CrossRef]
- Hawley, S.A. Reversible pressure-temperature denaturation of chymotrypsinogen. Biochemistry 1971, 10, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Smeller, L. Pressure-temperature Phase Diagrams of Biomolecules. Biochim. Biophys. Acta 2002, 1595, 11–29. [Google Scholar] [CrossRef]
- Dubins, D.N.; Lee, A.; Macgregor, R.B.; Chalikian, T.V. On the Stability of Double Stranded Nucleic Acids. J. Am. Chem. Soc. 2001, 14, 9254–9259. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer. 2024. Available online: https://automeris.io/WebPlotDigitizer.html (accessed on 12 November 2023).
- Fung, A.; Li, P.; Godoy-Ruiz, R.; Sanchez-Ruiz, J.M.; Munoz, V. Expanding the Realm of Ultrafast Protein Folding: GpW, a Midsize Natural Single-Domain with α + β Topology that Folds Downhill. J. Am. Chem. Soc. 2008, 130, 7489–7495. [Google Scholar] [CrossRef]
- Garcia-Hernandez, E.; Hernandez-Arana, A.; Zubillaga, R.A.; Rojo-Domínguez, A. Spectroscopic and thermodynamic evidence for a complex denaturation mechanism of bovine β-lactoglobulin A. Biochem. Mol. Biol. Int. 1998, 45, 761–768. [Google Scholar]
- Mazurenko, S.; Kunka, A.; Beerens, K.; Johnson, C.M.; Damborsky, J.; Prokop, Z. Exploration of Protein Unfolding by Modelling Calorimetry Data from Reheating. Sci. Rep. 2017, 7, 16321. [Google Scholar] [CrossRef]
- Leharne, S. The physical chemistry of high-sensitivity differential scanning calorimetry of biopolymers. ChemTexts 2017, 3, 1. [Google Scholar] [CrossRef][Green Version]
- Muñoz, V.; Sanchez-Ruiz, J.M. Exploring protein-folding ensembles: A variable-barrier model for the analysis of equilibrium unfolding experiments. Proc. Natl. Acad. Sci. USA 2004, 101, 17646–17651. [Google Scholar] [CrossRef]
- Naganathan, A.N.; Sanchez-Ruiz, J.M.; Munoz, V. Direct Measurement of Barrier Heights in Protein Folding. J. Am. Chem. Soc. 2005, 127, 17970–17971. [Google Scholar] [CrossRef]
- Godoy-Ruiz, R.; Henry, E.R.; Kubelka, J.; Hofrichter, J.; Munoz, V.; Sanchez-Ruiz, J.M.; Eaton, W.A. Estimating Free-Energy Barrier Heights for an Ultrafast Folding Protein from Calorimetric and Kinetic Data. J. Phys. Chem. B 2008, 112, 5938–5949. [Google Scholar] [CrossRef] [PubMed]
- Bruscolini, P.; Naganathan, A.N. Quantitative Prediction of Protein Folding Behaviors from a Simple Statistical Model. J. Phys. Chem. 2011, 133, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.M.; Lemaire, S.D.; Jacquot, J.P.; Makhatadze, G.I. Difference in the Mechanisms of the Cold and Heat Induced Unfolding of Thioredoxin h from Chlamydomonas reinhardtii: Spectroscopic and Calorimetric Studies. Biochemistry 2000, 39, 11154–11162. [Google Scholar] [CrossRef] [PubMed]
- Badasyan, A.; Valant, M.; Grdadolnik, J.; Uversky, V.N. The Finite Size Effects and Two-State Paradigm of Protein Folding. Int. J. Mol. Sci. 2021, 22, 2184–2191. [Google Scholar] [CrossRef] [PubMed]

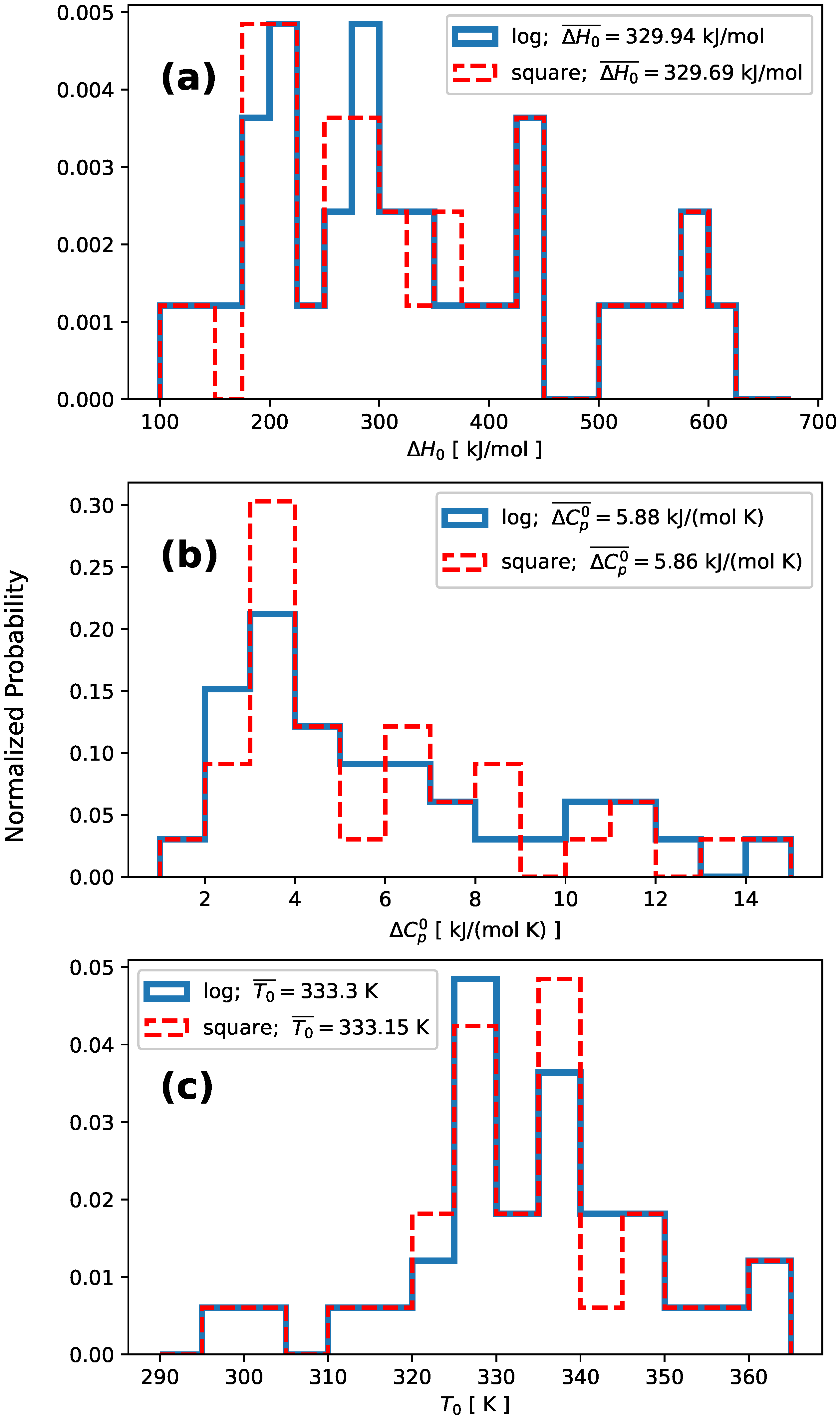
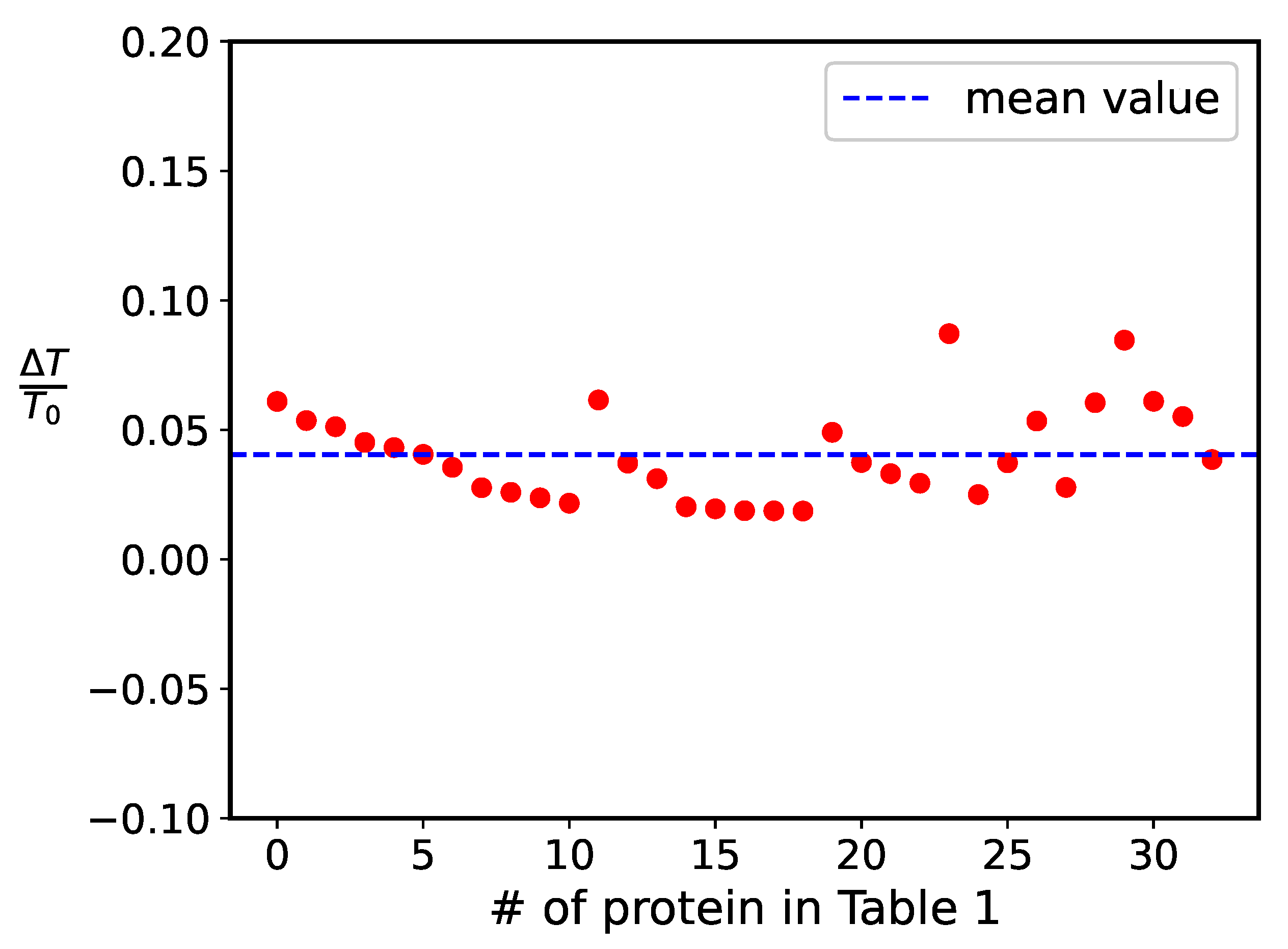
| # | Protein | Reference | N | 2-State (square) | 2-State (log ) | ||
|---|---|---|---|---|---|---|---|
| 1 | gpW | Ref. [17], Figure 4a | 62 | : 338 (0.2) K : 185 (3.8) kJ/mol : 3.3 (8.8) kJ/(mol K) | 0.897 | : 338 (0.2) K : 184.1 (3.3) kJ/mol : 3.4 (7.6) kJ/(mol K) | 0.908 |
| 2 | ubiquitin pH 2.0 | Ref. [5], Figure 1b | 76 | : 328 (0.02) K : 203.9 (0.4) kJ/mol : 3.96 (1) kJ/(mol K) | 0.995 | : 328 (0.02) K : 203.3 (0.4) kJ/mol : 4.2 (1.3) kJ/(mol K) | 0.998 |
| 3 | ubiquitin pH 2.5 | Ref. [5], Figure 1b | 76 | : 333 (0.02) K : 214 (0.5) kJ/mol : 4.1 (1.3) kJ/(mol K) | 0.994 | : 333 (0.02) K : 216 (0.5) kJ/mol : 4 (2) kJ/(mol K) | 0.995 |
| 4 | ubiquitin pH 3.0 | Ref. [5], Figure 1b | 76 | : 346 (0.03) K : 253.2 (0.8) kJ/mol : 3.9 (3.7) kJ/(mol K) | 0.988 | : 346 (0.03) K : 254.5 (0.7) kJ/mol : 3.85 (3.1) kJ/(mol K) | 0.990 |
| 5 | ubiquitin pH 3.5 | Ref. [5], Figure 1b | 76 | : 357 (0.05) K : 274.6 (1.4) kJ/mol : 4 (6.6) kJ/(mol K) | 0.969 | : 357 (0.04) K : 274.86 (1.2) kJ/mol : 4 (5.7) kJ/(mol K) | 0.972 |
| 6 | ubiquitin pH 4.0 | Ref. [5], Figure 1b | 76 | : 362 (0.03) K : 295.4 (0.7) kJ/mol : 3.5 (4.7) kJ/(mol K) | 0.992 | : 362 (0.02) K : 296.7 (0.6) kJ/mol : 3.4 (4.6) kJ/(mol K) | 0.992 |
| 7 | barnase pH 1.8 | Ref. [5], Figure 1a | 110 | : 295 (0.02) K : 271.4 (0.5) kJ/mol : 6.2 (2.9) kJ/(mol K) | 0.996 | : 295 (0.02) K : 275.96 (0.5) kJ/mol : 5.6 (3.6) kJ/(mol K) | 0.993 |
| 8 | barnase pH 2.5 | Ref. [5], Figure 1a | 110 | : 303 (0.009) K : 363 (0.3) kJ/mol : 5.7 (3.2) kJ/(mol K) | 0.996 | : 303 (0.007) K : 364 (0.3) kJ/mol : 5.5 (2.7) kJ/(mol K) | 0.997 |
| 9 | barnase pH 2.8 | Ref. [5], Figure 1a | 110 | : 314 (0.008) K : 402.8 (0.3) kJ/mol : 3.15 (7) kJ/(mol K) | 0.995 | : 314 (0.007) K : 403.2 (0.3) kJ/mol : 3.07 (6.7) kJ/(mol K) | 0.996 |
| 10 | barnase pH 3.45 | Ref. [5], Figure 1a | 110 | : 320 (0.009) K : 448 (0.4) kJ/mol : 6 (4.6) kJ/(mol K) | 0.992 | : 320 (0.008) K : 448 (0.4) kJ/mol : 6 (4.1) kJ/(mol K) | 0.993 |
| 11 | barnase pH 5.5 | Ref. [5], Figure 1a | 110 | : 329 (0.007) K : 504.33 (0.4) kJ/mol : 3.9 (7.3) kJ/(mol K) | 0.994 | : 329 (0.006) K : 504.67 (0.3) kJ/mol : 3.8 (7.2) kJ/(mol K) | 0.994 |
| 12 | Sox-5 | Ref. [5], Figure 3a | 81 | : 315 (0.1) K : 185.5 (2.3) kJ/mol : 4.7 (3.2) kJ/(mol K) | 0.893 | : 315 (0.1) K : 170 (3) kJ/mol : 5.7 (4) kJ/(mol K) | 0.932 |
| 13 | bovine -lactoglobulin A | Ref. [18], Figure 2a | 162 | : 352 (0.01) K : 313.87 (0.3) kJ/mol : 3 (3.6) kJ/(mol K) | 0.995 | : 352 (0.01) K : 314.95 (0.3) kJ/mol : 2.9 (3.3) kJ/(mol K) | 0.995 |
| 14 | S44[A] mutant of T4 lysozyme | Ref. [4], Figure 3a | 164 | : 327 (0.02) K : 350.5 (0.8) kJ/mol : 8.8 (3.5) kJ/(mol K) | 0.948 | : 327 (0.02) K : 349 (0.8) kJ/mol : 9.03 (3.1) kJ/(mol K) | 0.949 |
| 15 | pseudo-WT T4 lysozyme pH 2.8 | Ref. [4], Figure 2 | 164 | : 323 (0.008) K : 528.7 (0.5) kJ/mol : 10.9 (3.5) kJ/(mol K) | 0.988 | : 323 (0.007) K : 529.3 (0.4) kJ/mol : 10.8 (3.3) kJ/(mol K) | 0.989 |
| 16 | pseudo-WT T4 lysozyme pH 3.0 | Ref. [4], Figure 2 | 164 | : 327 (0.007) K : 557.1 (0.4) kJ/mol : 8.4 (4.6) kJ/(mol K) | 0.991 | : 327 (0.006) K : 557.45 (0.4) kJ/mol : 8.34 (4.4) kJ/(mol K) | 0.991 |
| 17 | pseudo-WT T4 lysozyme pH 3.3 | Ref. [4], Figure 2 | 164 | : 332 (0.006) K : 588 (0.4) kJ/mol : 11.6 (3.8) kJ/(mol K) | 0.993 | : 332 (0.005) K : 587.85 (0.3) kJ/mol : 11.44 (3.4) kJ/(mol K) | 0.993 |
| 18 | pseudo-WT T4 lysozyme pH 3.5 | Ref. [4], Figure 2 | 164 | : 335 (0.006) K : 593.7 (0.4) kJ/mol : 13 (3.2) kJ/(mol K) | 0.993 | : 335 (0.005) K : 595 (0.3) kJ/mol : 12.75 (3) kJ/(mol K) | 0.994 |
| 19 | pseudo-WT T4 lysozyme pH 3.7 | Ref. [4], Figure 2 | 164 | : 337 (0.008) K : 601 (0.5) kJ/mol : 14.8 (3.8) kJ/(mol K) | 0.991 | : 338 (0.007) K : 602.69 (0.4) kJ/mol : 14.4 (3.4) kJ/(mol K) | 0.992 |
| 20 | metmyoglobin pH 3.9 | Ref. [3], Figure 3a | 153 | : 333 (0.05) K : 230 (1.3) kJ/mol : 6.8 (2) kJ/(mol K) | 0.972 | : 333 (0.04) K : 225.57 (1.2) kJ/mol : 7.33 (2) kJ/(mol K) | 0.988 |
| 21 | metmyoglobin pH 4.08 | Ref. [3], Figure 3a | 153 | : 340 (0.02) K : 297.5 (0.6) kJ/mol : 7 (2) kJ/(mol K) | 0.994 | : 340 (0.02) K : 302 (0.7) kJ/mol : 6.67 (2.7) kJ/(mol K) | 0.994 |
| 22 | metmyoglobin pH 4.2 | Ref. [3], Figure 3a | 153 | : 345 (0.01) K : 344 (0.4) kJ/mol : 6.6 (2.3) kJ/(mol K) | 0.998 | : 345 (0.01) K : 346.5 (0.4) kJ/mol : 6.37 (2.5) kJ/(mol K) | 0.997 |
| 23 | metmyoglobin pH 4.44 | Ref. [3], Figure 3a | 153 | : 349 (0.02) K : 391.3 (0.6) kJ/mol : 7.7 (2.7) kJ/(mol K) | 0.991 | : 349 (0.02) K : 394.7 (0.8) kJ/mol : 7.28 (5.7) kJ/(mol K) | 0.989 |
| 24 | mutant SpA(L20A + Y15W) | Ref. [19], Figure 6 | 58 | : 324 (0.09) K : 107.8 (2) kJ/mol : 3 (2) kJ/(mol K) | 0.975 | : 327 (0.09) K : 124.6 (1.5) kJ/mol : 2.5 (2.9) kJ/(mol K) | 0.954 |
| 25 | Lysozyme pH 2.5 | Ref. [12], Figure 8 | 129 | : 335 (0.006) K : 441.6 (0.3) kJ/mol : 11.1 (1.6) kJ/(mol K) | 0.996 | : 335 (0.006) K : 444.85 (0.3) kJ/mol : 10.6 (1.7) kJ/(mol K) | 0.995 |
| 26 | Apolipoprotein A-1 | Ref. [12], Figure 7 | 245 | : 326 (0.04) K : 315 (1.5) kJ/mol : 8 (2.4) kJ/(mol K) | 0.825 | : 325 (0.03) K : 289.8 (1) kJ/mol : 11.3 (1.4) kJ/(mol K) | 0.942 |
| 27 | ubiquitin | Ref. [20], Figure 1 | 76 | : 329 (0.009) K : 201.5 (0.2) kJ/mol : 2.9 (0.8) kJ/(mol K) | 0.999 | : 329 (0.004) K : 204.6 (0.08) kJ/mol : 2.66 (0.4) kJ/(mol K) | 0.999 |
| 28 | thioredoxin | Ref. [21], Figure 2a | 104 | : 362 (0.02) K : 431.5 (0.7) kJ/mol : 4.6 (9.8) kJ/(mol K) | 0.996 | : 362 (0.02) K : 433.33 (0.7) kJ/mol : 4.3 (10.3) kJ/(mol K) | 0.995 |
| 29 | Cold shock protein CspB | Ref. [22], Figure 2 | 67 | : 327 (0.05) K : 177.5 (0.8) kJ/mol : 2.3 (3.4) kJ/(mol K) | 0.996 | : 327 (0.04) K : 179.55 (0.8) kJ/mol : 2.2 (3.5) kJ/(mol K) | 0.995 |
| 30 | villin headpiece | Ref. [23], Figure 3 | 35 | : 339 (0.2) K : 132.7 (2.9) kJ/mol : 1.6 (8.4) kJ/(mol K) | 0.955 | : 340 (0.2) K : 133.4 (2.4) kJ/mol : 1.64 (7.4) kJ/(mol K) | 0.960 |
| 31 | gpW | Ref. [24], Figure 1a | 58 | : 338 (0.2) K : 184.5 (3.5) kJ/mol : 3.3 (8.4) kJ/(mol K) | 0.911 | : 338 (0.2) K : 183.95 (3) kJ/mol : 3.37 (7.2) kJ/(mol K) | 0.920 |
| 32 | SH3 | Ref. [24], Figure 1b | 57 | : 339 (0.02) K : 202.8 (0.4) kJ/mol : 2.4 (1.9) kJ/(mol K) | 0.998 | : 340 (0.009) K : 204.8 (0.2) kJ/mol : 2.3 (1.1) kJ/(mol K) | 0.999 |
| 33 | thioredoxin h | Ref. [25], Figure 1b | 113 | : 335 (0.01) K : 288 (0.4) kJ/mol : 3.3 (3.2) kJ/(mol K) | 0.996 | : 335 (0.01) K : 288.84 (0.3) kJ/mol : 3.22 (2.7) kJ/(mol K) | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeritsyan, K.; Badasyan, A. Differential Scanning Calorimetry of Proteins and the Two-State Model: Comparison of Two Formulas. Biophysica 2024, 4, 227-237. https://doi.org/10.3390/biophysica4020016
Yeritsyan K, Badasyan A. Differential Scanning Calorimetry of Proteins and the Two-State Model: Comparison of Two Formulas. Biophysica. 2024; 4(2):227-237. https://doi.org/10.3390/biophysica4020016
Chicago/Turabian StyleYeritsyan, Knarik, and Artem Badasyan. 2024. "Differential Scanning Calorimetry of Proteins and the Two-State Model: Comparison of Two Formulas" Biophysica 4, no. 2: 227-237. https://doi.org/10.3390/biophysica4020016
APA StyleYeritsyan, K., & Badasyan, A. (2024). Differential Scanning Calorimetry of Proteins and the Two-State Model: Comparison of Two Formulas. Biophysica, 4(2), 227-237. https://doi.org/10.3390/biophysica4020016







