Low-Energy Electron Generation for Biomolecular Damage Inquiry: Instrumentation and Methods
Abstract
1. Introduction—Importance of Electron Interactions with Biological Systems
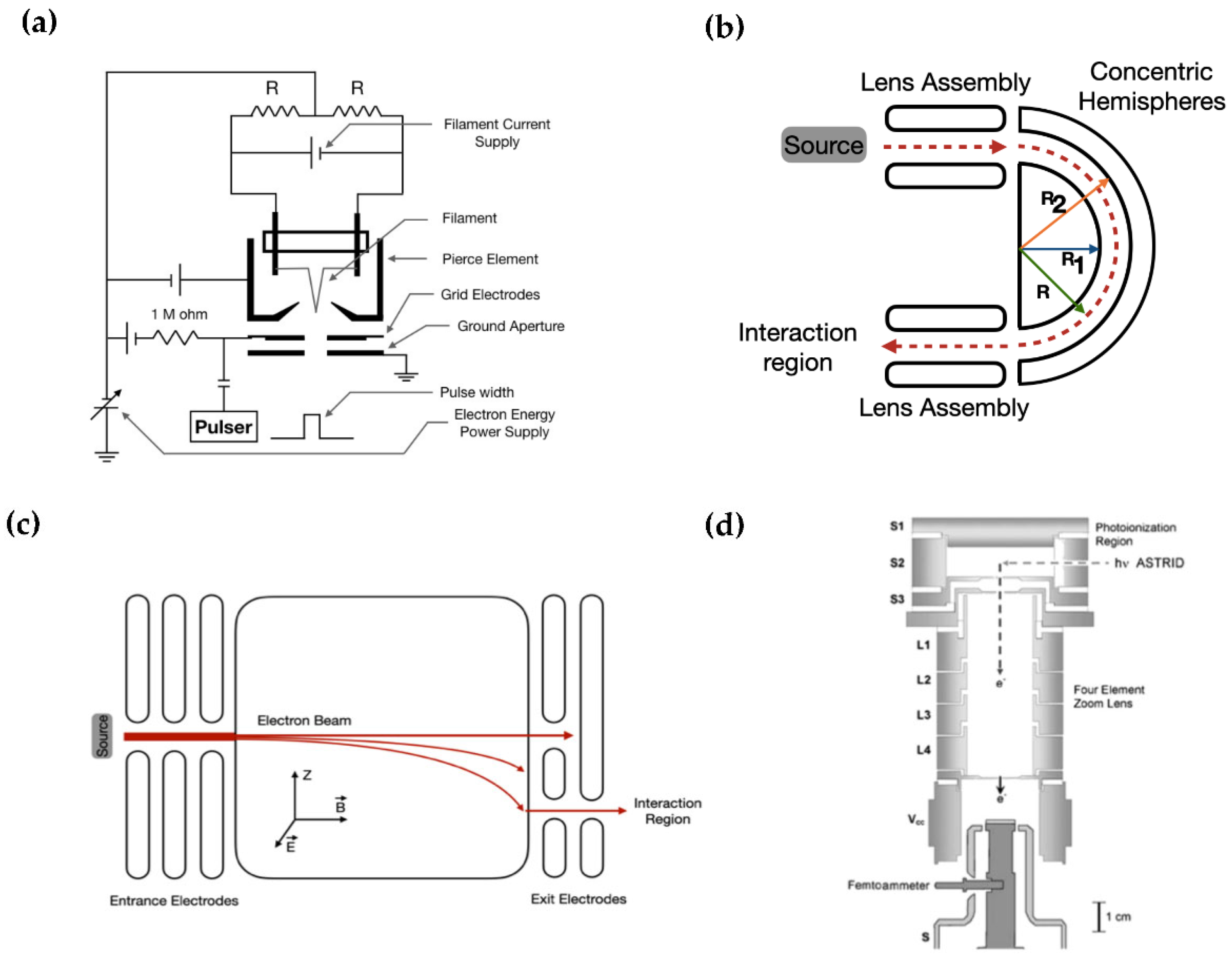
2. Sources of Low Energy Electrons (LEEs) in Vacuum
2.1. Common Sources of LEEs
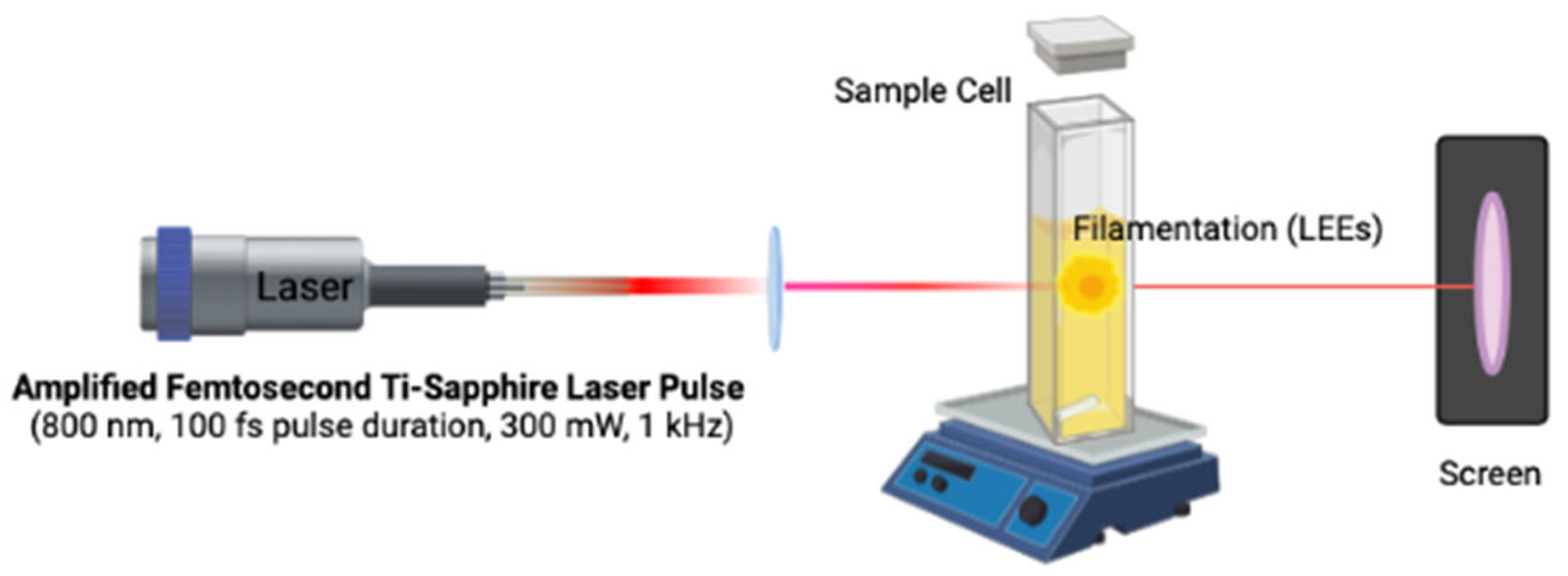
2.2. Alternative and Unconventional Sources
3. Recent Developments of LEE Sources to Approach Cellular Conditions
3.1. X-ray Photoelectron Spectroscopy at Near Ambient Pressure (XPS-NAP)
3.2. X-ray Interaction with Metal at Standard Ambient Temperature and Pressure (SATP)
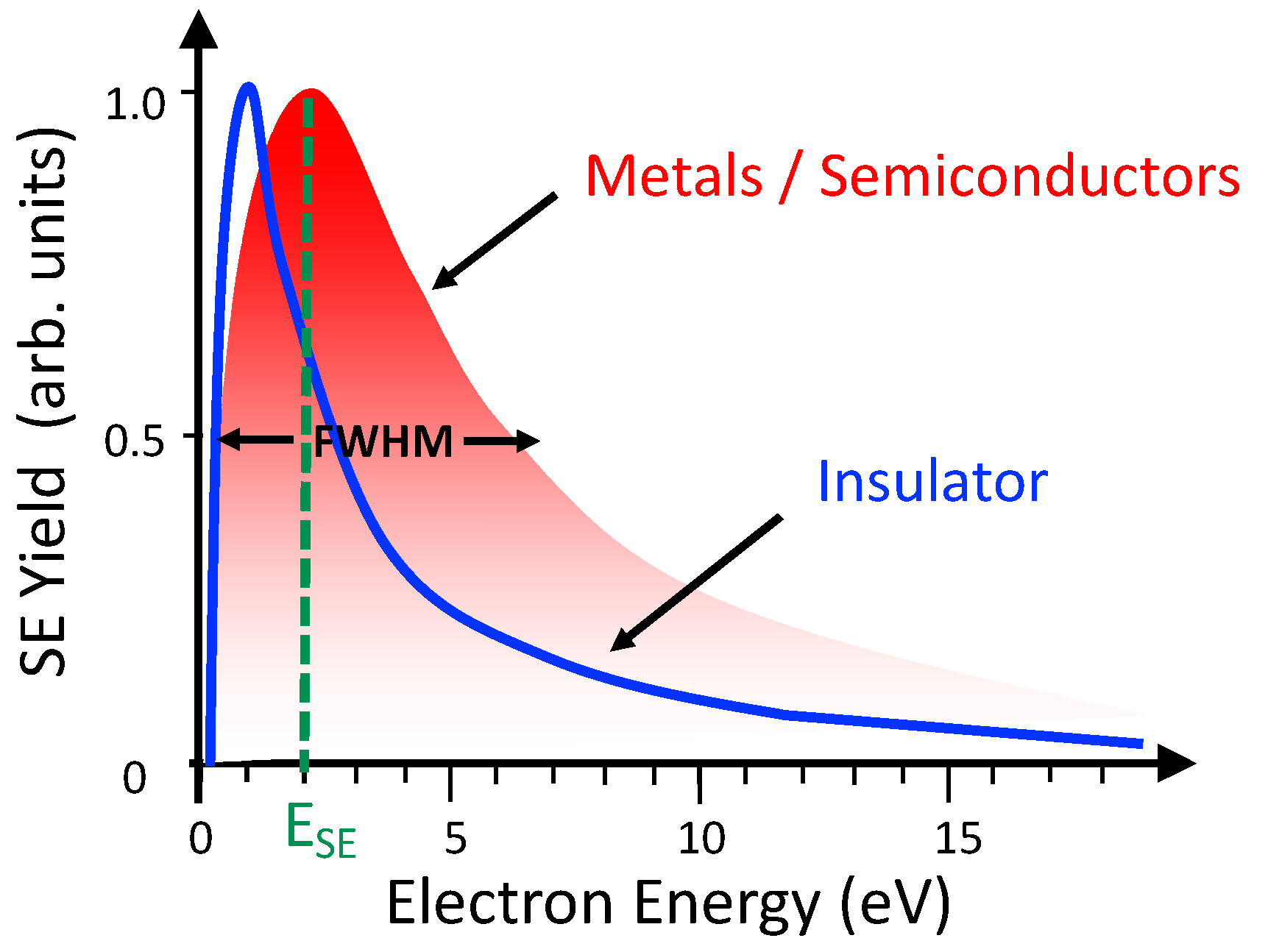
3.2.1. Secondary Electron Emission from Metals
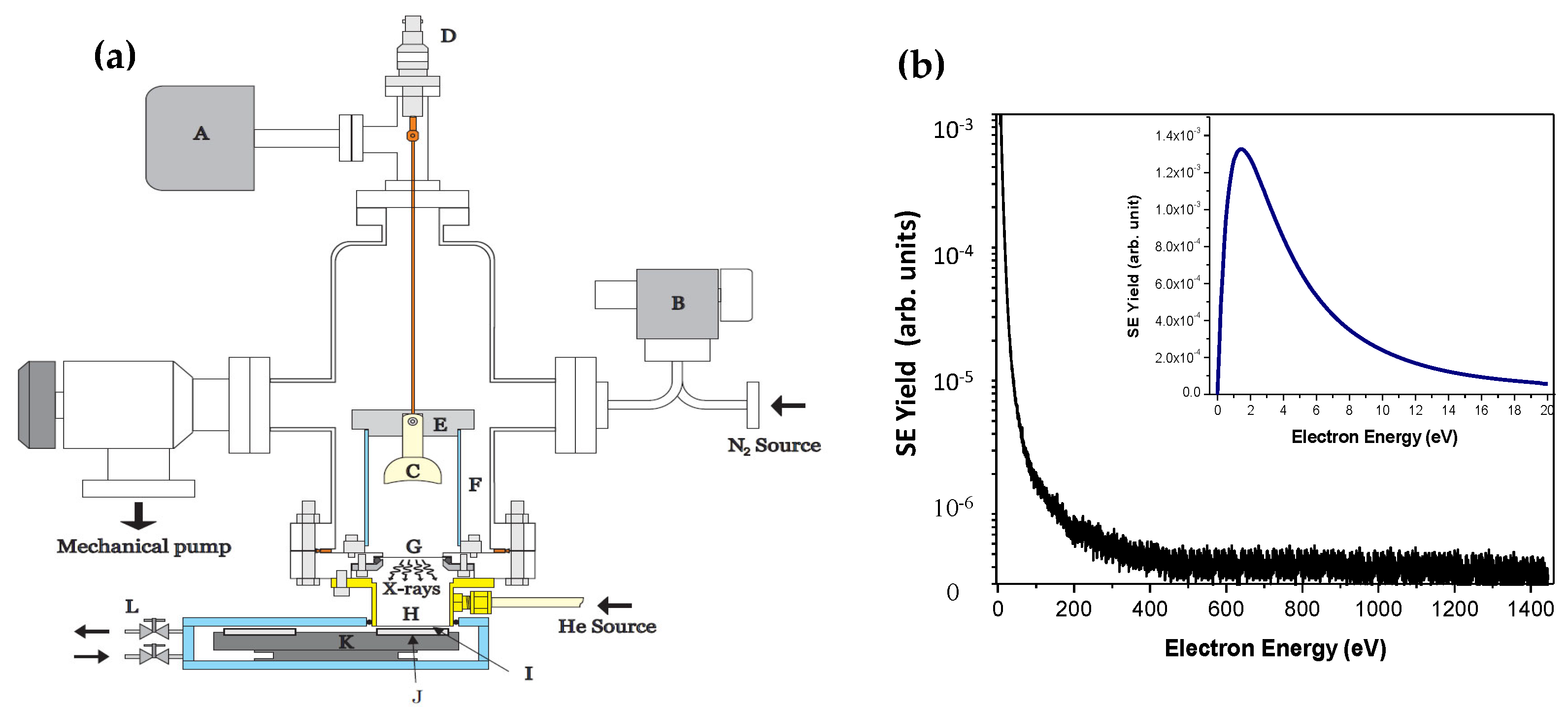
3.2.2. Experimental Setup and Irradiation Conditions
4. Ultimate Sources for Studies under Cellular Conditions
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AE | Auger electron |
| AuNP | gold nanoparticle |
| CISS | chiral induced spin selectivity |
| DEA | dissociative electron attachment |
| ELS | electron loss spectroscopy |
| FLF | femtosecond laser filamentation |
| FWHM | full width at half-maximum |
| HEE | high-energy electron |
| HEM | hemispherical electron monochromator |
| HREEL | high-resolution electron energy loss |
| ICD | intermolecular Coulomb decay |
| IORT | intra-operative radiotherapy |
| LC | liquid chromatography |
| LEE | low energy electron |
| LINAC | linear accelerator |
| MS/MS | tandem mass spectrometry |
| MRI | magnetic resonance imaging |
| NAP | near ambient pressure |
| p | pressure |
| PDT | photodynamic therapy |
| Pt-drugs | platinum-based chemotherapeutic agents |
| RF | radio-frequency |
| ROS | reactive oxygen species |
| RT | radiation therapy |
| SATP | standard ambient temperature and pressure |
| SE | secondary electron |
| TEM | trochoidal electron monochromator |
| THF | tetrahydrofuran |
| TOF | time of flight |
| UHV | ultra-high vacuum |
| UV | ultraviolet |
| VMAT | volumetric modulated arc therapy |
| VHEE | very high-energy electrons |
| XPS | X-ray photoelectron spectroscopy |
References
- Navarro, J. A History of the Electron: J. J. and G. P. Thomsons, 1st ed.; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: A review. World J. Radiol. 2016, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Fertey, J.; Thoma, M.; Beckmann, J.; Bayer, L.; Finkensieper, J.; Reißhauer, S.; Berneck, B.S.; Issmail, L.; Schönfelder, J.; Casado, J.P.; et al. Automated application of low energy electron irradiation enables inactivation of pathogen- and cell-containing liquids in biomedical research and production facilities. Sci. Rep. 2020, 10, 12786. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, C.; Schönfelder, J.; Schwarz, W.; Funk, R.H.W. Surface modification of polyurethane and silicone for therapeutic medical technics by means of electron beam. Surf. Coat. Technol. 2010, 205, 1618–1623. [Google Scholar] [CrossRef]
- Gotzmann, G.; Portillo, J.; Wroski, S.; Kohl, Y.; Gorjup, E.; Schuck, H.; Rögner, F.H.; Müller, M.; Chaberny, I.F.; Schönfelder, J.; et al. Low-energy electron-beam treatment as alternative for on-site sterilization of highly functionalized medical products: A feasibility study. Radiat. Phys. Chem. 2018, 150, 9–19. [Google Scholar] [CrossRef]
- Akbarpoor, R.; Khaledi, N.; Wang, X.; Samiei, F. Optimization of low-energy electron beam production for superficial cancer treatments by Monte Carlo code. J Can. Res. Ther. 2019, 15, 475–479. [Google Scholar] [CrossRef]
- Amgarou, K.; Lacoste, V.; Martin, A. Experimental characterization of the neutron spectra generated by a high-energy clinical LINAC. Nucl. Instrum. Methods Phys. Res. Section A Accel. Spectrom. Detectors Assoc. Equip. 2011, 629, 329–336. [Google Scholar] [CrossRef]
- Banaee, N.; Goodarzi, K.; Nedaie, H.A. Neutron contamination in radiotherapy processes: A review study. J Radia. Res. 2021, 62, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Very High Energy Electron Beam Radiotherapy Workshop (VHEE’2020); CERN: Zurich, Switzerland, 2020.
- Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 2008, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pilar, A.; Gupta, M.; Laskar, S.G.; Laskar, S. Intraoperative radiotherapy: Review of techniques and results. Ecancermedicalscience 2017, 11, 750. [Google Scholar] [CrossRef]
- Sethi, A.; Chinsky, B.; Gros, S.; Diak, A.; Emami, B.; Small, W.J. Tissue inhomogeneity corrections in low-kV intra-operative radiotherapy (IORT). Transl. Cancer Res. 2015, 4, 182–188. [Google Scholar] [CrossRef]
- Papiez, L.; DesRosiers, C.; Moskvin, V. Very high energy electrons (50–250 MeV) and radiation therapy. Technol. Cancer Res. Treat. 2002, 1, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Eriksson, K.; Hynning, E.; Hancock, S.L.; Hiniker, S.M.; Bazalova-Carter, M.; Wong, T.; Le, Q.T.; Loo, B.W.; Maxim, P.G. Very high-energy electron (VHEE) beams in radiation therapy; Treatment plan comparison between VHEE, VMAT, and PPBS. Med. Phys. 2017, 44, 2544–2555. [Google Scholar] [CrossRef] [PubMed]
- Palma, B.; Bazalova-Carter, M.; Hårdemark, B.; Hynning, E.; Qu, B.; Loo, B.W.; Maxim, P.G. Assessment of the quality of very high-energy electron radiotherapy planning. Radiother. Oncol. 2016, 119, 154–158. [Google Scholar] [CrossRef]
- Maxim, P.; Loo, B.W. Pluridirectional High-Energy Agile Scanning Electron Radiotherapy (PHASER): Extremely Rapid Treatment for Early Lung Cancer; Defense Technical Information Center Annual Report; Stanford University: Stanford, CA, USA, 2014. [Google Scholar]
- Geddes, C.G.R.; Toth, C.; van Tilborg, J.; Esarey, E.; Schroeder, C.B.; Bruhwiler, D.; Nieter, C.; Cary, J.; Leemans, W.P. High-quality electron beams from a laser wakefield accelerator using plasma-channel guiding. Nature 2004, 431, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Labate, L.; Palla, D.; Panetta, D.; Avella, F.; Baffigi, F.; Brandi, F.; Di Martino, F.; Fulgentini, L.; Giulietti, A.; Köster, P.; et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: Feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci. Rep. 2020, 10, 17307. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, H.; Zhang, D.; Liu, L.; Han, Y. Low-dose electron microscopy imaging of electron beam-sensitive crystalline materials. Acc. Mater. Res. 2022, 3, 552–564. [Google Scholar] [CrossRef]
- Ptasińska, S. A missing puzzle in dissociative electron attachment to biomolecules: The detection of radicals. Atoms 2021, 9, 77. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S.; Sanche, L. Transient anions in radiobiology and radiotherapy: From gaseous biomolecules to condensed organic and biomolecular solids. In Radiation Effects in Materials; Monteiro, W.A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 179–230. [Google Scholar] [CrossRef]
- Gorfinkiel, J.D.; Ptasińska, S. Electron scattering from molecules and molecular aggregates of biological relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef]
- Sevilla, M.D.; Bernhard, W.A. Mechanisms of direct radiation damage to DNA. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences, 1st ed.; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 191–201. [Google Scholar]
- Uehara, S.; Nikjoo, H.; Goodhead, D.T. Comparison and assessment of electron cross sections for Monte Carlo track structure codes. Radiat. Res. 1999, 152, 202–213. [Google Scholar] [CrossRef]
- Wishart, J.F.; Rao, B.M.S. Recent Trends in Radiation Chemistry; World Scientific: Singapore, 2010. [Google Scholar] [CrossRef]
- O’Neill, P. Radiation-induced damage in DNA. In Radiation Chemistry: Present Status and Future Trends; Jonah, C.D., Rao, B.S.M., Eds.; Elsevier Sciences: Amsterdam, The Netherland, 2001; pp. 585–622. [Google Scholar]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef] [PubMed]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Hearst, J.E.; Vinograd, J. The net hydration of deoxyribonucleic acid. Proc. Natl. Acad. Sci. USA 1961, 47, 825–830. [Google Scholar] [CrossRef]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984. [Google Scholar]
- Jeffrey, G.; Saenger, W. Hydration Bonding in Biological Structures; Springer: New York, NY, USA, 1991. [Google Scholar]
- Ptasińska, S.; Varella, M.T.N.; Khakoo, M.A.; Slaughter, D.S.; Denifl, S. Electron scattering processes: Fundamentals, challenges, advances, and opportunities. Eur. Phys. J. D 2022, 76, 179. [Google Scholar] [CrossRef]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Ann. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Głuch, K.; Fedor, J.; Matt-Leubner, S.; Echt, O.; Stamatovic, A.; Probst, M.; Scheier, P.; and Märk, T.D. Kinetic-energy release in coulomb explosion of metastable C3H52+. J. Chem. Phys. 2003, 118, 3090–3095. [Google Scholar] [CrossRef]
- Chakraborty, D.; Nag, P.; and Nandi, D. A new time of flight mass spectrometer for absolute dissociative electron attachment cross-section measurements in gas phase. Rev. Sci. Instr. 2018, 89, 025115–025123. [Google Scholar] [CrossRef]
- Moradmand, A.; Williams, J.B.; Landers, A.L.; Fogle, M. Momentum-imaging apparatus for the study of dissociative electron attachment dynamics. Rev. Sci. Instr. 2013, 84, 033104–033110. [Google Scholar] [CrossRef]
- Kumar, S.V.K.; Pota, T.; Peri, D.; Dongre, A.D.; Rao, B.J. Low energy electron induced damage to plasmid DNA pQE30. J. Chem. Phys. 2012, 137, 045101. [Google Scholar] [CrossRef] [PubMed]
- Panelli, G.; Moradmand, A.; Griffin, B.; Swanson, K.; Weber, T.; Rescigno, T.N.; McCurdy, C.W.; Slaughter, D.S.; Williams, J.B. Investigating resonant low-energy electron attachment to formamide: Dynamics of model peptide bond dissociation and other fragmentation channels. Phys. Rev. Res. 2021, 3, 013082. [Google Scholar] [CrossRef]
- Dawley, M.M.; Pirim, C.; Orlando, T.M. Radiation processing of formamide and formamide: Water ices on silicate grain analogue. J. Phys. Chem. A 2014, 118, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Kuyatt, C.E.; Simpson, J.A. Electron monochromator design. Rev. Sci. Instr. 1967, 38, 103–111. [Google Scholar] [CrossRef]
- Purcell, E.M. The focusing of charged particles by a spherical condenser. Phys. Rev. 1938, 54, 818–826. [Google Scholar] [CrossRef]
- Čížek, M.; Horáček, J.; Sergenton, A.C.; Popović, D.B.; Allan, M.; Domcke, W.; Leininger, T.; Gadea, F.X. Inelastic low-energy electron collisions with the HBr and DBr molecules: Experiment and theory. Phys. Rev. A 2001, 63, 062710. [Google Scholar] [CrossRef]
- Schürmann, R.; Tsering, T.; Tanzer, K.; Denifl, S.; Kumar, S.V.K.; Bald, I. Resonant formation of strand Breaks in sensitized oligonucleotides induced by low-energy electrons (0.5–9 eV). Angew. Chem. Int. Ed. 2017, 56, 10952–10955. [Google Scholar] [CrossRef] [PubMed]
- Dawley, M.M.; Tanzer, K.; Cantrell, W.A.; Plattner, P.; Brinkmann, N.R.; Scheier, P.; Denifl, S.; Ptasińska, S. Electron ionization of the nucleobases adenine and hypoxanthine near the threshold: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 25039–25053. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, P.; Alizadeh, E.; Mauracher, A.; Märk, T.D.; Scheier, P. Detailed dissociative electron attachment studies on the amino acid proline. Int. J. Mass Spectrom. 2008, 277, 274–278. [Google Scholar] [CrossRef]
- Ptasińska, S.; Alizadeh, E.; Sulzer, P.; Abouaf, R.; Mason, N.J.; Märk, T.D.; Scheier, P. Negative ion formation by low energy electron attachment to gas-phase 5-nitrouracil. Int. J. Mass Spectrom. 2008, 277, 291–295. [Google Scholar] [CrossRef]
- Panajotović, R.; Michaud, M.; Sanche, L. Cross sections for low-energy electron scattering from adenine in the condensed phase. Phys. Chem. Chem. Phys. 2007, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Panajotović, R.; Sanche, L. From DNA to nucleic bases—The effects of low-energy electron impact. J. Phys. Conf. Ser. 2007, 88, 012074. [Google Scholar] [CrossRef]
- Stamatovic, A.; Schulz, G.J. Trochoidal electron monochromator. Rev. Sci. Instr. 1968, 39, 1752–1753. [Google Scholar] [CrossRef]
- Stamatovic, A.; Schulz, G.J. Characteristics of the trochoidal electron monochromator. Rev. Sci. Instr. 1970, 41, 423–427. [Google Scholar] [CrossRef]
- Balog, R.; Cicman, P.; Jones, N.C.; Field, D. Spontaneous dipole alignment in films of N2O. Phys. Rev. Lett. 2009, 102, 073003–073007. [Google Scholar] [CrossRef]
- Bass, A.D.; Sanche, L. Absolute and effective cross-sections for low-energy electron-scattering processes within condensed matter. Radiat. Environ. Biophys. 1998, 37, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Naaman, R.; Sanche, L. Low-energy electron transmission through thin-film molecular and biomolecular solids. Chem. Rev. 2007, 107, 1553–1579. [Google Scholar] [CrossRef]
- Rezaee, M.; Cloutier, P.; Bass, A.D.; Michaud, M.; Hunting, D.J.; Sanche, L. Absolute cross section for low-energy-electron damage to condensed macromolecules: A case study of DNA. Phys. Rev. E 2012, 86, 031913. [Google Scholar] [CrossRef]
- Hoffmann, S.V.; Lunt, S.L.; Jones, N.C.; Field, D.; Ziesel, J.-P. An undulator-based spherical grating monochromator beamline for low energy electron-molecule scattering experiments. Rev. Sci. Instr. 2002, 73, 4157–4163. [Google Scholar] [CrossRef]
- Brunkow, E.; Jones, E.R.; Batelaan, H.; Gay, T.J. Femtosecond-laser-induced spin-polarized electron emission from a GaAs tip. Appl. Phys. Lett. 2019, 114, 073502. [Google Scholar] [CrossRef]
- Machacek, J.R.; Gay, T.J.; Buckman, S.J.; Hodgman, S.S. A high-resolution, variable-energy electron beam from a Penning–Malmberg (Surko) buffer-gas trap. Eur. Phys. J. D 2022, 76, 33. [Google Scholar] [CrossRef]
- Marler, J.P.; Surko, C.M. Systematic comparison of positron- and electron-impact excitation of the ν3 vibrational mode of CF4. Phys. Rev. A 2005, 72, 062702. [Google Scholar] [CrossRef]
- Seiler, H. Secondary electron emission in the scanning electron microscope. J. App. Phys. 1983, 54, R1–R18. [Google Scholar] [CrossRef]
- Kemp, M.A.; Kovaleski, S.D.; Steinfelds, E.V.; Prelas, M.A.; Loyalka, S.K.; Miraglia, P.Q. Transmission and secondary electron emission of 20–50 keV electrons through aluminium foils. J. Phys. D Appl. Phys. 2007, 40, 284. [Google Scholar] [CrossRef]
- Mayne, K.I. Polarized electron beams. Contemp. Phys. 1969, 10, 387–412. [Google Scholar] [CrossRef]
- Ratliff, J.M.; Rutherford, G.H.; Dunning, F.B.; Walters, G.K. Electron exchange in collisions with O2 and NO. Phys. Rev. A 1989, 39, 5584–5587. [Google Scholar] [CrossRef] [PubMed]
- Dapor, M. Polarized electron beams elastically scattered by atoms as a tool for testing fundamental predictions of quantum mechanics. Sci Rep. 2018, 8, 5370. [Google Scholar] [CrossRef]
- Keramati, S.; Brunner, W.; Gay, T.J.; Batelaan, H. Non-poissonian ultrashort nanoscale electron pulses. Phys. Rev. Lett. 2021, 127, 180602. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.T.; Celotta, R.J.; Wang, G.C.; Unertl, W.N.; Galejs, A.; Kuyatt, C.E.; Mielczarek, S.R. The GaAs spin polarized electron source. Rev. Sci. Instr. 1980, 51, 478–499. [Google Scholar] [CrossRef]
- Gay, T.J. Physics and technology of polarized electron scattering from atoms and molecules. In Advances in Atomic, Molecular, and Optical Physics; Arimondo, E., Berman, P.R., Lin, C.C., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 57, pp. 157–247. [Google Scholar] [CrossRef]
- McCarter, J.L.; Poelker, M.; Gay, T.J.; Clayburn, N.B.; Dreiling, J.M.; Ryan, D.M.; Afanasev, A.; Kechiantz, A.; Hansknecht, J. Two Novel Approaches for Electron Beam Polarization from Unstrained GaAs. In Proceedings of the XVth International Workshop on Polarized Sources, Targets, and Polarimetry (PSTP2013)—Electron Sources III, Charlottesville, VA, USA, 9 September 2013. [Google Scholar] [CrossRef]
- Dreiling, J.M.; Gay, T.J. Chirally sensitive electron-induced molecular breakup and the Vester-Ulbricht hypothesis. Phys. Rev. Lett. 2014, 113, 118103. [Google Scholar] [CrossRef]
- Dreiling, J.M.; Lewis, F.W.; Gay, T.J. Spin-polarized electron transmission through chiral halocamphor molecules. J. Phys. B At. Mol. Opt. Phys. 2018, 51, 21LT01. [Google Scholar] [CrossRef]
- Rays, K.; Ananthaveld, P.; Waldeckand, H.; Naaman, R. Asymmetric scattering of polarized electrons by organized organic films of chiral molecules. Science 1999, 283, 814–816. [Google Scholar] [CrossRef]
- Göhler, B.; Hamelbeck, V.; Markus, T.Z.; Kettner, M.; Hanne, G.F.; Vager, Z.; Naaman, R.; Zacharias, H. Spin selectivity in electron transmission through self-assembled monolayers of dsDNA. Science 2011, 331, 894–897. [Google Scholar] [CrossRef]
- Rosenberg, R.A.; Symonds, J.M.; Kalyanaraman, V.; Markus, T.; Elyahu, N.; Orlando, T.M.; Naaman, R.; Medina, E.A.; López, F.A.; Mujica, V. Kinetic energy dependence of spin filtering of electrons transmitted through organized layers of DNA. J. Phys. Chem. C 2013, 117, 22307–22313. [Google Scholar] [CrossRef]
- Reiker, T.; Liu, Z.; Winter, C.; Kleimeier, N.F.; Zhang, D.; Zacharias, H. Dynamics in electronically excited states of Diketopyrrolopyrrole-Thiophene conjugated polymer thin films. J. Phys. Chem. C 2021, 125, 5572–5580. [Google Scholar] [CrossRef]
- Stemer, D.M.; Abendroth, J.M.; Cheung, K.M.; Ye, M.; El Hadri, M.S.; Fullerton, E.E.; Weiss, P.S. Differential charging in photoemission from mercurated DNA monolayers on ferromagnetic films. Nano Lett. 2020, 20, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, D.H.; Naaman, R.; Paltiel, Y. The spin selectivity effect in chiral materials. APL Mater. 2021, 9, 040902. [Google Scholar] [CrossRef]
- Schaible, M.J.; Rosenberg, R.A.; Kundu, S.; Orlando, T.M. Electron spin-polarization dependent damage to chiral amino acid l-Histidine. J. Phys. Chem. Lett. 2020, 11, 10182–10187. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.A.; Rozhkova, E.A.; Novosad, V. Investigations into spin- and unpolarized secondary electron-induced reactions in self-assembled monolayers of cysteine. Langmuir 2021, 37, 2985–2992. [Google Scholar] [CrossRef]
- Pal, C.; Majumder, S. Manipulating electron-spin polarization using cysteine–DNA chiral conjugates. J. Chem. Phys. 2022, 156, 164704. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Kitajima, M.; Hirano, Y.; Odagiri, T.; Kato, H.; Kawahara, H.; Hoshino, M.; Tanaka, H.; Ito, K. Development of a new set-up for cold electron collision experiment utilizing the threshold photoelectrons. J. Phys. Conf. Series 2009, 194, 042010. [Google Scholar] [CrossRef]
- Hotop, H.; Ruf, M.W.; Allan, M.; Fabrikant, I.I. Resonance and threshold phenomena in low-energy electron collisions with molecules and clusters. In Advances in Atomic, Molecular, and Optical Physics, 1st ed.; Bederson, B., Walther, H., Eds.; ScienceDirect: Novosibirsk, Russia, 2003; Volume 49, pp. 85–216. [Google Scholar] [CrossRef]
- Schröer, C.D.; Rudenko, A.; Dorn, A.; Moshammer, R.; Ullrich, J. Status of the pulsed photoelectron source for atomic and molecular collision experiments. In Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors, and Associated Equipment, 1st ed.; Rachek, I.A., Terekhov, A.S., Toporkov, D.K., Eds.; ScienceDirect: Novosibirsk, Russia, 2005; Volume 536, pp. 312–318. [Google Scholar] [CrossRef]
- Jones, N.C.; Hoffmann, S.V.; Field, D. Chiral recognition in electron scattering by S- and R-2-butanol. Mol. Phys. 2015, 113, 2197–2203. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S. Recent advances in plasma-based cancer treatments: Approaching clinical translation through an intracellular view. Biophysica 2021, 1, 48–72. [Google Scholar] [CrossRef]
- Kolobov, V.; Godyak, V. Electron kinetics in low-temperature plasmas. Phys. Plasmas 2019, 26, 060601. [Google Scholar] [CrossRef]
- von Woedtke, T.; Laroussi, M.; Gherardi, M. Foundations of plasmas for medical applications. Plasma Sources Sci. Technol. 2022, 31, 054002. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-temperature plasma for biology, hygiene, and medicine: Perspective and roadmap. IEEE Trans. Rad. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Jung, J.; Lee, M.-Y.; Hwang, J.-G.; Lee, M.-H.; Kim, M.-S.; Lee, J.; Chung, C.-W. Low-energy electron beam generation in inductively coupled plasma via a DC biased grid. Plasma Sources Sci. Technol. 2022, 31, 025002. [Google Scholar] [CrossRef]
- Meesat, R.; Belomuaddine, H.; Allard, J.F.; Tanguay-Renaud, C.; Lemay, R.; Brastaviceanu, T.; Tremblay, L.; Paquette, B.; Wagner, R.; Jay-Gerin, J.P.; et al. Cancer radiotherapy based on femtosecond IR laser-beam filamentation yielding ultra-high dose rates and zero entrance dose. Proc. Natl. Acad. Sci. USA 2012, 109, E2508–E2513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sevilla, M.D. Role of excited states in low-energy electron (LEE) induced strand breaks in DNA model systems: Influence of aqueous environment. Chem. Phys. Chem. 2009, 10, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Siefermann, K.R.; Lin, Y.; Lugovoy, E.; Link, O.; Faubel, M.; Buck, U.; Winter, B.; Abel, B. Binding energies, lifetimes and implications of bulk and interface solvated electrons in water. Nat. Chem. 2010, 2, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Stypczyńska, A.; Nixon, T.; Mason, N.J.; Klyachko, D.V.; Sanche, L. X-ray induced damage in DNA monitored by X-ray photoelectron spectroscopy. J. Chem. Phys. 2008, 129, 065102. [Google Scholar] [CrossRef]
- Gomes, P.J.; Ferraria, A.M.; Botelho do Rego, A.M.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Energy thresholds of DNA damage induced by UV radiation: An XPS study. J. Phys. Chem. B 2015, 119, 5404–5411. [Google Scholar] [CrossRef]
- McKee, A.D.; Schaible, M.J.; Rosenberg, R.A.; Kundu, S.; Orlando, T.M. Low energy secondary electron induced damage of condensed nucleotides. J. Chem. Phys. 2019, 150, 204709. [Google Scholar] [CrossRef]
- Kundu, S.; Schaible, M.J.; McKee, A.D.; Orlando, T.M. Direct damage of deoxyadenosine monophosphate by low-energy electrons probed by X-ray photoelectron spectroscopy. J. Phys. Chem. B 2020, 124, 1585–1591. [Google Scholar] [CrossRef]
- Salmeron, M.; Schlögl, R. Ambient pressure photoelectron spectroscopy: A new tool for surface science and nanotechnology. Surf. Sci. Rep. 2008, 63, 169–200. [Google Scholar] [CrossRef]
- Schnadt, J.; Knudsen, J.; Johansson, N. Present and new frontiers in materials research by ambient pressure x-ray photoelectron spectroscopy. J. Phys. Condens. Matter. 2020, 32, 413003. [Google Scholar] [CrossRef]
- Patel, D.I.; Roychowdhury, T.; Jain, V.; Shah, D.; Avval, T.G.; Chatterjee, S.; Bahr, S.; Dietrich, P.; Meyer, M.; Thißen, A.; et al. Introduction to near-ambient pressure x-ray photoelectron spectroscopy characterization of various materials featured. Surf. Sci. Spectra 2019, 26, 016801. [Google Scholar] [CrossRef]
- Hahn, M.B.; Dietrich, P.M.; Radnik, J. In situ monitoring of the influence of water on DNA radiation damage by near-ambient pressure X-ray photoelectron spectroscopy. Commun. Chem. 2021, 4, 1. [Google Scholar] [CrossRef]
- Kjærvik, M.; Schwibbert, K.; Dietrich, P.M.; Thissen, A.; Unger, W.E.S. Surface characterisation of Escherichia coli under various conditions by near- ambient pressure XPS. Surf. Interface Anal. 2018, 50, 996–1000. [Google Scholar] [CrossRef]
- Patel, D.I.; Shah, D.; Bahr, S.; Dietrich, P.M.; Meyer, M.; Thißen, A.; Linford, M.R. Water vapor, by near-ambient pressure XPS. Surf. Sci. Spectra 2019, 26, 014026. [Google Scholar] [CrossRef]
- Agostinelli, S. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Section A Accel. Spectrom. Detectors Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Ptasińska, S.; Sanche, L. Dissociative electron attachment to hydrated single DNA strands. Phys. Rev. E 2007, 75, 031915. [Google Scholar] [CrossRef]
- Orlando, T.M.; Oh, D.; Chen, Y.; Alexandrov, A. Low-energy electron diffraction and induced damage in hydrated DNA. J. Chem. Phys. 2008, 128, 195102. [Google Scholar] [CrossRef] [PubMed]
- Grieves, G.A.; McLain, J.L.; Orlando, T.M. Low-energy electron-stimulated reactions in nanoscale water films and water-DNA interfaces. In Charged Particle and Photon Interactions with Matter, Recent Advances, Applications, and Interfaces, 1st ed.; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 473–501. [Google Scholar]
- Sidorov, A.N.; Orlando, T.M. Monolayer graphene platform for the study of DNA damage by low-energy electron irradiation. Phys. Chem. Lett. 2013, 4, 2328–2333. [Google Scholar] [CrossRef]
- Brun, E.; Cloutier, P.; Sicard-Roselli, C.; Fromm, M.; Sanche, L. Damage induced to DNA by low-energy (0−30 eV) electrons under vacuum and atmospheric conditions. J. Phys. Chem. B 2009, 113, 10008–10013. [Google Scholar] [CrossRef]
- Nikjoo, H.; Lindborg, L. RBE of low energy electrons and photons. Phys. Med. Biol. 2010, 55, R65–R109. [Google Scholar] [CrossRef]
- Farhataziz; Rodgers, M.A.J. Radiation Chemistry, Principles and Applications, 1st ed.; VCH: New York, NY, USA, 1987. [Google Scholar] [CrossRef]
- Alizadeh, E.; Cloutier, P.; Hunting, D.J.; Sanche, L. Soft X-rays and low energy electrons induced damage to DNA in N2 and O2 Atmospheres. J Phys. Chem. B 2011, 115, 4523–4531. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Low-energy-electron interactions with DNA: Approaching cellular conditions with atmospheric experiments. Eur. Phys. J. D 2014, 68, 1–13. [Google Scholar] [CrossRef]
- Golding, C.G.; Lamboo, L.L.; Beniac, D.R.; Booth, T.F. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci. Rep. 2016, 6, 26516. [Google Scholar] [CrossRef] [PubMed]
- Rösler, M.; Brauer, W.; Devooght, J.; Dehaes, J.C.; Dubus, A.; Cailler, M.; Ganachaud, J.P. Particle Induced Electron Emission I; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kitamura, H. Thermalization dynamics of primary and secondary electrons in metals. J. Electron Spectrosc. Relat. Phenom. 2019, 232, 45–52. [Google Scholar] [CrossRef]
- Huels, M.A.; Boudaïffa, B.; Cloutier, P.; Hunting, D.J.; Sanche, L. Single, double, and multiple double strand breaks induced in DNA by 3−100 eV electrons. J. Am. Chem. Soc. 2003, 125, 4467–4477. [Google Scholar] [CrossRef]
- Śmiałek, M.A.; Jones, N.C.; Balog, R.; Mason, N.J.; Field, D. The influence of the substrate temperature on the preparation of DNA films for studies under vacuum conditions. Eur. Phys. J. D 2011, 62, 197–203. [Google Scholar] [CrossRef]
- Hoshi, M.; Goodhead, D.T.; Brenner, D.J.; Bance, D.A.; Chmielewski, J.J.; Paciotti, M.A.; Bradbur, J.N. Dosimetry comparison and characterisation of an Al K ultrasoft x-ray beam from an MRC cold-cathode source. Phys. Med. Biol. 1985, 30, 1029–1041. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanz, A.G.; Garcia, G.; Sanche, L. Radiation damage to DNA: The indirect effect of low energy electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanche, L. The role of humidity and oxygen level on damage to DNA induced by soft X-rays and low-energy electrons. J. Phys. Chem. C 2013, 117, 22445–22453. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanz, A.G.; Madugundu, G.S.; Garcia, G.; Wagner, J.R.; Sanche, L. Thymidine induced decomposition by LEEs and soft X-rays under N2 and O2 atmospheres. Radiat. Res. 2014, 181, 629–640. [Google Scholar] [CrossRef]
- Choofong, S.; Cloutier, P.; Sanche, L.; Wagner, J.R. Base release and modification in solid-phase DNA exposed to low-energy electrons. Radiat. Res. 2016, 186, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Alizadeh, E.; Cloutier, P.; Hunting, D.J.; Sanche, L. A single ultra-LEE (0.5 eV) can induce a double strand break in DNA modified by Pt chemotherapeutic drugs. Chem. Med. Chem. 2014, 9, 1145–1149. [Google Scholar] [CrossRef]
- Rezaee, M.; Hill, R.P.; Jaffray, D.A. The exploitation of low-energy electrons in cancer treatment. Radiat Res. 2017, 188, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanopartides. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Aguerri, A.R.; Filipovic, N. Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 2013, 15, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Gokhberg, K.; Kolorenč, P.; Kuleff, A.I.; Cederbaum, L.S. Site- and energy-selective slowelectron production through intermolecular Coulombic decay. Nature 2014, 505, 661–663. [Google Scholar] [CrossRef]
- Antosh, M.P.; Wijesinghe, D.D.; Shrestha, S.; Lanou, R.; Huang, Y.H.; Hasselbacher, T.; Fox, D.; Nerettia, N.; Sun, S.; Katenkaf, N.; et al. Enhancement of radiation effect on cancer cells by gold-Phlip. Proc. Natl. Acad. Sci. USA 2015, 112, 5372–5376. [Google Scholar] [CrossRef] [PubMed]
- Pomplun, E.; Booz, J.; Charlton, D.E. A Monte Carlo simulation of Auger cascades. Radiat. Res. 1987, 111, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Pronschinske, A.; Pedevilla, P.; Murphy, C.J.; Lewis, E.A.; Lucci, F.R.; Brown, G.; Pappas, G.; Michaelides, A.; Sykes, E.C.H. Enhancement of low-energy electron emission in 2D radioactive films. Nat. Mat. 2015, 14, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Santra, R.; Zobeley, J.; Cederbaum, L.S.; Moiseyev, N. Interatomic coulombic decay in van der Waals clusters and impact of nuclear motion. Phys. Rev. Lett. 2000, 85, 4490–4493. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, T.; Hergenhahn, U.; Winter, B.; Dörner, R.; Frühling, U.; Demekhin, F.V.; Gokhberg, K.; Cederbaum, L.S.; Ehresmann, A.; Knie, A.; et al. Interatomic and intermolecular Coulombic decay. Chem. Rev. 2020, 120, 11295–11369. [Google Scholar] [CrossRef]
- Mucke, M.; Braune, M.; Barth, S.; Förstel, M.; Lischke, T.; Ulrich, V.; Arion, T.; Becker, U.; Bradshaw, A.; Hergenhahn, U. A hitherto unrecognized source of low-energy electrons in water. Nat. Phys. 2010, 6, 143–146. [Google Scholar] [CrossRef]
- Grieves, G.A.; Orlando, T.M. Intermolecular coulomb decay at weakly coupled heterogeneous interfaces. Phys. Rev. Lett. 2011, 107, 016104. [Google Scholar] [CrossRef]
- Hergenhan, U. Production of low kinetic energy electrons and energetic ion pairs by intermolecular Coulombic decay. Int. J. Radiat. Biol. 2012, 88, 871–883. [Google Scholar] [CrossRef]
- Märk, T.D.; Scheier, P. Ionization dynamics: Unexpected electrons. Nat. Phys. 2010, 6, 82–83. [Google Scholar] [CrossRef]
- Seo, S.J.; Han, S.M.; Cho, J.H.; Hyodo, K.; Zaboronok, A.; You, H.; Peach, K.; Hill, M.A.; Kim, J.K. Enhanced production of reactive oxygen species by Gadolinium Oxide nanoparticles under core-inner-shell excitation by proton or monochromatic X-ray irradiation: Implication of the contribution from the interatomic de-excitation-mediated nanoradiator effect to dose enhancement. Radiat. Environ. Biophys. 2015, 54, 423–431. [Google Scholar]
- Ren, X.; Wang, E.; Skitnevskaya, A.D.; Trofimov, A.B.; Gokhberg, K.; Dorn, A. Experimental evidence for ultrafast intermolecular relaxation processes in hydrated biomolecules. Nat. Phys. 2018, 14, 1062–1067. [Google Scholar] [CrossRef]
- Houde, D.; Meesat, R.; Allard, J.F.; Brastaviceanu, T. Method of generating low-energy secondary electrons for applications in biological sciences, radiochemistry, and chemistry of polymers and physics of radiotherapy. US patent Publication of US8884181B2, 23 July 2014. [Google Scholar]
- Schmitt-Sody, A.; Kurz, H.G.; Bergé, L.; Skupin, S.; Polynkin, P. Picosecond laser filamentation in air. New J. Phys. 2016, 18, 093005. [Google Scholar] [CrossRef]
- Couairon, A.; Mysyrowicz, A. Femtosecond filamentation in transparent media. Phys. Rep. 2007, 441, 47–189. [Google Scholar] [CrossRef]
- Meesat, R.; Allard, J.F.; Houde, D.; Tremblay, L.; Khalil, A.; Jay-Gerin, J.P.; Lepage, M. Femtosecond laser pulse filamentation characterized by polymer gel dosimetry and Fricke dosimetry. J. Phys. Conf. Ser. 2010, 250, 012077. [Google Scholar] [CrossRef]
- Wang, E.; Ren, X.; Baek, W.Y.; Rabus, H.; Pfeifer, P.; Dorn, A. Water acting as a catalyst for electron-driven molecular break-up of tetrahydrofuran. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pshenichnyuk, S.A.; Modelli, A.; Komolov, A.S. Interconnections between dissociative electron attachment and electron-driven biological processes. Int. Rev. Phys. Chem. 2018, 37, 125–170. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh, E.; Chakraborty, D.; Ptasińska, S. Low-Energy Electron Generation for Biomolecular Damage Inquiry: Instrumentation and Methods. Biophysica 2022, 2, 475-497. https://doi.org/10.3390/biophysica2040041
Alizadeh E, Chakraborty D, Ptasińska S. Low-Energy Electron Generation for Biomolecular Damage Inquiry: Instrumentation and Methods. Biophysica. 2022; 2(4):475-497. https://doi.org/10.3390/biophysica2040041
Chicago/Turabian StyleAlizadeh, Elahe, Dipayan Chakraborty, and Sylwia Ptasińska. 2022. "Low-Energy Electron Generation for Biomolecular Damage Inquiry: Instrumentation and Methods" Biophysica 2, no. 4: 475-497. https://doi.org/10.3390/biophysica2040041
APA StyleAlizadeh, E., Chakraborty, D., & Ptasińska, S. (2022). Low-Energy Electron Generation for Biomolecular Damage Inquiry: Instrumentation and Methods. Biophysica, 2(4), 475-497. https://doi.org/10.3390/biophysica2040041





