1. Introduction
Environmental pollution caused by metallurgical industry waste is a major modern problem. Tailings are one of the large-scale and underutilized types of mining waste [
1,
2]. Tailings occupy large territories, fine waste particles fall into water bodies, and wind blows them off to considerable distances.
However, the tailings generated from ore processing may have promising applications, including the production of permeable bricks and their use as industrial additives. Therefore, future studies should focus on developing technologies to extract valuable components, such as silicon and nonferrous metals from tailings, thereby increasing their economic attractiveness [
3,
4].
2. Literature Review
Modern studies have shown that SiO
2 recovery from secondary sources expands the raw material base and has a significant potential for industrial applications. As noted in [
5], processing industrial waste for silica recovery helps solve environmental problems and opens up opportunities for developing a green economy. Environmental sustainability is a key factor that has stimulated research in this area. A previous study [
6] provided evidence of significant reduction in waste disposal by recycling waste and biomass to produce SiO
2. These processes can be integrated into the existing production cycles to reduce harmful environmental impacts. Thus, the tailings of processing plants are technogenic deposits with significant potential for processing and extracting beneficial components that could significantly replenish the national mineral resource base.
Thermal treatment is one of the technologies used for processing silicon-containing waste, which makes it possible to obtain pure silicon dioxide with minimal carbon dioxide emissions. Thus, in studies [
7,
8,
9,
10,
11,
12], silicon dioxide was precipitated after processing mineral high-silicon ore and phosphorus production slags [
9]. The main disadvantages of these processes are the low recovery of silicon into a solution, the lack of information on the recovery of rhenium, which is often an associated metal, and the complexity of processing owing to the need for an additional leaching operation.
Hydrometallurgical processing methods are also under development to extract silicon from the ash and waste generated during silicon production [
13,
14]. To decompose high-silica bauxite, a sequential combined hydrochemical alkaline method for alumina production, the Bayer process, has been developed [
15,
16].
Papers [
17,
18] have described a thermochemical enrichment method for high-silica alumina-containing raw materials. The preliminary chemical enrichment technique proposed in [
19] is of particular interest for hydroalkali treatments. Chemical enrichment consists of autoclave treatment with a caustic sodium solution of sulfide copper ore of the Konyrat deposit (Balkhash, Karaganda region, Kazakhstan) with a composition of Cu—0.32 wt. %, SiO
2—68.35 wt. %, Al
2O
3—10.88 wt. %, Fe—3.1 wt. %, S—2.35 wt. %, and Re—1.0 g/t. To reduce the concentration of SiO
2, the concentrate underwent autoclave desiliconization at an alkali concentration of 160 g/L,–id, a solid ratio of 5:1, and a temperature of 230 °C. The disadvantages of this method are the low degree of desiliconization of concentrates (57.10% SiO
2) and the lack of complex recovery of other nonferrous metals, such as rhenium [
19].
In addition, methods for processing copper concentrates, including sintering the concentrate with alkali and producing white soot, have been previously developed [
20,
21,
22], but these methods did not include the extraction of the associated metals.
The analysis of the literature shows that given the assessment of the efficiency of various options for processing alumina-containing materials (sintering, acid treatment, and hydro-alkali treatment), preference should be given to the alkaline hydrochemical method of decomposition as it is more economical, excluding high-temperature sintering, and less energy-intensive. In contrast to similar studies, the processing of raw materials with the complex extraction of silicon and rhenium in a solution is proposed.
Thus, the development of an efficient technology for ore processing with the associated extraction of rhenium is urgently required. The basis for solving the urgent problem of creating a scientific basis for the complex processing technology of sub-standard concentrates is the process of thermochemical decomposition-sintering, which makes it possible to reveal and transfer valuable components (Si and Re) into water-soluble compounds in one process.
This study aims to investigate the possibility of thermochemical enrichment and the opening of low-grade copper tailings of processing plants with the transfer of silicon and rhenium in the form of silicate- and perrhenate-ions into a solution with the output of a multifactor multiplicative model and tabular nomograms.
In contrast to similar methods, this study proposes the extraction of silicon and rhenium from a rough concentrate obtained after the flotation enrichment of waste copper-containing tailings using low-temperature sintering and sodium hydroxide as a reagent that forms a chemical compound with silicon dioxide with further water leaching. A feature of this direction is the possibility of complex processing owing to the optimal combination of flotation and chemical enrichment, which provides the maximum interdependent recovery of metals (Si and Re). The essential characteristic features are the sequence of technological operations, conditions of rough concentrate sintering, and silicon and rhenium recovery, with further enrichment of the copper cake (through the removal of waste rock) after water leaching. This study uses process operations with multifactor dependencies based on tabular nomograms.
3. Materials and Methods
Rough copper concentrate obtained by flotation from waste copper-containing tailings was used for the experiments. The enrichment of mature tailings via flotation has been investigated in previous studies [
21,
22,
23].
Figure 1 shows the technological scheme and the conditions of the laboratory experiments. The primary components in the copper-containing tailings were copper—0.14 wt. %, rhenium—1.51 g/t, sulfur—0.13 wt. %, and total iron—2.10 wt. %. The rock components included silicon dioxide—59.12 wt. %, aluminum oxides—11.15 wt. %, calcium—4.84 wt., and magnesium—2.01 wt. %.
Ore mineralization, mainly represented by iron hydroxides and oxides, was insignificant. Much smaller amounts of copper oxide were formed in the malachite form; copper sulfides in the form of chalcopyrite, chalcosine + digenite + covellite, bornite, and pyrite were even rarer.
Ore mineralization was as follows.
Major: iron hydroxides and oxides (goethite, hematite).
Rarely occurring (in descending order): malachite, chalcopyrite, chalcosine + digenite, covelline, bornite, pyrite, magnetite, and covelline.
All ore minerals were almost 100% represented in concretions containing rock minerals or as inclusions. Separate ore mineral grains are extremely rare.
The size of copper minerals varied from 0.001 to 0.07 mm:
- -
malachite—0.01 mm to 0.05 mm;
- -
sulfide copper minerals—from 0.001 mm to 0.07 mm.
During the closed experiment, based on the developed technological scheme and optimized reagent mode, copper concentrate with a yield of 1.53% was obtained and used for further studies.
Table 1 and
Table 2 list the chemical and phase compositions of the concentrate samples, respectively.
The concentrate contained 88.347% Cu of sulfide minerals and 11.653% Cu of oxidized minerals.
Using a Bruker D2 Phaser diffractometer (step size of 1 s, scanning speed of 120°/min), the phase composition of the concentrate was determined: quartz (SiO2)—39.4%; albite (K[AlSi3O8])—28.1%; chalcopyrite (CuFeS2)—8.7%; anorthite (Ca[Al2Si2O8])—20.0%; and calcite (CaCO3)—3.7%.
Studies on concentrate sintering with alkali occurred in the temperature range t from 300 °C to 500 °C, and sintering duration τ was from 30 min to 120 min; the ratio of caustic alkali NaOH to concentrate was γNaOH = 0.5–2 units at a constant layer height in the crucible
h = 0.03 m; NaNO
2 was added at 0.1% of the concentrate mass to improve oxidation processes in the intervals of the reaction of the interaction of caustic alkali with base minerals, as well as based on the stoichiometry of the reaction [
24]. The following interactions express the main sintering reactions.
The main experiment followed these conditions: the sample mass was 100 g (with a class yield of −0.071 + 0–80%), t was 350 °C, τ was 90 min, and γNaOH was 2:1.
Water leaching of the sinter occurred at a temperature of 60 °C, liquid–solid ratio of 3:1, and duration of 60 min. Under these conditions, copper is extracted (by 5%) into an aqueous solution. However, in our case, when the precipitant reagent was added, copper did not enter the solution. Further studies should include experiments in this regard. The obtained silicate solution was used to produce commercial products [
22,
24]. Technical reagents were used in all experiments.
Design of experiments for the sequential study of the acting factors involved using the method [
25] and obtaining a multiplicative multifactor Protodyakonov–Malyshev model. The model generalized partial functions through their normalization by a general value and combined them into a multifactor dependence of the Protodyakonov equation type. For a more economical and uniform representation of the generalized function in the entire multifactor space within the actual limits of the change and influence of each factor, further adaptation of the method was expressed in such an organization of the experiment when the normalization of partial dependencies occurred according to the results of the main experiment
me, which entered each partial dependence. In this case, the total number of experiments is reduced by subtracting one factor. After obtaining this equation, it can be used for the construction of multifactor tabular nomograms with the selection of areas of acceptable and unacceptable combinations of factor levels, and thus, for process control [
24].
When deriving the equation, the nonlinear multiple correlation coefficient
R and its significance
tR expressed by the known formulas [
26] were used to test its adequacy:
where
ye,i—experimental value,
yc,i—calculated value,
ye,av—average experimental value,
n—the number of independent (not repeated) experimental data,
k—the number of acting factors, and (
n −
k − 1)—the number of degrees of freedom of the adequacy variance.
Our research highlights the main problems of silicon and rhenium recovery from final copper-containing tailings. After selecting suitable research methods, we performed concentrate thermochemical enrichment and water leaching while obtaining multiplicative multifactor Protodyakonov–Malyshev models for Si and Re. As a result, we recovered silicon up to 85% and rhenium up to 98% into a solution and obtained multifactor tabular nomograms, which included the complete number of combinations of all factors and levels among themselves. The effectiveness of the selected methods of thermochemical treatment was confirmed, and the scientific novelty and practical significance of the study were established.
4. Results and Discussion
To recover the target components and obtain marketable products, tests on the thermochemical enrichment of rough concentrates with alkali, followed by water leaching, were conducted. The concentrate was mixed with sodium hydroxide at a given ratio (according to the reaction stoichiometry), after which the material under study was placed in a furnace and heated to a given temperature and duration.
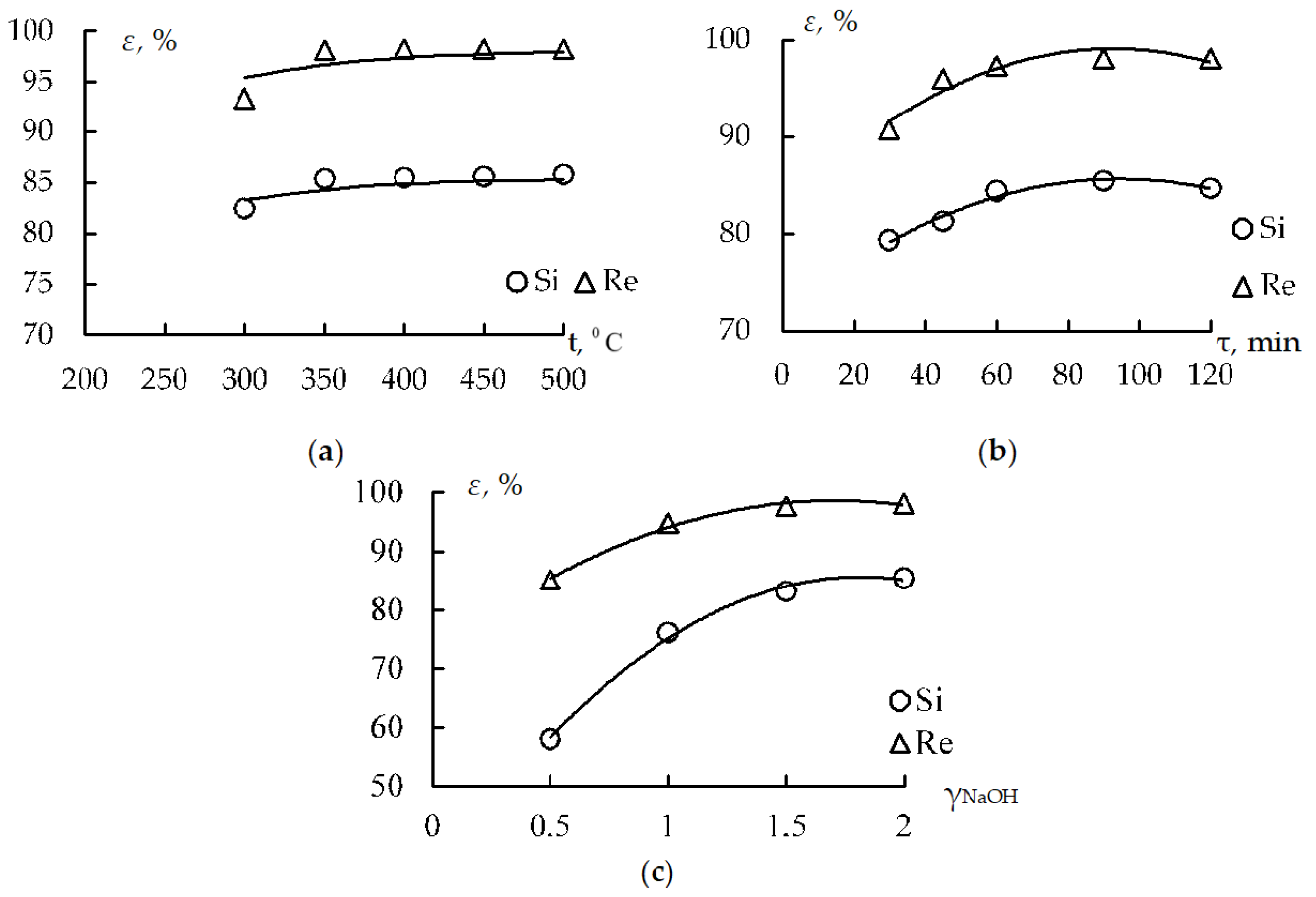
Figure 2 shows dot plots with the approximation of partial dependencies.
Table 3 presents the experimental results of the thermochemical enrichment of the concentrate with the alkali. According to the X-ray phase analysis performed using a D2 Phaser, the concentrate after thermochemical enrichment contained Na
4SiO
4—sodium silicate and NaAlSiO
4—sodium aluminosilicate.
The stage of the preliminary desiliconization of the rough concentrate yielded up to 85% of silica and 98% of rhenium in the solution. The silicate solution obtained under optimal conditions had the following composition: Na2O = 126.5 g/L, SiO2 = 112.8 g/L, and Al2O3 = 4.0 g/L.
After water leaching, the solution was used to obtain marketable products such as white soot and ammonium perrhenate. By increasing the leaching time from 30 min to 120 min, the extraction of silicon and rhenium into the solution increased from 79.32% to 85.37%, and 90.68% to 98.06%, respectively. According to the results of a series of experiments, the optimum sintering time was 90 min, because a further increase in time did not significantly affect the process.
Table 4 shows the partial equations for silicon and rhenium recovery into the solution, whose adequacy was determined using Equations (1) and (2).
The multifactor equation for silicon and rhenium extraction into the solution includes significant partial functions for temperature, duration, and alkali-to-concentrate ratio normalized to the central calculated value. As the partial functions (
Table 4) in the multifactor equation are combined as a product, the overall mean value should be calculated as the geometric mean. The generalized equations for silicon and rhenium extraction into solution are as follows:
The obtained values of the nonlinear multiple correlation coefficients and their significance, according to Equations (1) and (2), indicate the high adequacy of the multifactor equation. This makes it possible to use these equations to calculate the multifactor space of indicators of silicon and rhenium extraction into the solution with the variation in technologically possible limits of change of each significant factor and by selecting, in these space areas, acceptable and unacceptable values of indicators; in this case, 70–80% (for acceptable values) and below 70% and above 80% (for unacceptable values). Moreover, such a space can be represented on the plane in tabular numerical form for any number of factors.
Table 5 presents a three-factor numerical space for Si and Re recovery rates. Combinations of factor levels that provide an acceptable recovery of 80% (green) are highlighted (based on the data obtained in [
20]). This table can be used as a flowchart for the recovery of the joint silicon and rhenium.
Table 5 shows that the thermochemical enrichment is inexpedient at a sintering duration of 30 min, a temperature of 300 °C, and a ratio of alkali to the concentrate in the range from 0.5 to 2. Simultaneously, the high values are in the area of increased sintering temperatures and extended duration, which may not be economically efficient because of excessive heating costs. Therefore, such a flow chart helps find an acceptable control solution in the sufficiently extensive zones of permissible and optimum indicators.
During the stage of preliminary desiliconization of the rough concentrate, up to 85% of silicon and 98% of rhenium passed into the solution. Under optimal conditions, the silicate solution had the following composition: Na
2O = 126.5 g/L, SiO
2 = 112.8 g/L, Al
2O
3 = 4.0 g/L. The solution was then passed to obtain white soot and for the sorption extraction of rhenium [
27,
28,
29]. After water leaching, the cake is extracted from the target components. As shown in
Table 3, after water leaching, the cake yield decreased an average of 20%, thereby increasing the content of valuable components; for example, copper, from 4.857%, as shown in
Table 1, to 6.5%, as shown in
Table 6.
The studies on thermochemical enrichment applied to the concentrate from the final tailings with sodium hydroxide at a temperature of 300–350 °C and subsequent water leaching with the extraction of silicon and rhenium in the solution made it possible to extract valuable components without using high-temperature pyrometallurgical processes.
The introduction of an integrated processing of copper-containing tailings, including sintering followed by water leaching, opens up new opportunities for the complete use of resources and minimization of harmful environmental impacts. Further studies are required to optimize these technologies to improve their efficiency and expand their applications, including the production of silicon, which is in high demand in various industries.
5. Conclusions
The optimal sintering conditions (temperature 350 °C, duration 90 min) promoted the extraction of 84.44% of silicon and 97.44% of rhenium from the rough concentrate into the solution. After water leaching, the cake was subjected to the further extraction of target components. This degree of extraction reduces the need for fresh raw materials and positively affects the production environmental performance because it minimizes the volume of industrial waste disposal.
The partial dependencies of silicon and rhenium extraction into the solution were constructed based on multifactor experiments on the thermochemical enrichment of rough copper concentrates. In this study, mathematical Protodyakonov–Malyshev models of these processes and multifactor nomograms over a wide range of duration temperatures and the ratio of alkali to the concentrate with the determination of maximum recovery rates were developed. The developed multifactor models make it possible to establish optimal intervals of changes in the concentrate sintering parameters, providing high recovery rates (up to 85% of silicon and 98% of rhenium) in the subsequent water leaching.
5.1. Theoretical Significance
The obtained multiplicative multifactor Protodyakonov–Malyshev models can be used to calculate the multifactor space of indicators of silicon and rhenium extraction into the solution with the variation in technologically possible limits of change of each significant factor and with the selection of areas of acceptable and unacceptable values in this space.
The multifactor tabular representation of the summarizing function is the most illustrative and easily formalized representation of computer execution. Therefore, it is worthwhile to conduct independent theoretical and practical studies.
5.2. Practical Significance
Copper concentrator tailings currently occupy a large area owing to the absence of an efficient processing technology. The combined method, which includes flotation and thermochemical processes, allows for the recovery of valuable components, reduces the overall processing costs, and improves the economic profitability of the process. Industrialization of this technology has the potential to be an essential step in sustainable waste management and the provision of secondary raw materials for various industries, including electronics and construction.
The residue obtained after water leaching of the concentrate was sent for further extraction of the target components.
5.3. Strengths and Limitations of the Study
Strengths. This method of processing copper-containing final tailings aims to improve the environment by eliminating dumps in processing plants.
Limitations. The sintering of the rough copper concentrate should occur at a temperature not lower than 300 °C because of the crystallization of NaOH. After thermochemical enrichment, water leaching should occur at temperatures no higher than 60 °C because the solution coagulates.
5.4. Recommendations and Further Actions
Further studies will involve the multifactor analysis of technological operations by identifying zones of optimal process regimes and optimizing leaching technology with further theoretical justification of the processes to increase their efficiency and expand their application, including the production of silicon demanded in various industries.
Author Contributions
Conceptualization, L.K.; methodology, L.K. and G.M.; software, A.M.; validation, Y.K.; formal analysis, G.M. and L.K.; investigation, Y.K. and A.M.; resources, L.K.; data curation, L.K.; writing—original draft preparation, L.K., G.M., Y.K. and A.M.; writing—review and editing, L.K., G.M., Y.K. and A.M.; visualization, G.M.; supervision, L.K.; project administration, L.K.; funding acquisition, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed under the grant project AP 19675340, funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Lyutsiya Karimova, Guldana Makasheva, and Adilet Magaz were employed by Metallurgy Laboratory of LLP “Innovation” and LLP “KazHydroMed”, Author Yelena Kharchenko was employed by Metallurgy Laboratory of LLP “Innovation” and Department of Metallurgy and Materials Science, Non-Profit Joint Stock Company “Karaganda Industrial University”. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Masloboev, V.A.; Seleznev, S.G.; Makarov, D.V.; Svetlov, A.V. Assessment of ecological hazard of storage of wastes from mining and processing of copper-nickel ores. Sib. Branch Russ. Acad. Sci. 2014, 3, 138–153. (In Russian) [Google Scholar]
- Rylnikova, M.V.; Yun, A.B.; Terentyeva, I.V. Second breath of Zhezkazgan. Min. Ind. 2015, 3, 32–34. (In Russian) [Google Scholar]
- Ma, S.; Xing, P.; Li, H.; Wang, C.; Liu, M.; Xu, B. Efficient and Emission-Reduced Recovery of High-Purity Copper from Waste Enameled Copper Wires. Resour. Conserv. Recycl. 2024, 212, 107903. [Google Scholar] [CrossRef]
- Guj, P.; Schodde, R. Will Future Copper Resources and Supply Be Adequate to Meet the Net Zero Emission Goal? Geosyst. Geoenviron. 2024, 4, 100320. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, J.; Wang, Z. Green Process Innovation, Green Product Innovation and Its Economic Performance Improvement Paths: A Survey and Structural Model. J. Environ. Manag. 2021, 297, 113282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Green Chemistry: An Approach Towards Eco-Friendly Environment. In Proceedings of the International Conference on Recent Trends in Green Chemistry, Athens, Greece, 28–30 September 2021. [Google Scholar] [CrossRef]
- Bochevskaya, Y.G.; Karshigina, Z.B.; Sargelova, E.A.; Abisheva, Z.S. Deposition of amorphous silicon dioxide from silicate solutions obtained after processing of mineral high-silica ore. J. Sci. Educ. 2017, 36, 18–23. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Bochevskaya, E.G.; Karshigina, Z.B.; Zagorodnaya, A.N.; Frangulidi, L.H.; Sharipova, A.S. A Process for Producing “White Soot” from Calcium Silicate Slags Phosphorus Production. Patent 24434 RK, 15 July 2011. (In Russian). [Google Scholar]
- Karshigina, Z.B.; Abisheva, Z.S.; Bocevskaya, E.G.; Akchil, A.; Bakhireva, N.A. Extraction of rare earth metals from slags of phosphorus production and obtaining silicate solution. Integr. Use Miner. Raw Mater. 2016, 3, 3–8. Available online: https://official.satbayev.university/download/document/7167/%D0%92%D0%95%D0%A1%D0%A2%D0%9D%D0%98%D0%9A-2016%20%E2%84%963.pdf (accessed on 17 February 2025).
- Medyankina, I.S.; Pasechnik, L.A. Kinetics of silica leaching by ammonium hydrofluoride from iron ore tailings. Theor. Found. Chem. Eng. 2024, 58, 62–67. [Google Scholar] [CrossRef]
- Kutischeva, E.S.; Usoltseva, I.O.; Perederin, Y.V. Methods of obtaining highly dispersed silicon dioxide. Polzunov Bull. 2021, 2, 188–193. [Google Scholar] [CrossRef]
- Panina, O.D.; Perederin, Y.V.; Usoltseva, I.O. Study of the influence of various factors on the value of the specific surface of silicon dioxide. In Proceedings of the VI All-Russian Conference “Chemistry and Chemical Technology: Achievements and prospects; Tomsk, Russia, 15–19 May 2023; Kuzbass State Technical University Named After T.F. Gorbachev: Kemerovo, Russia, 2023; pp. 116–118. Available online: http://earchive.tpu.ru/handle/11683/76626 (accessed on 17 February 2025). (In Russian).
- Sazhin, S. New Hydrochemical Methods of Complex Processing of Aluminosilicates and High-Silicon Bauxites; Metallurgy: Moscow, Russia, 1988. (In Russian) [Google Scholar]
- Abisheva, Z.S.; Zagorodnaya, A.N.; Bochevskaya, E.G.; Frangulidi, L.H.; Baskakova, G.A.; Sapukov, I.A.; Kokoveshnikova, T.A. Possibility of using wastes of chemical and metallurgical enterprises of Kazakhstan for the production of precipitated silicon dioxide. Integr. Use Miner. Raw Mater. 2006, 2, 70–75. [Google Scholar]
- Loginova, I.V.; Kyrchikov, A.V.; Lebedev, V.A.; Ordon, S.F. Investigation into the Question of Complex Processing of Bauxites of the Srednetimanskoe Deposit. Russ. J. Non-Ferr. Met. 2013, 54, 143–147. [Google Scholar] [CrossRef]
- Loginova, I.V.; Shoppert, A.A.; Chaikin, L.I. Extraction of Rare-Earth Metals during the Systematic Processing of Diaspore-Boehmite Bauxites. Metallurgist 2016, 60, 198–203. [Google Scholar] [CrossRef]
- Karshigina, Z.B. Complex Processing of Silicon-Containing Minerals and Technogenic Formations by Obtaining Precipitated Silicon Dioxide and Extracting Rare-Earth Metals. Ph.D. Thesis, Satbayev University, Almaty, Kazakhstan, 2016. [Google Scholar]
- Sadyralieva, U.J. Chemical enrichment of nepheline syenites to obtain concentrate of rare-earth elements. Izv. Vuzov Kyrg. 2015, 2, 45–47. [Google Scholar]
- Gizatullina, D.R.; Katkeeva, G.L.; Oskembekov, I.M.; Akubaeva, M.A.; Gainz, L.V. Development of the technology of autoclave desiliconization of rough concentrate from off-balance copper ores. In Proceedings of the II International Scientific and Practical Conference “Theoretical and Applied Aspects of Modern Science”, Belgorod, Russia, 31 August 2014; Volume 1, pp. 41–44. [Google Scholar]
- Shoppert, A.A.; Karimova, L.M.; Zakharyan, D.V. Novel Method for Comprehensive Processing of Low-Grade Copper Concentrate. Solid State Phenom. 2018, 284, 856–862. [Google Scholar] [CrossRef]
- Yussupov, K.; Aben, E.; Akhmetkanov, D.; Abenk, K.; Yussupova, S. Investigation of the Solid Oxidizer Effect on the Metal Geotechnology Efficiency. Min. Miner. Depos. 2023, 17, 12–17. [Google Scholar] [CrossRef]
- Akishev, K.; Aryngazin, K.; Tleulessov, A.; Bulyga, L.; Stanevich, V. The use of simulation modeling in calculating the productivity of the technological system for the production of building products with fillers from man-made waste. News Natl. Acad. Sci. Repub. Kazakhstan 2024, 4, 22–32. [Google Scholar] [CrossRef]
- Karimova, L.M.; Kairalapov, Y.T.; Makasheva, G.K.; Kharchenko, Y.M. Studying the thermochemical characteristics of sintering with alkali concentrate from waste copper tailings. Izv. Vyss. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnologiya 2024, 67, 72–79. [Google Scholar] [CrossRef]
- Karimova, L.; Makasheva, G.; Malyshev, V.; Kharchenko, Y.; Kairalapov, Y. Permissible Extrapolation Justification of the Multiplicative Multifactorial Model and Its Application to the White Soot Production Technology. HighTech Innov. J. 2024, 5, 663–676. [Google Scholar] [CrossRef]
- Malyshev, V.P. Kinetic and technological analysis of generalizing mathematical models of chemical and metallurgical processes. Rep. Natl. Acad. Sci. Repub. Kazakhstan 2008, 2, 13–18. (In Russian) [Google Scholar]
- Novy Semester. Multiple Correlation Coefficient and Coefficient of Determination. Available online: https://math.semestr.ru/regress/multiple-correlation.php# (accessed on 12 January 2025).
- Troshkina, I.D. Rhenium; Great Russian Encyclopedia: Moscow, Russia, 2015. [Google Scholar]
- Palant, A.A.; Troshkina, I.D.; Chekmarev, A.M.; Kostylev, A.I. Rhenium Technology; OOO Galleya Print: Moscow, Russia, 2015. [Google Scholar]
- Karimov, N.M.; Petukhov, O.F. Study of the rhenium sorption technology from carbonate solutions on activated carbon. Int. Sci. J. Internauka 2020, 16, 10–13. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).