Comprehensive Efficiency Analysis of Ethanol–Gasoline Blends in Spark Ignition Engines
Abstract
1. Introduction
- Higher octane number: Ethanol’s higher octane rating provides greater resistance to knocking, allowing the engine to operate at higher compression ratios and/or more advanced ignition timings, thereby improving combustion efficiency [4,5]. This directly contributes more efficient conversion of the fuel’s chemical energy into useful work [3,4,5].
- Oxygen content: Ethanol contains oxygen, contributing to a more complete combustion process, particularly under certain operating conditions [3,10]. This leads to better combustion efficiency and potentially higher BTE [3,4]. The flame quenching distance for E10 is greater than the flame quenching distance of E30; therefore, more of the introduced fuel can participate in the combustion process, thus increasing the fuel conversion rate and the BTE [20,21]. The combustion efficiency of any fuel is based on the work of the combustion divided by the mass and the heating value of the fuel [22]. The oxygen content within ethanol’s molecular structure also promotes a more complete combustion process, which is beneficial for achieving higher combustion efficiency and, subsequently, better thermal efficiency [1].
- BSFC of E30 is anticipated to increase due to its reduced lower heating value (LHV) compared to E10.
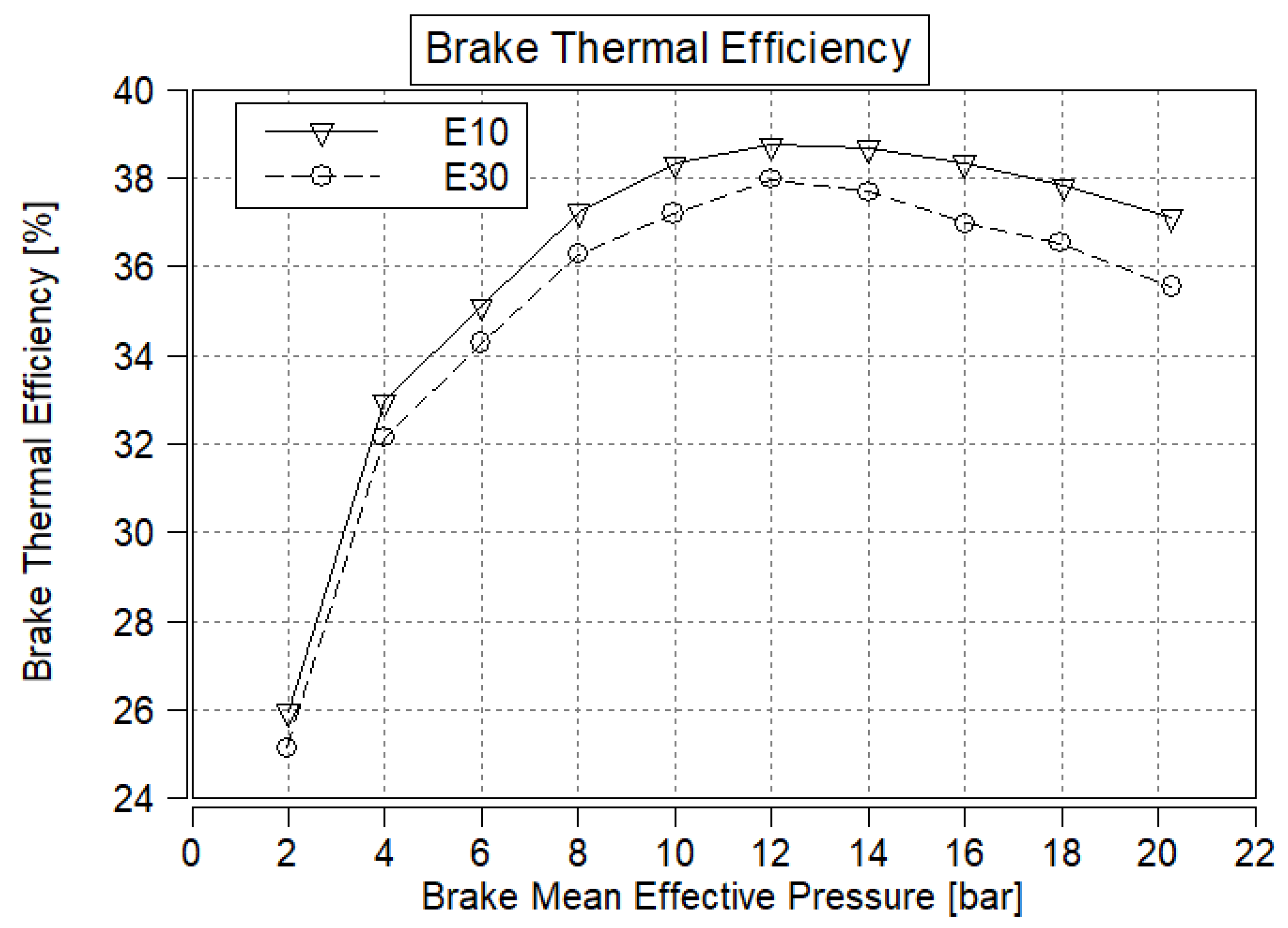
- BTE is not expected to significantly increase because the potential benefits of ethanol (such as enhanced anti-knocking performance and quicker combustion) cannot be fully utilized if the overall octane number is not higher than that of the reference fuel.
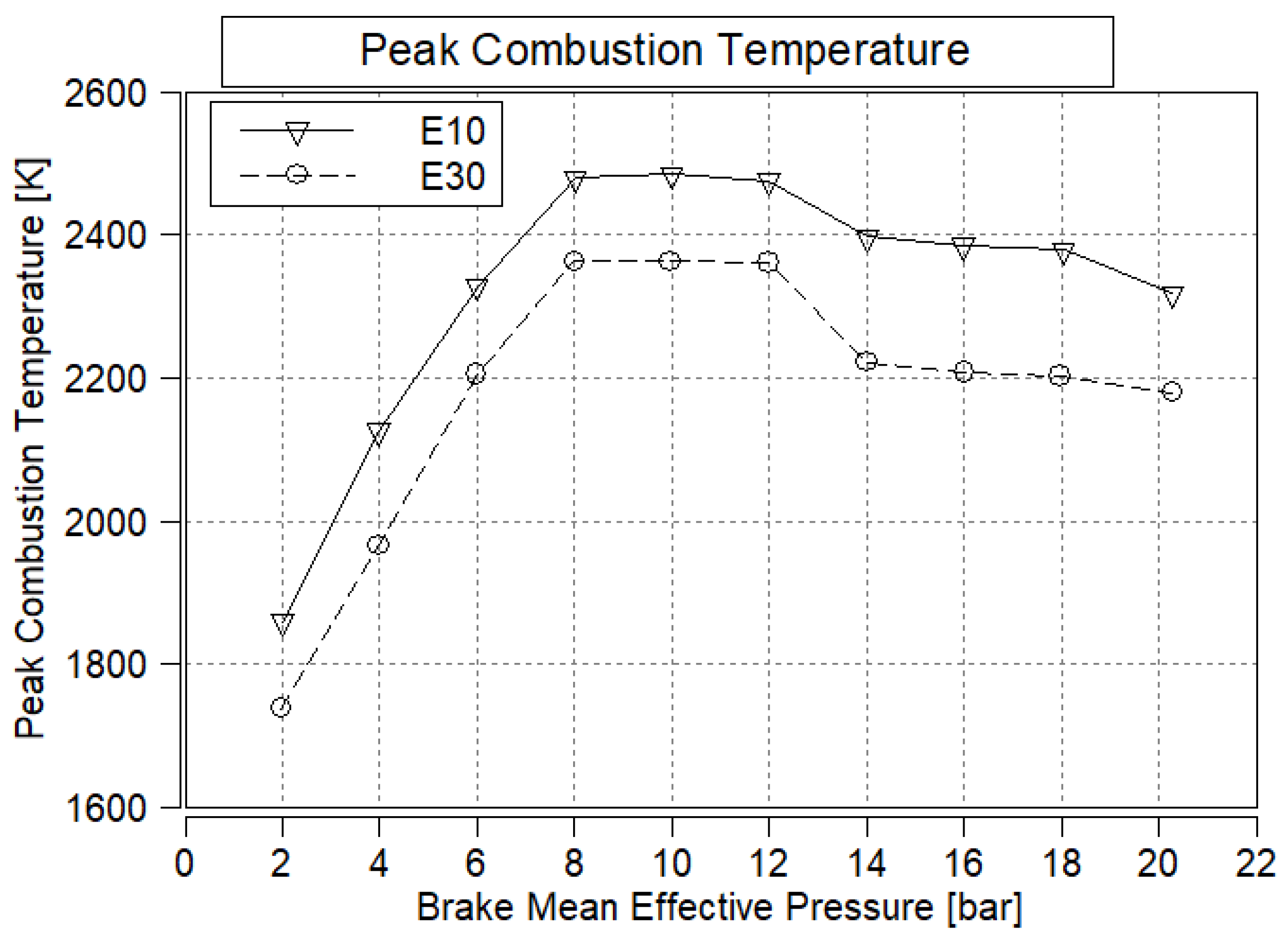
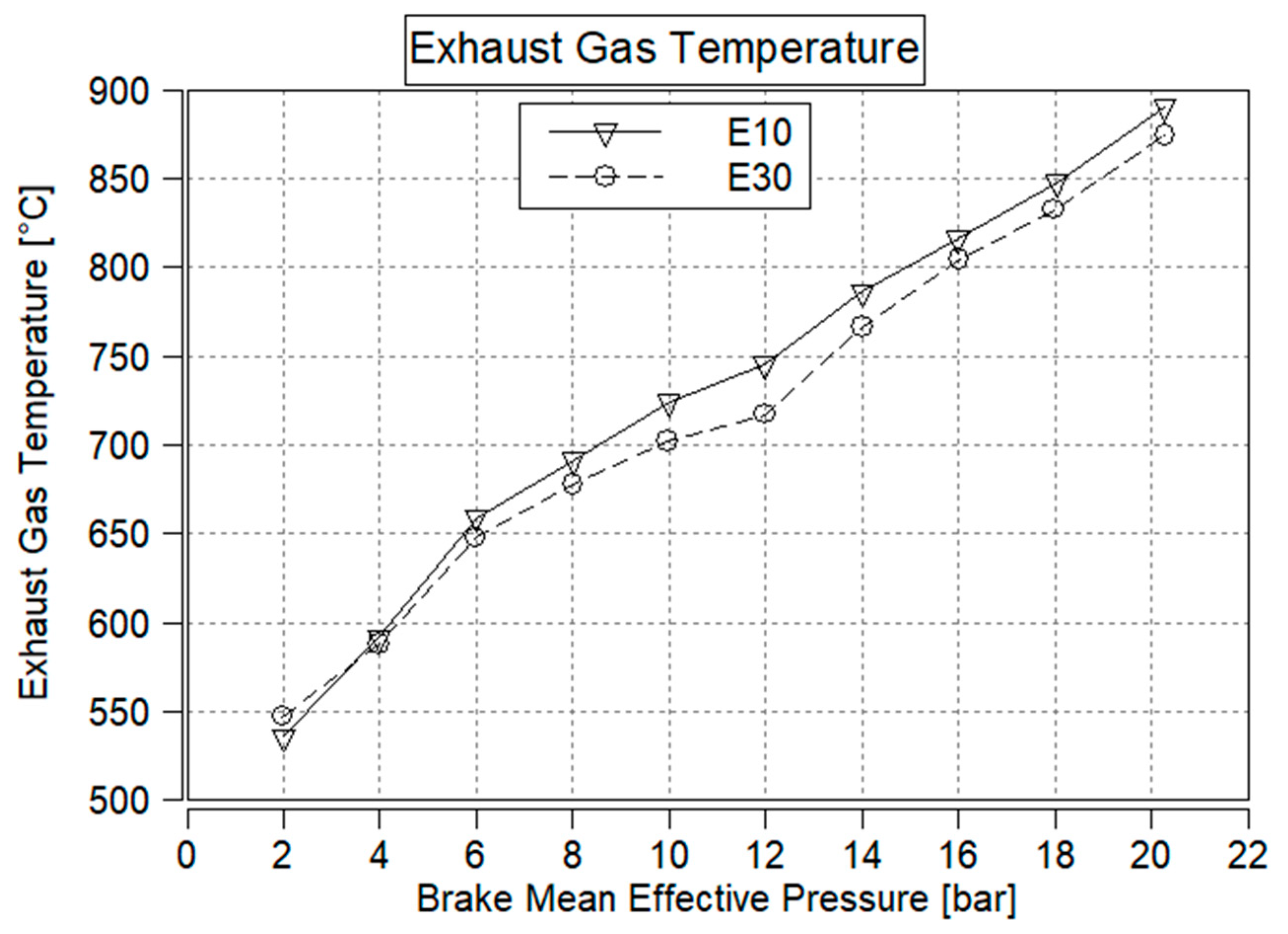
- EGT and peak combustion temperature are expected to decrease due to the higher latent heat of vaporization of ethanol, which effectively reduces the combustion temperature.
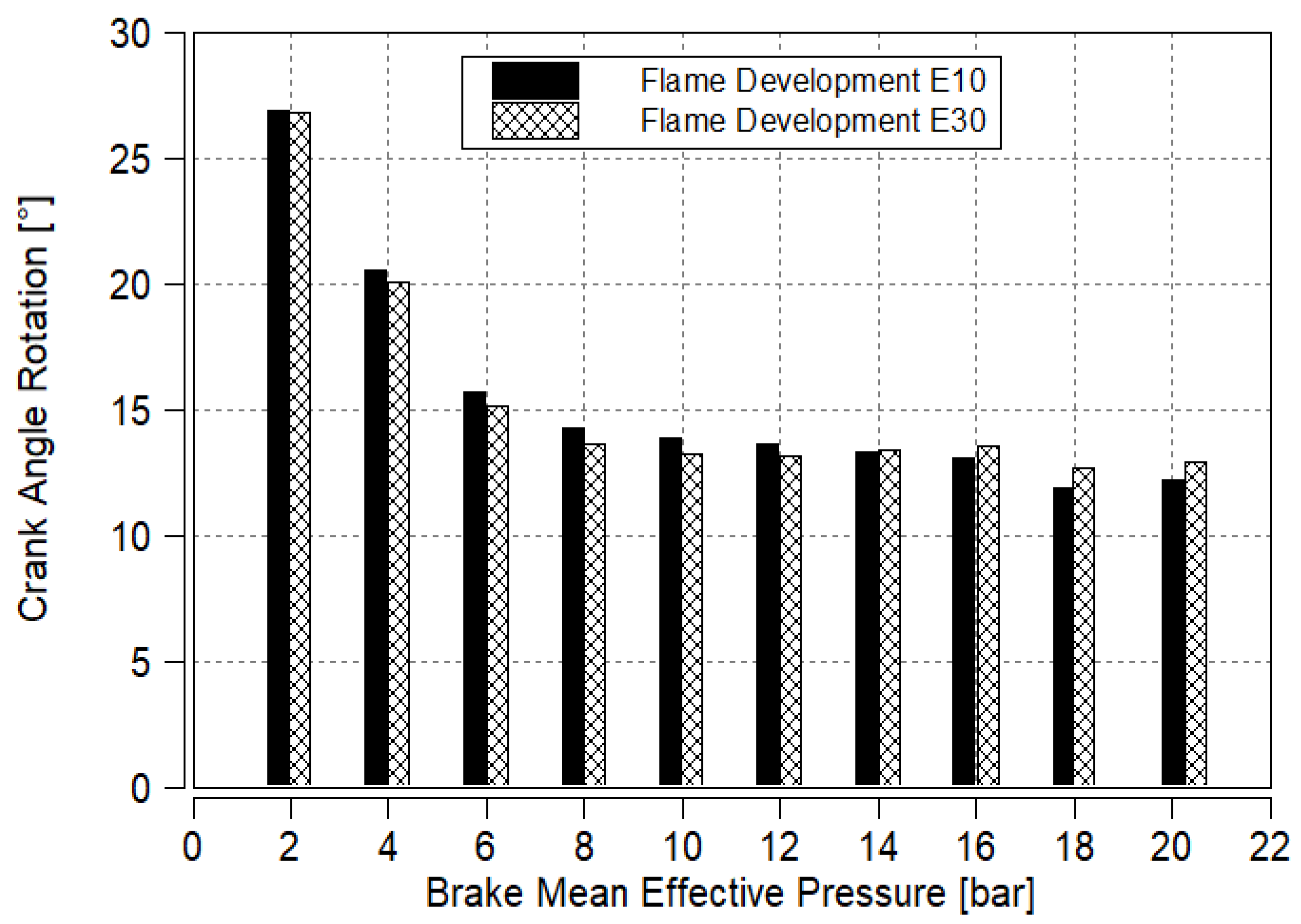
- A comprehensive combustion analysis will also be conducted to evaluate in-cylinder pressure, flame development duration and combustion duration, providing deeper insights into the combustion process of E30 compared to E10.
2. Materials and Methods
2.1. Measurement Methodology—Steady-State Measurement
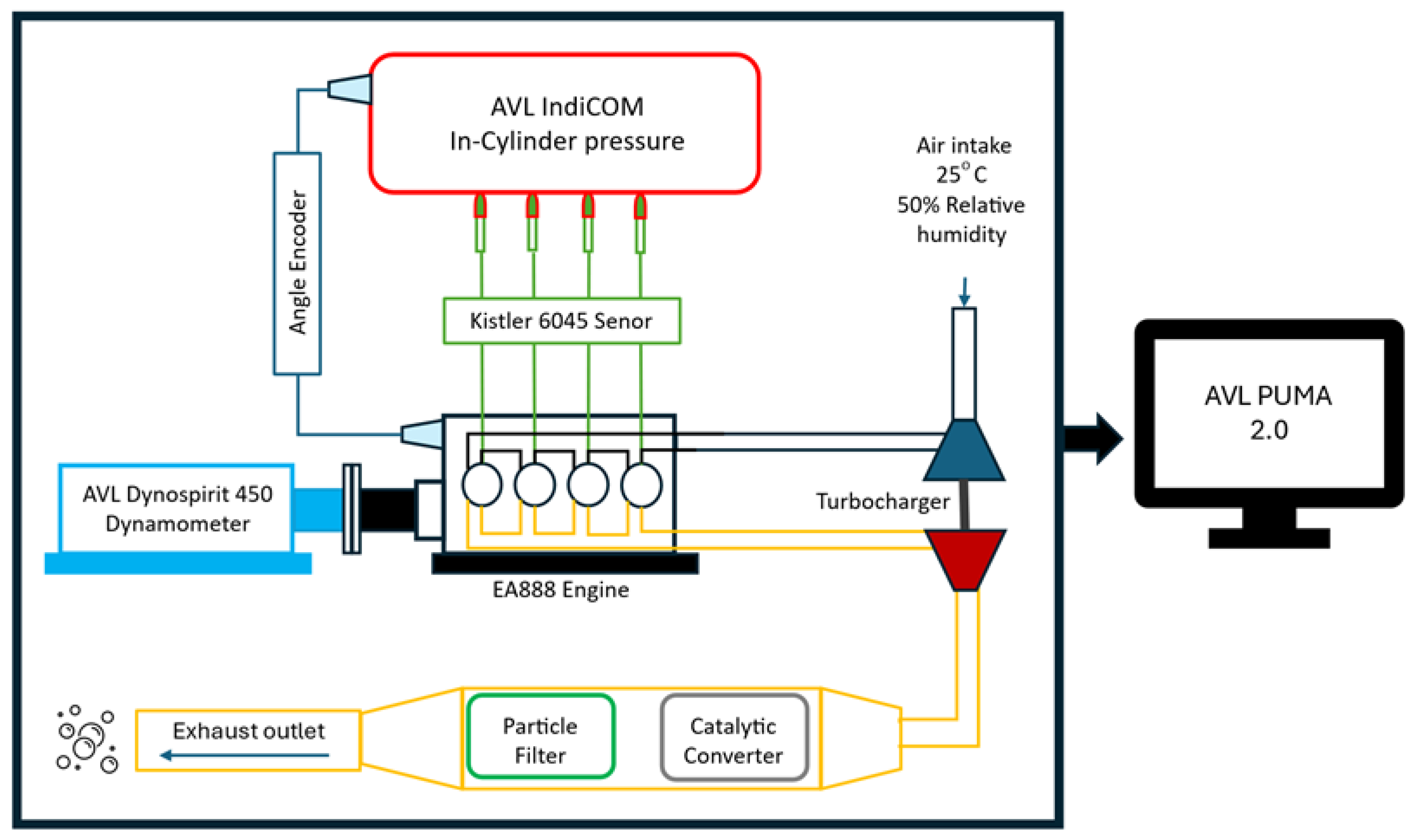
2.2. Experimental Setup
2.2.1. Test Case
2.2.2. Test Bench Properties and Measurement Environment
2.2.3. In-Cylinder Pressure Measurement
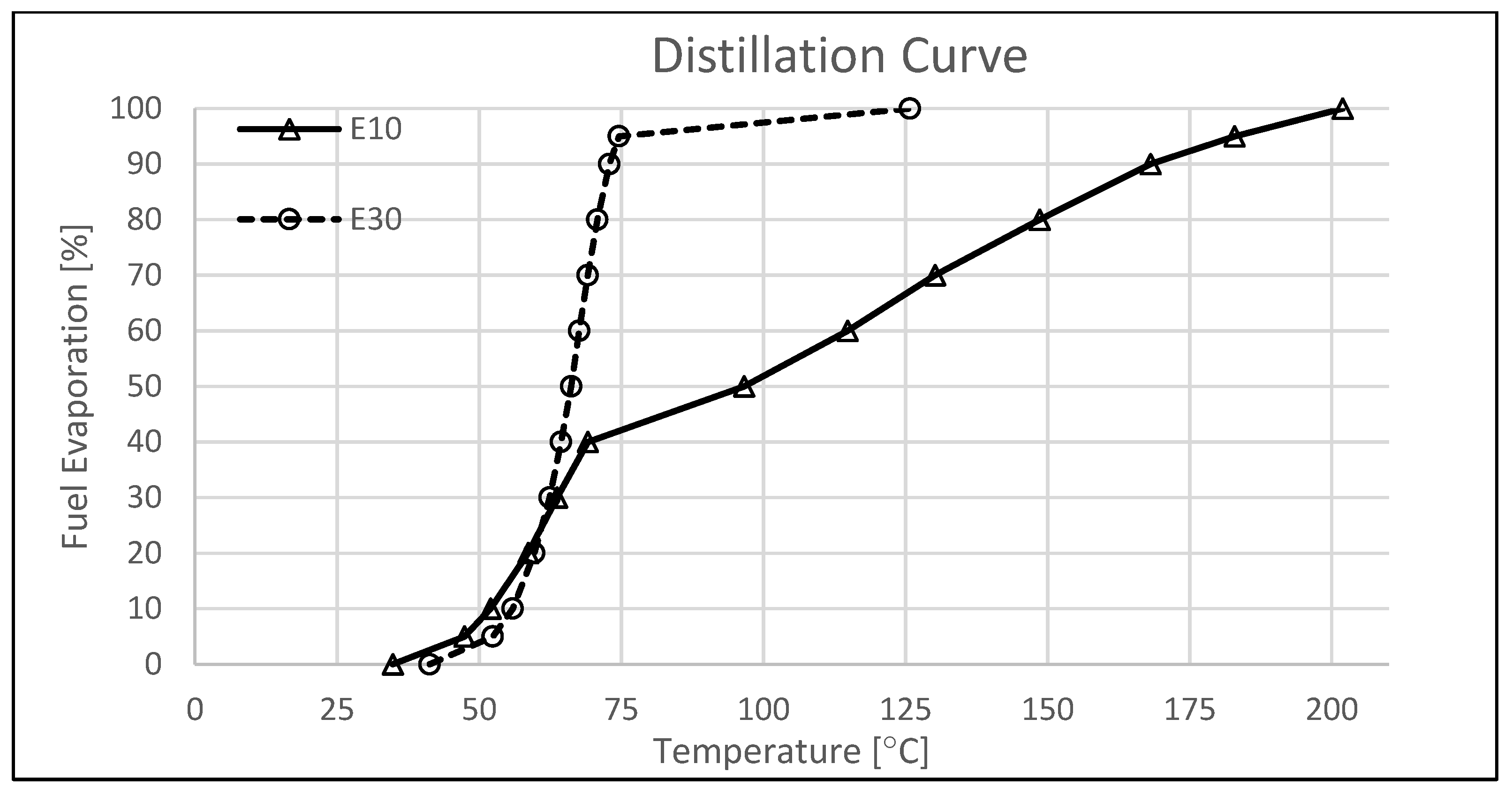
2.3. Fuels
3. Results
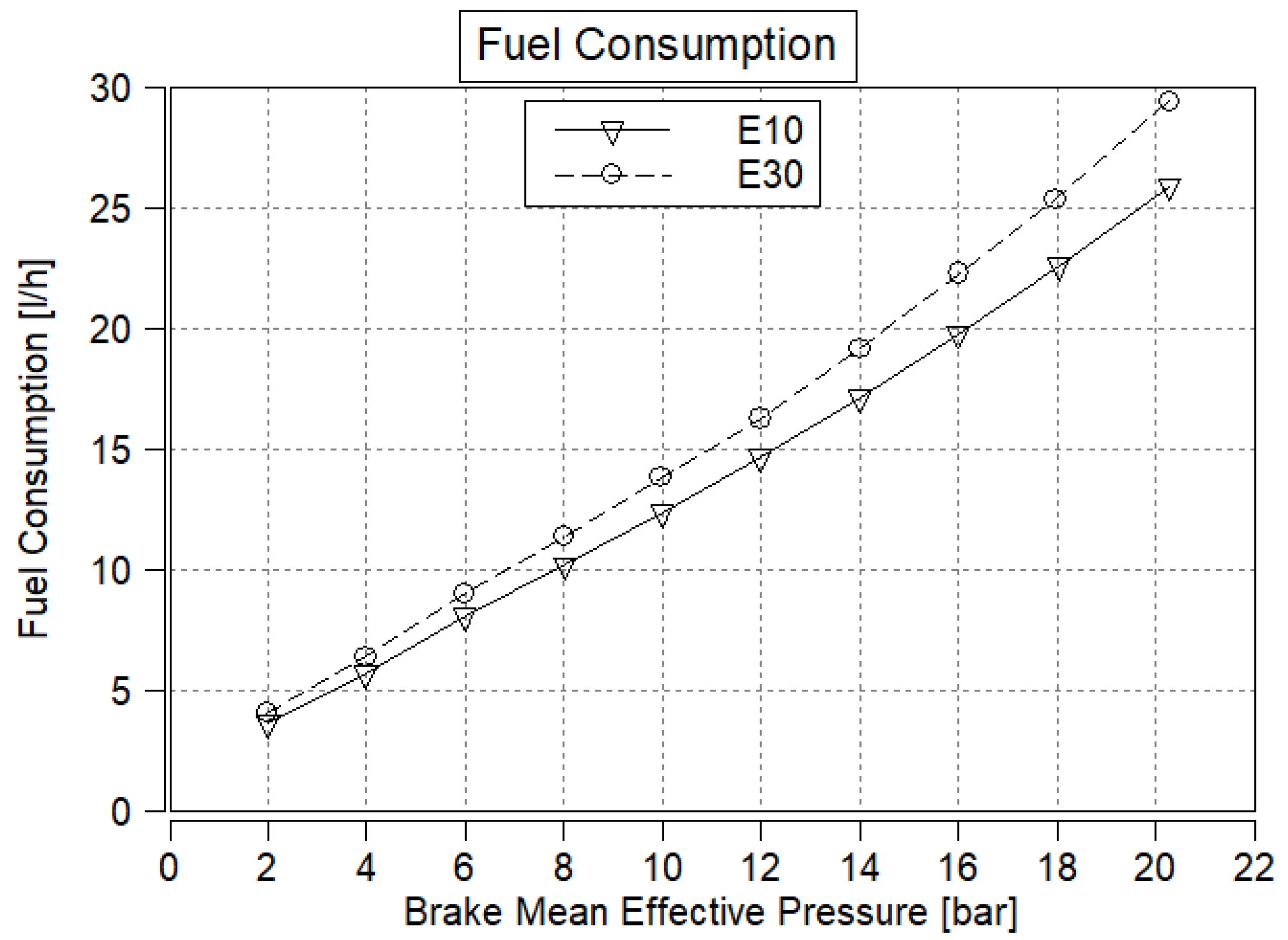
3.1. Fuel Consumption
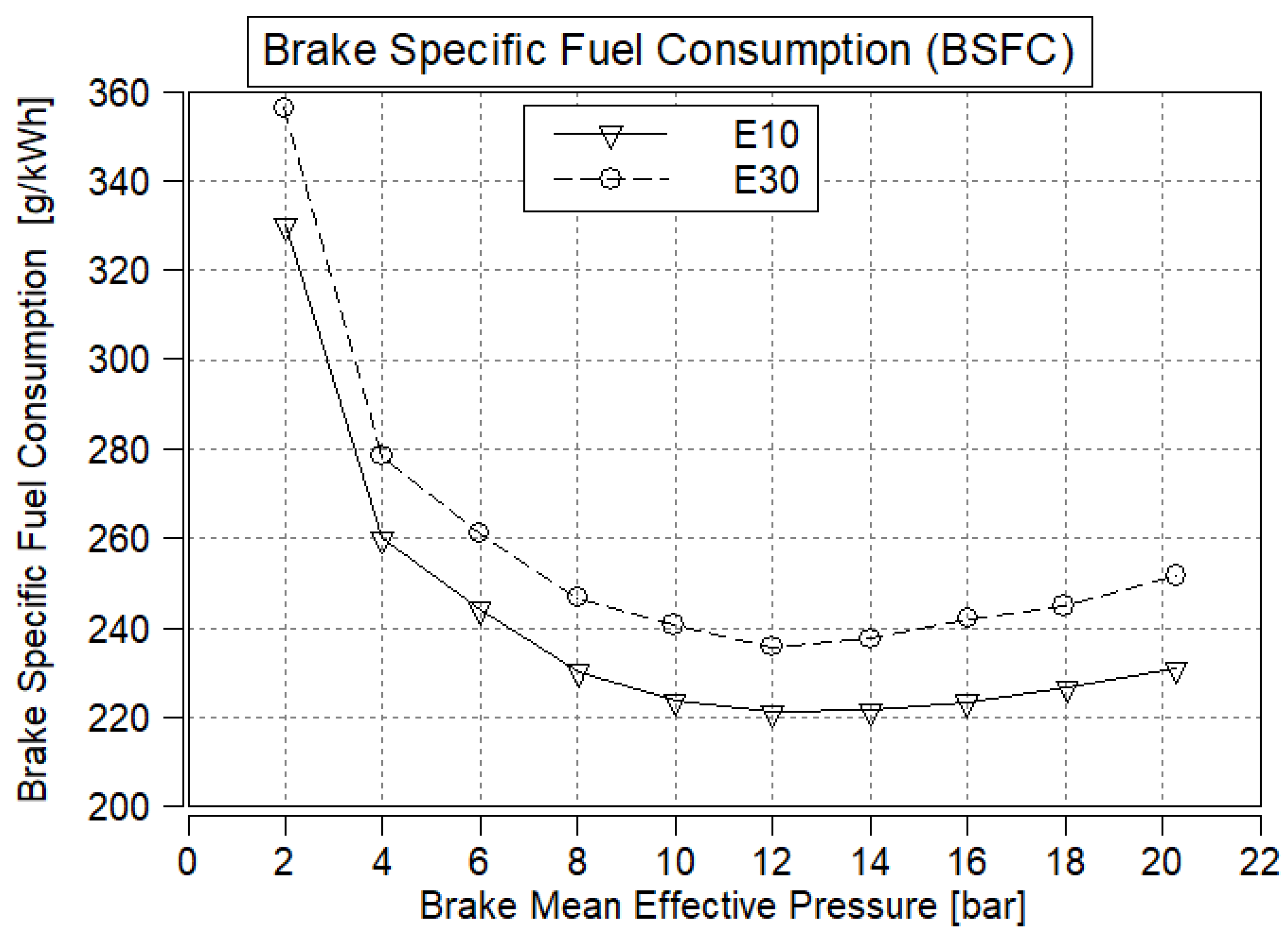
3.2. Brake-Specific Fuel Consumption
3.3. Brake Thermal Efficiency
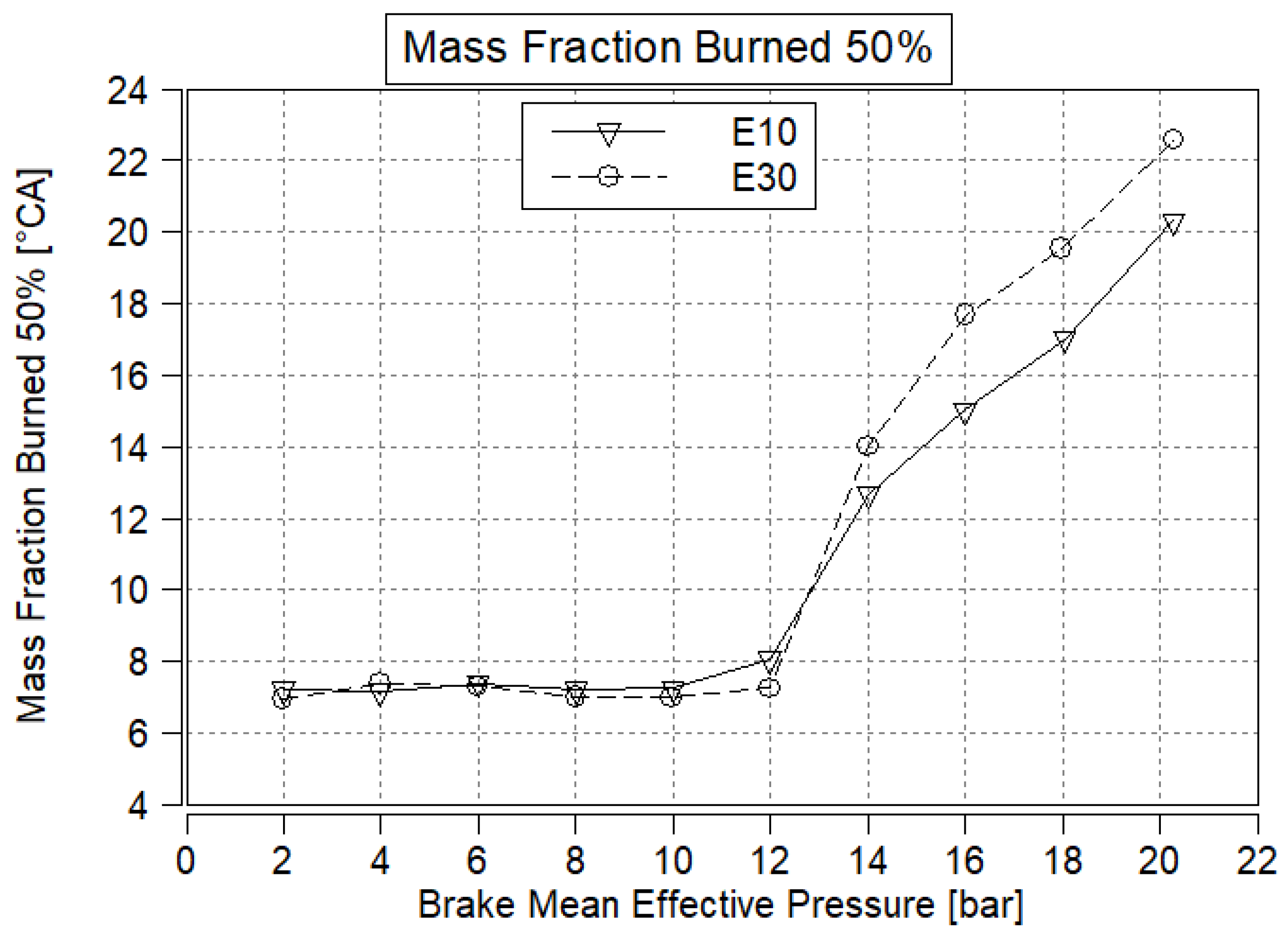
3.4. Mass Fraction Burned 50%
3.5. Flame Development
3.6. Rapid Burning Phase
3.7. Peak Combustion Pressure
3.8. Peak Combustion Temperature
3.9. Exhaust Gas Temperature
4. Conclusions
Future Research Directions
- Analyze the exhaust gas emissions of different ethanol–gasoline fuel blends under steady-state operating conditions to assess ethanol’s potential emission reduction effect in SI engines.
- Detailed combustion analysis under identical operating points to investigate cycle variation and rate of heat release.
- Measure fuel consumption and cumulated emission output under dynamic engine operations that resemble real driving conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFR | Air–Fuel Ratio |
| aTDC | After Top Dead Center |
| AVL | (Brand name, not spelled out, refers to AVL List GmbH) |
| BMEP | Brake Mean Effective Pressure |
| BSFC | Brake-Specific Fuel Consumption |
| BTE | Brake Thermal Efficiency |
| CA° | Crank Angle Degree |
| E10 | Gasoline with 10% ethanol by volume |
| E30 | Gasoline with 30% ethanol by volume |
| EGT | Exhaust Gas Temperature |
| HBM | (Brand name, not spelled out, refers to Hottinger Brüel & Kjær) |
| HCCI | Homogeneous Charge Compression Ignition |
| LHV | Lower Heating Value |
| MFB10 | Mass Fraction Burned 10% |
| MFB50 | Mass Fraction Burned 50% |
| MFB90 | Mass Fraction Burned 90% |
| MON | Motor Octane Number |
| RON | Research Octane Number |
| SI | Spark Ignition Engine |
References
- García, M.A.; Villalba, J.; Castaño, M.R.; Bailera, M. Performance and Emissions of Spark-Ignition Internal Combustion Engine Operating with Bioethanol-Gasoline Blends at High Altitudes Under Low- and High-Speed Conditions. Energies 2025, 18, 1401. [Google Scholar]
- Maurya, R.K.; Agarwal, A.K. Experimental study of combustion and emission characteristics of ethanol fuelled port injected homogeneous charge compression ignition (HCCI) combustion engine. Appl. Energy 2011, 88, 1169–1180. [Google Scholar] [CrossRef]
- Chansauria, P.; Mandloi, R.K. Effects of Ethanol Blends on Performance of Spark Ignition Engine—A Review. Mater. Today Proc. 2018, 5, 4066–4077. [Google Scholar]
- Doğan, B.; Erol, D.; Yaman, H.; Kodanli, E. The effect of ethanol-gasoline blends on performance and exhaust emissions of a spark ignition engine through exergy analysis. Appl. Therm. Eng. 2017, 120, 433–443. [Google Scholar] [CrossRef]
- Tibaquirá, J.E.; Huertas, J.I.; Ospine, S.; Quirama, L.F.; Nino, J.E. The Effect of Using Ethanol-Gasoline Blends on the Mechanical, Energy and Environmental Performance of In-Use Vehicles. Energies 2018, 11, 221. [Google Scholar]
- Goldember, J.; Coelho, S.T.; Guardabassi, P. The sustainability of ethanol production from sugarcane. Energy Policy 2008, 36, 2086–2097. [Google Scholar] [CrossRef]
- Schifter, I.; Diaz, L.; Rodriguez, R.; Gómez, J.P.; Gonzalez, U. Combustion and emission behaviour for ethanol-gasoline blends in a single cylinder engine. Fuel 2011, 90, 3586–3592. [Google Scholar]
- Johansen, L.C.R.; Hemdal, S.; Denbratt, I. Comparison of E10 and E85 spark ignited stratified combustion and soot formation. Fuel 2017, 205, 11–23. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, J.; Zhang, Z.; Zhong, W.; Zhao, Z.; Zhang, J. Effect of Different Ratios of Gasoline-Ethananol Blend Fuels on Combustion Enhancement and Emission Reduction in Electronic Fuel Injection Engine. Polymers 2023, 15, 3932. [Google Scholar] [CrossRef]
- Iodice, P.; Langella, G.; Amoresano, A. Ethanol in gasoline fuel blends: Effect on fuel consumption and engine out emissions of SI engines in cold operating conditions. Appl. Therm. Eng. 2018, 130, 1081–1089. [Google Scholar] [CrossRef]
- Anderson, J.E.; DiCicco, D.M.; Ginder, J.M.; Kramer, U.; Leone, T.G.; Rayne-Pablo, H.E.; Wallington, T.J. High octane number ethanol-gasoline blends: Quantifying the potential benefits in the United States. Fuel 2012, 97, 585–594. [Google Scholar]
- Al-Hasan, M. Effect of ethanol-unleaded gasoline blends on engine performance and exhaust emission. Energy Convers. Manag. 2003, 44, 1547–1561. [Google Scholar]
- Chhalotre, S.; Baredar, P.V.; Soni, S. Investigation of the Effects of Gasoline—Ethanol Fuel Blend on Throttle Response and Intake Air Temperature in a Naturally Aspirated S.I. Engine. Int. J. Mech. Prod. Eng. Res. Dev. 2018, 8, 445–454. [Google Scholar]
- Turner, J.W.G.; Lewis, A.G.J.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.C.P.; Edwards, P.P. Alcohol Fuels for Spark-Ignition Engines: Performance, Efficiency, and Emission Effects at Mid to High Blend Rates for Ternary Mixtures. Energies 2020, 13, 6390. [Google Scholar] [CrossRef]
- Graham, L.A.; Belisle, S.L.; Baas, C.-L. Emissions from light duty gasoline vehicles operating on low blend ethanol gasoline and E85. Atmos. Environ. 2008, 42, 4498–4516. [Google Scholar]
- Gumus, M.; Sayin, C.; Canakci, M. The impact of fuel injection pressure on the exhaust emissions of a direct injection diesel engine fueled with biodiesel-diesel fuel blends. Fuel 2012, 95, 486–494. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, D.; Huang, Z. Spray properties of alternative fuels: A comparative analysis of ethanol-gasoline blends and gasoline. Fuel 2007, 86, 1645–1650. [Google Scholar] [CrossRef]
- Phuangwongtrakul, S.; Wechsatol, W.; Sethaput, T.; Suktang, K.; Wongwises, S. Experimental study on sparking ignition engine performance for optimal mixing ratio of ethanol-gasoline blended fuels. Appl. Therm. Eng. 2016, 100, 869–879. [Google Scholar]
- Turner, D.; Xu, H.; Cracknell, R.F.; Natarajan, V.; Chen, X. Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine. Fuel 2011, 90, 1999–2006. [Google Scholar] [CrossRef]
- Suckart, D.; Linse, D.; Schutting, E.; Eichlseder, H. Experimental and simulative investigation of flame–wall interactions and quenching in spark–ignition engines. Automot. Engine Technol. 2017, 2, 25–38. [Google Scholar]
- Yang, Q.; Liu, Z.; Hou, X.; Sjörberg, M.; Villeumier, D.; Liu, C.; Liu, F. Measurement of laminar flame speeds and flame instability analysis of E30-air premixed flames at elevated temperatures and pressures. Fuel 2020, 259, 116223. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 1st ed.; McGraw-Hill, Inc.: New York, NY, USA; St. Louis, MO, USA; San Francisco, CA, USA; Auckland, New Zealand, 1988; p. 85. [Google Scholar]
- Szybist, J.P.; Busch, S.; McCormick, R.L.; Pihl, J.A.; Splitter, D.A.; Ratcliff, M.A.; Kolodziej, C.P.; Storey, J.M.E.; Moses-DeBusk, M.; Vuilleumier, D.; et al. What fuel properties enable higher thermal efficiency in spark-ignited engines? Prog. Energy Combust. Sci. 2021, 82, 100876. [Google Scholar] [CrossRef]
- Farooq, S.; Kumar, D.V. Experimental Study on Performance, Emissions and Combustion Characteristics of PFI Spark Ignition Engine Fueled with E30 Equivalent Binary and Ternary GEM Blends. INCAS Bull. 2020, 12, 101–112. [Google Scholar] [CrossRef]
- Rimkus, A.; Pukalskas, S.; Mejeras, G.; Nagurnas, S. Impact of Bioethanol Concentration in Gasoline on SI Engine Sustainability. Sustainability 2024, 16, 2397. [Google Scholar] [CrossRef]
- Schifter, I.; Diaz, L.; Gómez, J.P.; Gonzalez, U. Combustion characterization in a single cylinder engine with mid-levels hydrated ethanol-gasoline blended fuels. Fuel 2013, 103, 292–298. [Google Scholar] [CrossRef]
- Yeliana, W.J.; Michalek, D.; Naber, J. Property Determination For Ethanol-Gasoline Blends With Application to Mass Fraction Burn Analysis In a Spark Ignition Engine. J. KONES Powertrain Transp. 2008, 15, 553–561. [Google Scholar]
- Morey, F.; Seers, P. Comparison of cycle-by-cycle variation of measured exhaust-gas temperature and in-cylinder pressure measurements. Appl. Therm. Eng. 2010, 30, 487–491. [Google Scholar] [CrossRef]
- Pandey, V.; Gupta, V.K. Technical Assessment of Performance & Emission Characteristics of an SI Engine using Ethanol-Gasoline Blended Fuel. Int. J. Eng. Res. Technol. 2016, 5, 422–426. [Google Scholar]
- Doğan, B.; Yeşilyurt, M.K.; Erol, D.; Çakmak, A. A Study Toward Analyzing the Energy, Exergy and Sustainability Index Based on Performance and Exhaust Emission Characteristics of a Spark-Ignition Engine Fueled with the Binary Blends of Gasoline and Methanol or Ethanol. Int. J. Eng. Res. Dev. 2020, 12, 529–548. [Google Scholar] [CrossRef]
- EN 228:2025; Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2025.
- Hellier, P.; Talibi, M.; Eveleigh, A.; Ladommatos, N. An overview of the effects of fuel molecular structure on the combustion and emission characteristics of compression ignition engines. J. Automob. Eng. 2017, 232, 90–105. [Google Scholar] [CrossRef]



| Specification | Details |
|---|---|
| Configuration | Inline 4-cylinder (Audi Hungaria Zrt., Győr, Hungary) |
| Displacement | 1984 cm3 |
| Valvetrain | 16 valves, dual overhead camshaft, variable valve lift and timing |
| Fuel System | Direct injection |
| Aspiration | Turbocharged with electronically controlled wastegate |
| Bore | 82.5 mm |
| Stroke | 92.8 mm |
| Compression ratio | 12.2:1 |
| Maximum power output | 150 kW @ 5000–6000 rpm |
| Maximum torque | 320 Nm @ 2000–4000 rpm |
| Test cell temperature | 22 °C |
| Intake air temperature | 25 °C |
| Intake air relative humidity | 50% |
| Charge air temperature after the intercooler | 30 °C |
| Coolant temperature at the inlet to the engine | 60 °C |
| Property | E10 | E30 |
|---|---|---|
| Research octane number [-] | 96.5 | 93.9 |
| Motor octane number [-] | 85.1 | 85.0 |
| Density [kg/m3] | 753.6 | 722.6 |
| Lower heating value [MJ/kg] | 42.04 | 40.23 |
| Stoichiometric air–fuel ratio [-] | 14.02 | 13.04 |
| Calorific value of stoichiometric mixture [MJ/kg] | 2.80 | 2.87 |
| Carbon content [m/m%] | 83.27 | 73.50 |
| Hydrogen content [m/m%] | 13.32 | 14.79 |
| Oxygen content [m/m%] | 3.41 | 11.70 |
| Ethanol content [v/v%] | 8.9 | 30.6 |
| Olefin content [v/v%] | 13.9 | 0.1 |
| Aromatic content [v/v%] | 29.4 | 1.6 |
| Initial boiling point [°C] | 34.8 | 41.3 |
| Final boiling point [°C] | 201.9 | 125.8 |
| Vapor pressure [kPa] | 63.0 | 57.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, Á.I.; Mursi, Z.T.; Wégerer, A.; Nagy, G. Comprehensive Efficiency Analysis of Ethanol–Gasoline Blends in Spark Ignition Engines. Eng 2025, 6, 256. https://doi.org/10.3390/eng6100256
Szabó ÁI, Mursi ZT, Wégerer A, Nagy G. Comprehensive Efficiency Analysis of Ethanol–Gasoline Blends in Spark Ignition Engines. Eng. 2025; 6(10):256. https://doi.org/10.3390/eng6100256
Chicago/Turabian StyleSzabó, Ádám István, Zaid Tharwat Mursi, Anna Wégerer, and Gábor Nagy. 2025. "Comprehensive Efficiency Analysis of Ethanol–Gasoline Blends in Spark Ignition Engines" Eng 6, no. 10: 256. https://doi.org/10.3390/eng6100256
APA StyleSzabó, Á. I., Mursi, Z. T., Wégerer, A., & Nagy, G. (2025). Comprehensive Efficiency Analysis of Ethanol–Gasoline Blends in Spark Ignition Engines. Eng, 6(10), 256. https://doi.org/10.3390/eng6100256









