Abstract
Due to the environmental impact of using fossil fuels, alternatives for the generation of biofuels are being studied. An option for this problem is to obtain biodiesel from recycled vegetable oil. Studies show that basic homogeneous catalysis has advantages such as speed over other types of catalysis. However, most of these studies are conducted with unused or little-used oils. Therefore, this study aims to obtain biodiesel from recycled vegetable oil collected from municipal oil collection centers by transesterification applying NaOH or KOH as catalysts. The used oil was filtered, washed, and dried to remove impurities. The transesterification reaction catalyzed with NaOH and KOH was carried out; each catalyst was tested at two concentrations: 0.5% and 1% w/w. All reactions were carried out at 55 °C, 350 rpm, methanol, and alcohol/oil ratio of 6/1 for 1.5 h. The best yield was found with the KOH with a concentration of 0.5%. The biodiesel obtained presented the following properties: density of 0.8807 g/mL, a viscosity of 4.694 mm2/s, an acid number of 0.355 mg KOH/g, and corrosion 1a, a calorific value of 39,726 J/g, and a FAME of 93%.
1. Introduction
Nowadays, fossil fuels and nuclear power provide 90% of the energy used each year globally [1]. However, according to the British energy company BP, world reserves in 2020 will be enough to supply 50 years of demand according to consumption and production patterns; this is an indication that this resource is limited. In addition, studies have detected that the areas where oil and natural gas are extracted are affected, giving rise to environmental problems [2].
In recent years, the use of waste has gained importance as an energy source, because it is renewable and respects the environmental approach [1]. This is how the alternative of obtaining biodiesel from recycled vegetable oil was born. Using used oil enters a philosophy of integrating waste into the productive and economic cycle [3]. This type of waste has a high content of different fatty acids, making it suitable for being treated in a transesterification process. Due to the massive consumption of oils, it is a residue that is readily available. This is the case in Ecuador, where annually, 9.45 million gallons of used vegetable oil are discarded [4]. In addition, another advantage of using used oil as a raw material is that it provides an alternative to waste that is often disposed of inappropriately due to the lack of options for use. In fact, it is common for used oil to be disposed of in drains, rivers, and on the ground, among others, without prior treatment [5].
Transesterification is an alcoholysis reaction since fat or oil intervenes with short-chain alcohol to produce esters and glycerol in the presence of a catalyst to accelerate the reaction rate and improve yield [6,7]. This reaction consists of three consecutive and reversible stages, transforming triglycerides into diglycerides, monoglycerides, and glycerin [8]. The process is shown in Figure 1.
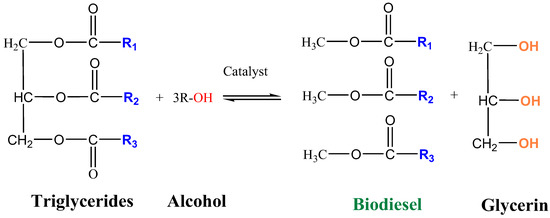
Figure 1.
Transesterification reaction.
Generally, this reaction can occur through basic, acidic, and enzymatic catalysis. Acid catalysis is slower, with a mean time of 4 h for a yield of ~99% [9]. This has the advantage of being more suitable for glycerol, which possesses free fatty acids and a higher aqueous content, thus preventing the saponification reaction [10] from occurring. On the other hand, the use of enzymatic catalysts at room temperature has the drawback of reaction time, with a mean of 104 h for an efficiency of ~95% [9]. It also presents problems regarding separation and reuse, making the process very expensive [11]. Ahead of these two catalysts are basic catalysts, which can achieve a high biodiesel production (~99% yield) in a short time (mean of 2 h). However, the main disadvantage is that a saponification reaction can occur.
Basic catalysis is less corrosive and can be up to 400 times faster than acid catalysis, although glycerols and alcohols must be anhydrous to avoid saponification reactions, which decrease yield [10]. In addition, basic catalysis can be carried out under moderate pressure and temperature conditions [12]. The alcohols commonly used to produce biodiesel are ethanol and methanol, with the latter being the most used. This is due to its physicochemical properties, so it is possible to work with a lower amount of alcohol, lower temperature, and reaction time, obtaining a higher conversion efficiency [13]. The catalysts that are commonly used in the homogeneous basic transesterification process are sodium and potassium methoxide and hydroxide, with the latter being more soluble in methanol, facilitating the transesterification reaction [14].
Table 1 presents the studies starting from recycled vegetable oil used in cooking. The most widely used catalyst is KOH. In the case of the concentration, this ranges between 0.5 and 1% w/w; in most cases, the reaction temperature is 60 °C. The yields vary from 79% to 99%.

Table 1.
Conditions of the transesterification reaction with vegetable oil used in cooking.
The use of biodiesel has the advantage of reducing CO2 emissions by 78% compared to petroleum diesel. With respect to degradability, it is considered highly degradable, as it is similar to dextrose [22]. However, regarding mechanical performance, the amount of oxygen it contains causes greater fuel consumption. Another of the main disadvantages is the low stability to oxidation stabilization, which causes rapid degradation, with a maximum storage time of 6 months.
Despite this subject having been widely studied, the biodiesel is generally made with used oil obtained from a kitchen, which does not represent what can be found in collection centers. Therefore, this study has the purpose of obtaining biodiesel from recycled vegetable oil provided by a municipal company in Cuenca, ETAPA EP [23], using two types of basic catalysts at different concentrations to optimize the transesterification reaction.
2. Materials and Methods
2.1. Pretreatment and Characterization of Recycled Vegetable Oil
The raw material use to obtain biodiesel was recycled vegetable oil, which was collected by ETAPA from different collection points in Cuenca. Then, it was transferred to the Ucubamba wastewater plant, where it underwent separation treatment by density between solid material and oil. Finally, it was deposited in a storage tank, from which 6 L were withdrawn for this investigation. To remove solid residues and moisture from the oil, it was filtered with Whatman No. 40 filter paper. Then, it was washed with water at 40 °C, and finally decanted at 110 °C for 4 h.
Once the pretreatment of the recycled vegetable oil had been carried out, the main physicochemical characteristics were determined: the density by means of the pycnometer method at 25 °C, based on the standard NTE INEN 35 (2012) [24]. The percentage of fatty acids and free acidity was determined by dissolving the sample in a 95% (v/v) solution of diethyl ether and ethanol in a 1:1 ratio with phenolphthalein as an indicator, and its assessment was performed using a 0.1 N sodium hydroxide solution, based on the standard NTE INEN 38 (1973) [25]. The humidity was determined by working with 5 g of sample in an oven at 103 ± 2 °C. First, the sample is heated for 1 h and cooled; then, the time was reduced to 30 min in the oven until the difference between the results of two successive weighing operations did not exceed 0.002 g. This process is based on the standard NTE INEN 39 (1973) [26]. Finally, the determination of the kinematic viscosity was carried out by applying the glass capillary viscometer method at 40 °C, according to the standard ASTM D445 (2021) [27]. To determine FAMEs of recycled vegetable oil and biodiesel, we worked with the Agilent Technologies 6890 N network CG systems gas chromatograph, with an injector at 250 °C, Split 40:1, injection volume of one . The column was an Aplent CP7487 (60 m × 250 μm × 2 μm). The oven conditions were: 120 °C (4 min), 5 °C to 230 °C, 230 °C (10 min). The detector temperature was 275 °C. The N2 gas temperature was 175 °C with a flow of 0.6 .
2.2. Transesterification Reaction with Base Catalysis
Alkaline methoxide was prepared by mixing methanol with a basic catalyst. Methoxide was ready for each catalyst, NaOH or KOH, for each concentration: 0.5% and 1% w/w about the weight of the oil with methanol at 30 °C. For the transesterification reaction, the recycled vegetable oil was heated to 30 °C, and the alkaline methoxide was added, maintaining an alcohol/oil ratio of 6/1. The mixture was stirred at 350 rpm at 55 °C for 1.5 h. Each assay was performed in triplicate, and a blank was made without a catalyst. Once the reaction was finished, the product was decanted for 1 h, during which the formation of 2 phases was observed: glycerin and biodiesel. These phases were separated, and the biodiesel obtained was washed with plenty of water and stirred for 5 min to remove traces of the catalyst, glycerin, or soap that could be formed during the reaction. Washing was carried out until a pH of approximately 7 was reached. Subsequently, the biodiesel was separated from the water and heated in an oven at 110 °C for 4 h. Finally, it was filtered through Whatman No. 40 paper.
2.3. Biodiesel Characterization
The properties of density, kinematic viscosity, and acidity index of biodiesel were determined by the standards already mentioned in Section 2.1 for recycled vegetable oil. To obtain the calorific power, the calorimetric bomb (IKA C200) was used, in which 0.5 g of sample was worked. In the case of corrosion to copper, this was determined with the method described in the standard ASTM D130 (2019) [28]. Finally, the biodiesel yield percentage was obtained from the final volume obtained compared to the volume of used recycled vegetable oil.
2.4. Statistical Analysis
An experimental design was used that consisted of varying the type of catalyst and the concentration of the respective catalyst at two levels. Once the normality and homoscedasticity of the data had been verified, an ANOVA analysis was performed to determine if there were statistically significant differences between yields and the different characteristics at a significance level of 5%. In cases where the differences were significant, a post hoc analysis was performed. For these analyses, R and R-Studio were used.
3. Results and Discussion
3.1. Characteristics of Recycled Vegetable Oil
The initial color of the recycled vegetable oil was dark brown, while the odor was sui generis. Once the pre-treatment of the raw material was carried out, a light brown color was obtained, but the smell remained.
In Table 2 the physical characteristics of the recycled vegetable oil are shown. The density of 0.942 ± 0.018 of the recycled vegetable oil was higher than that described in other research [29,30]. This is because the other investigations used unused oil or domestic used oil, while in this study, used oil from different sources was used, including domestic oil reused several times and oil used in restaurants, among others. Regarding the percentage of free fatty acids, a value of 0.311 ± 0.003% was obtained. This value is below the 4% maximum recommended value [31]. Therefore, the sample complies with and can be subjected to a transesterification process. On the other hand, the free acidity was higher than that indicated in NTE INEN 34 [32] for blends of edible vegetable oils, which is an indication that this oil has undergone various frying processes. The value was less than 0.5%, the maximum recommended value, since higher values generate problems in transesterification [33]. For kinematic viscosity, a value of 39.870 ± 0.010 was reported, which is lower than the values reported in other studies [34,35].

Table 2.
Physical characterization of recycled vegetable oil.
The components were obtained by running a standard sample. The components that were found in the highest percentages were oleic acid (C18:1), linoleic acid (C18:2), and palmitic acid, with 30.233%, 26.286%, and 19.152%, respectively, as can be seen in Table 3. The results registered indicate that these fatty acids are found in greater quantities in edible vegetable oils, with palm oil, corn, sunflower, soybean, and lard being the most frequently consumed [36]. Therefore, this real source of oil includes animal fat.

Table 3.
Chromatograph results: recycled vegetable oil.
3.2. Characteristics of the Biodiesel Obtained
All the parameters studied in the biodiesel presented normality and homoscedasticity. According to the ANOVA results, no statistically significant differences were found, p > 0.05, in the density, calorific power, kinematic viscosity, acidity index, and percentage of FAMEs of the different biodiesel obtained with the catalysts and concentrations studied. All the values that obtained in each of the experiments are presented in Table S1. In a synthesized way, the characteristics of biodiesel are presented in Table 4.

Table 4.
Biodiesel physicochemical characteristics: NaOH and KOH with different concentrations.
The standard that governs the quality of biodiesel in Ecuador is the standard NTE INEN 2482 [37]. Meanwhile, at the international level, biodiesel is governed by the norms ASTM D6751 [38] and EN14214 [39]. The average density of biodiesel was obtained as 0.8798 ± 0.0011 , which is a value similar to that of other researchers [29,30]. The mean density values are all within the ranges established by the standards.
Biodiesel generally has a lower calorific value than conventional diesel, with values that vary between 39.6 and 39.9 [40]. While conventional diesel obtained with unused oil has a value of 43 . In this study, the mean calorific value was 39.748 ± 0.1727 . Other studies using used oil also had lower values, close to the value in this study [40,41]. The reason for the lower calorific value is that the biodiesel produced has a lower degree of unsaturation and a lower number of carbons than diesel [42]. This unsaturation increases when the source is used oil.
The decrease in the viscosity value makes it possible to confirm the conversion of vegetable oil to esters [43]. In this case, a decrease was observed, since the viscosity of the raw material was 39.870 , and the value obtained from biodiesel was 4.682 ± 0.0580 . It is stated that the viscosity of the product meets and is within the ranges established by the regulations.
The acid number in biodiesel measures the free fatty acids and their degradation. In turn, the average value of this property in biodiesel obtained from recycled cooking oil is 0.345 [31]. In this case, a value very close to that reported was obtained, since the acidity index was 0.350 ± 0.120 . On the other hand, the standards establish the maximum value of acidity that biodiesel can have. According to the Ecuadorian standard NTE INEN 2482 [37] and the European standard EN 14214 [39], the maximum value of the acid value is 0.5 . Meanwhile, the ASTM D6751 [38] standard allows a maximum value of 0.8 . Taking these limits into account, the acidity index obtained is below the value established by the different standards.
The method used to determine copper corrosion was based on the ASTM D130 [28] standard. The result was obtained by comparing the color of each copper strip with the color scale given by the same standard. In this case, all the samples presented the same value on the scale. Therefore, it was not analyzed statistically. The results are presented visually in Figure 2.

Figure 2.
(a) Corrosion to copper caused by biodiesel obtained with 0.5% NaOH; (b) corrosion to copper caused by biodiesel obtained with 1% NaOH; (c) corrosion to copper caused by biodiesel obtained with 0.5% KOH; (d) corrosion to copper caused by biodiesel obtained with 1% KOH.
In all of the study cases, the copper sheets presented slight tarnishing. This made it possible to determine the scale in which they were found, this being in “1a”. For this property, the NTE INEN 2482 and ASTM D6751 standards establish that the maximum degree of corrosion is 3 [37,38]. Meanwhile, standard EN 14214 establishes a maximum of 1 [39]. In the first case, the biodiesel obtained is below the limit; however, in the case of standard EN 14214, it is above the limit [39]. This indicates that the biodiesel obtained is not corrosive to engines.
For the purposes of analysis, the percentage of linolenic acid is a parameter that must be considered, and our determinations are presented in Table 5. As indicated by standard EN 14214 and NTE INEN 2482, the minimum total ester content is 96.5% and the maximum linolenic acid methyl ester content is 12% [37,39]. When analyzing the average of 92.53 ± 3.629% of the content of FAMEs present in the samples, a value lower than that requested was registered. The linolenic acid methyl ester content with an average of 2.535 ± 0.092% complied with the established norms. In the study of biodiesel production from used cooking oil, an ester content of 94.21% and 96.15% was obtained [21]; these values are higher than the average obtained in this study. These differences are due to the operating conditions that were applied in each investigation.

Table 5.
Percentage of C18:3 present in biodiesel.
Comparing the two concentrations of NaOH (0.5% and 1%) as a catalyst, it was found that the concentration of 0.5% gave a higher yield. When using KOH in two concentrations (0.5% and 1%), there was no statistically significant variation in its performance, since, in this case, yields of 93.19 ± 1.669% and 93.31 ± 3.022% were obtained.
On the other hand, statistically significant differences were found in the performance between the different types of catalyst and concentration, p (5.42 × 10−5) < 0.00, wherein the post hoc with Holm adjustment method demonstrated that there were significant differences between the following yields: (1) KOH and NaOH at 0.5% (p = 0.008) with KOH was the one with the highest yield. (2) In the case of 0.5% KOH and 1% NaOH (p = 0.00012), the yields obtained were 85.96 ± 2.115% and 78.18% ± 0.934 for concentrations of 0.5% and 1% NaOH, respectively. (3) Then, 1% KOH and 0.5% NaOH (p = 0.008). (4) KOH and 1% NaOH (p = 0.00012). KOH is, in all cases, the one with the highest yield. This is because KOH presents a higher solubility in methanol than NaOH. The ANOVA results for each of the properties are shown in Table S2.
The yield obtained when using 1% NaOH as a catalyst is lower than that reported in similar research, where a yield of 93.75% was achieved [14]. Results with 0.5 NaOH were also lower than those reported in other studies. However, the results obtained with 1% KOH were quite similar to those found in similar studies [17]. When using 0.5% KOH, the yield was 93.19 ± 3.022%, a value higher than the yields reported in research that used the same catalyst and concentration [7]. This could be because, in this study, oil was used that was made up of a mixture of several highly saturated sources. In addition, variables such as temperature, reaction time, agitation, alcohol/oil ratio, and even the type of raw material can affect biodiesel production yield.
In Figure 3, the process diagram is presented using the catalyst that gave the best result.
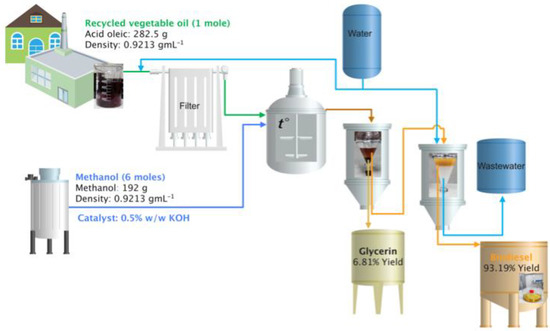
Figure 3.
Process diagram.
4. Conclusions
The physicochemical properties of the recycled vegetable oil, after pretreatment, were: density of 0.942 ± 0.018 , 0.311 ± 0.003% of free fatty acids, acid value of 0.3102 ± 0.003 , humidity of 0.024 ± 0.021% and viscosity of 39.870 ± 0.010 . In addition, the chromatographic analysis showed that oleic acid C18:1 accounted for the highest proportion, at 30.233%. This value, and the molar ratio 6:1 alcohol/oil previously established, allowed us to determine that for every 68.712 g of methanol, 101.1 g of recycled vegetable oil was used. The ANOVA analysis with a 0.05 level of significance determined that the properties of density, calorific value, viscosity, acidity index, and composition of methyl esters did not have statistically significant differences. Meanwhile, the corrosion of copper presented no variability between catalysts or concentrations. Otherwise, it occurred with the yield percentage in which a post hoc analysis was carried out, with 0.5% KOH being determined as the catalyst and optimal concentration. The properties of the biodiesel obtained with KOH at 0.5% concentration, selected for its higher performance, were: density of 0.881 ± 0.001 , viscosity of 4.694 ± 0.077 , acidity index 0.355 ± 0.086 , corrosion 1a and 93.084 ± 0.957% of FAMEs, and calorific value 39,726 ± 364.91 . When analyzing these properties, in the case of the composition of FAMEs, the requirements of EN 14214 and NTE INEN 2482 standards were not met. The calorific value for its part is not established in the standards analyzed. However, the values reported by similar studies were reached. As for the other properties, they comply with the provisions of the ASTM D6751, NTE INEN 2482, and EN14214 regulations.
These results prove that biodiesel obtained from used oil from various sources can be converted into good-quality biodiesel, which can be used as a lubricant or as a fuel blended with diesel or biodiesel of higher calorific value. This strategy encourages the revaluation of spent oil waste for its successful conversion into energy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/eng4010056/s1, Table S1: Characteristics of the biodiesel obtained; Table S2: Statistics and ANOVA table of the biodiesel obtained with each catalyst and concentration; Figure S1: Plot of main effects and interactions for performance; Figure S2: boxplot.
Author Contributions
Conceptualization, V.P.-V.; methodology, V.P.-V., J.R. and L.B.; validation, J.R. and L.B.; formal analysis, J.R. and L.B.; investigation, J.R. and L.B.; writing—original draft preparation, J.R., L.B., E.A.L.-M. and V.P.-V.; writing—review and editing, V.P.-V. and E.A.L.-M.; supervision, V.P.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martínez Ullate, P. Biocombustibles y su Mercado Internacional; Universidad Nacional de Rosario: Rosario, Argentine, 2016; Available online: https://rephip.unr.edu.ar/xmlui/bitstream/handle/2133/11365/Paula%20Mart%c3%adnez%20Ullate%20-%20Trabajo%20Final.pdf?sequence=3&isAllowed=y (accessed on 11 May 2022).
- British Petroleum (BP). Statistical Review of World Energy 2021, 70th ed.; British Petroleum: London, UK, 2021; Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 24 July 2022).
- Piña Contreras, S. Análisis de la Esterificación por Catálisis Ácida Homogénea Como Pretratamiento de Aceite Modelo Acidificado; Universidad Michoacana de San Nicolás de Hidalgo: Morelia, Mexico, 2020; Available online: http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/bitstream/handle/DGB_UMICH/4925/FIQ-M-2020-1218.pdf?sequence=1&isAllowed=y (accessed on 22 June 2022).
- ARC. Aceite Reciclado de Cocina. 2021. Available online: https://www.arc.ec/2186/0/sobre-nosotros (accessed on 28 July 2022).
- Pantoja Cabrera, D.A. Caracterización de las Propiedades Fisicoquímicas de Biodiesel Extraído de Aceite Reciclado de Origen Vegetal; Universidad de San Francisco de Quito: Quito, Ecuador, 2018; Available online: https://repositorio.usfq.edu.ec/bitstream/23000/7140/1/137034.pdf (accessed on 18 May 2022).
- Castillo Fernández, K. Caracterización Teórica de Parámetros del Biodiésel y Estudio de Algunas de Sus Emisiones; Universidad Politécnica de Madrid: Madrid, Spain, 2018; Available online: https://oa.upm.es/53357/1/TFG_KEVIN_CASTILLO_FERNANDEZ.pdf (accessed on 21 June 2022).
- Miranda Peña, N.A. Obtención del Biodiesel a Partir del Aceite Vegetal Residual Mediante el Proceso de Transesterificación a Nivel Laboratorio, en Sullana, 2019–2020; Universidad Nacional de Piura: Castilla, Peru, 2021; Available online: https://repositorio.unp.edu.pe/bitstream/handle/20.500.12676/3178/IQUI-MIR-PEN-2021.pdf?sequence=1&isAllowed=y (accessed on 17 July 2022).
- Guerrero De La, B.N.; Kocher Solano, K.N. Obtención de Éster Metílico (Biodiesel), Mediante Reacción de Transesterificación del Aceite de Moringa Oleífera; Universidad de Guayaquil, Facultad de Ingeniería Química: Guayaquil, Ecuador, 2019; Available online: http://repositorio.ug.edu.ec/handle/redug/45466 (accessed on 23 June 2022).
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Medina Pérez, E.; Ruiz Domínguez, M.R.; Morales Espinoza, J.; Cerezal Mezquita, P. Evaluación del perfil de ácidos grasos de isochrysis galbana mediante el uso de métodos ácidos y alcalinos de transesterificación. Inf. Técnico 2019, 83, 66–75. [Google Scholar] [CrossRef]
- Diniz Vicente Pardal, A.C. Obtención de Biodiesel por Transesterificación de Aceites Vegetales: Nuevos Métodos de Síntesis. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 2012. [Google Scholar]
- De La Cruz López, C.J.; Trujillo Luna, C.A. Obtención de Biodiésel a Partir de Aceite Comestible Residual del Comedor de la UNAC; Universidad Nacional de Callao: Callao, Peru, 2017. [Google Scholar]
- Zannol, C.V. Factibilidad Económica de Producción de Biodiesel a Partir de Microalgas en Argentina; Instituto Tecnológico de Buenos Aires: Buenos Aires, Argentine, 2016. [Google Scholar]
- Flores Patiño, E.E. Obtención y Caracterización de Biodiesel a Partir de Aceite Vegetal Usado y su Efecto en Las Presentaciones de un MCIA; Universidad de Guanajuato: Guanajuato, Mexico, 2017. [Google Scholar]
- Caro Becerra, J.L.; Castellanos Rangel, L.; Romero Gonzalez, F.; Ruiz Morales M del, R. Generación de biodiesel a partir de residuos de aceites, utilizando un reactor con PLC para la automatización del proceso; Revista de Energía Química y Física. 2017, pp. 4–11, 16–27. Available online: https://www.ecorfan.org/bolivia/researchjournals/Energia_Quimica_y_Fisica/vol4num11/Revista_de_Energ%C3%ADa_Qu%C3%ADmica_y_F%C3%ADsica_V4_N11_3.pdf (accessed on 17 February 2022).
- Rodríguez Rodríguez, D.A.; Riesco Avila, J.M.; Malagón Romero, D.H.M. Obtención de biodiésel a partir de mezclas de aceite de cocina usado y aceite de higuerilla por transesterificación. Jóvenes En La Cienc. 2016, 2, 1850–1854. [Google Scholar]
- Tovar Torres, M.L.T. Análisis de parámetros para la producción de biodiesel a partir de aceite de cocina usado. Rev. EJE Eng. J. ECCI 2019, 1, 28–33. [Google Scholar] [CrossRef]
- López, L.; Bocanegra, J.; Malagón Romero, D. Obtención de biodiesel por transesterificación de aceite de cocina usado. Ing. Y Univ. 2015, 19, 155–172. [Google Scholar]
- Verdesoto Cozzarelli, C.E.; Verduga Loza, K.E. Evaluación Técnica de un Biocombustible (Biodiésel) Obtenido por Catálisis Homogénea y Transesterificación de Aceites Usados. Bachelor’s Thesis, Universidad de Guayaquil. Facultad de Ingeniería Química, Guayaquil, Ecuador, 2021. [Google Scholar]
- Argumedo Negrete, A. Diseño y Construcción de un Reactor BATCH Para la Producción de Biodiesel Como Combustible de Origen Orgánico Producido a Partir de Aceites Vegetales Variando la Relación Molar Aceite/Alcohol; Universidad de Pamplona—Facultad de Ingenieras y Arquitectura: Pamplona, Colombia, 2019. [Google Scholar]
- Acevedo Páez, J.C.; Urbina Suárez, N.A.; Acevedo Rodríguez, A.Z.; Becerra Orozco, L.C. Estudio de la producción de biodiesel por procesos químicos y enzimáticos a partir de aceite de cocina usado. Aibi Rev. De Investig. Adm. E Ing. 2019, 7, 20–26. [Google Scholar]
- Avellaneda Vargas, F. Producción y Caracterización de Biodiesel de Palma y de Aceite Reclicado Mediante un Proceso Batch y un Proceso Continuo con un Reactor Helicoidal; Universitat Rovira I Virgili: Tarragona, Spain, 2010. [Google Scholar]
- Available online: https://www.etapa.net.ec/informacion/saneamiento/plantas-de-tratamiento-de-aguas-residuales-ucubamba (accessed on 25 February 2023).
- NTE INEN. Grasas y Aceites de Origen Animal y Vegetal. Determinación de la Densidad Relativa; Instituto Ecuatoriano de normalización: Quito, Ecuador, 2012. [Google Scholar]
- NTE INEN. Grasas y Aceites Comestibles. Determinación de la Acidez; Instituto Ecuatoriano de normalización: Quito, Ecuador, 1973. [Google Scholar]
- NTE INEN. Grasas y Aceites Comestibles. Determinación de la Pérdida por Calentamiento; Instituto Ecuatoriano de normalización: Quito, Ecuador, 1973. [Google Scholar]
- ASTM. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); American Society for Testing and Materials: Philafelphia, PA, USA, 2021. [Google Scholar]
- ASTM. Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test; ASTM: Philafelphia, PA, USA, 2019. [Google Scholar]
- Zarate Gamarra, J.A.; Luján Rojas, J.M.; Llaque Fernández, G.I. Índice de Aceites Residuales de Cocina Para la Producción de Biodiésel en las Provincias de Lima y Trujillo; Universidad Privada del Norte: Peruterui, Peru, 2022. [Google Scholar] [CrossRef]
- Alvarado Pacheco, J.J.; Canul Ku, J.A.; Quijano Carreón, F.N.; Gamboa Quijano, Y.J.; Herrera Chalé, F.G. Aprovechamiento de aceite residual doméstico de Puerto Progreso, Yucatán, como recurso para la producción de un biodiesel. Rinderesu 2021, 5, 205–215. [Google Scholar]
- Díaz Alvarez, M.C.; Guerrero Arrelucea, S.M. Influencia del Índice de Acidez en el Poder Calorífico del Biodiesel, Obtenido a Partir de Aceites Reciclados de Cocina; Repositorio Institucional—UNS: Chimbote, Peru, 2018. [Google Scholar]
- NTE INEN. Mezclas de Aceites Vegetales Comestibles. Requisitos; Quito, Ecuador. Instituto Ecuatoriano de normalización: Quito, Ecuador, 1985. [Google Scholar]
- Barbosa Reina, C.; Jiménez Ramírez, L.N.; Pedraza Morales, N. Obtención de biodiesel (etil-éster) mediante catálisis básica a nivel planta piloto derivado de aceites usados de la industria alimenticia. Publ. E Investig. 2014, 8, 99–116. [Google Scholar] [CrossRef]
- Gaspar Ñaña, F.J.; Zorrilla Cavero, P.C. Evaluación de la Dosis de Na(OH) y Metanol en la Producción de Biodiésel a Partir de los Aceites Usados de las Pollerías; Universidad Nacional del Centro del Perú: Huancayo, Peru, 2019. [Google Scholar]
- Quispe Puma, K.Y. Obtención de Biodiesel a Partir de la Mezcla de Aceite Doméstico Residual y Aceite de Soya en la Región del Cusco—2020; Repositorio Institucional—UCV: Latitude, Longitude, 2021. [Google Scholar]
- Alarcón Tarira, M.M.; Romero Mosquera, R.L. Estudio y Diseño de un Sistema de Recolección de Aceite Vegetal Usado Para el Sector Comercial y Residencial del Norte de la Ciudad de Guayaquil. Available online: https://dspace.ups.edu.ec/handle/123456789/210482021 (accessed on 17 July 2022).
- NTE INEN. Biodiesel. Requisitos; Instituto Ecuatoriano de normalización: Quito, Ecuador, 2009. [Google Scholar]
- ASTM. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuel; The American Society for Testing and Materials: Philafelphia, PA, USA, 2020. [Google Scholar]
- EN. Automotive Fuels. Fatty Acid Methyl Esters (FAME) for Diesel Engines. Requirements and Test Methods; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- Gorky, G.; Rivera, A.; Morales, A. Efecto del Poder Calorífico en la Relación de Combustión del Motor con Distintos Tipos de Biodiesel; Quito, Ecuador, 2018; Volume 2, pp. 20–24. Available online: https://nexoscientificos.vidanueva.edu.ec/index.php/ojs/article/view/16/16 (accessed on 19 July 2022)ISSN 2773-7489.
- Rodríguez, D.A.R.; Ávila, J.M.R.; Romero, D.M. Obtención de Biodiesel a Partir de Mezclas de Aceite de Cocina Usado y Aceite de Higuerilla; Universidad de Santo Tomas: Bogota, Colombia, 2017. [Google Scholar]
- Sánchez Domínguez, S.M.; Torres Aldaco, A.; Lugo Leyte, R.; Cervantes Ruiz, J.; Torres González, E.V. Caracterización de Propiedades Físicas del Biodiesel a Partir de Aceite de Coco y Medición del Poder Calorífico; Memorias del XXXI Congreso Nacional De Termodinámica: Durango, Mexico, 2016. [Google Scholar]
- Zamora Burbano, Á.M. Evaluación del Aceite de Lupino Andino (Lupinus mutabilis L.) Cómo Alternativa Energética Para la Producción del Biodiesel; Universidad de las Fuerzas Armadas: Sangolqui, Ecuador, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).