Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review
Abstract
:1. Introduction
2. Review Objectives
- (i)
- Explain the rationale for reprocessing tailings for their REE content;
- (ii)
- Reconcile and discuss all the key findings emerging from the comprehensive fundamental investigations carried out on complex low-grade tailings;
- (iii)
- Summarize the response of valuable REE and gangue minerals in the real plant tailings during froth flotation, magnetic, and gravity separation methods;
- (iv)
- Discuss the identified challenges and opportunities in the use of tailings as a secondary resource for REE minerals beneficiation.
3. Beneficiation Studies
3.1. Rationale
3.2. Physicochemical and Mineralogical Characteristics of the Tailings
- What are the REE-bearing minerals in the tailings?
- What are the gangue minerals present in the tailings, and what are their physical and chemical characteristics?
- What REE–gangue mineralization and associations are present in the tailings?
3.3. Froth Flotation
3.4. Gravity Separation
3.5. Magnetic Separation
3.6. Flotation of Magnetic Separation Products
3.7. REE Minerals Grade–Recovery Relationship
4. Discussion
4.1. Identified Challenges and Limitations
4.2. Future Considerations
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. The upgrading of rare earth oxides from iron-oxide silicate rich tailings: Flotation performance using sodium oleate and hydroxamic acid as collectors. Adv. Powder Technol. 2018, 29, 3163–3172. [Google Scholar] [CrossRef]
- Moran-Palacios, H.; Ortega-Fernandez, F.; Lopez-Castaño, R.; Alvarez-Cabal, J.V. The Potential of Iron Ore Tailings as Secondary Deposits of Rare Earths. Appl. Sci. 2019, 9, 2913. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. The Occurrence States of Rare Earth Elements Bearing Phosphorite Ores and Rare Earth Enrichment Through the Selective Reverse Flotation. Minerals 2019, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Ni, S.; Chen, Q.; Gao, Y.; Guo, X.; Sun, X. Recovery of rare earths from industrial wastewater using extraction-precipitation strategy for resource and environmental concerns. Miner. Eng. 2020, 151, 106315. [Google Scholar] [CrossRef]
- Golev, A.; Scott, M.; Erskine, P.; Ali, S.; Ballantyne, G. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B. Beneficiation of Rare Earth Elements Minerals from Tailings as an Analogue of Complex Low Grade Ores, in Future Indutries Institute. PhD. Thesis, University of South Australia, Adelaide, Australia, 2019. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Johansson, N.; Krook, J.; Eklund, M.; Berglund, B. An integrated review of concepts and initiatives for mining the technosphere: 533 towards a new taxonomy. J. Clean. Prod. 2013, 55, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Krook, J.; Baas, L. Getting serious about mining the technosphere: A review of recent landfill mining and urban mining research. J. Clean. Prod. 2013, 55, 1–9. [Google Scholar] [CrossRef]
- Cossu, R.; Williams, I.D. Urban mining: Concepts, terminology, challenges. Waste Manag. 2015, 45, 1–3. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, L. Urban mining of e-waste is becoming more cost-effective than virgin mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Bugnosen, E. Country case study on artisanal and small-scale mining: Philippines. Min. Miner. Sustain. Dev. 2001, 83, 1–8. [Google Scholar]
- Fleming, C.A.; Brown, J.A.; Botha, M. An economic and environmental case for re-processing gold tailings in South Africa. Tech. Bull. 2010, 3, 1–12. [Google Scholar]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Cheng, T.C.; Kassimi, F.; Zinck, J.M. A holistic approach of green mining innovation in tailings reprocessing and repurposing. In Proceedings of the Tailings & Mine Waste 2016, Keystone, CO, USA, 2–5 October 2016. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Geoscience-Australia. Australia’s Identified Mineral Resources 2011; Geoscience Australia: Canberra, Australia, 2012; pp. 72–78. [Google Scholar]
- Geoscience-Australia. Australia’s Identified Mineral Resources 2013; Geoscience Australia: Canberra, Australia, 2014; pp. 107–115. [Google Scholar]
- Zhang, B.; Liu, C.; Li, C.; Jiang, M. A novel approach for recovery of rare earths and niobium from Bayan Obo tailings. Miner. Eng. 2014, 65, 17–23. [Google Scholar] [CrossRef]
- Grauch, R.I.; Verplanck, P.L.; Seeger, C.M.; Budahn, J.R.; Van Gosen, B.S. Chemistry of Selected Core Samples, Concentrate, Tailings, and Tailings Pond Waters: Pea Ridge Iron (-Lanthanide-Gold) Deposit, Washington County, Missouri; US Geological Survey: Reston, VA, USA, 2010; Volume 1080, p. 15. [Google Scholar]
- Vierrether, C.W.; Cornell, W.L. Rare-Earth Occurrences in the Pea Ridge Tailings; US Department of the Interior, Bureau of Mines: Spokane, WA, USA, 1993; Volume 9453. [Google Scholar]
- Hotta, Y.; Kojima, S. Policy Framework for International Collaboration towards Sustainable Resource Circulation and Management in Asia. In Greening Governance in Asia-Pacific; Institute for Global Environmental Strategies: Hayama, Japan, 2012; Volume 159. [Google Scholar]
- Lee, I.-S.; Kang, H.-Y. A review on the direction of the framework act on resource circulation for establishing a resource circulation society. J. Korean Inst. Resour. Recycl. 2016, 25, 82–91. [Google Scholar]
- Hamilton, J.L.; Wilson, S.A.; Morgan, B.; Harrison, A.L.; Turvey, C.C.; Paterson, D.J.; Dipple, G.M.; Southam, G. Accelerating mineral carbonation in ultramafic mine tailings via direct CO2 reaction and heap leaching with potential for base metal enrichment and recovery. Econ. Geol. 2020, 115, 303–323. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. The Use of Mining Tailings as Analog of Rare Earth Elements Resources: Part 1—Characterization and Preliminary Separation. Miner. Process. Extr. Met. Rev. 2021, 1–15. [Google Scholar] [CrossRef]
- Owen, J.R.; Kemp, D.; Lébre, É.; Svobodova, K.; Murillo, G.P. Catastrophic tailings dam failures and disaster risk disclosure. Int. J. Disaster Risk Reduct. 2020, 42, 101361. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Shengo, L. Review of practices in the managements of mineral wastes: The case of waste rocks and mine tailings. Water Air Soil Pollut. 2021, 232, 1–31. [Google Scholar] [CrossRef]
- Xu, D.-M.; Zhan, C.L.; Liu, H.X.; Lin, H.Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; Pownceby, M.I. Characterisation of a ferruginous rare earth bearing lateritic ore and implications for rare earth mineral processing. Miner. Eng. 2019, 134, 23–36. [Google Scholar] [CrossRef]
- Filippov, L.O.; Dehaine, Q.; Filippova, I.V. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production–Part 3: Processing of fines using gravity and flotation. Miner. Eng. 2016, 95, 96–106. [Google Scholar] [CrossRef]
- Faris, N.; Ram, R.; Tardio, J.; Bhargava, S.; McMaster, S.; Pownceby, M.I. Application of ferrous pyrometallurgy to the beneficiation of rare earth bearing iron ores–a review. Miner. Eng. 2017, 110, 20–30. [Google Scholar] [CrossRef]
- Wills, B. Comminution in the minerals industry-an overview. Miner. Eng. 1990, 3, 3–5. [Google Scholar] [CrossRef]
- Stanujkic, D.; Zavadskas, E.K.; Karabasevic, D.; Milanovic, D.; Maksimovic, M. An approach to solving complex decision-making problems based on IVIFNs: A case of comminution circuit design selection. Miner. Eng. 2019, 138, 70–78. [Google Scholar] [CrossRef]
- Cleary, P.W.; Delaney, G.W.; Sinnott, M.D.; Cummins, S.J.; Morrison, R.D. Advanced comminution modelling: Part 1—Crushers. Appl. Math. Model. 2020, 88, 238–265. [Google Scholar] [CrossRef]
- Owusu, K.B.; Karageorgos, J.; Greet, C.; Zanin, M.; Skinner, W.; Asamoah, R.K. Predicting mill feed grind characteristics through acoustic measurements. Miner. Eng. 2021, 171, 107099. [Google Scholar] [CrossRef]
- Marion, C.; Grammatikopoulos, T.; Rudinsky, S.; Langlois, R.; Williams, H.; Chu, P.; Awais, M.; Gauvin, R.; Rowson, N.A.; Waters, K.E. A mineralogical investigation into the pre-concentration of the Nechalacho deposit by gravity separation. Miner. Eng. 2018, 121, 1–13. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Abaka-Wood, G.B.; Quast, K.; Zanin, M.; Addai-Mensah, J.; Skinner, W. A study of the feasibility of upgrading rare earth elements minerals from iron-oxide-silicate rich tailings using Knelson concentrator and Wilfley shaking table. Powder Technol. 2019, 344, 897–913. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Magnetic separation of monazite from mixed minerals. In Chemeca 2016: Chemical Engineering-Regeneration, Recovery and Reinvention; Engineers Australia: Melbourne, Australia, 2016; p. 596. [Google Scholar]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of rare earth elements minerals from iron oxide–silicate rich tailings—Part 1: Magnetic separation. Miner. Eng. 2019, 136, 50–61. [Google Scholar] [CrossRef]
- Xiong, W.; Deng, J.; Chen, B.; Deng, S.; Wei, D. Flotation-magnetic separation for the beneficiation of rare earth ores. Miner. Eng. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Yang, X.; Satur, J.V.; Sanematsu, K.; Laukkanen, J.; Saastamoinen, T. Beneficiation studies of a complex REE ore. Miner. Eng. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Van Gerven, T.; Jones, P.T.; et al. REE recovery from end-of-life NdFeB permanent magnet scrap: A critical review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of rare earth elements minerals from iron oxide–silicate rich tailings—Part 2: Froth flotation separation. Miner. Eng. 2019, 142, 105888. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of fine particles: A review. Miner. Process. Extr. Metall. Rev. 2021, 42, 473–483. [Google Scholar] [CrossRef]
- Li, H.; Feng, Q.; Yang, S.; Ou, L.; Lu, Y. The entrainment behaviour of sericite in microcrystalline graphite flotation. Int. J. Miner. Process. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Hoang, D.H.; Heitkam, S.; Kupka, N.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Froth properties and entrainment in lab-scale flotation: A case of carbonaceous sedimentary phosphate ore. Chem. Eng. Res. Des. 2019, 142, 100–110. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Influence of aggregation/dispersion state of hydrophilic particles on their entrainment in fine mineral particle flotation. Miner. Eng. 2021, 166, 106835. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Liu, Q. Entrainment of Gangue Minerals in Froth Flotation: Mechanisms, Models, Controlling Factors, and Abatement Techniques—A Review. Min. Met. Explor. 2020, 38, 673–692. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Song, S.; Liu, Y. Reducing the entrainment of gangue fines in low grade microcrystalline graphite ore flotation using multi-stage grinding-flotation process. Minerals 2017, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xing, Y.; Wang, J. Mechanism of the combined effects of air rate and froth depth on entrainment factor in copper flotation. Physicochem. Probl. Miner. Process. 2020, 56, 43–53. [Google Scholar]
- Oberteuffer, J. Magnetic separation: A review of principles, devices, and applications. IEEE Trans. Magn. 1974, 10, 223–238. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Ou, L. Recovery enhancement of ultrafine wolframite through hydrophobic flocs magnetic separation. Miner. Process. Extr. Metall. Rev. 2017, 38, 298–303. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Fosu, S.; Addai-Mensah, J.; Skinner, W. Flotation recovery of rare earth oxides from hematite–quartz mixture using sodium oleate as a collector. Miner. Eng. 2019, 141, 105847. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Selective flotation of rare earth oxides from hematite and quartz mixtures using oleic acid as a collector. Int. J. Miner. Process. 2017, 169, 60–69. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of selective flotation recovery of rare earth oxides from hematite and quartz using hydroxamic acid as a collector. Adv. Powder Technol. 2018, 29, 1886–1899. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Li, Y.; Gao, P. Beneficiation of an iron ore fines by magnetization roasting and magnetic separation. Int. J. Miner. Process. 2017, 168, 102–108. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, Y.; Sun, Y.; Lv, Y.; Li, Y.; Tang, Z. A novel method for iron recovery from iron ore tailings with pre-concentration followed by magnetization roasting and magnetic separation. Miner. Process. Extr. Metall. Rev. 2020, 41, 117–129. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Yuan, S.; Yin, H. High-efficiency extraction of iron from early iron tailings via the suspension roasting-magnetic separation. Powder Technol. 2021, 379, 466–477. [Google Scholar] [CrossRef]
- Bisaka, K.; Thobadi, I.; Pawlik, C. Extraction of rare-earth elements from iron-rich rare-earth deposits. In Proceedings of the Hydrometallurgy Conference 2016: Sustainable Hydrometallurgical Extraction of Metals, Cape Town, South Africa, 1–3 August 2016. [Google Scholar]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Leaching of rare earths from bauxite residues using imidazolium based ionic liquids. In Proceedings of the ERES2014: 1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014. [Google Scholar]
- Tohar, S.; Yunus, M.M. Mineralogy and BCR sequential leaching of ion-adsorption type REE: A novelty study at Johor, Malaysia. Phys. Chem. Earth Parts A/B/C 2020, 120, 102947. [Google Scholar] [CrossRef]
- Prameswara, G.; Trisnawati, I.; Mulyono, P.; Prasetya, A.; Petrus, H.T.B.M. Leaching Behaviour and Kinetic of Light and Heavy Rare Earth Elements (REE) from Zircon Tailings in Indonesia. JOM 2021, 73, 988–998. [Google Scholar] [CrossRef]
- Peelman, S.; Sun, Z.H.; Sietsma, J.; Yang, Y. Leaching of rare earth elements: Review of past and present technologies. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–334. [Google Scholar]
- Middleton, A.; Park, D.M.; Jiao, Y.; Hsu-Kim, H. Major element composition controls rare earth element solubility during leaching of coal fly ash and coal by-products. Int. J. Coal Geol. 2020, 227, 103532. [Google Scholar] [CrossRef]
- Binnemans, K.; Pontikes, Y.; Jones, P.T.; Van Gerven, T.; Blanpain, B. Recovery of rare earths from industrial waste residues: A concise review. In Proceedings of the 3rd International Slag Valorisation Symposium: The Transition to Sustainable Materials Management, Leuven, Belgium, 19–20 March 2013. [Google Scholar]
- Yahorava, V.; Bazhko, V.; Freeman, M. Viability of phosphogypsum as a secondary resource of rare earth elements. In Proceedings of the XXVIII International Mineral Processing Congress, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Aliedeh, M.A.; Jarrah, N.A. Application of full factorial design to optimize phosphogypsum beneficiation process (P2O5 Reduction) by using sulfuric and nitric acid solutions. In Proceedings of the Sixth Jordanian International Chemical Engineering Conference, Amman, Jordan, 12–14 March 2012; pp. 1–10. [Google Scholar]
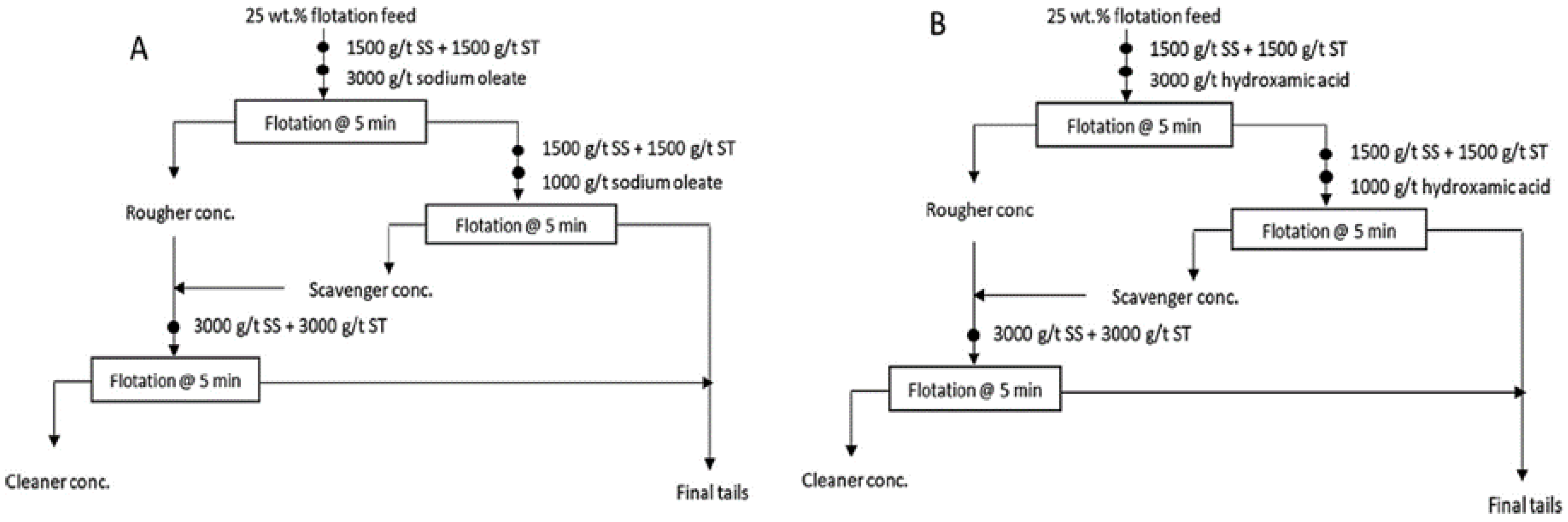
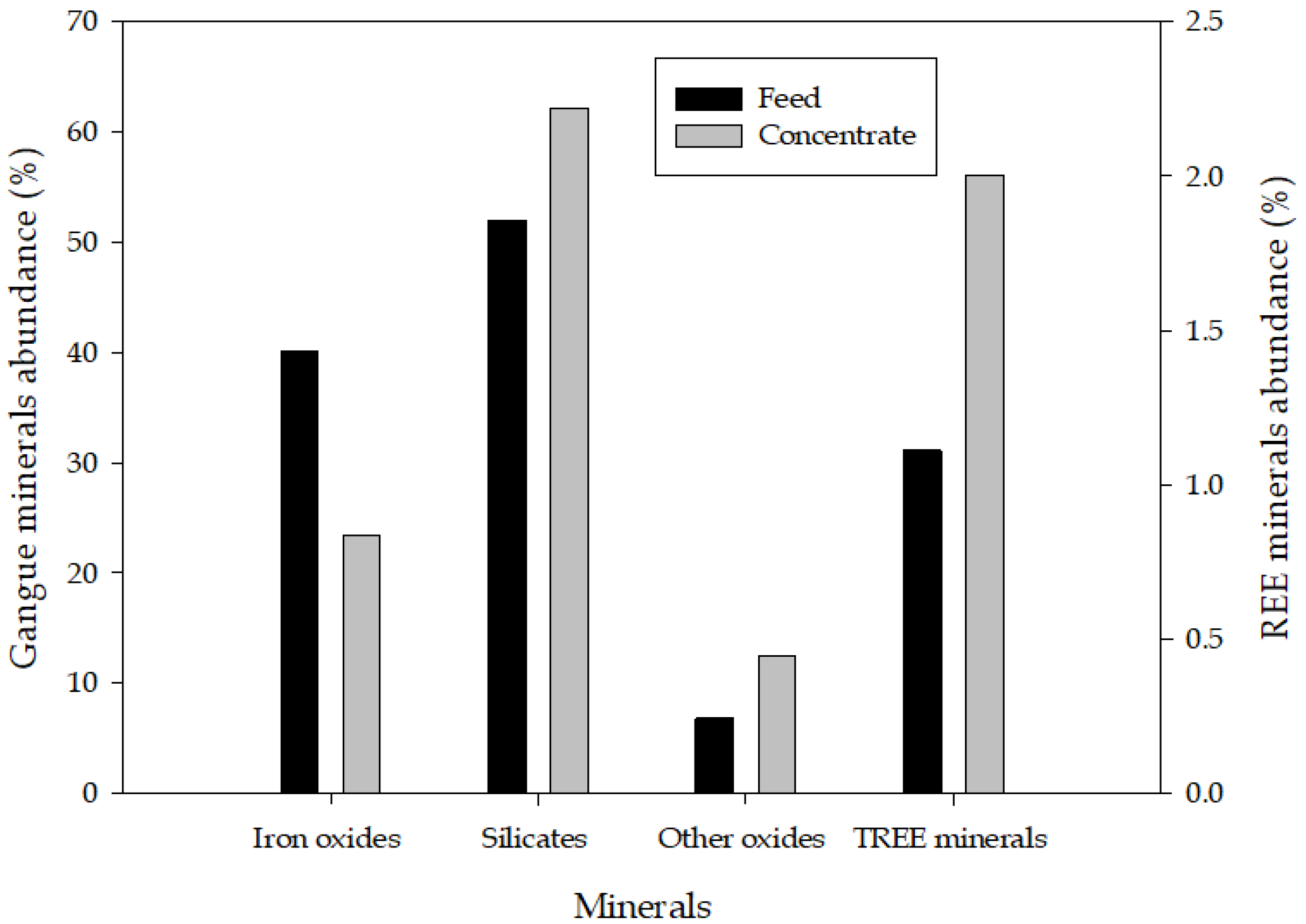
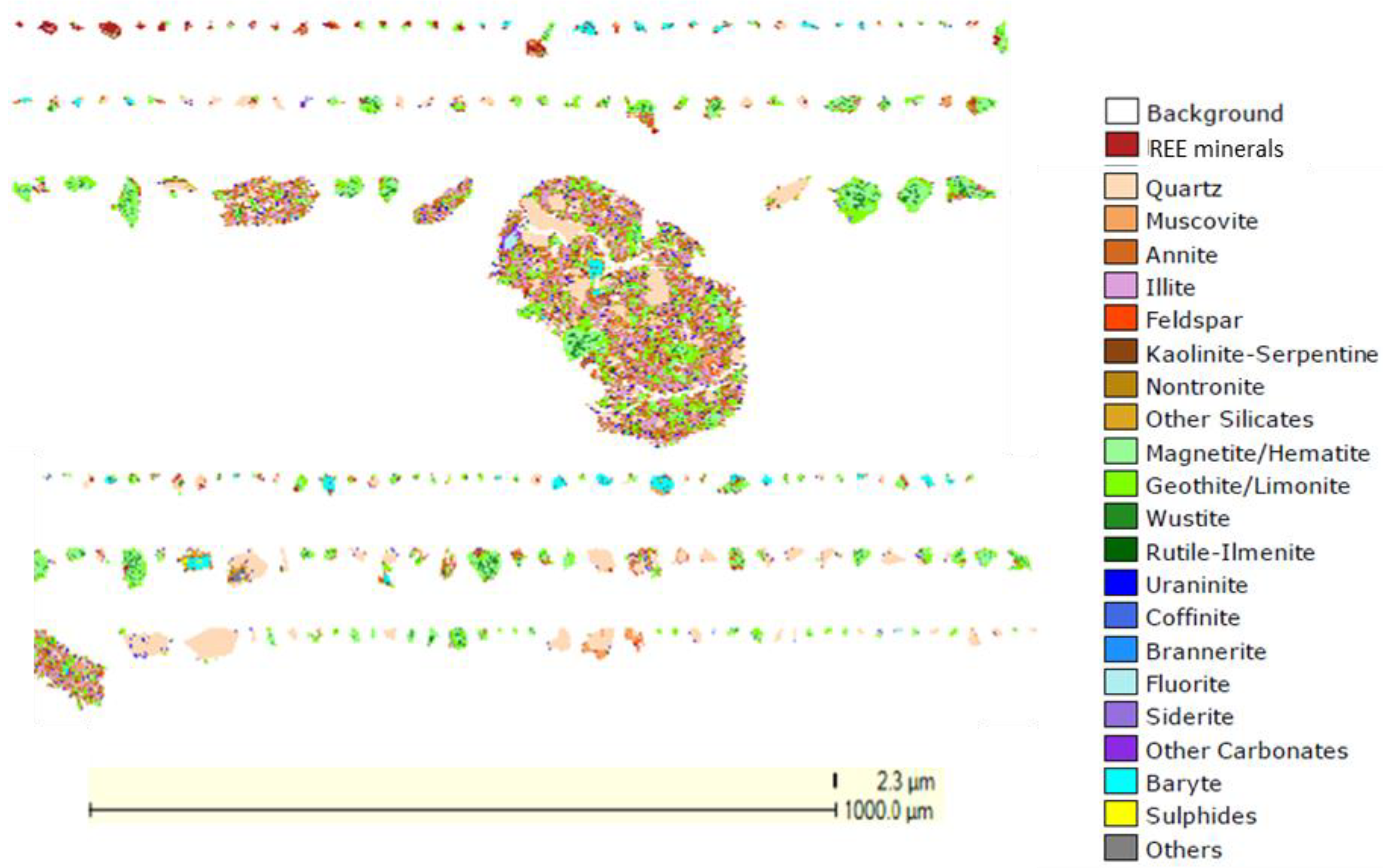

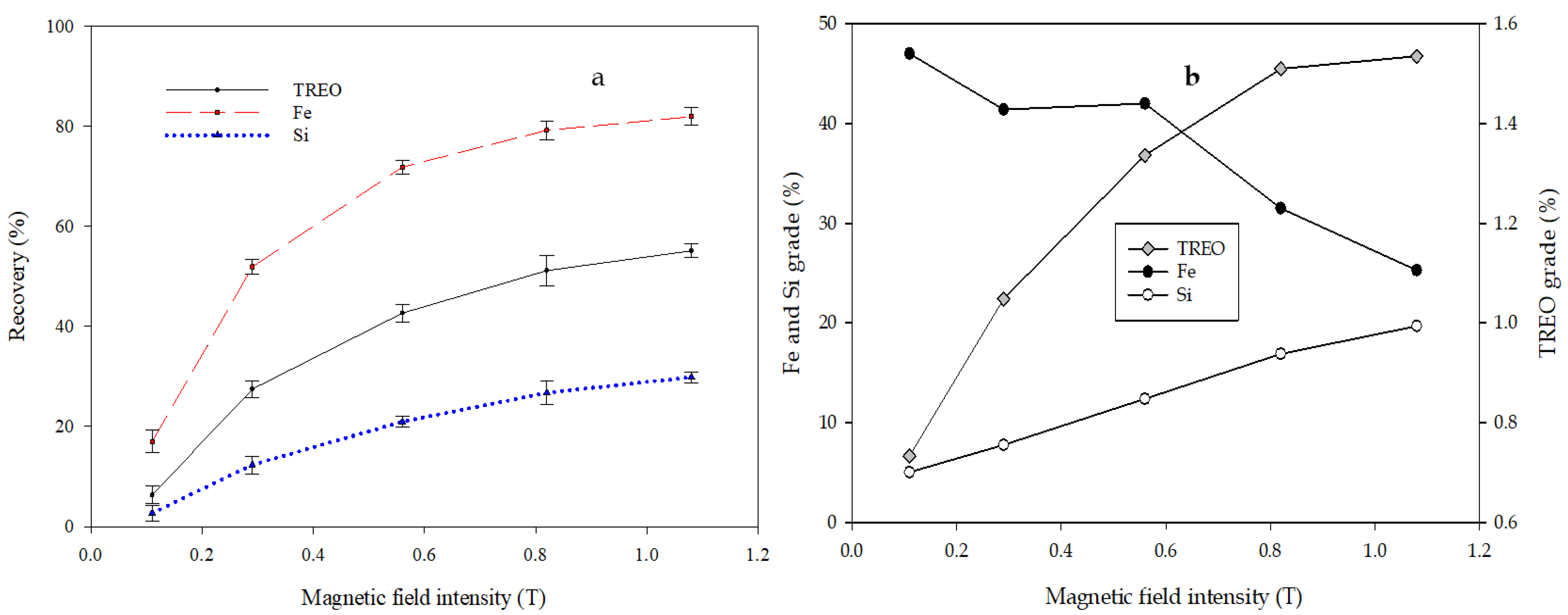


| Rare Earth Oxides | Content (%) | Gangue Elements | Content (%) |
|---|---|---|---|
| La2O3 | 0.35 | Al | 3.9 |
| CeO | 0.52 | Ca | 1.71 |
| Pr6O11 | 0.05 | Fe | 26.2 |
| Nd2O3 | 0.13 | Mg | 0.15 |
| Y2O3 | 0.02 | P | 0.15 |
| TREO | 1.07 | Si | 18.6 |
| Minerals | Relative Abundance (%) |
|---|---|
| REE minerals (bastnäsite, monazite, florencite, and others) | 1.11 |
| Iron oxide (hematite, goethite) | 40.15 |
| Quartz | 29.53 |
| Muscovite | 8.82 |
| Annite | 4.76 |
| Other silicates | 8.84 |
| Other oxides | 6.79 |
| Total | 100 |
| Flowsheet | TREO Dist. (%) | TREO Grade (%) |
|---|---|---|
| Feed | 100 | 1.07 |
| A (sodium oleate + sodium silicate + starch) | 63 | 2.25 |
| B (hydroxamic acid + sodium silicate + starch) | 60 | 1.99 |
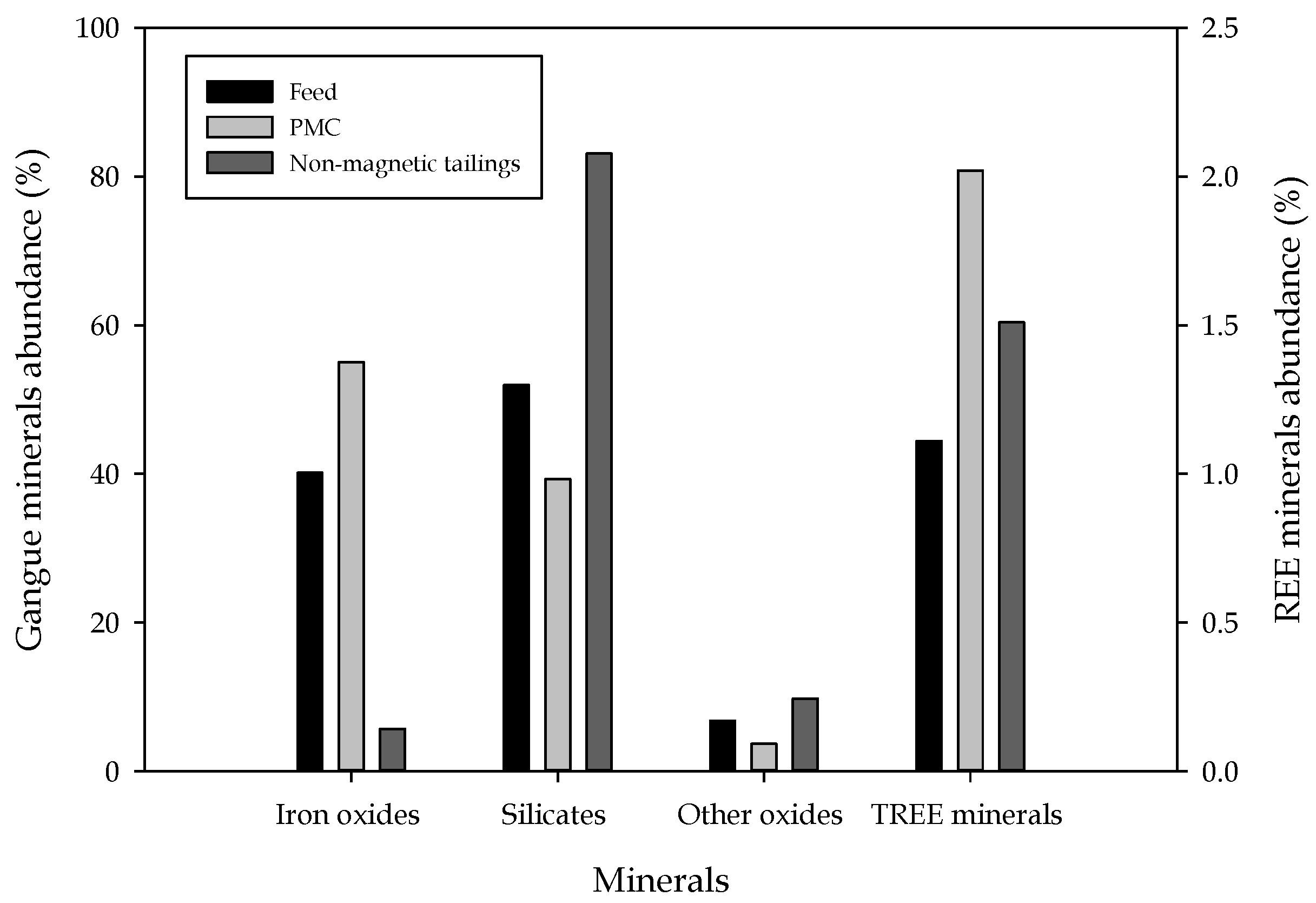
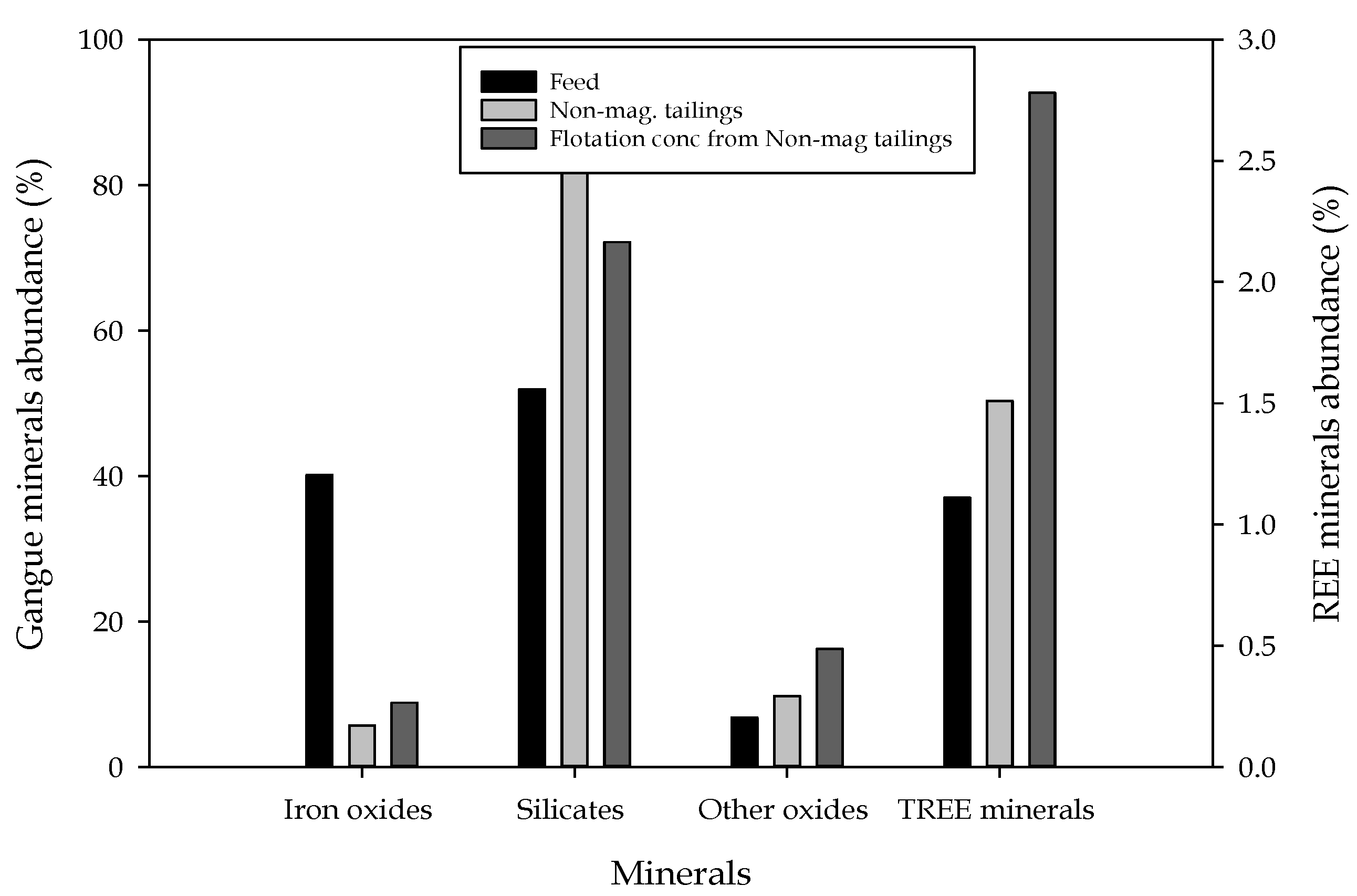
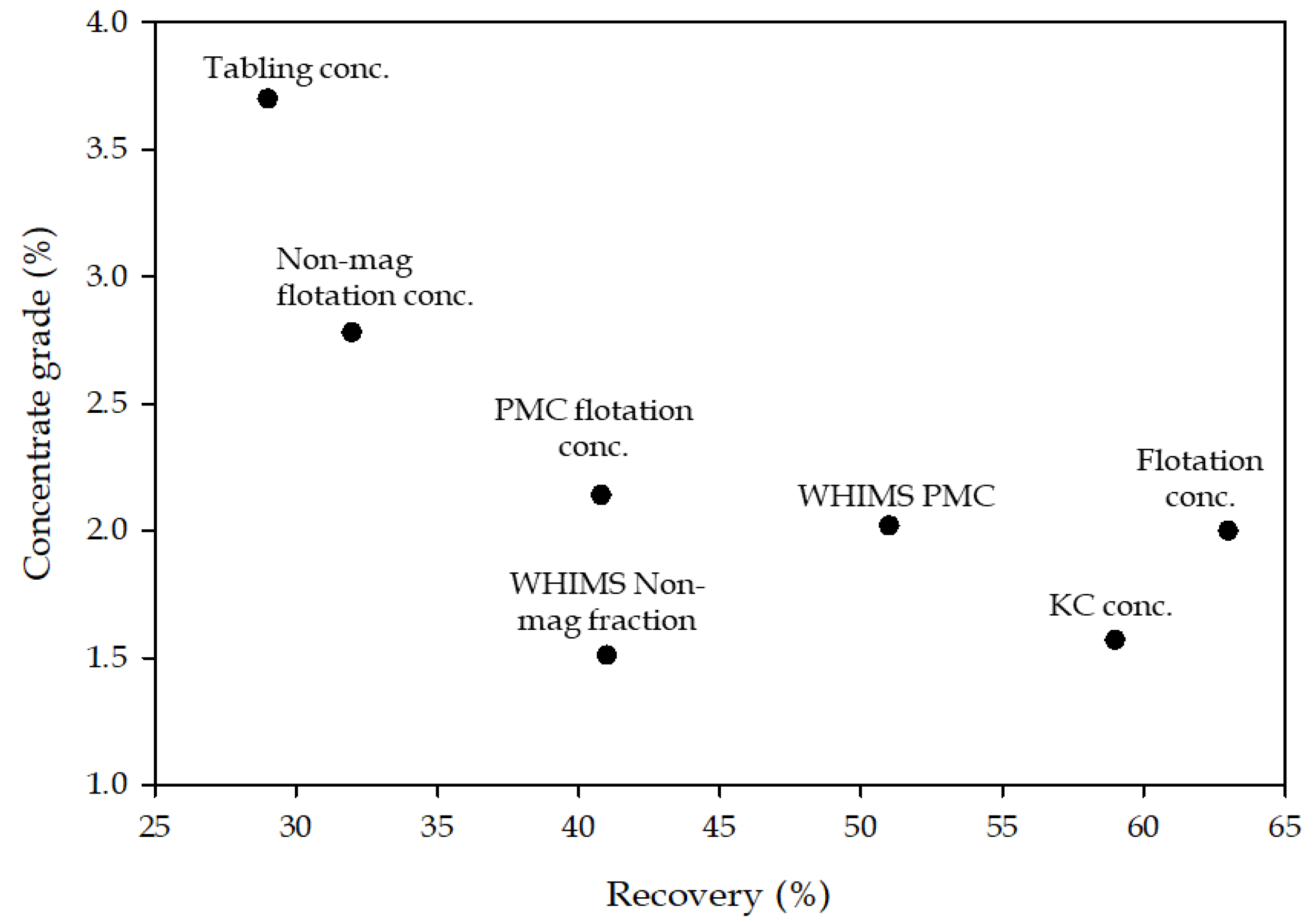
| Product | REE Minerals | Iron Oxides | Silicates | Others | Total |
|---|---|---|---|---|---|
| Feed | 1.11 | 40.15 | 51.95 | 6.79 | 100 |
| KC conc | 1.74 | 48.92 | 43.93 | 5.42 | 100 |
| Tabling conc | 3.70 | 72.23 | 19.62 | 4.45 | 100 |
| Flotation conc. (hydroxamic acid) | 2.00 | 23.46 | 62.13 | 12.41 | 100 |
| WHIMS concentrate (PMC) | 2.02 | 55.01 | 39.29 | 3.68 | 100 |
| Nonmagnetic tails (NMT) | 1.51 | 5.69 | 83.07 | 9.74 | 100 |
| Flotation conc from PMC | 2.14 | 77.01 | 15.86 | 4.99 | 100 |
| Flotation conc from NMT | 2.78 | 8.82 | 72.17 | 16.23 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaka-Wood, G.B.; Ehrig, K.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review. Eng 2022, 3, 259-275. https://doi.org/10.3390/eng3020020
Abaka-Wood GB, Ehrig K, Addai-Mensah J, Skinner W. Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review. Eng. 2022; 3(2):259-275. https://doi.org/10.3390/eng3020020
Chicago/Turabian StyleAbaka-Wood, George Blankson, Kathy Ehrig, Jonas Addai-Mensah, and William Skinner. 2022. "Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review" Eng 3, no. 2: 259-275. https://doi.org/10.3390/eng3020020
APA StyleAbaka-Wood, G. B., Ehrig, K., Addai-Mensah, J., & Skinner, W. (2022). Recovery of Rare Earth Elements Minerals from Iron-Oxide-Silicate-Rich Tailings: Research Review. Eng, 3(2), 259-275. https://doi.org/10.3390/eng3020020








