1. Introduction
Industrial production continuously generates a large quantity of waste residues, which occupies a considerable amount of land resources and presents the risk of environmental pollution [
1,
2]. The data indicate that in 2023, the output of general industrial solid waste in China was as high as 4.28 billion tons. However, the disposal volume was merely 870 million tons, and the comprehensive utilization volume was only 2.57 billion tons, with a comprehensive utilization rate of merely 60%. The comprehensive utilization of industrial waste residues has become an essential component of China’s sustainable development strategy. Utilizing industrial waste residues as a substitute for cement is a crucial approach for waste residue reuse [
3], which can reduce the consumption of cement and lower costs, and generate significant economic and environmental benefits [
4].
Numerous scholars [
5,
6,
7] have carried out relevant studies on the utilization of silicate solid waste material (SISWM) in industrial waste residues. In the field of soil solidification technology, the electro calcium carbonate—fly ash composite solidifying agent can be used to treat sludge [
8]. The regulatory effect of the alkali activator on the hydration products has been revealed [
9], providing a theoretical basis for the development of soil solidifying agents. In the field of soil improvement, a composite curing agent composed of phosphogypsum, blast furnace slag, and sodium silicate can be employed to partially replace cement for soil stabilization [
10]. Additionally, alkali-activated fly ash has been demonstrated to effectively solidify clay [
11]. The underlying reaction mechanism involves the formation of aluminosilicate gel through the activation of reactive silicon-aluminum components under highly alkaline conditions. One research has proved that the phosphogypsum—steel slag—blast furnace slag system can effectively solidify low liquid limit silty clay [
12], and the co-generated effect of flocculent C-S-H gel and needle-like calcium aluminosilicate was confirmed through microscopic observation.
Alkali activation of SISWM is commonly carried out with NaOH solutions, Na
2SiO
3 solutions and cement [
13,
14,
15,
16]. The method of activating the cementitious activity of steel slag through sodium hydroxide has been proven effective [
17], and the technology of using sodium hydroxide to adjust the modulus of sodium silicate to optimize the performance of the composite alkali activator has also been developed [
18].When alkaline activators such as sodium hydroxide are applied in the preparation of slag-based cementitious materials [
19], they exhibit remarkable high compressive strength and erosion resistance. The research on the influence of pore structure on the performance of solidified soil indicates that the soil strengthened by the activation of slag with sodium hydroxide has a smaller porosity [
20]. The process of preparing geopolymer by simultaneously activating fly ash with sodium silicate and sodium hydroxide solution has been successfully achieved [
21].
The above-mentioned researches on the reuse of SISWM are limited to the addition of alkali activators such as cement, Na
2O·nSiO
2 solutions, and MgO. It is well known that the OH
− required for alkali activation is merely provided by the hydration reaction of cement or strong alkali aqueous solutions. However, there are no relevant research on improving the properties of solidified soil through the collaboration of cement with alkali activators, SISWM, and sulfate solid waste material (SUSWM). Desulfurization gypsum and other sulfate solid waste materials can interact with cement [
22], slag and other silicate solid waste materials have potential pozzolanic activity and are prone to pozzolanic reactions and hydration reactions under alkaline conditions [
23]. The hydration products formed after pozzolanic reactions and hydration reactions can act as the soil skeleton, filling the soil pores and cementing other substances [
24].
In this study, industrial wastes, such as ordinary Portland cement (OPC), calcium based alkaline activator (CAA), SISWM and SUSWM, were selected to produce a solidifying agent for reinforcing silt. Orthogonal and comparative experiments were conducted to evaluate the mechanical strength and water stability of the stabilized silt and to determine the optimal proportions of each component in the solidifying agent. A series of indoor tests, including unconfined compressive strength (UCS) test, water stability (WS) test, scanning electron microscopy (SEM) test, energy dispersive spectroscopy (EDS) test, and X-ray diffraction (XRD) test, were conducted on silt samples solidified with the solidifying agent using the optimal mixing ratios of OPC, CAA, SISWM, and SUSWM to clarify the evolution of mineral composition and microstructure of solidified silt.
3. Results and Discussion
3.1. Analysis of Orthogonal Experiment
Figure 3 shows that the UCS of each group improves as the OPC dosage increases by 1%, with the 3rd group exhibiting the greatest improvement, reaching 1.86 times the UCS at a 5% OPC dosage. This suggests that in group O
6L
1S
3G
2, OPC actively participates in hydration reactions and works synergistically with other admixtures. The lowest improvement is observed in the 9th group, indicating that in group O
5L
3S
3G
2, CAA, SISWM, and SUSWM are already capable of undergoing effective hydration reactions, and an increase in OPC dosage does not significantly enhance the UCS of the solidified silt.
The WS coefficients of the 5th, 7th, 9th group with 5% OPC dosage are below 80% (see
Figure 4), failing to meet the specification requirements. In the O
6 group, except the O
6L
3S
1G
3 group (i.e., the 7th group), the WS coefficients of all other groups are greater than 80%, which indicates that in the O
6L
3S
1G
3 group, a low dosage of SISWM results in inadequate performance in terms of WS requirements.
The statistical analysis results of the unconfined compressive strength and water stability coefficient of the stabilized soil are summarized in
Table 7,
Table 8,
Table 9 and
Table 10. These tables list, for each level, the total values (K
1, K
2, K
3) of the unconfined compressive strength or water stability coefficient, the corresponding average values (K
1-avg, K
2-avg, K
3-avg), and the range R of the average values (K-avg). The range R serves as an indicator to evaluate the influence of each experimental factor on the enhancement of the unconfined compressive strength or water stability coefficient. A larger range R indicates a more significant effect of the factor.
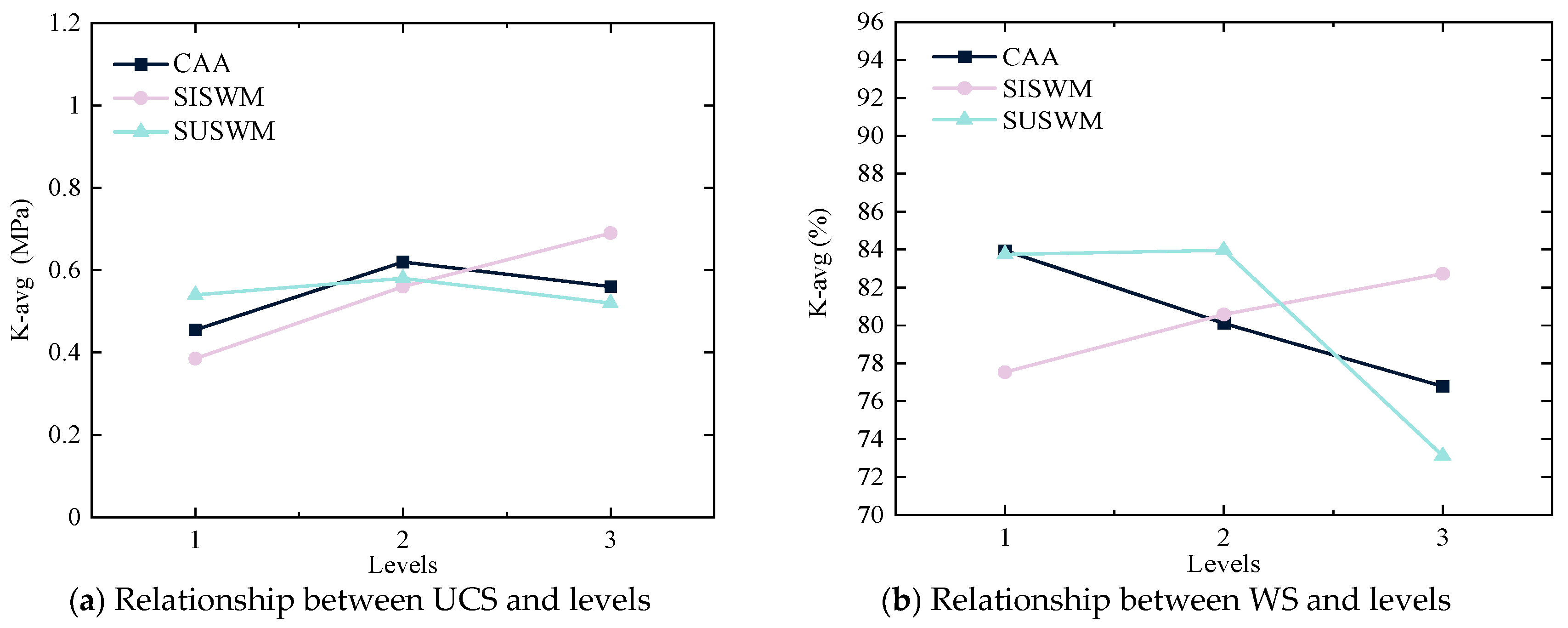
Through comparative analysis of the range values R in
Table 7 and
Table 9, it is evident that the dosage of SISWM is the primary factor influencing the unconfined compressive strength of the stabilized soil in the orthogonal experiment. Furthermore, according to the data in
Table 8, under a 5% OPC dosage, the dosage of SUSWM predominantly affects the water stability of the stabilized soil. Similarly, as shown in
Table 10, when the OPC dosage is 6%, the dosage of SISWM becomes the main influencing factor on water stability.
Figure 5 and
Figure 6 show that the UCS and WS of the solidified silt are directly proportional to the dosage of SISWM and inversely proportional to the dosage of SUSWM. The amount of hydration products generated through OH
− hydrolysis increases with higher SISWM dosage, leading to an increase in the UCS of the solidified silt. However, a high dosage of SUSWM may cause excess SUSWM to remain between particles in the solidified silt, which can disrupt the pore structure and reduce the UCS of the material.
When the OPC dosage is 5%, the UCS of the solidified silt first increases and then decreases with the increase of CAA dosage, while the WS coefficient decreases with increasing CAA dosage. When the OPC dosage is 6%, the UCS of the solidified silt decreases with the increase of CAA dosage, and the WS coefficient first increases and then decreases with increasing CAA dosage. The results show that when the dosage of OPC is low, CAA enhances the concentration of OH− in the solidified silt, thereby accelerating the hydrolysis of SISWM. However, when the OPC dosage is increased, excessive addition of SUSWM can cause alkaline substances to be precipitated, which will affect the subsequent reaction of SISWM in the components and lead to a decrease in the UCS of solidified silt.
3.2. Analysis of Control Group
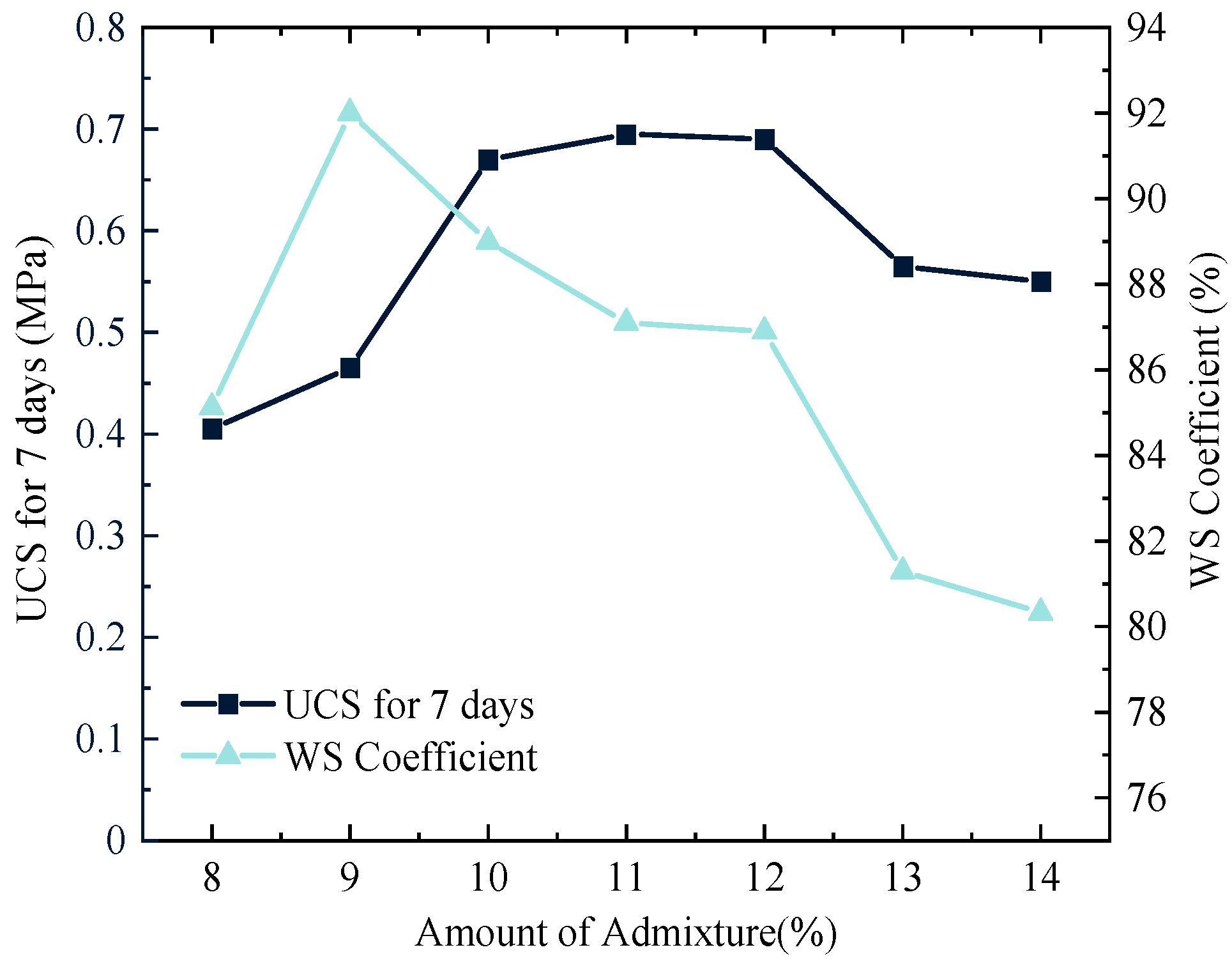
Figure 7 shows that the UCS and WS of solidified silt first increase and then decrease with the increase of OPC dosage. When the OPC dosage is 11%, the UCS reaches a maximum value of 1.39 MPa. When the OPC dosage is 9%, the WS coefficient reaches a maximum value of 92%. Excessive OPC dosage may result in surplus material accumulating within the pores of the solidified silt, which can hinder effective bonding between silt particles and potentially reduce both the UCS and WS of the material [
29].
In general, following the requirements of the UCS and WS, the group O6L1S3G2 demonstrates an optimal performance, i.e., the UCS and WS coefficients in the group O6L1S3G2 are 1.54 times and 1.0246 times those of the solidified silt by using OPC, respectively.
3.3. Analysis of SEM Tests
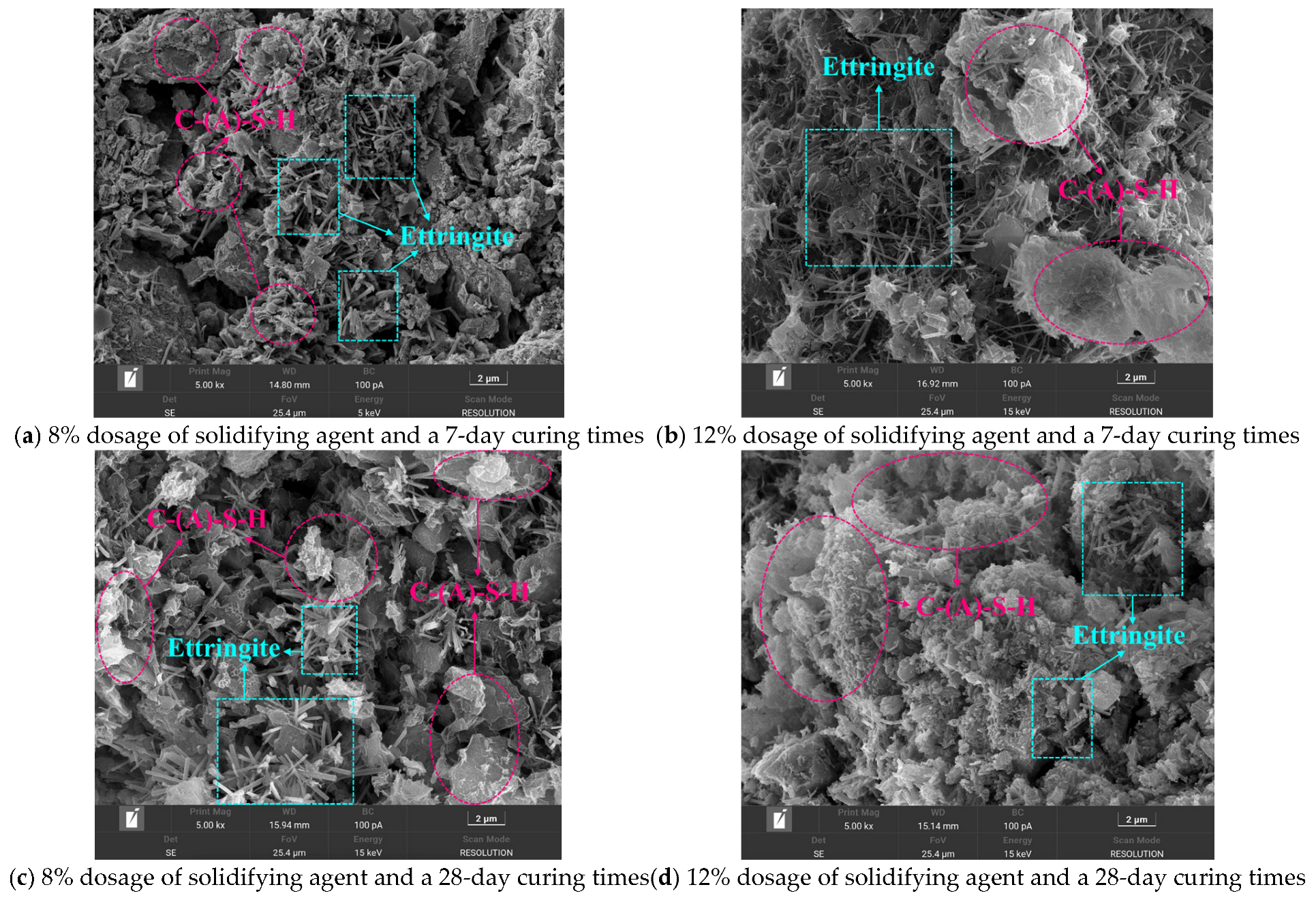
The test specimens from the O
6L
1S
3G
2 group were selected for SEM analysis (see
Figure 8), pink circular annotations were employed to indicate the presence of hydrated calcium silicate (C-(A)-S-H) (aluminate), while blue rectangular markers were utilized to identify the location of ettringite crystals. C-(A)-S-H typically adheres to the surfaces of silt particles and exhibits a blocky morphology. Ettringite is commonly distributed within the pore spaces between silt particles and displays a radiating needle-like or rod-like structure. SEM observations enabled the acquisition of microstructural images of the stabilized soil under varying dosages and curing times.
Figure 8 shows that after a curing period of 7 days, more hydrated calcium silicate (C-(A)-S-H) (aluminate) and ettringite crystals are observed in the solidified silt treated with a solidifying agent dosage of 12% compared to the solidified silt treated with an 8% dosage of solidifying agent. More hydrated calcium silicate (C-(A)-S-H) (aluminate) and ettringite crystals are observed in the solidified silt with a 28-day curing times compared to the solidified silt with a 7-day curing times.
3.4. Analysis of EDS Tests
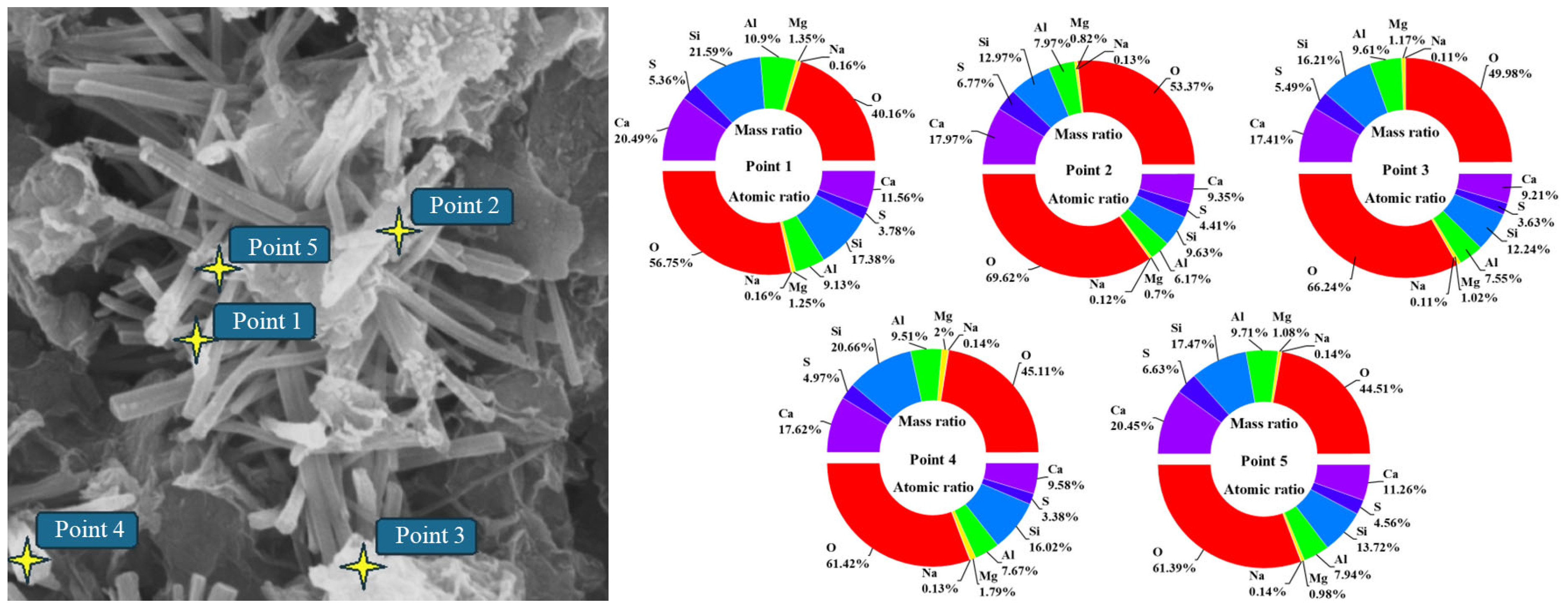
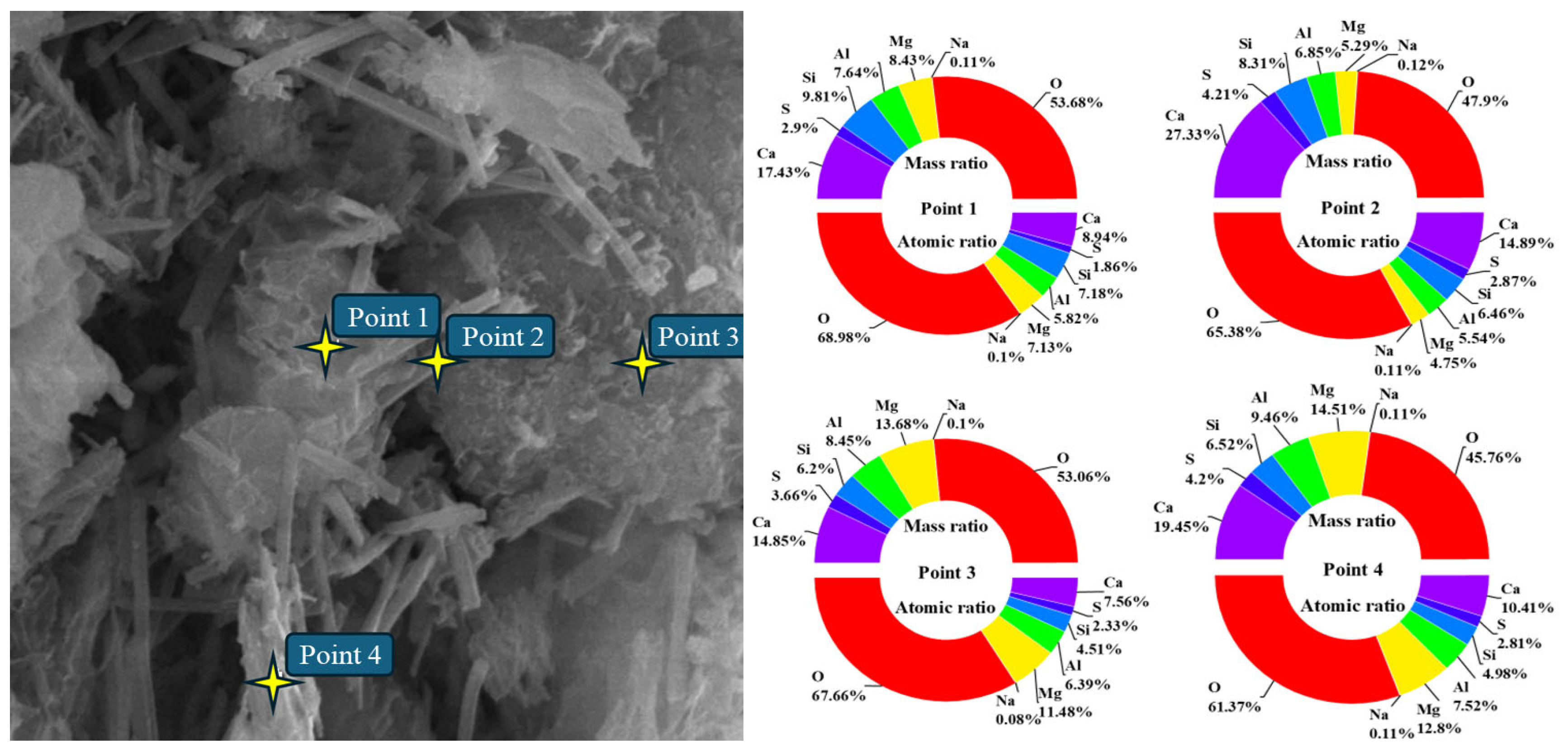
Figure 9 shows the EDS point schematic and elemental analysis results of solidified silt with a curing period of 28 days and a curing agent dosage of 8%. The upper section of the pie chart illustrates the mass ratio of each element at the designated position, whereas the lower section depicts the atomic ratio of each element.
The main hydration products are rod-shaped and flocculent. Therefore, points 3 and 5 (flocculent hydration products) and points 1, 2, and 4 (needle-shaped hydration products) are selected as energy spectrum points. The elemental analysis results show that the main elements of the hydration products are O, Al, Si, and Ca. The proportion of the atomic ratio of Ca to the atomic ratio of Si and the proportion of the atomic ratio of Ca to the atomic ratio of Al fluctuate around 1. Combined with the hydration reaction of OPC and the hydration products produced by alkali excitation of SISWM, the crystal may be C-S-H(I). It also shows that a small amount of sulfur is found in the hydration products, indicating that the SUSWM is involved in the hydration reaction.
Figure 10 shows the EDS point schematic and elemental analysis results of solidified soil with a 28-day curing period and a 12% curing agent dosage, and the main hydration products present are in the form of blocks. Therefore point 1 (smaller volume block hydration products), points 2 and 4 (rod-shaped hydration products), and point 3 (dense block hydration products) are selected as energy spectrum points. According to the elemental analysis results, the main elements of the hydration products are still O, Al, Mg, Si, Ca. At points 1 and 3, the proportion of the atomic ratio of Ca to the atomic ratio of Si fluctuates around 2, indicating that the blocky hydration product may be C-S-H(II). At points 2 and 4, the proportion of the atomic ratio of Ca to the atomic ratio of Si is greater than 2, indicating the formation of crystals composed of multiple hydration products. Besides, there is still a significant amount of magnesium in the hydration products, indicating that MgO in the SISWM also participates in the hydration reaction.
3.5. Analysis of XRD Tests
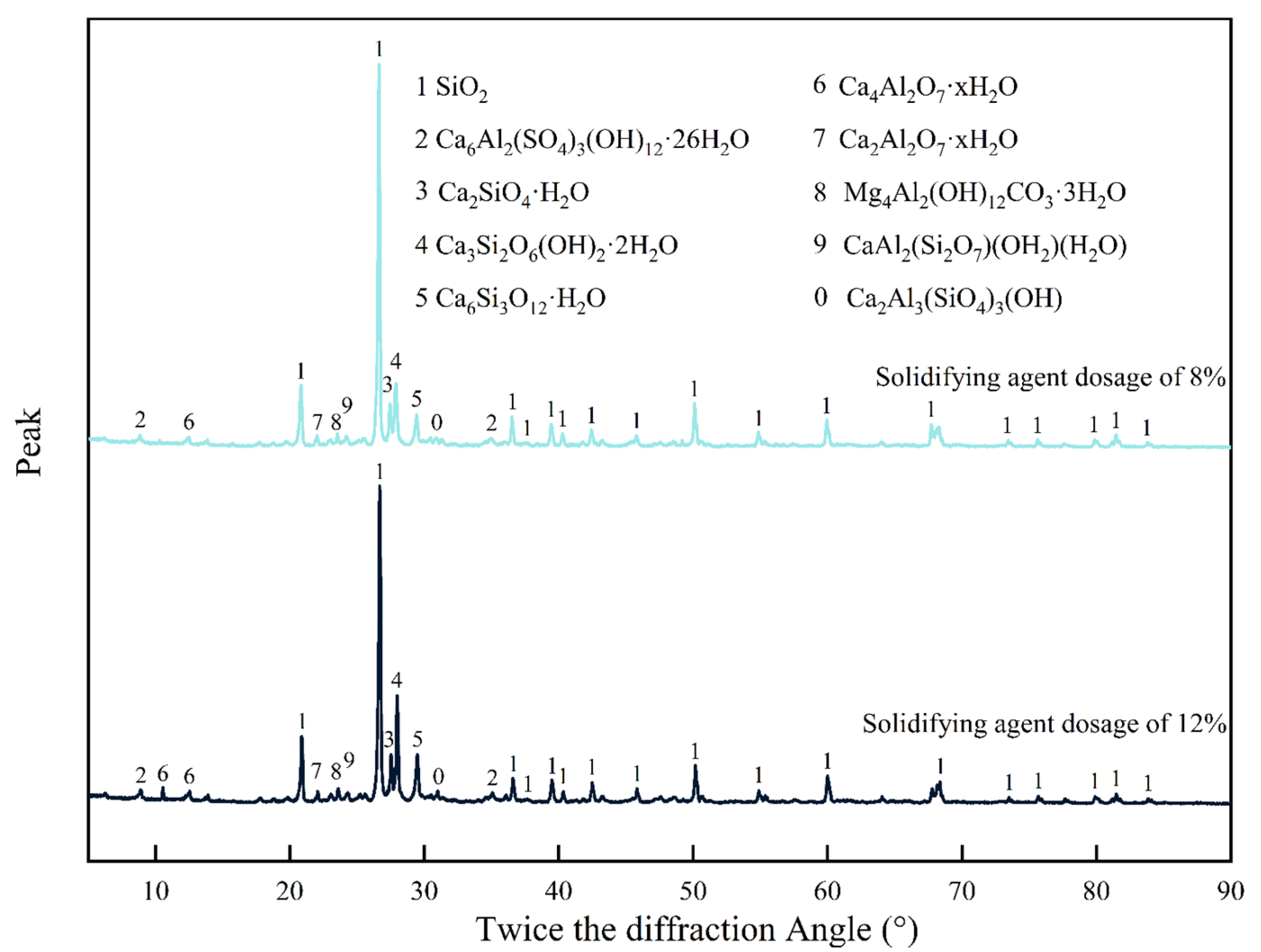
Figure 11 shows the XRD of solidified silt with a curing period of 28 days and solidifying agent dosages of 8% and 12%, respectively, at the diffraction angles in the range of 0° to 90°. In
Figure 11, numbers 0 to 9 are employed to denote different reaction products present in the stabilized silt, and these indicators are annotated at the apex of the corresponding diffraction peaks. The primary component of natural silt is quartz, therefore, the diffraction peak of SiO
2 is the most pronounced in the XRD pattern. The intensity relationships of the diffraction peaks discussed in the following analyses do not pertain to the intensity of the diffraction peak of SiO
2.
In the solidified silt, the type I C-S-H (Ca3Si2O6(OH)2·2H2O) and type II C-S-H (Ca6Si3O12·H2O, Ca2SiO4·H2O) have the highest diffraction peak, when the dosages of solidifying agent are 8% and 12%, respectively. Diffraction peaks of hydration products such as ettringite (Ca6Al2(SO4)3(OH)12·26H2O), hydrotalcite (Mg4Al2(OH)12CO3·3H2O), and hard columnar stone (CaAl2(Si2O7)(OH2)·H2O) are also investigated. This indicates that the hydration reaction of the solidifying agent mainly produces hydrated calcium silicate (aluminate) crystals C-(A)-S-H and ettringite. The peaks of type I and type II C-S-H significantly increase with the increase of curing agent dosage.
3.6. Analysis of Curing Mechanism
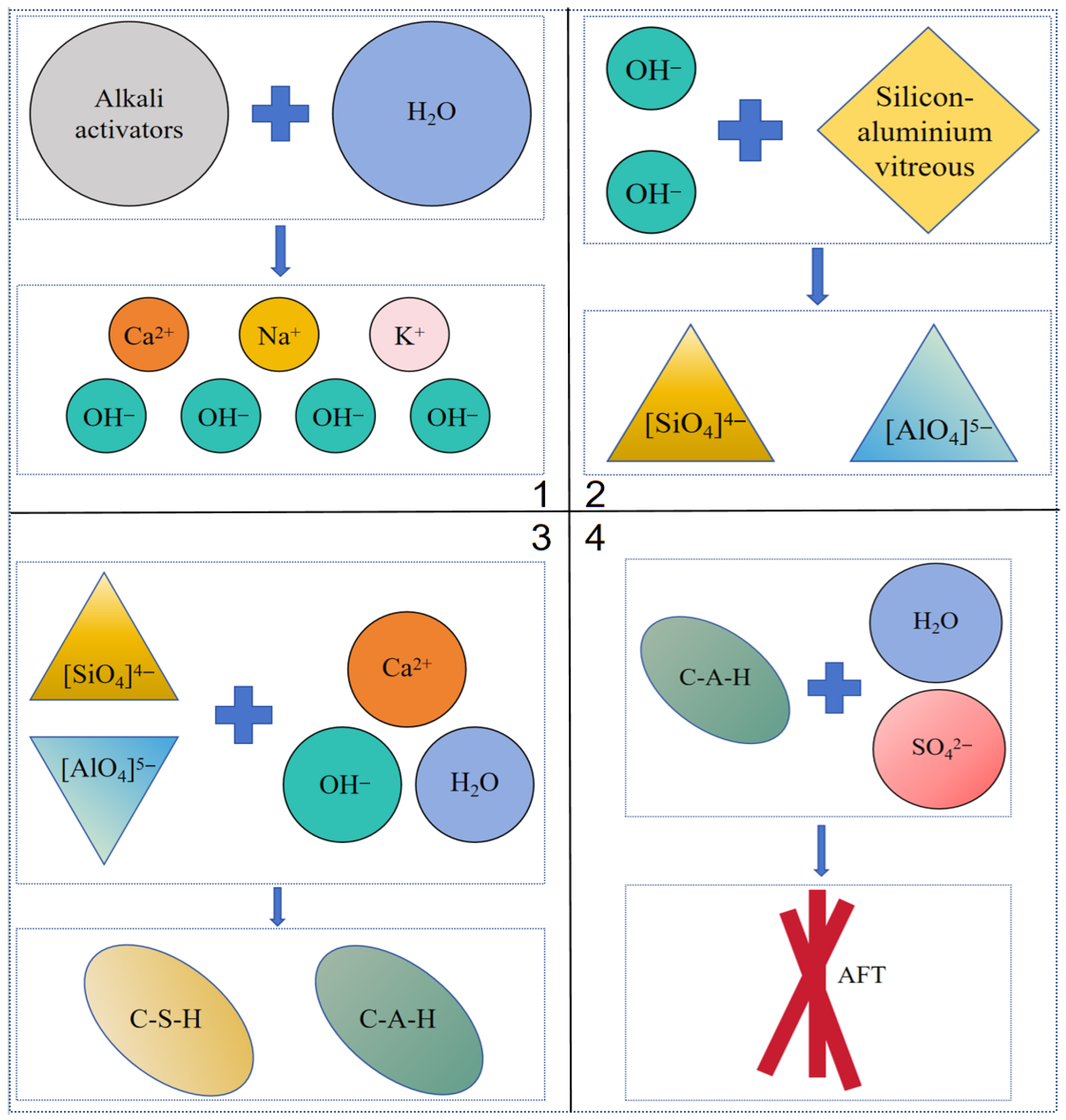
In the group O6L1S3G2, diffraction peaks of hydration products such as type I and type II C-S-H, ettringite, hydrotalcite, and andalusite are observed, indicating the reaction between OPC with CAA and SISWM to form C-S-H gels and C-A-H gels. Furthermore, the gels reacts with Ca(OH)2, SO42− to produce ettringite. The specific reaction process is outlined as follows.
The reaction between CAA and water generates a significant amount of OH
−, which subsequently corrode the silicon aluminum glass phase structure in the SISWM, and depolymerizing a large amount of [SiO
4]
4− and [AlO
4]
5− tetrahedra [
30]. The reaction equations are illustrated in Equations (1)–(3).
The active [SiO
4]
4− and [AlO
4]
5− monomers and the network modifier ions (i.e., Ca
2+, K
+, Na
+) undergo polycondensation to generate (C-S-H) gels and (C-A-H) gels [
30]. The reaction equations are illustrated in Equations (4) and (5).
Hydrated calcium aluminate (C-A-H) reacts with sulfate ions (SO
42−) in SUSWM to form hydrated calcium sufflaminate (ettringite) [
31]. The reaction equations are illustrated in Equation (6).
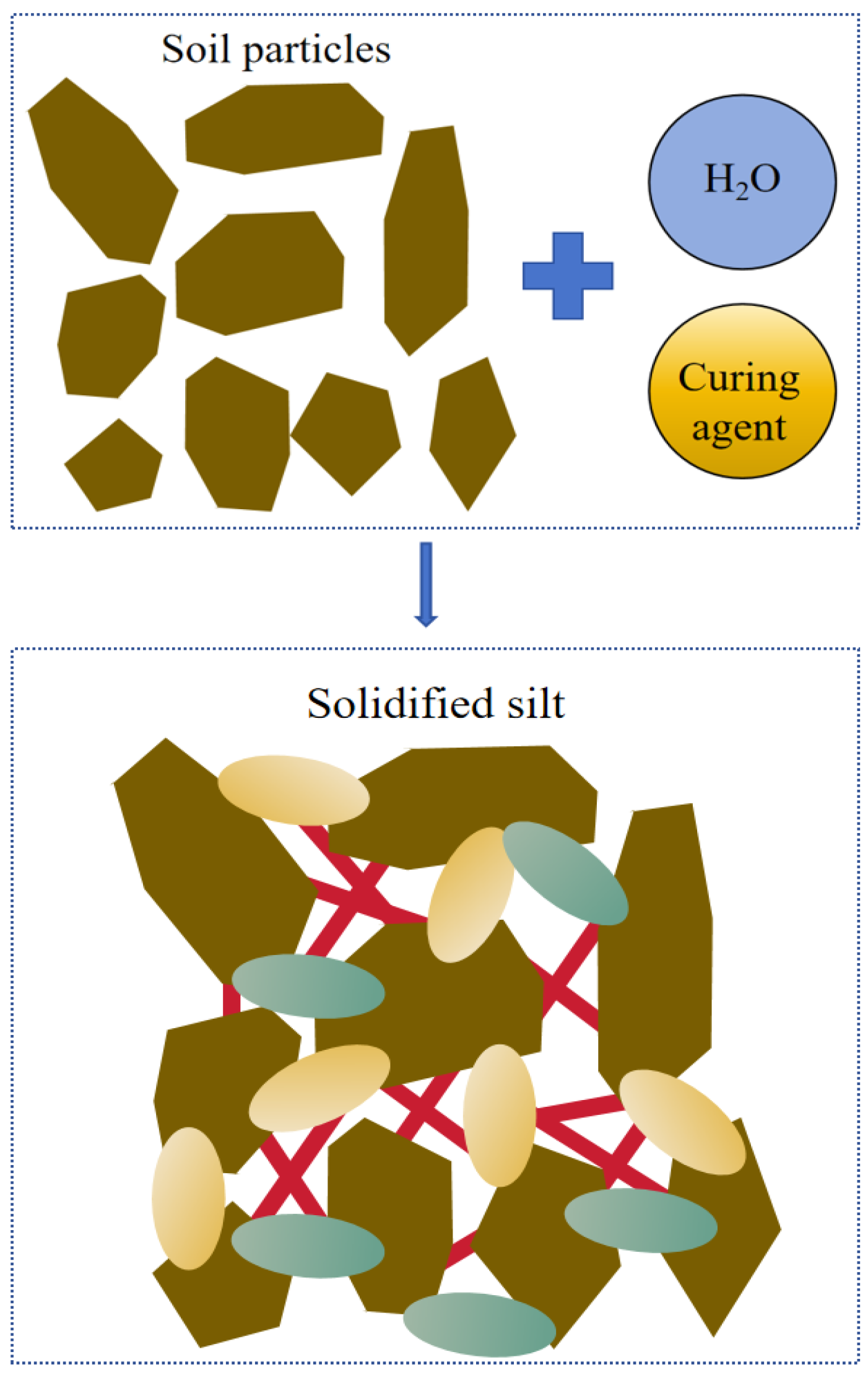
The strength of the solidified silt is attributed to the formation of C-A-H and C-S-H gels, while its WS is derived from the development of columnar crystals. The silt particles are encapsulated and interconnected by the C-(A)-S-H gel produced during the hydration reaction, leading to the formation of aggregates and thereby improving the cementation performance between soil particles. The residual pores among the particles are gradually filled by columnar crystals, which transform into smaller pores. This evolution significantly enhances the WS of the solidified silt. Macroscopically, these microstructural changes manifest as an increase in both strength and water stability of the solidified silt. The reaction process and solidification mechanism are schematically illustrated in
Figure 12 and
Figure 13.
4. Discussions
Based on the XRD and SEM analyses, it is evident that an increase in the dosage of the curing agent or the curing period of the stabilized silt promotes the hydration reaction of the curing agent, resulting in the formation of greater amounts of C-(A)-S-H. This reaction product encapsulates silt particles and facilitates aggregate formation, thereby enhancing the interparticle bonding capacity and ultimately improving the strength of the stabilized silt. The remaining pores in the silt will gradually be filled with columnar crystals such as hydrotalcite, hard spar, and ettringite, transforming into small pores and significantly improving the WS of the solidified silt.
The solidifying agent developed through the aforementioned research utilizes industrial by-products as primary raw materials throughout its lifecycle, thereby reducing the extraction of natural resources and minimizing solid waste disposal. This approach effectively lowers carbon emissions by approximately 40% to 60%. Moreover, the alkali activation process can be completed at relatively low temperatures, resulting in energy consumption that is only one-third of that required for OPC. From an economic perspective, the solidified silt treated with this solidifying agent demonstrates UCS exceeding that of conventional cement-stabilized silt, while the cost of the solidifying agent itself is 12.78% lower than that of OPC. Consequently, the overall cost is significantly reduced. Furthermore, this environmentally friendly technology aligns with national carbon reduction policies, offering both environmental sustainability and economic viability. However, this study did not address certain critical properties of solidifying agent, such as durability and shrinkage behavior. Future research should focus on these aspects to further enhance the engineering applicability and performance evaluation of the developed solidifying agent.
5. Conclusions
The present study investigates the impact of four materials, namely OPC, CAA, SISWM, and SUSWM, on the strength and durability of solidified silt through orthogonal experiments and comparative experiments. The mechanical properties, durability, microstructure, and hydration products of the solidified silt are analyzed through a series of laboratory tests, and based on these findings, the solidification mechanism of the solidifying agent is analyzed. The following conclusions are drawn:
The main factor affecting the UCS and WS of solidified silt is SISWM. Based on property such as strength and durability, the group O6L1S3G2 demonstrates an optimal performance, with the ratio of solidifying agent is OPC: CAA: SISWM: SUSWM = 6:1:3:2. At 12% dosage of the solidifying agent, the UCS reaches 1.065 MPa, representing a 1.54-fold increase compared to OPC solidified silt. Additionally, the WS coefficient is measured at 89.09%, which is 2.46% higher than that of the OPC solidified silt.
The primary hydration products of solidifying agents include type I and type II C-S-H, as well as C-A-H, ettringite, hydrotalcite, and hard spar.
The C-(A)-S-H formed during the hydration reaction envelops silt particles, forming aggregates, which enhance the bonding performance between particles and improve the UCS of the solidified silt. The residual pores within the silt will gradually be filled with columnar crystals such as hydrotalcite, hard spar, and ettringite, which transform into smaller pores and significantly enhance the WS of the solidified silt.