Pelvic Neuroanatomy in Colorectal Surgery: Advances in Nerve Preservation for Optimized Functional Outcomes
Abstract
1. Introduction
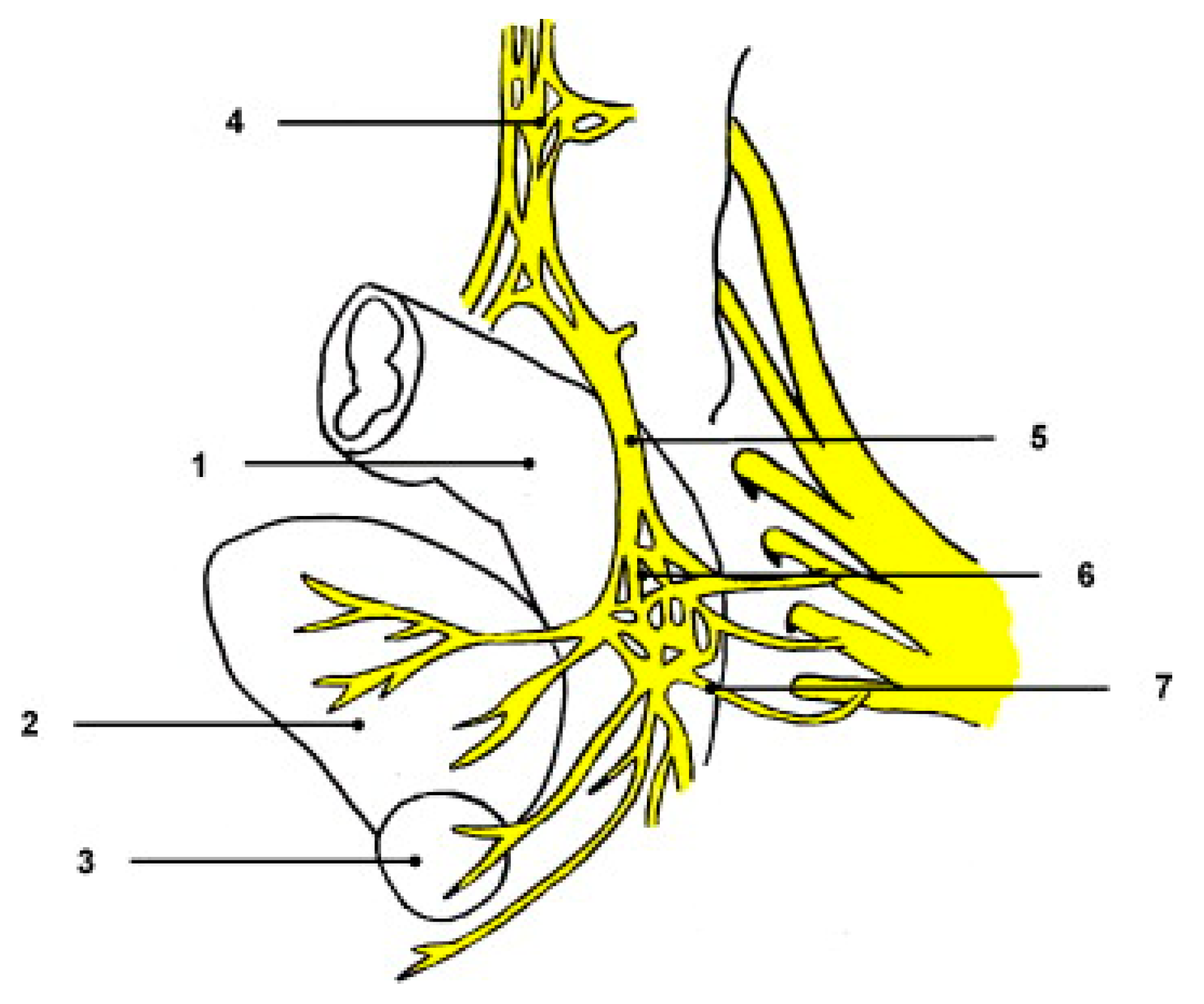
2. Pelvic Neuroanatomy
2.1. Pelvic Autonomic Nervous System
2.2. Specific Nerve Structures
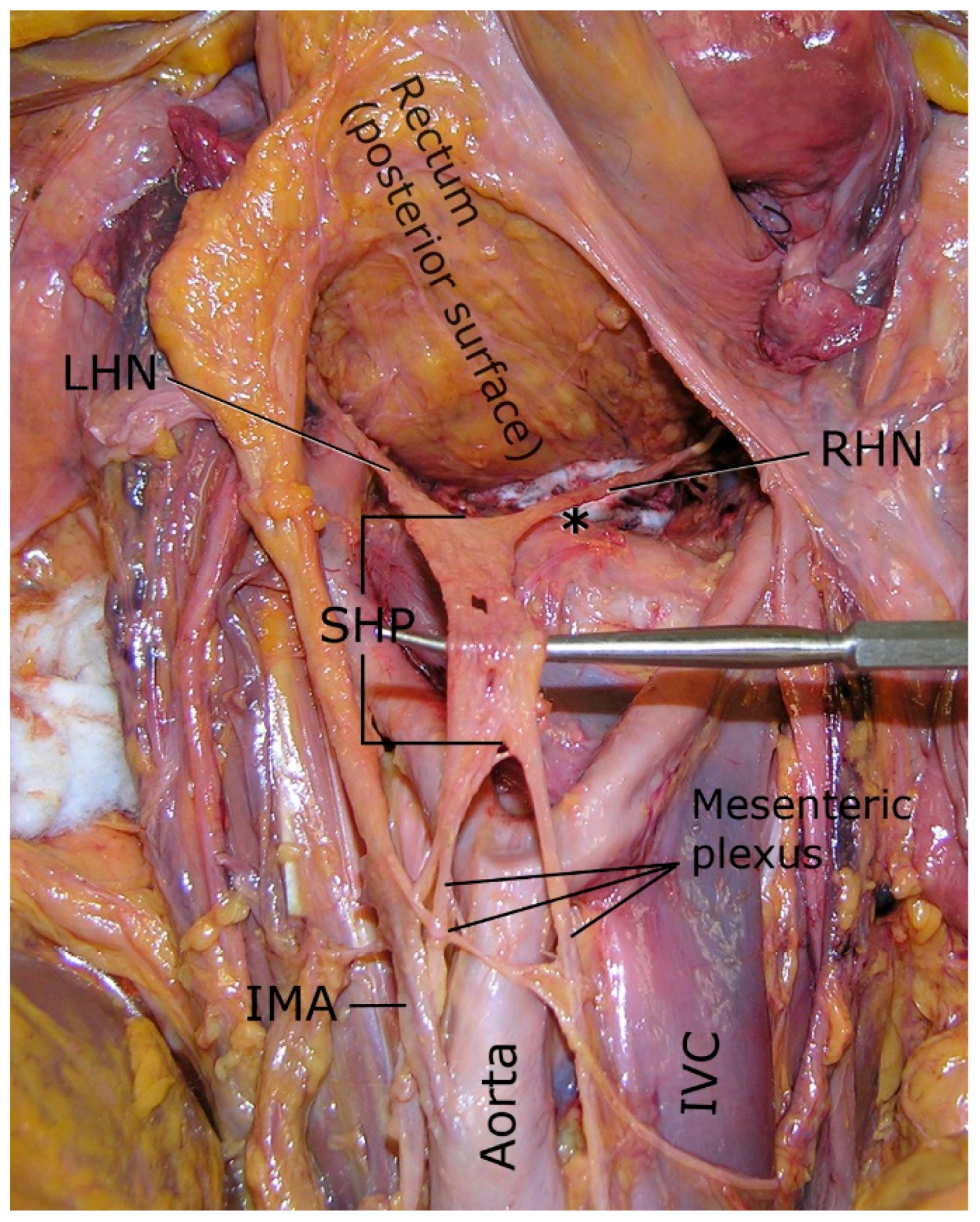
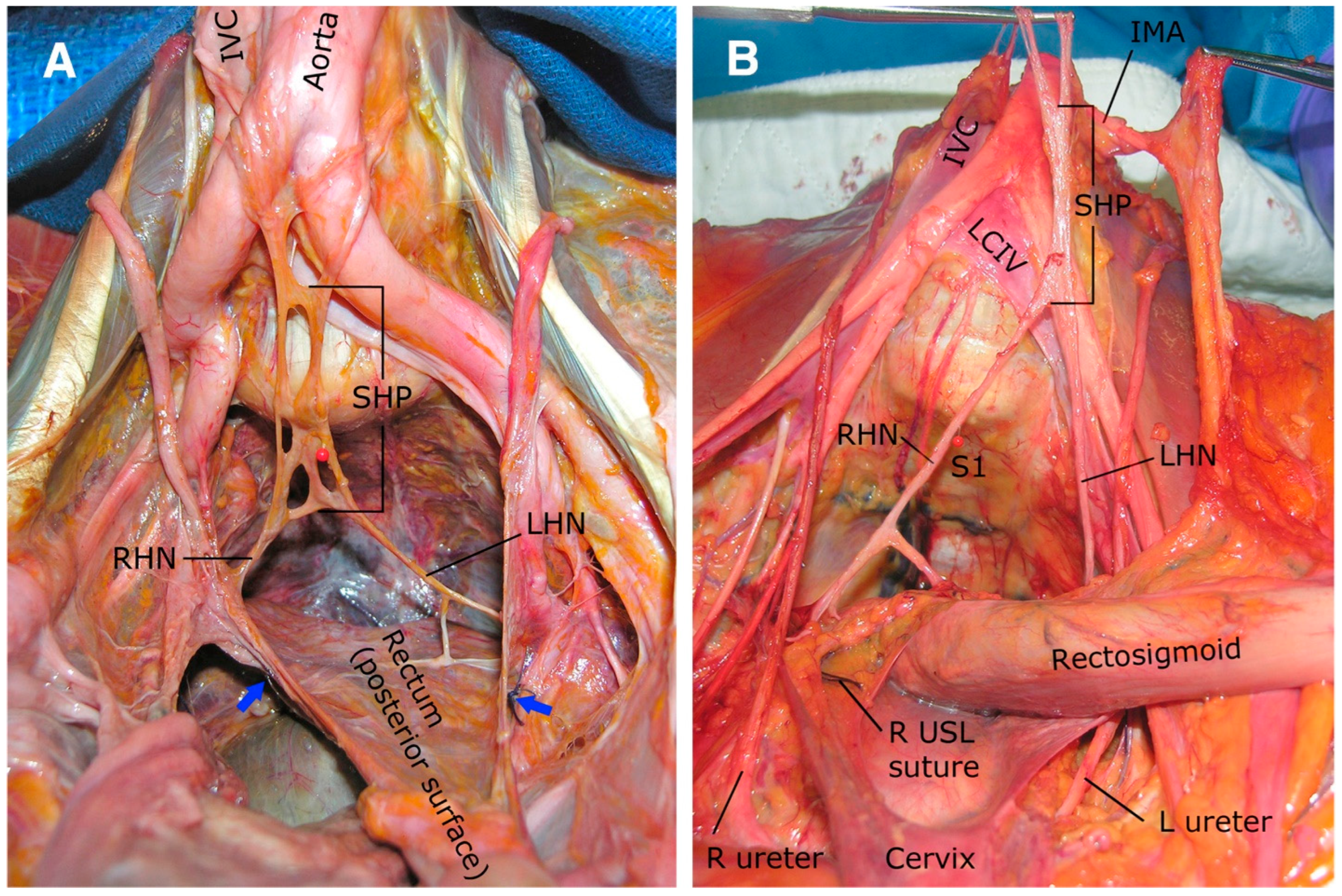
2.2.1. Superior Hypogastric Plexus (SHP)
2.2.2. Hypogastric Nerves
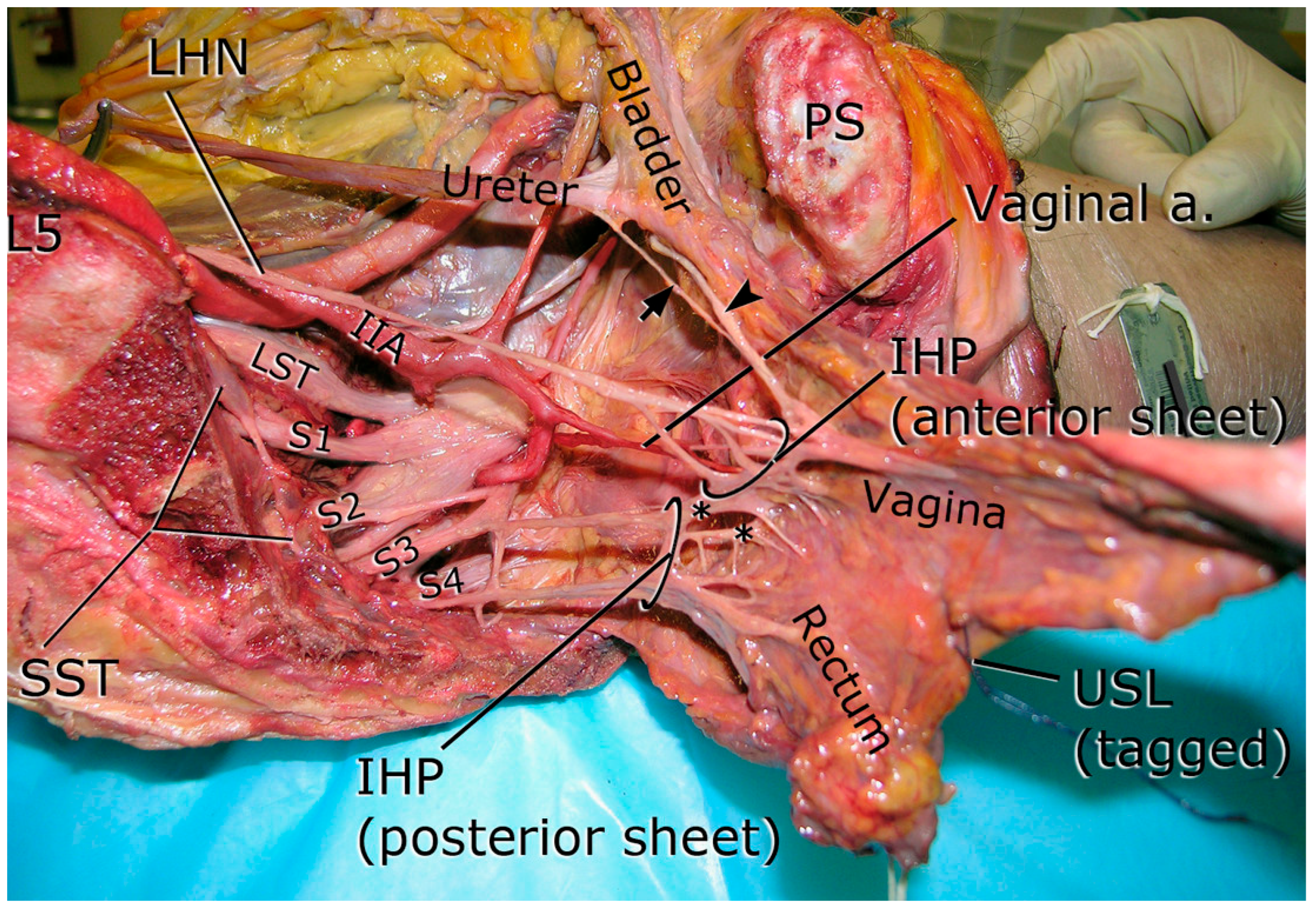
2.2.3. Pelvic Splanchnic Nerves
2.2.4. Inferior Hypogastric Plexus (IHP)
2.3. Splanchnic Innervation
2.4. Advanced Imaging Correlates
Quantitative Identification of Pelvic Autonomic Nerves: Imaging vs. Intraoperative
3. Surgical Challenges: Identification and Preservation of Pelvic Nerves
3.1. Nerve-Sparing Surgical Technique in Colorectal Surgery (Stepwise Protocol)
3.2. Intraoperative Identification Techniques
3.3. Variability in Anatomy and Technical Considerations
3.4. Nerve-Sparing Dissection Techniques
3.5. Integrating Preoperative Imaging into Surgical Planning
4. Functional Outcomes and Correlation with Nerve Preservation
4.1. Continence and Sexual Function Outcomes
4.2. Evidence from Comparative Studies
4.3. Role of Rehabilitation and Adjunct Therapies
4.4. Future Directions for Outcome Improvement
5. Integration of Anatomical Knowledge with Surgical Practice
5.1. Limitations of Current Literature
5.2. Translational Implications and Future Research Areas
5.3. Comparative Surgical Anatomy and Organ-Specific Nerve-Sparing Techniques
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Kim, N.K. Outcome of lateral pelvic lymph node dissection with total mesorectal excision in treatment of rectal cancer: A systematic review and meta-analysis. Surgery 2021, 169, 1005–1015. [Google Scholar] [CrossRef]
- Giglia, M.; Stein, S. Overlooked Long-Term Complications of Colorectal Surgery. Clin. Colon Rectal Surg. 2019, 32, 204–211. [Google Scholar] [CrossRef]
- Saito, S.; Fujita, S.; Mizusawa, J.; Kanemitsu, Y.; Saito, N.; Kinugasa, Y.; Akazai, Y.; Ota, M.; Ohue, M.; Komori, K.; et al. Male sexual dysfunction after rectal cancer surgery: Results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan Clinical Oncology Group Study JCOG0212. Eur. J. Surg. Oncol. 2016, 42, 1851–1858. [Google Scholar] [CrossRef]
- Sienkiewicz-Zawilińska, J.; Zawiliński, J.; Kaythampilla, L.N.; Jankiel, M.; Urbaniak, J.; Bereza, T.; Kowalski, W.; Loukas, M.; Walocha, J. Autonomic nervous system of the pelvis—General overview. Folia Med. Cracov. 2018, 58, 21–44. [Google Scholar] [CrossRef]
- Azaïs, H.; Collinet, P.; Delmas, V.; Rubod, C. Rapport anatomique du ligament utérosacré et du nerf hypogastrique pour la chirurgie des lésions d’endométriose profonde. Gynécologie Obs. Fertil. 2013, 41, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Aurore, V.; Röthlisberger, R.; Boemke, N.; Hlushchuk, R.; Bangerter, H.; Bergmann, M.; Imboden, S.; Mueller, M.D.; Eppler, E.; Djonov, V. Anatomy of the female pelvic nerves: A macroscopic study of the hypogastric plexus and their relations and variations. J. Anat. 2020, 237, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Iwanaga, J.; Chapman, J.R.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. Superior Hypogastric Plexus and Its Surgical Implications During Spine Surgery: A Review. World Neurosurg. 2018, 120, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Cho, K.H.; Hieda, K.; Kim, J.H.; Murakami, G.; Abe, S.; Matsubara, A. Composite nerve fibers in the hypogastric and pelvic splanchnic nerves: An immunohistochemical study using elderly cadavers. Anat. Cell Biol. 2015, 48, 114. [Google Scholar] [CrossRef]
- Muraoka, K.; Morizane, S.; Hieda, K.; Honda, M.; Sejima, T.; Murakami, G.; Abe, S.; Takenaka, A. Site-dependent differences in the composite fibers of male pelvic plexus branches: An immunohistochemical analysis of donated elderly cadavers. BMC Urol. 2018, 18, 47. [Google Scholar] [CrossRef]
- Alsaid, B.; Bessede, T.; Karam, I.; Abd-Alsamad, I.; Uhl, J.; Benoît, G.; Droupy, S.; Delmas, V. Coexistence of adrenergic and cholinergic nerves in the inferior hypogastric plexus: Anatomical and immunohistochemical study with 3D reconstruction in human male fetus. J. Anat. 2009, 214, 645–654. [Google Scholar] [CrossRef]
- Han, F.; Zhong, G.; Zhi, S.; Han, N.; Jiang, Y.; Tan, J.; Zhong, L.; Zhou, S. Artificial Intelligence Recognition System of Pelvic Autonomic Nerve During Total Mesorectal Excision. Dis. Colon Rectum 2025, 68, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Chen, L.; Zhang, W. Robotic-assisted colorectal surgery in colorectal cancer management: A narrative review of clinical efficacy and multidisciplinary integration. Front. Oncol. 2025, 15, 1–14. [Google Scholar] [CrossRef]
- McKechnie, T.; Khamar, J.; Chu, C.; Hatamnejad, A.; Jessani, G.; Lee, Y.; Doumouras, A.; Amin, N.; Hong, D.; Eskicioglu, C. Robotic versus laparoscopic colorectal surgery for patients with obesity: An updated systematic review and meta-analysis. ANZ J. Surg. 2025, 95, 675–689. [Google Scholar] [CrossRef]
- Kutlu, B.; Guner, M.A.; Akyol, C.; Gungor, Y.; Benlice, C.; Arslan, M.N.; Açar, H.İ.; Kuzu, M.A. Comprehensive anatomy of the superior hypogastric plexus and its relationship with pelvic surgery landmarks: Defining the safe zone around the promontory. Tech. Coloproctol. 2022, 26, 655–664. [Google Scholar] [CrossRef]
- Rocher, G.; Azaïs, H.; Favier, A.; Uzan, C.; Castela, M.; Moawad, G.; Lavoué, V.; Morandi, X.; Nyangoh Timoh, K.; Canlorbe, G. Relationships between pelvic nerves and levator ani muscle for posterior sacrocolpopexy: An anatomic study. Surg. Radiol. Anat. 2022, 44, 891–898. [Google Scholar] [CrossRef]
- Almughamsi, A.M.; Elhassan, Y.H. Understanding the anatomical basis of anorectal fistulas and their surgical management: Exploring different types for enhanced precision and safety. Surg. Today 2025, 55, 457–474. [Google Scholar] [CrossRef]
- Ripperda, C.M.; Jackson, L.A.; Phelan, J.N.; Carrick, K.S.; Corton, M.M. Anatomic relationships of the pelvic autonomic nervous system in female cadavers: Clinical applications to pelvic surgery. Am. J. Obstet. Gynecol. 2017, 216, e1–e388. [Google Scholar] [CrossRef]
- Ghignone, F.; Hernandez, P.; Mahmoud, N.N.; Ugolini, G. Functional recovery in senior adults undergoing surgery for colorectal cancer: Assessment tools and strategies to preserve functional status. Eur. J. Surg. Oncol. 2020, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bislenghi, G. Post-Operative Functional Outcomes in Early Age Onset Rectal Cancer. Front. Oncol. 2022, 12, 868359. [Google Scholar] [CrossRef] [PubMed]
- Garoufalia, Z.; Wexner, S.D. Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. J. Clin. Med. 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Lamblin, A.; Mariette, C.; Pocard, M. Sexual and urinary dysfunction after proctectomy for rectal cancer. J. Visc. Surg. 2010, 147, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Roche, F.; Pichot, V.; Mouhli-Gasmi, L.; Monier, M.; Barthélémy, J.C.; Berger, M.; Celle, S.; Chouchou, F. Anatomy and physiology of the autonomic nervous system: Implication on the choice of diagnostic/monitoring tools in 2023. Rev. Neurol. 2024, 180, 42–52. [Google Scholar] [CrossRef]
- Sekiyama, K.; Fujii, S.; Mandai, M. Anatomical location of the surgically identifiable bladder branch of the inferior hypogastric plexus for nerve-sparing radical hysterectomy. Gynecol. Oncol. Rep. 2023, 46, 101152. [Google Scholar] [CrossRef]
- Bankenahally, R.; Krovvidi, H. Autonomic nervous system: Anatomy, physiology, and relevance in anaesthesia and critical care medicine. BJA Educ. 2016, 16, 381–387. [Google Scholar] [CrossRef]
- Abdelli, A.; Tillou, X.; Alves, A.; Menahem, B. Genito-urinary sequelae after carcinological rectal resection: What to tell patients in 2017. J. Visc. Surg. 2017, 154, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.L.D.; Tsukada, Y.; Ito, M. Essential anatomy for total mesorectal excision and lateral lymph node dissection, in both trans-abdominal and trans-anal perspective. Surgeon 2021, 19, e462–e474. [Google Scholar] [CrossRef]
- Bazira, P.J. Anatomy of the lower urinary tract. Surgery 2022, 40, 489–500. [Google Scholar] [CrossRef]
- Alkatout, I.; Wedel, T.; Pape, J.; Possover, M.; Dhanawat, J. Review: Pelvic nerves—From anatomy and physiology to clinical applications. Transl. Neurosci. 2021, 12, 362–378. [Google Scholar] [CrossRef]
- Kraima, A.C.; van Schaik, J.; Susan, S.; van de Velde, C.J.H.; Hamming, J.F.; Lakke, E.A.J.F.; DeRuiter, M.C. New insights in the neuroanatomy of the human adult superior hypogastric plexus and hypogastric nerves. Auton. Neurosci. 2015, 189, 60–67. [Google Scholar] [CrossRef]
- Paraskevas, G.; Tsitsopoulos, P.; Papaziogas, B.; Natsis, K.; Martoglou, S.; Stoltidou, A.; Kitsoulis, P. Variability in superior hypogastric plexus morphology and its clinical applications: A cadaveric study. Surg. Radiol. Anat. 2008, 30, 481–488. [Google Scholar] [CrossRef]
- Mantzaris, G.; Rodolakis, A.; Vlachos, G.; Athanasiou, S.; Theocharis, S.; Sotiripoulou, C.H.M.; Antsaklis, A. Magnifying lenses assisted nerve-sparing radical hysterectomy and prevention of nerve plexus trauma. Int. J. Gynecol. Cancer 2008, 18, 868–875. [Google Scholar] [CrossRef]
- Craven, J. Lumbar and sacral plexuses. Anaesth. Intensive Care Med. 2004, 5, 108. [Google Scholar] [CrossRef]
- Sharabi, A.F.; Carey, F.J. Anatomy, Abdomen and Pelvis, Splanchnic Nerves; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Wijsmuller, A.R.; Giraudeau, C.; Leroy, J.; Kleinrensink, G.J.; Rociu, E.; Romagnolo, L.G.; Melani, A.G.F.; Agnus, V.; Diana, M.; Soler, L.; et al. A step towards stereotactic navigation during pelvic surgery: 3D nerve topography. Surg. Endosc. 2018, 32, 3582–3591. [Google Scholar] [CrossRef] [PubMed]
- Kraima, A.C.; Derks, M.; Smit, N.N.; van de Velde, C.J.H.; Kenter, G.G.; DeRuiter, M.C. Careful Dissection of the Distal Ureter Is Highly Important in Nerve-sparing Radical Pelvic Surgery. Int. J. Gynecol. Cancer 2016, 26, 959–966. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, B.; Zhong, J.; Zhong, Y.; Zhi, S.; Shen, J.; Zhou, S.; Tan, J.; Huang, J.; Zhu, J.; et al. Magnetic resonance neuroimaging promotes the preservation of pelvic autonomic nerves in laparoscopic total mesorectal excision: A comparative study. Ann. Transl. Med. 2021, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.O.; Mille, E.; Virzi, A.; Marret, J.-B.; Peyrot, Q.; Delmonte, A.; Berteloot, L.; Gori, P.; Blanc, T.; Grevent, D.; et al. Integrating tractography in pelvic surgery: A proof of concept. J. Pediatr. Surg. Case Rep. 2019, 48, 101268. [Google Scholar] [CrossRef]
- Pilania, K.; Jankharia, B.; Kazi, A.; Sankhe, S. Enhanced diagnostic value of adding 3D MR neurography sequence to routine lumbar spine MRI protocol: A retrospective study. J. Clin. Orthop. Trauma 2025, 65, 102990. [Google Scholar] [CrossRef] [PubMed]
- Weissman, E.; Boothe, E.; Wadhwa, V.; Scott, K.; Chhabra, A. Magnetic Resonance Neurography of the Pelvic Nerves. Semin. Ultrasound CT MRI 2017, 38, 269–278. [Google Scholar] [CrossRef]
- Jin, H.; Zheng, L.; Lu, L.; Cui, M. Near-infrared intraoperative imaging of pelvic autonomic nerves: A pilot study. Surg. Endosc. 2022, 36, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, Z.; Tao, L.; Tang, M.; Xu, Y.; Pan, Y.; Zhang, K.; Qiu, X.; Lv, F. 3D MR neurography with gadolinium contrast to improve the visualization of pelvic nerves and the branches. Front. Physiol. 2024, 15, 1394431. [Google Scholar] [CrossRef] [PubMed]
- Kneist, W.; Ghadimi, M.; Runkel, N.; Moesta, T.; Coerper, S.; Benecke, C.; Kauff, D.W.; Gretschel, S.; Gockel, I.; Jansen-Winkeln, B.; et al. Pelvic Intraoperative Neuromonitoring Prevents Dysfunction in Patients With Rectal Cancer. Ann. Surg. 2023, 277, e737–e744. [Google Scholar] [CrossRef]
- Kim, N.K. Anatomic Basis of Sharp Pelvic Dissection for Curative Resection of Rectal Cancer. Yonsei Med. J. 2005, 46, 737. [Google Scholar] [CrossRef]
- Fang, J.; Wei, B.; Zheng, Z.; Xiao, J.; Han, F.; Huang, M.; Xu, Q.; Wang, X.; Hong, C.; Wang, G.; et al. Preservation versus resection of Denonvilliers’ fascia in total mesorectal excision for male rectal cancer: Follow-up analysis of the randomized PUF-01 trial. Nat. Commun. 2023, 14, 6667. [Google Scholar] [CrossRef]
- Wei, B.; Zheng, Z.; Fang, J.; Xiao, J.; Han, F.; Huang, M.; Xu, Q.; Wang, X.; Hong, C.; Wang, G.; et al. Effect of Denonvilliers’ Fascia Preservation Versus Resection During Laparoscopic Total Mesorectal Excision on Postoperative Urogenital Function of Male Rectal Cancer Patients. Ann. Surg. 2021, 274, e473–e480. [Google Scholar] [CrossRef]
- Choi, G.-S.; Kim, H.J. The role of lateral pelvic lymph node dissection in advanced rectal cancer: A review of current evidence and outcomes. Ann. Coloproctol. 2024, 40, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Phang, P.T. Total mesorectal excision: Technical aspects. Can. J. Surg. 2004, 47, 130–137. [Google Scholar] [PubMed]
- Sato, S.; Kagoshima, H.; Shiozawa, M.; Nukada, S.; Iguchi, K.; Mikayama, Y.; Oshima, T.; Numata, M.; Tamagawa, H.; Rino, Y.; et al. Automated non-invasive identification of pelvic autonomic nerves with a handheld Raman spectrometer and potential application to nerve-sparing colorectal surgery: A preliminary study in surgical specimens. Transl. Cancer Res. 2021, 10, 3921–3929. [Google Scholar] [CrossRef]
- Kauff, D.W.; Kempski, O.; Koch, K.P.; Huppert, S.; Hoffmann, K.P.; Lang, H.; Kneist, W. Continuous intraoperative monitoring of autonomic nerves during low anterior rectal resection: An innovative approach for observation of functional nerve integrity in pelvic surgery. Langenbeck’s Arch. Surg. 2012, 397, 787–792. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Sugihara, K. Topology of the Fascial Structures in Rectal Surgery: Complete Cancer Resection and the Importance of Avoiding Autonomic Nerve Injury. Semin. Colon Rectal Surg. 2010, 21, 95–101. [Google Scholar] [CrossRef]
- Wei, H.B.; Fang, J.F.; Zheng, Z.H.; Wei, B.; Huang, J.L.; Chen, T.F.; Huang, Y.; Lei, P.R. Effect of preservation of Denonvilliers’ fascia during laparoscopic resection for mid-low rectal cancer on protection of male urinary and sexual functions. Medicine 2016, 95, e3925. [Google Scholar] [CrossRef]
- Galema, H.A.; Meijer, R.P.J.; Lauwerends, L.J.; Verhoef, C.; Burggraaf, J.; Vahrmeijer, A.L.; Hutteman, M.; Keereweer, S.; Hilling, D.E. Fluorescence-guided surgery in colorectal cancer; A review on clinical results and future perspectives. Eur. J. Surg. Oncol. 2022, 48, 810–821. [Google Scholar] [CrossRef]
- Chew, M.-H.; Yeh, Y.-T.; Lim, E.; Seow-Choen, F. Pelvic autonomic nerve preservation in radical rectal cancer surgery: Changes in the past 3 decades. Gastroenterol. Rep. 2016, 4, 173–185. [Google Scholar] [CrossRef]
- Bertrand, M.M.; Macri, F.; Mazars, R.; Droupy, S.; Beregi, J.P.; Prudhomme, M. MRI-based 3D pelvic autonomous innervation: A first step towards image-guided pelvic surgery. Eur. Radiol. 2014, 24, 1989–1997. [Google Scholar] [CrossRef]
- Kneist, W.; Kauff, D.W.; Juhre, V.; Hoffmann, K.P.; Lang, H. Is intraoperative neuromonitoring associated with better functional outcome in patients undergoing open TME? Eur. J. Surg. Oncol. 2013, 39, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chen, W.; Huang, L.; Ni, J.; Ding, W.; Yin, L. The anatomic basis of total mesorectal excision. Clin. Sci. 2011, 201, 537–543. [Google Scholar] [CrossRef]
- Gaessler, J.; Anderhuber, F.; Kuchling, S.; Pilsl, U. Topography of the pelvic autonomic nerves—An anatomical study to facilitate nerve-preserving total mesorectal excision. Acta Chir. Belg. 2022, 122, 396–402. [Google Scholar] [CrossRef]
- Fleming, C.A.; Cullinane, C.; Lynch, N.; Killeen, S.; Coffey, J.C.; Peirce, C.B. Urogenital function following robotic and laparoscopic rectal cancer surgery: Meta-analysis. Br. J. Surg. 2021, 108, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Thiel, P.; Kobylianskii, A.; McGrattan, M.; Lemos, N. Entrapped by pain: The diagnosis and management of endometriosis affecting somatic nerves. Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 95, 102502. [Google Scholar] [CrossRef] [PubMed]
- Godolias, P.; Tataryn, Z.L.; McCullough, B.J.; Abdul-Jabbar, A.; Gerstmeyer, J.R.; Plümer, J.; Cibura, C.; Koutras, C.; Heep, H.; Dudda, M.; et al. Lumbosacral plexus 3D printing with dissection validation—A baseline study with regards to lateral spine surgery. Interdiscip. Neurosurg. 2023, 31, 101666. [Google Scholar] [CrossRef]
- Ortega, M.V.; Bordeianou, L. Sexual and urinary dysfunction after proctectomy with or without abdominoperineal resection: Incidence and treatment. Semin. Colon Rectal Surg. 2021, 32, 100848. [Google Scholar] [CrossRef]
- Ye, L.; Huang, M.; Huang, Y.; Yu, K.; Wang, X. Risk factors of postoperative low anterior resection syndrome for colorectal cancer: A meta-analysis. Asian J. Surg. 2022, 45, 39–50. [Google Scholar] [CrossRef]
- Burch, J.; Swatton, A.; Taylor, C.; Wilson, A.; Norton, C. Managing Bowel Symptoms After Sphincter-Saving Rectal Cancer Surgery: A Scoping Review. J. Pain Symptom Manage. 2021, 62, 1295–1307. [Google Scholar] [CrossRef]
- Campelo, P.; Barbosa, E. Functional outcome and quality of life following treatment for rectal cancer. J. Coloproctology 2016, 36, 251–261. [Google Scholar] [CrossRef]
- Kang, S.-B.; Cho, J.R.; Jeong, S.-Y.; Oh, J.H.; Ahn, S.; Choi, S.; Kim, D.-W.; Lee, B.H.; Youk, E.G.; Park, S.C.; et al. Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): A long-term prospective, multicentre, cohort study. Lancet Reg. Health-West. Pacific 2021, 6, 100087. [Google Scholar] [CrossRef]
- Wong, P.C.; Chow, F.C.L.; Law, W.L.; Foo, C.C. Anorectal and urogenital functional outcome after robotic and transanal total mesorectal excision for rectal cancer: A propensity score-matched analysis. Tech. Coloproctol. 2025, 29, 141. [Google Scholar] [CrossRef]
- Habeeb, T.A.A.M.; Podda, M.; Chiaretti, M.; Kechagias, A.; Lledó, J.B.; Kalmoush, A.-E.; Mustafa, F.M.; Nassar, M.S.; Labib, M.F.; Teama, S.R.A.; et al. Comparative study of laparoscopic ventral mesh rectopexy versus perineal stapler resection for external full-thickness rectal prolapse in elderly patients: Enhanced outcomes and reduced recurrence rates—A retrospective cohort study. Tech. Coloproctol. 2024, 28, 48. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Lei, Y.; Zhang, H.; Xie, J.; Zhu, S.; Jiang, J.; Li, J.; Yi, B. Evaluation of effect of robotic versus laparoscopic surgical technology on genitourinary function after total mesorectal excision for rectal cancer. Int. J. Surg. 2022, 104, 106800. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Zhao, W.; Mei, S.; Li, Y.; Liu, Q. Efficacy of ICG-guided bilateral lateral lymph node dissection in rectal cancer. Clin. Surg. Oncol. 2025, 4, 100079. [Google Scholar] [CrossRef]
- Pal, A. The digital horizon in colorectal cancer surgery: A narrative review. Laparosc. Endosc. Robot. Surg. 2025, 8, 67–72. [Google Scholar] [CrossRef]
- Yao, S.; Lan, H.; Han, Y.; Mao, C.; Yang, M.; Zhang, X.; Jin, K. From organ preservation to selective surgery: How immunotherapy changes colorectal surgery? Surg. Open Sci. 2023, 15, 44–53. [Google Scholar] [CrossRef]
- Mansouri, V.; Beheshtizadeh, N.; Gharibshahian, M.; Sabouri, L.; Varzandeh, M.; Rezaei, N. Recent advances in regenerative medicine strategies for cancer treatment. Biomed. Pharmacother. 2021, 141, 111875. [Google Scholar] [CrossRef]
- Erozkan, K.; Gorgun, E. Robotic colorectal surgery and future directions. Am. J. Surg. 2024, 230, 91–98. [Google Scholar] [CrossRef]
- Ruo, L.; Guillem, J.G. Surgical management of primary colorectal cancer. Surg. Oncol. 1998, 7, 153–163. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Jia, X.; Qu, Y.; Li, M.; Cao, C.; Zhang, Z.; Qu, Q.; Luo, S.; Tang, J.; et al. NIR-II fluorescence imaging-guided colorectal cancer surgery targeting CEACAM5 by a nanobody. eBioMedicine 2023, 89, 104476. [Google Scholar] [CrossRef]
- Sun, J.-X.; Xu, J.-Z.; An, Y.; Ma, S.-Y.; Liu, C.-Q.; Zhang, S.-H.; Luan, Y.; Wang, S.-G.; Xia, Q.-D. Future in precise surgery: Fluorescence-guided surgery using EVs derived fluorescence contrast agent. J. Control. Release 2023, 353, 832–841. [Google Scholar] [CrossRef]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: Systematic review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef]
- Jafari, M.D.; Lee, K.H.; Halabi, W.J.; Mills, S.D.; Carmichael, J.C.; Stamos, M.J.; Pigazzi, A. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg. Endosc. 2013, 27, 3003–3008. [Google Scholar] [CrossRef]
- Maas, C.P.; Trimbos, J.B.; Deruiter, M.C.; Van De Velde, C.J.H.; Kenter, G.G. Nerve sparing radical hysterectomy: Latest developments and historical perspective. Crit. Rev. Oncol. Hematol. 2003, 48, 271–279. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Armbrust, R.; Neymeyer, J.; Miranda, A.; Muallem, J. Nerve Sparing Radical Hysterectomy: Short-Term Oncologic, Surgical, and Functional Outcomes. Cancers 2020, 12, 483. [Google Scholar] [CrossRef]
- Nally, D.M.; Kavanagh, D.O.; Winter, D.C. Close rectal dissection in benign diseases of the rectum: A review. Surg. 2019, 17, 119–126. [Google Scholar] [CrossRef] [PubMed]
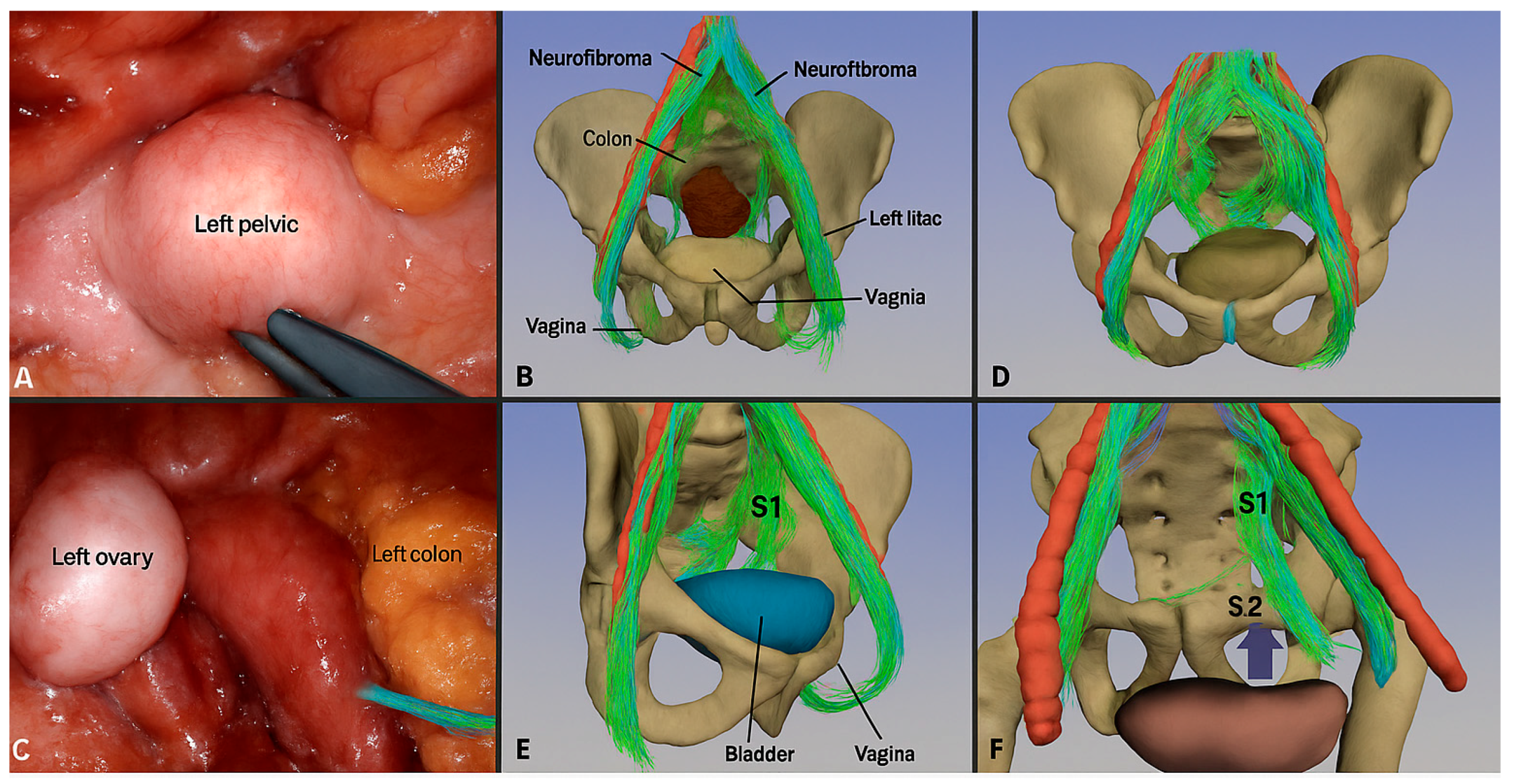

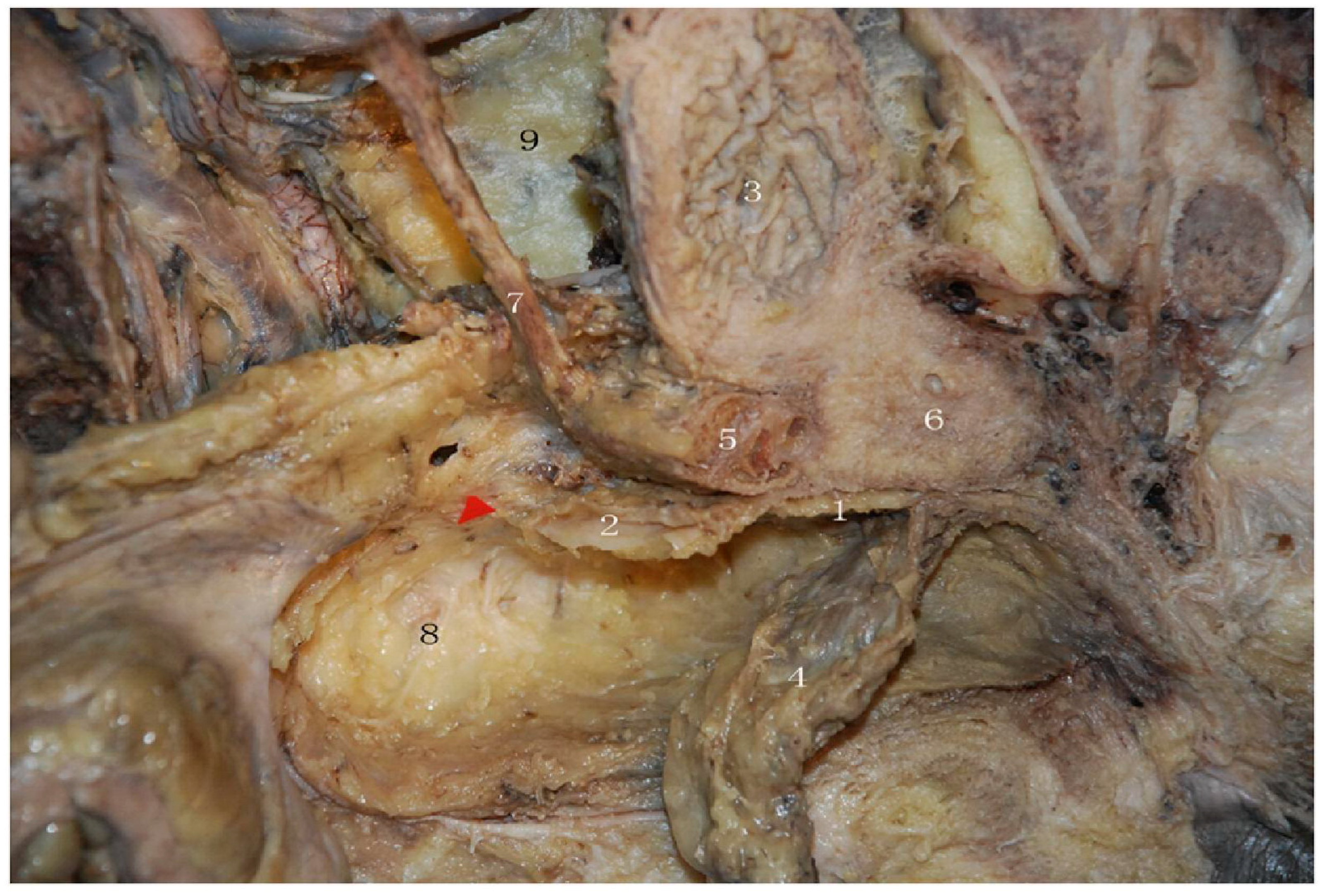
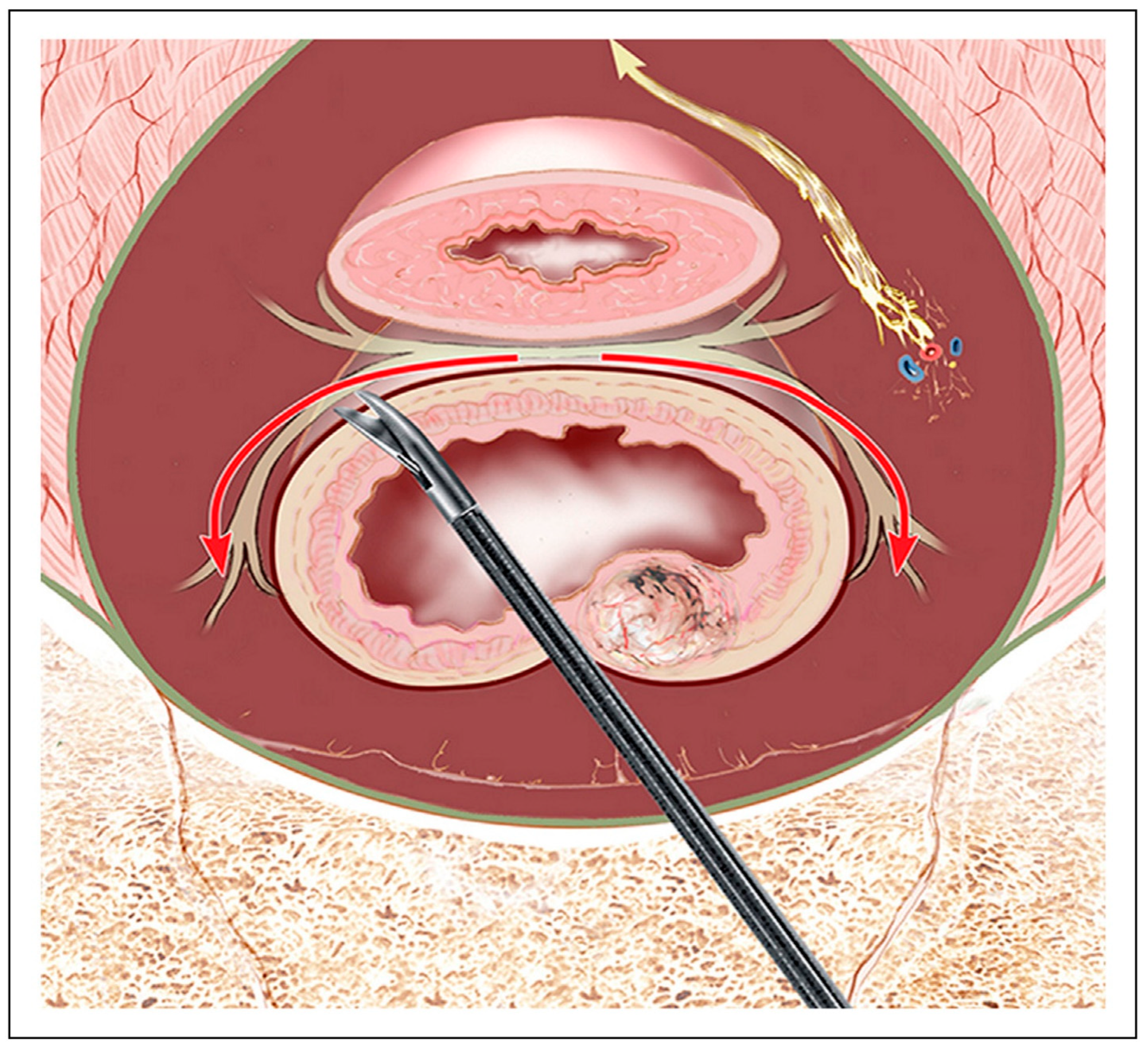
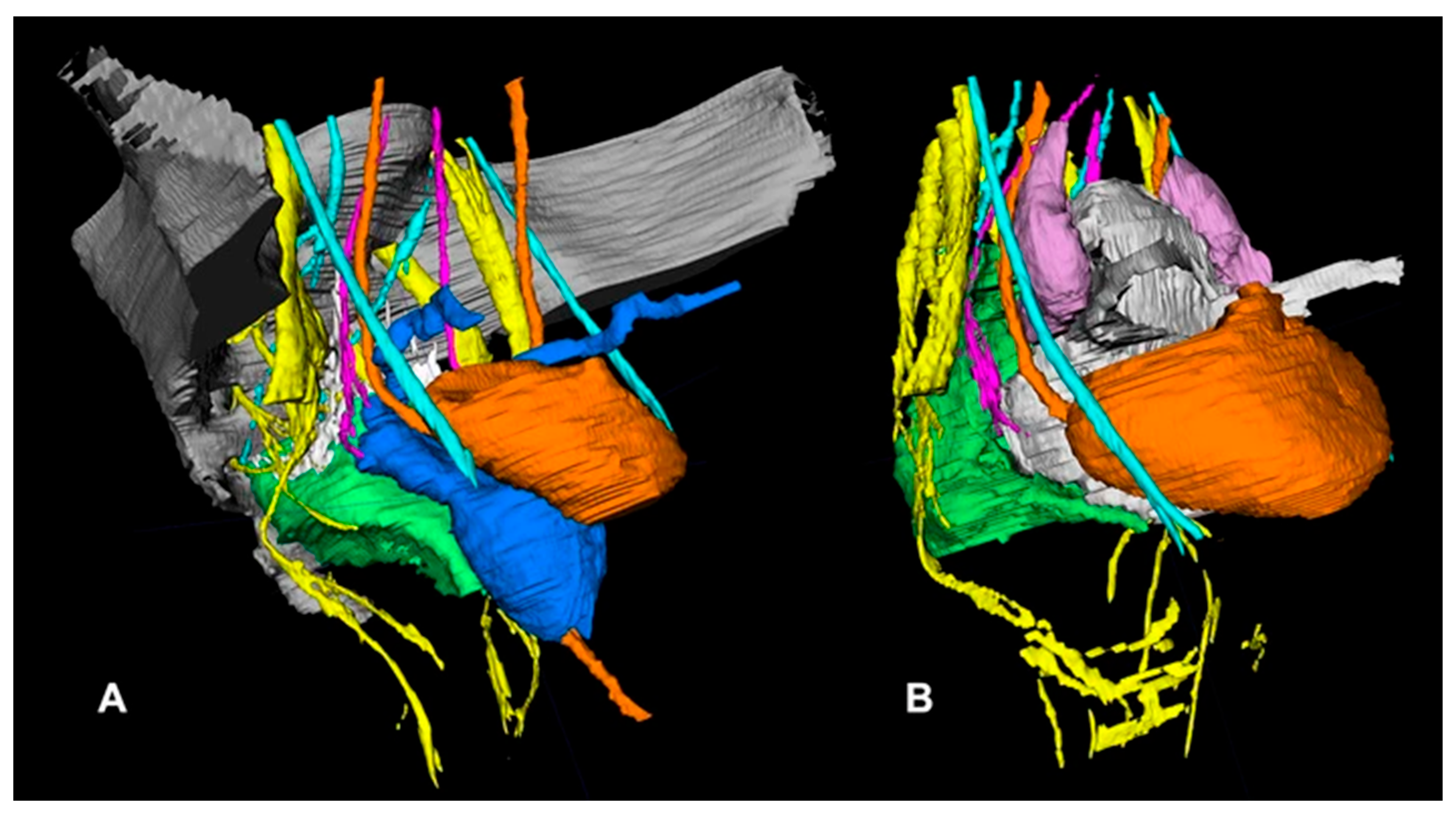
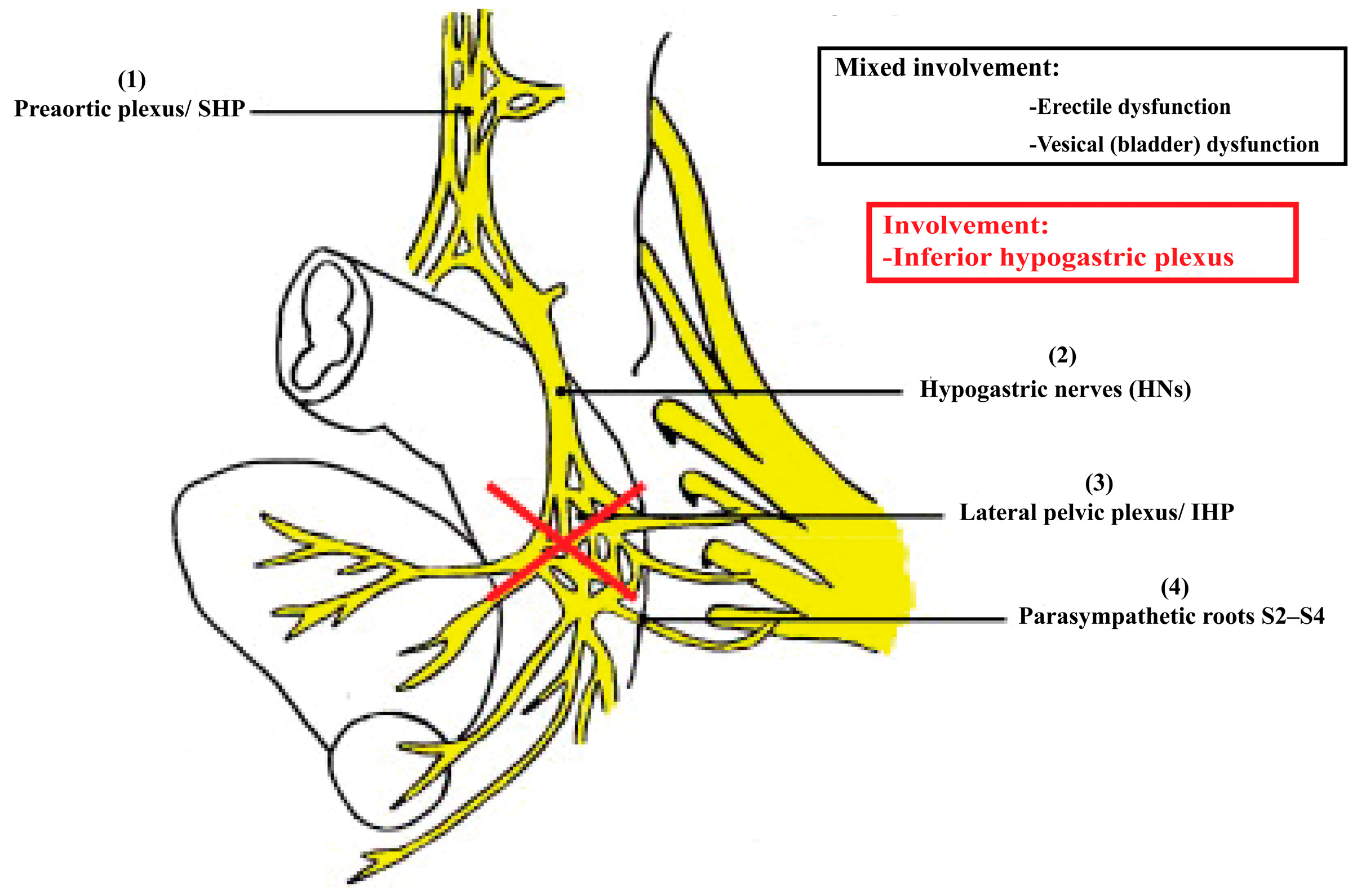
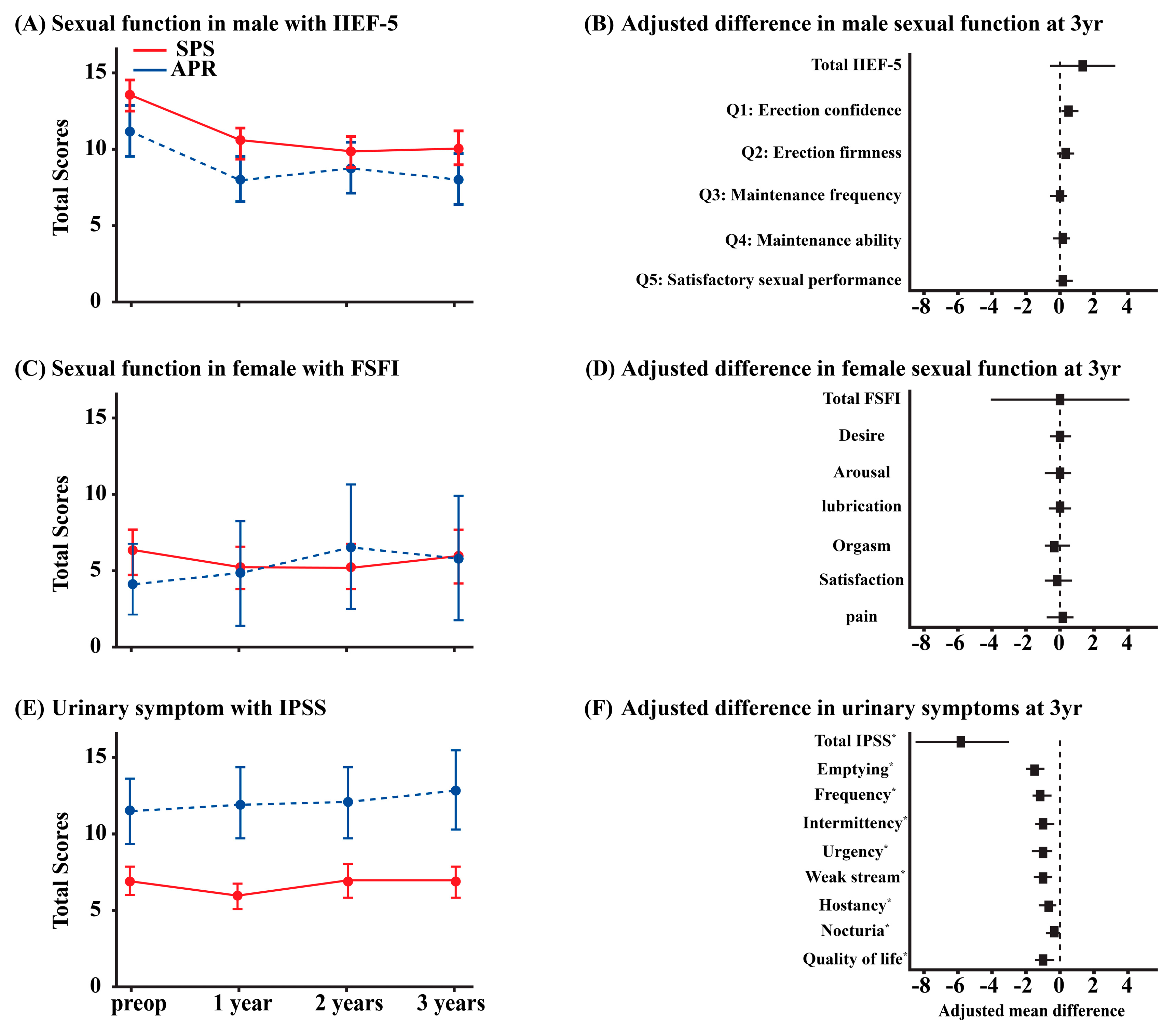
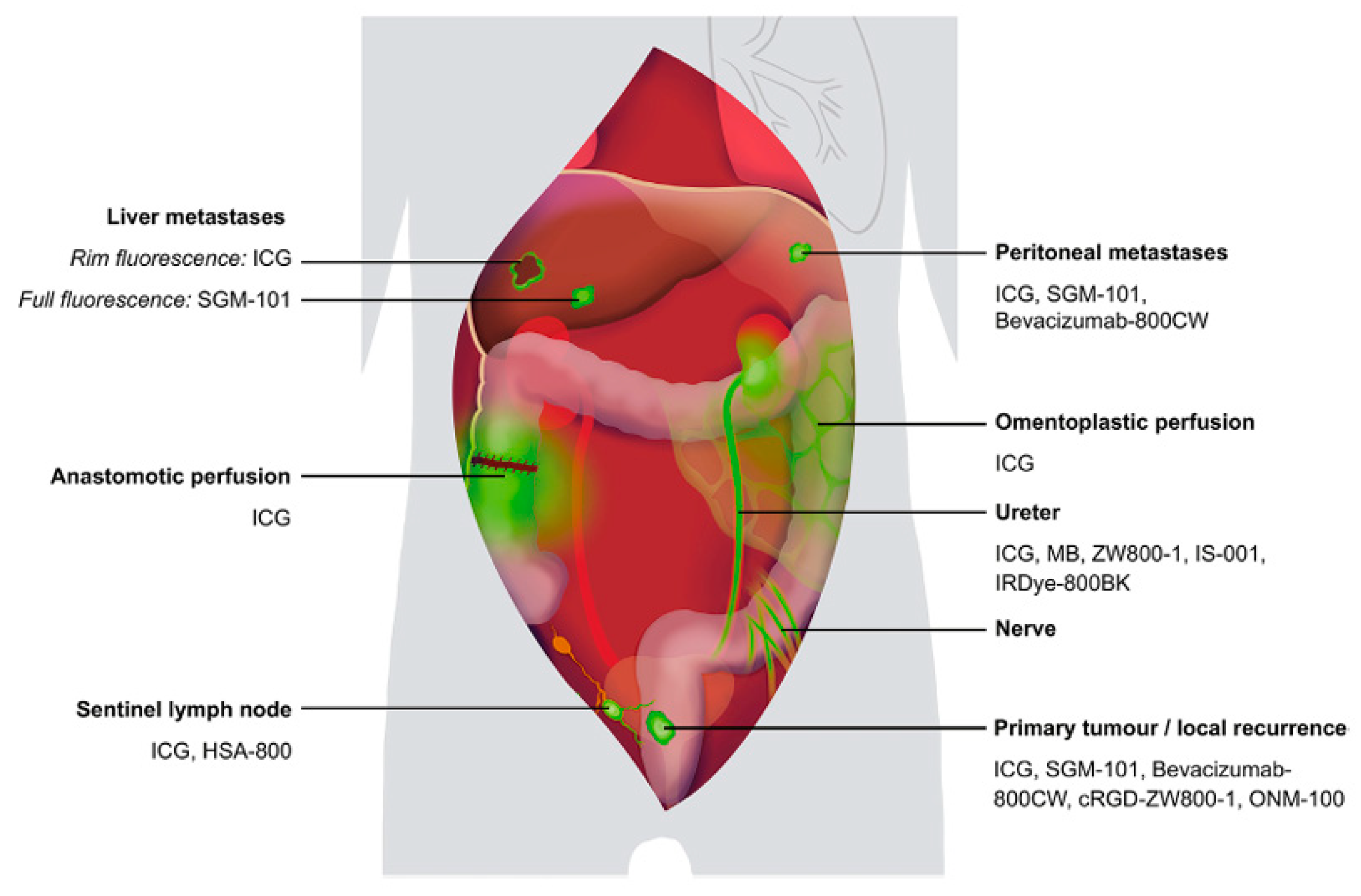
| Function | Sympathetic (Thoracolumbar)—Via SHP/HN/IHP | Parasympathetic (Sacral)—Via PSN (S2–S4)/IHP | Referencs |
|---|---|---|---|
| Urinary bladder | Relaxes detrusor; contracts the bladder neck (internal urethral sphincter) during emission/ejaculation to prevent retrograde flow; denervation → retrograde ejaculation. | Contracts the detrusor; relaxes the internal sphincter → facilitates micturition. | [17,25,28] |
| Anorectal/rectum | Inhibits distal colonic/rectal peristalsis; contracts internal anal sphincter → promotes fecal continence. | Stimulates peristalsis; relaxes the internal anal sphincter → facilitates defecation. | [17,25,28] |
| Sexual/genital | Orgasmic emission/ejaculation in males (peristalsis of vas deferens/ejaculatory ducts; contraction of seminal vesicles and prostate; concurrent bladder-neck closure); uterovaginal contraction; post-arousal vasoconstriction. | Arousal responses (penile/clitoral erection via vasodilation; increased vaginal lubrication/secretions). | [25,26,28] |
| Nerve Pathway | Origin (Spinal Levels) | Function | References |
|---|---|---|---|
| Pelvic splanchnic nerves | S2–S4 | Parasympathetic; bladder contraction, rectal peristalsis, erection | [32,33,34] |
| Sacral splanchnic nerves | S1–S2 | Sympathetic; contraction of internal sphincters, emission | [26,33,37] |
| Lumbar splanchnic nerves | T12–L2 | Sympathetic; GI vasomotor tone, organ pain sensation | [34,35,39] |
| Hypogastric nerves | T10–L2 | Sympathetic; bladder neck tone, inhibits defecation reflex | [33,38,39] |
| Imaging Modality | Utility | References |
| 3T MRI T2-weighted | Visualize IHP & hypogastric nerves. | [34,39,40] |
| Diffusion Tensor Imaging | Map nerve tracts in 3D | [35,38] |
| MR Neurography (MRN) | High-resolution visualization of autonomic plexuses | [37,40] |
| Functional MRI (BOLD) | Assess neural activity post-surgery | [26,40] |
| Stereotactic Navigation | Intraoperative nerve-preserving guidance | [35,37] |
| Structure | MRN (Preop POV) | Intraop White-Light POV | NIR-ICG (Intraop) | Notes | References |
|---|---|---|---|---|---|
| Superior hypogastric plexus (SHP) | ~61% | ~58% | — | MRN visibility is roughly comparable to white-light. | [37] |
| Hypogastric nerve (HGN) | ~93% | ~81% | Plexus-level visible | MRN has a higher POV; NIR aids in plexus contrast. | [37,41] |
| Pelvic plexus / IHP (PP) | ~65% | ~44% | SBR ≈ 3.18 | MRN > intraop POV; NIR improves real-time contrast. | [37,41] |
| Pelvic splanchnic nerves (PSN) | ~93% | ~13% | — | Large gap favoring MRN; mapping helpful. | [37] |
| Neurovascular bundles (NVB) | ~61% | ~32% | — | MRN improves the odds of recognition. | [37] |
| Technique | Description | Functional Role | Reference |
|---|---|---|---|
| Visual Identification | Guided by anatomical landmarks and fascial planes | Basic nerve mapping during TME | [49,50] |
| Magnification & HD Imaging | Improves contrast between fascia and nerves | Enhances structural visualization | [50,52] |
| Near-Infrared Fluorescence (NIR) | Uses ICG or targeted agents for real-time visualization | Improves intraoperative nerve visibility | [41,53] |
| Targeted NIR Probes | Fluorophores conjugated to nerve-specific ligands (e.g., cRGD-ZW800-1) | Enhances selective nerve detection | [53] |
| Low-Voltage Nerve Stimulation | Applies 1–5 mA to assess nerve function | Differentiates sympathetic vs. parasympathetic nerves | [37,45] |
| Intraoperative Neuromonitoring | Real-time pressure/electrical feedback using pelvic sensors | Functional confirmation and nerve injury avoidance | [54,55,56] |
| Anatomical Structure | Common Variations | Surgical Implications | References |
| Superior Hypogastric Plexus | Diffuse or lateralized branching pattern | Increased risk of misidentification during high ligation | [45,50] |
| Hypogastric Nerves | Asymmetric descent, often with left-sided deviation | Elevated risk of nerve injury during left-sided mobilization | [45,50,55] |
| Pelvic Splanchnic Nerves | Origin from S1–S5; variable entry into the IHP | Requires flexible dissection strategy and cautious lateral mobilization | [51,52] |
| Inferior Hypogastric Plexus | Clustered vs. diffuse neural arrangement | Diffuse configuration increases difficulty of intraoperative identification and preservation | [57] |
| Denonvilliers’ Fascia | Thin or multilayered; adherent or separable from rectal wall | Alters anterior dissection plane; risk of nerve entrapment or fascial misidentification | [51,57] |
| Neurovascular Bundles | Sex- and age-related variation in thickness and definition | Thicker in males (easier to preserve); less distinct with aging or fibrosis | [37,49] |
| Post-radiation Fibrosis | Fascial plane distortion and obliteration of natural nerve landmarks | Requires sharper dissection, magnification, and higher vigilance | [37,54,55] |
| Dissection Plane/Step | Description | Nerve Structures Preserved | References |
| Posterior Dissection | Sharp dissection in holy plane between fascia propria and presacral fascia | Superior hypogastric plexus, hypogastric nerves | [45,51] |
| Lateral Dissection | Dissect on the outer surface of the visceral/mesorectal fascia (fascia propria); clip/divide nervi recti and middle rectal vessels at mesorectal entry (≈4 & 8 o’clock); avoid behind-parietal-fascia skiving or skeletonizing ureter/iliac vessels. For the final release adjacent to the IHP, prefer sharp (cold) dissection over energy. | IHP, PSN; limits traction/thermal injury and lets plexus recoil laterally. | [26,44,45] |
| Anterior Dissection | Dissect behind Denonvilliers’ fascia or septum | Cavernous nerves, neurovascular bundles | [26,54] |
| Robotic TME | Technical facilitator (articulation, tremor filtration, 3D vision) for precise plane work; randomized data (ROLARR) show no functional superiority vs. laparoscopy. | Facilitates nerve-sparing execution; functional advantage unproven in RCTs. | [54] |
| Energy Device Modulation | Use minimal thermal spread devices near nerve planes; at/just off the IHP and NVBs, complete dissection with sharp, non-thermal technique (cold scissors/knife) to avoid lateral heat conduction. | Prevent lateral heat-induced nerve injury. | [44,45,54] |
| Imaging Technique | Purpose | Nerve Structures Visualized | References |
|---|---|---|---|
| High-resolution T2 MRI | Identify mesorectal fascia and hypogastric plexus | SHP, IHP, HN, NVBs | [35,55] |
| MR Neurography (T1/T2 + DWI) | Enhanced visualization of autonomic plexus | HN, IHP, PSN, CN | [37,55] |
| Diffusion Tensor Imaging (DTI) | Map directional nerve tracts, 3D reconstruction | Sacral nerves, IHP | [35,61] |
| Stereotactic Navigation | Real-time nerve guidance during TaTME | SHP, HN, IHP, CN | [35,37,61] |
| Postoperative MRN | Assess nerve integrity and correlate with function | IHP, CN, NVB | [37,60] |
| Surgical Approach | Urinary Dysfunction | Sexual Dysfunction | References |
|---|---|---|---|
| Conventional (non-sparing) | 30–70% | 40–80% | [62,66] |
| Nerve-sparing laparoscopic | 10–30% | 15–30% | [62,66,68] |
| Robotic nerve-sparing (R-TME) | 8–20% | 10–25% | [63,68,69] |
| Intervention Type | Target Function | Reported Improvement | Reference |
| Pelvic floor rehab (biofeedback, NMES) | Bowel, Urinary | LARS ↓ 45%, continence ↑ 30–40% | [64] |
| Sacral nerve stimulation (SNS) | Bowel, Urinary | Wexner ↓ from 16 to 5; incontinence ↓ 75% | [22,64] |
| PDE5 inhibitors | Erectile Function | 60–70% success in partial nerve preservation | [62] |
| Tibial nerve stimulation (PTNS) | Mixed continence | Success rate 60–80% | [64] |
| Surgical Technique | Nerve Preservation Success (%) | Functional Recovery (%) | Complications (%) | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| Conventional Surgery | 45 | 65 | 15 | 85 | [70] |
| Nerve-Sparing Surgery | 80 | 90 | 10 | 90 | [71] |
| Robotic Surgery | 85 | 92 | 5 | 95 | [53] |
| Surgical Method | Nerve Preservation Success (%) | Functional Recovery (%) | Intraoperative Nerve Identification (%) | Postoperative Complications (%) | Reference |
|---|---|---|---|---|---|
| Conventional Surgery | 45 | 65 | 40 | 25 | [73] |
| Fluorescence-guided Surgery | 80 | 90 | 80 | 10 | [72] |
| Surgical Procedure | Key Neural Structures at Risk | Anatomical Course & Relations (Surgical Landmarks) | Critical Nerve-Sparing Maneuvers | References |
|---|---|---|---|---|
| Rectal Cancer Surgery (TME) | SHP, HNs, IHP, PSN |
|
| [81,82] |
| Radical Prostatectomy | IHP continuations (Neurovascular Bundles, NVB), Cavernous Nerves |
|
| [81] |
| Radical Hysterectomy (Nerve-Sparing) | IHP (especially bladder branches), HNs, PSN |
|
| [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almughamsi, A.M.; Elhassan, Y.H. Pelvic Neuroanatomy in Colorectal Surgery: Advances in Nerve Preservation for Optimized Functional Outcomes. Surgeries 2025, 6, 94. https://doi.org/10.3390/surgeries6040094
Almughamsi AM, Elhassan YH. Pelvic Neuroanatomy in Colorectal Surgery: Advances in Nerve Preservation for Optimized Functional Outcomes. Surgeries. 2025; 6(4):94. https://doi.org/10.3390/surgeries6040094
Chicago/Turabian StyleAlmughamsi, Asim M., and Yasir Hassan Elhassan. 2025. "Pelvic Neuroanatomy in Colorectal Surgery: Advances in Nerve Preservation for Optimized Functional Outcomes" Surgeries 6, no. 4: 94. https://doi.org/10.3390/surgeries6040094
APA StyleAlmughamsi, A. M., & Elhassan, Y. H. (2025). Pelvic Neuroanatomy in Colorectal Surgery: Advances in Nerve Preservation for Optimized Functional Outcomes. Surgeries, 6(4), 94. https://doi.org/10.3390/surgeries6040094






