Abstract
Background: Upper eyelid blepharoplasty is one of the most common aesthetic surgeries performed worldwide. The procedure consists of removing excess skin with or without muscle and/or fat from the upper eyelid by a transcutaneous approach and placement of a supratarsal crease. The surgery is performed in a cosmetically sensitive area and every attempt to avoid poor scar formation should be made. Methods: This review presents a conspectus of the existing medical literature regarding scar-avoiding strategies in upper blepharoplasty with the aim of contributing to the reduction in postoperative scar formation. The Medline, Embase, and Cochrane databases were searched on 2 September 2025. Results: The search yielded a total of 562 records, and, following screening, eleven publications were included. Conclusions: A systematic approach to pre-, intra-, and postoperative measures to minimize scarring are presented. There is a need to standardize scar assessment and reporting to facilitate inter-study comparison of effects, as well as prospective, randomized studies comparing suture materials and techniques.
1. Introduction
Although considered a normal process in tissue repair [1], scars after surgical procedures can be aesthetically unfavorable and cause significant morbidity for the patient [2,3,4,5]. In the periocular region, scar formation can have serious consequences as even a millimeter of malposition can affect the delicate balance in the three-dimensional forces that support eye position, eyelid function, eye movements, and ocular surface homeostasis. Excessive periocular scarring can cause ectropion, entropion, eyelid retraction, and facial asymmetry [6]. These conditions, if left untreated, can potentially lead to irreversible vision loss [6].
Physiological cutaneous wound healing is typically divided into three phases: hemostasis and inflammation, proliferation, maturation and remodeling [7,8]. According to the International Scar Classification of 2019, scars are classified into immature, mature, atrophic, hypertrophic, and keloid [9]. While immature and mature scars are phases of the normal scarring process, atrophic, hypertrophic, and keloid scars are undesirable scar variants. Immature scars histologically still contain a sizeable proportion of inflammatory cells, in addition to collagen [9]. A set time limit cannot be defined by which a scar ceases to be immature as this varies from patient to patient and even from scar to scar on the same patient. The resolution of erythema, however, is reported to be a useful marker of scar maturity; a process reported to take as much as one or more years [10]. Mature scars can be white or hyperpigmented depending on the patient’s skin color. Histologically, mature scars have a healed epithelium, but a disorganized collagen fiber structure. Additionally, the tissue in mature scars no longer has tissue edema as opposed to immature scars. Normal and abnormal wound healing are illustrated in Figure 1.
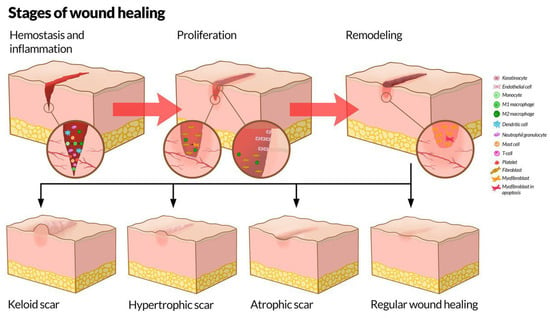
Figure 1.
The stages of wound healing. Top: The stages of wound healing, including hemostasis and inflammation, proliferation, and remodeling, as well as the most important involved cells at the different stages. Bottom: Physiological and pathological results of wound healing. Illustration by Kristin Skårdal.
Sometimes, scars become atrophic, i.e., they appear thinned or depressed. This can occur due to reduced collagen synthesis and an inappropriate inflammatory response during the maturation phase. If a suture is removed too early, the healing incision may not yet have adequate collagen deposition and strength; the edges can separate or retract, leading to loss of dermal volume or incomplete filling, which could manifest as a depression [11]. Incorrect suturing (e.g., uneven edge alignment, gaps, excessive tension that strangulates tissue, or too much tension in one area) might lead to localized ischemia or inadequate collagen deposition in that zone, again predisposing to a depressed defect.
Atrophic scars are caused by loss of dermal matrix and other extracellular matrix constituents through collagen breakdown. Atrophic scars are associated with a prolonged but weak inflammatory response without proper downregulation, steroid excess (e.g., Cushing syndrome), or exogenous steroid use [12]. Contrary to atrophic scars, in which collagen accumulation is low, hypertrophic scars are characterized by their distinct clinical appearance: elevated, wide, erythematous, stiff (potentially limiting mobility), and often also itchy/painful. Hypertrophic scars are further divided into linear and widespread, and some of the hypertrophic scars can cause contractures and functional impairment. While hypertrophic scars remain within the confines of the original scars, keloids can grow in a mushroom-like fashion well beyond the original scars. Both hypertrophic scars and keloids are more common in patients with higher Fitzpatrick skin types and who have some degree of genetic predisposition [13]. Keloids can be further classified as minor or major keloids [9].
Upper eyelid blepharoplasty is one of the most commonly performed cosmetic surgical procedures in both women and men, illustrated in Figure 2 [6,14]. The indications can be either functional or cosmetic. The former might be the case if a patient’s main complaint is restricted visual field due to excess skin in the upper eyelid. Clinical tests might include simple visual field examination or more advanced perimetry, depending on local guidelines. Manually lifting excess upper eyelid skin out of the way can also provide an indication of whether dermatochalasis is causing functional problems.
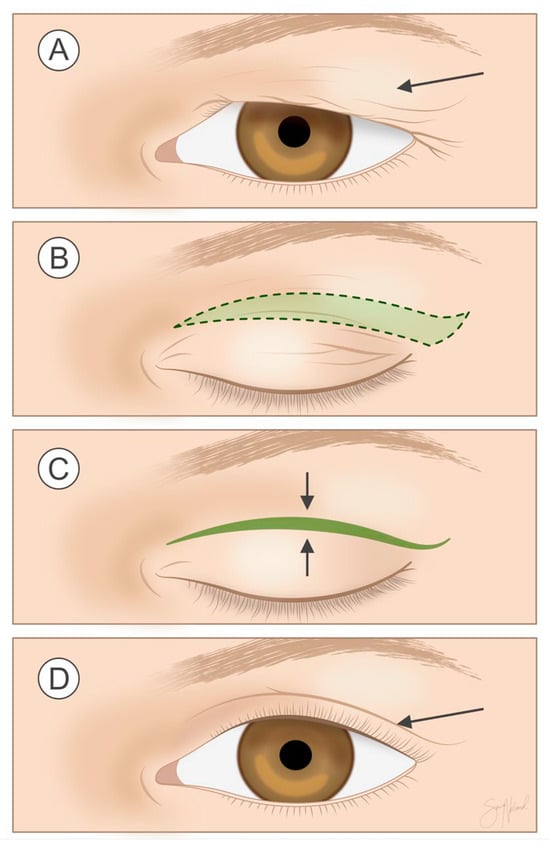
Figure 2.
Procedure at a glance: In upper eyelid blepharoplasty, redundant skin, with or without a strip of orbicularis oculi and/or preaponeurotic fat (A), is excised from the upper eyelid as marked in the figure (B), and the skin is then closed (C) so that the surgical scar (arrow) is hidden in the upper eyelid crease (D). Illustration by Sara Nøland.
The inferior incision line in upper eyelid blepharoplasty follows the supratarsal crease, which varies depending on age, sex, and ethnicity. The superior incision line is based on the amount of skin to be resected. Medially, the incision should not cross a hypothetical vertical line crossing the medial punctum. Laterally, the incision should not go beyond the lateral orbital rim. Pinching with forceps along the supratarsal crease, the superior incision line should be placed along the hypothetical line causing minimal eversion of the upper eyelid lashes. With advancing age there is a loss of subcutaneous fat, deepening of wrinkles, and eyelid fat prolapse. Thus, the excision of the underlying orbicularis oculi muscle depends on tissue fullness, age, and patient preferences. The excision of adipose tissue is generally limited to tissue prolapsed anterior to the orbital rim, unless the patient has very full upper lids in need of extensive debulking. Dermatochalasis can often occur in conjunction with eyebrow and lid ptosis. Their corrections are distinctive procedures and only considerations surrounding upper blepharoplasty for dermatochalasis are addressed here.
Even though postoperative scarring is one of the most common complications after upper eyelid blepharoplasty, it is less common than after many other surgical procedures [15,16]. Although, when it does occur, it can have devastating functional as well as cosmetic effects. While the underlying reasons are not fully understood, we hypothesize that the lack of scar formation may be due to the unique properties of the eyelid skin. As is well known, eyelid skin is among the thinnest in the human body and contains no subcutaneous fat [6]. Some evidence suggests that skin thickness is inversely associated with keloid formation [17]. Nonetheless, minimizing scarring and related complications remains a worthwhile goal, and arguably a professional duty for surgeons. This is especially important because correcting periorbital scars, when they do occur, is among the most challenging tasks in oculoplastic surgery. In the present review, we synthesize the scientific literature on strategies to reduce or minimize postoperative scarring in upper eyelid blepharoplasty. To the best of our knowledge, no such review has been published to date.
2. Methods
A literature search in Ovid Medline, Embase, and The Cochrane Database of Systematic Reviews was performed on 2 September 2025, using controlled vocabulary and text words expressing (blepharoplasty or dacryocystorhinostomy or eyelid surgery) AND (scar or keloid or cicatrix). No language restrictions were applied. A detailed search strategy and results are included in the Supplementary Materials. A total of 562 records were retrieved. These records were then screened manually. Records were excluded if upper blepharoplasty scarring was not discussed. Also excluded were patents, dissertations, and articles in languages other than English. Twenty-eight records remained eligible following the screening process and, finally, eleven articles were included after full-text evaluation (Figure 3).
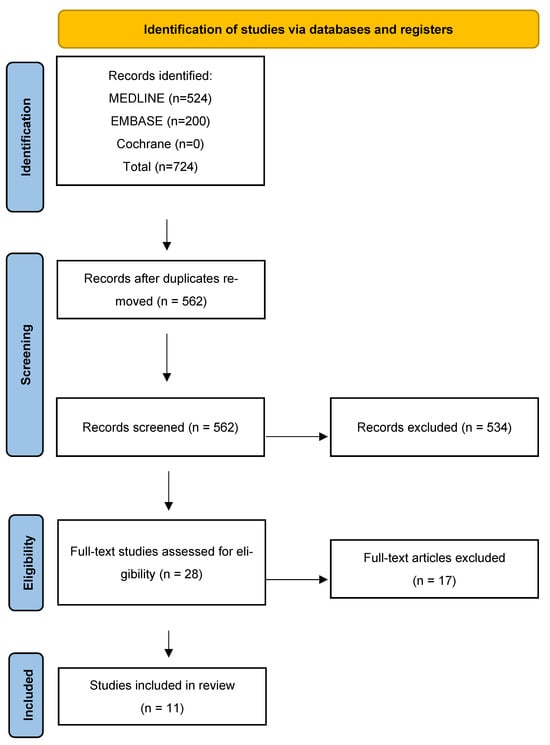
Figure 3.
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study identification, inclusion, and exclusion.
3. Results
Results are presented in Table 1. A total of eleven articles were included. The included number of participants varied from 26 to 866. Among the included studies, two were retrospective, one was cross-sectional, one was prospective, six were randomized, while one study did not report study design. Due to considerable heterogeneity concerning study design, intervention, and scar quantification, any meta-analysis was deemed impossible.

Table 1.
Studies evaluating scarring following blepharoplasty.
4. Discussion
4.1. A Systematic Approach to Addressing Surgical Scar Reduction
We deem the first and best opportunity to avoid or minimize scarring lies prior to making the incision. Hence, careful preoperative planning is key, taking into consideration factors such as the indications for surgery, comorbidities, medication use, and the patient’s unique periocular anatomy. In general, a history of hypertrophic scarring or keloid formation are risk factors for future incidents [28,29]. Whether this holds true regarding upper blepharoplasty, however, remains unknown. The incidence of hypertrophic scarring following eyelid procedures in general was recently found to be ~1% (36/3650 patients), with no reports in the literature of keloid formation after cosmetic eyelid procedures performed for aesthetic purposes only [30]. The diagnosis will dictate the type and size of incision and the extent of surgical trauma. While functional and aesthetic indications typically would not necessitate large incisions, oncologic processes could require a pre-defined clinical incisional margin to also be resected, depending on the pathology. The indication for surgery thus plays a role in the type and size of the incision, the surgical defect, and the ensuing scar. Comorbidities such as diabetes can impact wound healing, and various strategies can be employed to mitigate the risks associated with performing surgeries on patients with diabetes [31]. Smoking also impacts wound healing, and the patient should be strongly advised to permanently quit smoking prior to surgery [32]. Medications such as steroids can impact wound healing by interfering with inflammation, fibroblast proliferation, and collagen synthesis [32]. Although not directly related to scar formation, antiplatelet or anticoagulants increase the risk of perioperative bleeding, which, if not controlled, can lead to unfavorable scarring outcomes. Lastly, the patient’s age, race, previous surgical history, and skin condition impacts their unique periorbital physiology and anatomy, which requires a personalized approach to minimize postoperative scarring.
The second opportunity we consider to avoid or minimize scarring is during the intraoperative period, when the incision, suture materials, degree of iatrogenic trauma, and ensuing reconstruction should be methodically planned. Attention should be given to reconstruction of anatomic layers, maintenance of anatomical support structures, and skin closure with wound edge eversion.
The postoperative period is the third and final opportunity to minimize scarring and has been the focus of scar-reducing strategies for decades. Examples include petroleum jelly, negative pressure therapy, creams, silicone gel sheets, laser, dermabrasion, and glucocorticoid injections [33].
Acknowledging this systematic three-step approach in addressing scar reduction, the included studies in this review will now be discussed.
4.2. Preoperative Considerations in Minimizing Scarring
4.2.1. Incision Planning
Placement of incision and excision are crucial steps of preoperative planning. The incision for upper eyelid blepharoplasty is usually hidden in the natural upper eyelid skin crease (the skin crease separating the upper eyelid skin fold from the flat pretarsal component of the upper eyelid) [6]. Optimal preoperative markings are important. The shape and extent of the preoperative markings depend on age, amount of skin to be excised, and ethnicity [14,34]. A lenticular, or less commonly, trapezoid shape, is used. We recommend marking the incision with a thin-tipped surgical marker prior to injecting local anesthetic. Modifying established routines based on local considerations and individual anatomical characteristics can improve patient satisfaction and reduce the risk of unsightly scarring and other unwanted postoperative effects [24].
4.2.2. Hemostasis
Intra- and postoperative bleeding and edema increase the risk of wound dehiscence and, thus, of scarring [6]. Medications with anticoagulant properties should be noted and preferably discontinued preoperatively, if considered safe. Platelet inhibitors should be stopped 7–10 days prior to surgery [35]. It is important to note that not all patients consider over-the-counter medications such as aspirin relevant, and their use should be specifically queried. Non-steroidal anti-inflammatories may affect hemostasis and should be discontinued 3–4 days preoperatively. Warfarin should be stopped 4–5 days before the operation. Novel oral anticoagulants have differing half-lives and should be evaluated individually. No anticoagulant medication should be discontinued without consulting with the prescribing internist or cardiologist, and the risk versus benefits of discontinuation should be considered.
To achieve the greatest extent of hemostatic effects, it has been contended that local anesthetic agents should contain epinephrine (1/100,000) and be injected 10 min prior to making the first incision [6]. Novel research, however, indicates that maximum hypoperfusion from epinephrine is reached as early as two minutes following injection [36].
4.3. Intraoperative Considerations in Minimizing Scarring
4.3.1. Tranexamic Acid
Intraoperative bleeding may prolong the duration of surgery, healing, and recovery, as well as increase the use of intraoperative electrocautery, increasing scar formation [37]. The use of preoperative intravenously administered tranexamic acid (TXA) may reduce intraoperative blood loss as well as postoperative ecchymosis or edema in general surgery [38], facial plastic surgery [39], and blepharoplasty [40]. The safety and efficacy of subcutaneous or topical TXA administration in blepharoplasty, however, requires further consideration, although some studies have shown promising effects on other surgical procedures [41]. A recent study reported decreased postoperative edema and ecchymosis among patients administered subcutaneous TXA [40], another study found no difference in total blood loss or postoperative ecchymosis or hematoma [37], while a third study discovered increased bleeding among patients administered TXA in lidocaine with epinephrine when compared to lidocaine with epinephrine alone [42]. Taken together, TXA administered intravenously has superior efficacy compared to subcutaneous injection, but further studies are needed as the literature regarding subcutaneous and topical administration remains scarce.
4.3.2. Suture Materials and Suture Techniques
Several approaches, such as running or interrupted sutures and various suture materials exist and are reported interchangeably in the literature. There is, however, seemingly little scientific evidence available regarding the choice of suture materials and technique [14,34], with the choice often depending on the surgeon’s personal preference [6]. Only one study was identified examining this [16]. Based on the limited available evidence, running plain gut sutures cause the highest degree of erythema and scarring, while running locking polypropylene sutures cause more milia, and subcuticular polypropylene more standing cone deformities [16]. The reason for a higher degree of scarring with running plain gut is unknown but might be related to an increased degree of tissue reaction and inflammation [43]. There is a clear need for further research of this important topic.
4.3.3. Scalpel vs. Microdissection Needle
Various means of making skin incisions during blepharoplasty have been described, such as carbon dioxide laser, microdissection needle, and cold scalpel [44,45]. Despite the risk of incisional bleeding, the scalpel is often preferred as there has been concern of increased scarring from thermal damage following diathermy and the lack of tactile feedback from carbon dioxide lasers [45,46]. Indeed, histologic analysis revealed substantial thermal injury and necrosis resulting from carbon dioxide laser, and, to a lesser extent, microdissection needle use in specimens from upper blepharoplasty [18,47]. These changes were not found in samples excised with a cold scalpel. Conversely, no clinical differences in scar cosmesis or ecchymosis were noted when comparing microdissection needle to scalpel [18]. Only one clinical study comparing incisional instruments was identified [18], which highlights the need for further, prospective randomized studies.
4.4. Postoperative Considerations in Minimizing Scarring
Despite the importance of the postoperative period in cicatricial development and the plethora of suggested strategies to reduce postoperative scarring, no clear postoperative standard of care has been established for upper eyelid blepharoplasty [22,48]. The application of cold compresses for the first 48 h and elevation of the head to reduce swelling and ecchymoses are often advised [34]. The use of prophylactic antibiotics and corticosteroids vary. Tobramycin, chloramphenicol, or tobramycin/dexamethasone combination ointment are common choices [34,48]. Among the studies included herein, three applied topical antibiotics and none reported the use of topical corticosteroids as part of the standard postoperative regimen [20,21,22]. None of the included studies evaluated the effect of postoperative antibiotics on scar formation.
Hyaluronic acid, growth factors, and cytokines are available in commercial ointments and are involved in the wound-healing process and fetal scarless healing [49,50]. Based on the included studies, ointment containing growth factors and cytokines might lessen early phase erythema and pigmentation, but it does not seem to have an impact on the final scar [19]. Application of a silicone-based cream containing growth factors, hyaluronic acid, and vitamin C decreased the incidence of intralesional 5-fluorouracil/triamcinolone injections [21]. Interestingly, no clinical differences were found between onion extract, hydrocortisone, and petroleum jelly [20,22]. Nor were differences found between silicone gel without additional growth factors when compared to petrolatum ointment [26].
Thus, the use of scar creams during the wound-healing phase appears beneficial, as they may reduce initial redness and limit overall scar development. However, it remains uncertain whether any particular ointment is superior to others.
Wound dehiscence increases the risk of unfavorable scarring but is thankfully rare in upper blepharoplasty. The prevalence has been reported as 1–2% [51,52], at an average of nine days, postoperatively [51]. Risk factors include male gender, history of cigarette smoking, and fast-absorbing plain gut suture (compared to polypropylene) [51]. Interestingly, common risk factors such as age, hypertension, diabetes, and heart disease did not pose an increased risk of wound dehiscence. Based on surgeon assessment of wound tension, the addition of subcutaneous, buried sutures laterally might be preventative for lateral wound dehiscence [52].
4.5. Methodological Considerations and Limitations
The amount of scarring in the included studies is generally low, with a high degree of patient satisfaction. Lee et al. reported mild scarring among 0.6% of patients [53], while Joshi et al. reported unsightly scarring in 0.7% of patients [16]. The comparison of results across studies is hampered by the lack of standardized reporting. Among the nine included studies, nine different ways to quantify scar signs and symptoms were applied. With the exception of one study reporting 12-month follow-up [23], most of the included studies had short follow-up periods of 2–6 months. This timeframe is generally too short to make any final assessment of scars and decisions on necessary intervention. Future studies should strive to implement longer follow-up periods.
This review is subject to publication bias as all strategies to minimize scarring in upper eyelid blepharoplasty are not expected to have been published.
5. Conclusions and Future Perspectives
Scar formation following upper eyelid blepharoplasty is generally limited and the procedure has a high degree of patient satisfaction. Although it is difficult to completely avoid postoperative scarring, several pre-, intra- and postoperative measures can be employed by surgeons and patients in order to minimize the postoperative cicatricial burden. We recommend a systematic approach where the surgeon carefully evaluates these elements with the patient. Preoperative smoking cessation, evaluation of patient medications and incision planning, careful choice of suture materials and techniques, as well as minimizing postoperative swelling and risk of infection are of particular importance. Future studies should attempt to standardize scar assessment and reporting to facilitate inter-study comparison of effects. Moreover, additional research is needed concerning postoperative antibiotics, corticosteroids as well as suture techniques and suture materials. It is hoped that the discussion presented herein may aid surgeons to improve, modify, and even develop new and innovative techniques to minimize scarring and improve patient satisfaction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/surgeries6040093/s1.
Author Contributions
Conceptualization: F.A.F., A.Z.K., K.A.T., T.P.U., Investigation: F.A.F., A.Z.K., L.C.B.-A., Methodology: R.C.A., E.B., K.A.T., T.P.U., Project administration: F.A.F., T.P.U., Supervision: K.A.T., T.P.U., Writing—original draft: F.A.F., Writing—review & editing: F.A.F., A.Z.K., L.C.B.-A., R.C.A., E.B., K.A.T., T.P.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Marie Susanna Isachsen at the University of Oslo for her kind assistance with the literature search for this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayat, A.; McGrouther, D.A.; Ferguson, M.W. Skin scarring. BMJ 2003, 326, 88–92. [Google Scholar] [CrossRef]
- Goverman, J.; He, W.; Martello, G.; Whalen, A.; Bittner, E.; Schulz, J.; Gibran, N.; Herndon, D.; Suman, O.; Kowalske, K.; et al. The Presence of Scarring and Associated Morbidity in the Burn Model System National Database. Ann. Plast. Surg. 2019, 82, S162–S168. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Meyer, W.; Bishop, S.; Rosenberg, L.; Murphy, L.; Blakeney, P. Disfiguring burn scars and adolescent self-esteem. Burns 1999, 25, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Taal, L.; Faber, A.W. Posttraumatic stress and maladjustment among adult burn survivors 1 to 2 years postburn. Part II: The interview data. Burns 1998, 24, 399–405. [Google Scholar] [CrossRef]
- Nerad, J.A. Techniques in Ophthalmic Plastic Surgery: A Personal Tutorial; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Darby, I.A.; Desmouliere, A. Scar Formation: Cellular Mechanisms. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Teot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–26. [Google Scholar]
- Mustoe, T.A. International Scar Classification in 2019. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Teot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 79–84. [Google Scholar]
- Kant, S.; van den Kerckhove, E.; Colla, C.; van der Hulst, R.; Piatkowski de Grzymala, A. Duration of Scar Maturation: Retrospective Analyses of 361 Hypertrophic Scars Over 5 Years. Adv. Skin Wound Care 2019, 32, 26–34. [Google Scholar] [CrossRef]
- Weiss, E.T.; Chapas, A.; Brightman, L.; Hunzeker, C.; Hale, E.K.; Karen, J.K.; Bernstein, L.; Geronemus, R.G. Successful treatment of atrophic postoperative and traumatic scarring with carbon dioxide ablative fractional resurfacing: Quantitative volumetric scar improvement. Arch. Dermatol. 2010, 146, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauser, M.; Mayrhofer, M.; Kamolz, L.P.; Smolle, C. An Update on Molecular Mechanisms of Scarring-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 11579. [Google Scholar] [CrossRef]
- Téot, L.; Mustoe, T.A.; Middelkoop, E.; Gauglitz, G.G. Textbook on Scar Management: State of the Art Management and Emerging Technologies; Teot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Drolet, B.C.; Sullivan, P.K. Evidence-based medicine: Blepharoplasty. Plast. Reconstr. Surg. 2014, 133, 1195–1205. [Google Scholar] [CrossRef]
- Morax, S.; Touitou, V. Complications of blepharoplasty. Orbit 2006, 25, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Janjanin, S.; Tanna, N.; Geist, C.; Lindsey, W.H. Does suture material and technique really matter? Lessons learned from 800 consecutive blepharoplasties. Laryngoscope 2007, 117, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Dohi, T.; Wakai, H.; Quong, W.L.; Linh, N.D.T.; Usami, S.; Ogawa, R. In the face and neck, keloid scar distribution is related to skin thickness and stiffness changes associated with movement. Wound Repair. Regen. 2024, 32, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Arat, Y.O.; Sezenoz, A.S.; Bernardini, F.P.; Alford, M.A.; Tepeoglu, M.; Allen, R.C. Comparison of Colorado Microdissection Needle Versus Scalpel Incision for Aesthetic Upper and Lower Eyelid Blepharoplasty. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 430–433. [Google Scholar] [CrossRef]
- Murdock, J.; Sayed, M.S.; Tavakoli, M.; Portaliou, D.M.; Lee, W.W. Safety and efficacy of a growth factor and cytokine-containing topical product in wound healing and incision scar management after upper eyelid blepharoplasty: A prospective split-face study. Clin. Ophthalmol. 2016, 10, 1223–1228. [Google Scholar] [CrossRef]
- Owji, N.; Khademi, B.; Khalili, M.R. Effectiveness of Topical Onion Extract Gel in the Cosmetic Appearance of Blepharoplasty Scar. J. Clin. Aesthet. Dermatol. 2018, 11, 31–35. [Google Scholar]
- Kalasho, B.D.; Kikuchi, R.; Zoumalan, C.I. Silicone-Based Scar Cream for Post Upper Eyelid Blepharoplasty-associated Cicatricial and Hypertrophic Scarring. J. Drugs Dermatol. 2019, 18, 440–446. [Google Scholar]
- Owji, N.; Khalili, M.R.; Khademi, B.; Shirvani, M.; Sadati, M.S. Comparison of the Effectiveness of Onion Extract, Topical Steroid, and Petrolatum Emollient in Cosmetic Appearance of Upper Blepharoplasty Scar. J. Curr. Ophthalmol. 2020, 32, 408–413. [Google Scholar] [CrossRef]
- Hollander, M.H.J.; Delli, K.; Vissink, A.; Schepers, R.H.; Jansma, J. Patient-reported aesthetic outcomes of upper blepharoplasty: A randomized controlled trial comparing two surgical techniques. Int. J. Oral Maxillofac. Surg. 2022, 51, 1161–1169. [Google Scholar] [CrossRef]
- Dossan, A.; Doskaliyev, A.; Dzhumabekov, A.; Nuspekova, D. Patient Satisfaction and Scar Quality Following Upper Blepharoplasty Using a Simplified Preoperative Marking Technique. Plast. Aesthetic Nurs. 2023, 43, 131–135. [Google Scholar] [CrossRef]
- Guclu, E.S.; Ozer, O.; Celik, S.; Eroz, P.; Baysal, Z. Comparison of the Cosmetic Efficacy of Extractum Cepae and Silicone-Based Gel in Upper Blepharoplasty. J. Craniofacial Surg. 2024, 35, e658–e660. [Google Scholar] [CrossRef] [PubMed]
- Karanfilian, T.S.; Thuma, T.; Cheng, T.; Paramo, R.; Moon, J.Y.; Akella, S.; Barmettler, A. Efficacy of Silicone Gel Versus Placebo for Postsurgical Scars of the Eyelids: A Randomized, Controlled, Double-Blinded Study. Ophthalmic Plast. Reconstr. Surg. 2025, 45, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Erkan Pota, C.; Bilgic, A.; Cetinkaya Yaprak, A.; Ilhan, H.D.; Kahraman, U. Evaluation of periocular scars after blepharoplasty and external dacryocystorhinostomy according to Manchester and modified Vancouver scar score. Int. Ophthalmol. 2025, 45, 190. [Google Scholar] [CrossRef]
- Ogawa, R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2022, 149, 79e–94e. [Google Scholar] [CrossRef]
- Cho, M.Y.; Lee, S.G.; Kim, J.E.; Lee, Y.S.; Chang, H.S.; Roh, M.R. Analysis of Risk Factors to Predict Occurrence and Prognosis of Postsurgical Hypertrophic Scar Development: A Review of 4238 Cases. Yonsei Med. J 2023, 64, 687–691. [Google Scholar] [CrossRef]
- Anderson, L.; Vankawala, J.; Gupta, N.; Dorfman, R.; Pflibsen, L.; Vardanian, A.; Delong, M. Evaluation of the Risk of Hypertrophic Scarring and Keloid Following Eyelid Procedures: A Systematic Review. Aesthet. Surg. J. 2023, 43, 820–829. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Fan Chiang, Y.H.; Lee, Y.W.; Lam, F.; Liao, C.C.; Chang, C.C.; Lin, C.S. Smoking increases the risk of postoperative wound complications: A propensity score-matched cohort study. Int. Wound J. 2023, 20, 391–402. [Google Scholar] [CrossRef]
- Commander, S.J.; Chamata, E.; Cox, J.; Dickey, R.M.; Lee, E.I. Update on Postsurgical Scar Management. Semin. Plast. Surg. 2016, 30, 122–128. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Coberly, D.M.; Fagien, S.; Stuzin, J.M. Current concepts in aesthetic upper blepharoplasty. Plast. Reconstr. Surg. 2004, 113, 32e–42e. [Google Scholar] [CrossRef]
- Kim, C.; Pfeiffer, M.L.; Chang, J.R.; Burnstine, M.A. Perioperative Considerations for Antithrombotic Therapy in Oculofacial Surgery: A Review of Current Evidence and Practice Guidelines. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 226–233. [Google Scholar] [CrossRef]
- Bunke, J.; Merdasa, A.; Stridh, M.; Rosenquist, P.; Berggren, J.; Hernandez-Palacios, J.E.; Dahlstrand, U.; Reistad, N.; Sheikh, R.; Malmsjo, M. Hyperspectral and Laser Speckle Contrast Imaging for Monitoring the Effect of Epinephrine in Local Anesthetics in Oculoplastic Surgery. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, O.; Rosenfeld, E.; Kalderon, E.; Barazani, T.B.; Zloto, O.; Martinowitz, U.; Ben Simon, G.J.; Zilinsky, I. Subcutaneous tranexamic acid in upper eyelid blepharoplasty: A prospective randomized pilot study. Can. J. Ophthalmol. 2018, 53, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Heyns, M.; Knight, P.; Steve, A.K.; Yeung, J.K. A Single Preoperative Dose of Tranexamic Acid Reduces Perioperative Blood Loss: A Meta-analysis. Ann. Surg. 2021, 273, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Locketz, G.D.; Lozada, K.N.; Bloom, J.D. Tranexamic Acid in Aesthetic Facial Plastic Surgery: A Systematic Review of Evidence, Applications, and Outcomes. Aesthet. Surg. J. Open Forum 2020, 2, ojaa029. [Google Scholar] [CrossRef]
- Marous, C.L.; Farhat, O.J.; Cefalu, M.; Rothschild, M.I.; Alapati, S.; Wladis, E.J. Effects of Preoperative Intravenous Versus Subcutaneous Tranexamic Acid on Postoperative Periorbital Ecchymosis and Edema Following Upper Eyelid Blepharoplasty: A Prospective, Randomized, Double-Blinded, Placebo-Controlled, Comparative Study. Ophthalmic Plast. Reconstr. Surg. 2024, 40, 523–532. [Google Scholar] [CrossRef]
- Ausen, K.; Fossmark, R.; Spigset, O.; Pleym, H. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br. J. Surg. 2015, 102, 1348–1353. [Google Scholar] [CrossRef]
- Chaichumporn, T.; Kanokkangsadal, P.; Sarovath, A. Tranexamic Acid Subcutaneously Administered with Epinephrine and Lidocaine in Upper Blepharoplasty: A Randomized Double-Blind Control Trial. Aesthetic Plast. Surg. 2024, 48, 3076–3081. [Google Scholar] [CrossRef]
- Byrne, M.; Aly, A. The Surgical Suture. Aesthet. Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef]
- David, L.M.; Sanders, G. CO2 laser blepharoplasty: A comparison to cold steel and electrocautery. J. Dermatol. Surg. Oncol. 1987, 13, 110–114. [Google Scholar] [CrossRef]
- Rokhsar, C.K.; Ciocon, D.H.; Detweiler, S.; Fitzpatrick, R.E. The short pulse carbon dioxide laser versus the colorado needle tip with electrocautery for upper and lower eyelid blepharoplasty. Lasers Surg. Med. 2008, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Aird, L.N.; Bristol, S.G.; Phang, P.T.; Raval, M.J.; Brown, C.J. Randomized double-blind trial comparing the cosmetic outcome of cutting diathermy versus scalpel for skin incisions. Br. J. Surg. 2015, 102, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Carqueville, J.C.; Chesnut, C. Histologic Comparison of Upper Blepharoplasty Skin Excision Using Scalpel Incision Versus Microdissection Electrocautery Needle Tip Versus Continuous Wave CO2 Laser. Dermatol. Surg. 2021, 47, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Luecke, G.; Dozier, S.; Diven, D.G. A pilot study to investigate the efficacy of tobramycin-dexamethasone ointment in promoting wound healing. Dermatol. Ther. 2012, 2, 12. [Google Scholar] [CrossRef][Green Version]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Leung, A.; Crombleholme, T.M.; Keswani, S.G. Fetal wound healing: Implications for minimal scar formation. Curr. Opin. Pediatr. 2012, 24, 371–378. [Google Scholar] [CrossRef]
- Homer, N.A.; Zhou, S.; Watson, A.H.; Durairaj, V.D.; Nakra, T. Wound Dehiscence Following Upper Blepharoplasty: A Review of 2,376 Cases. Ophthalmic Plast. Reconstr. Surg. 2021, 37, S66–S69. [Google Scholar] [CrossRef]
- Kashkouli, M.B.; Jamshidian-Tehrani, M.; Sharzad, S.; Sanjari, M.S. Upper Blepharoplasty and Lateral Wound Dehiscence. Middle East. Afr. J. Ophthalmol. 2015, 22, 452–456. [Google Scholar] [CrossRef]
- Lee, Y.J.; Baek, R.M.; Chung, W.J. Nonincisional blepharoplasty using the debulking method. Aesthetic Plast. Surg. 2003, 27, 434–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).