A Novel Modification of Anconeus Muscle Flap for Extensor Digitorum Communis-Selective Lateral Epicondylitis: Preliminary Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
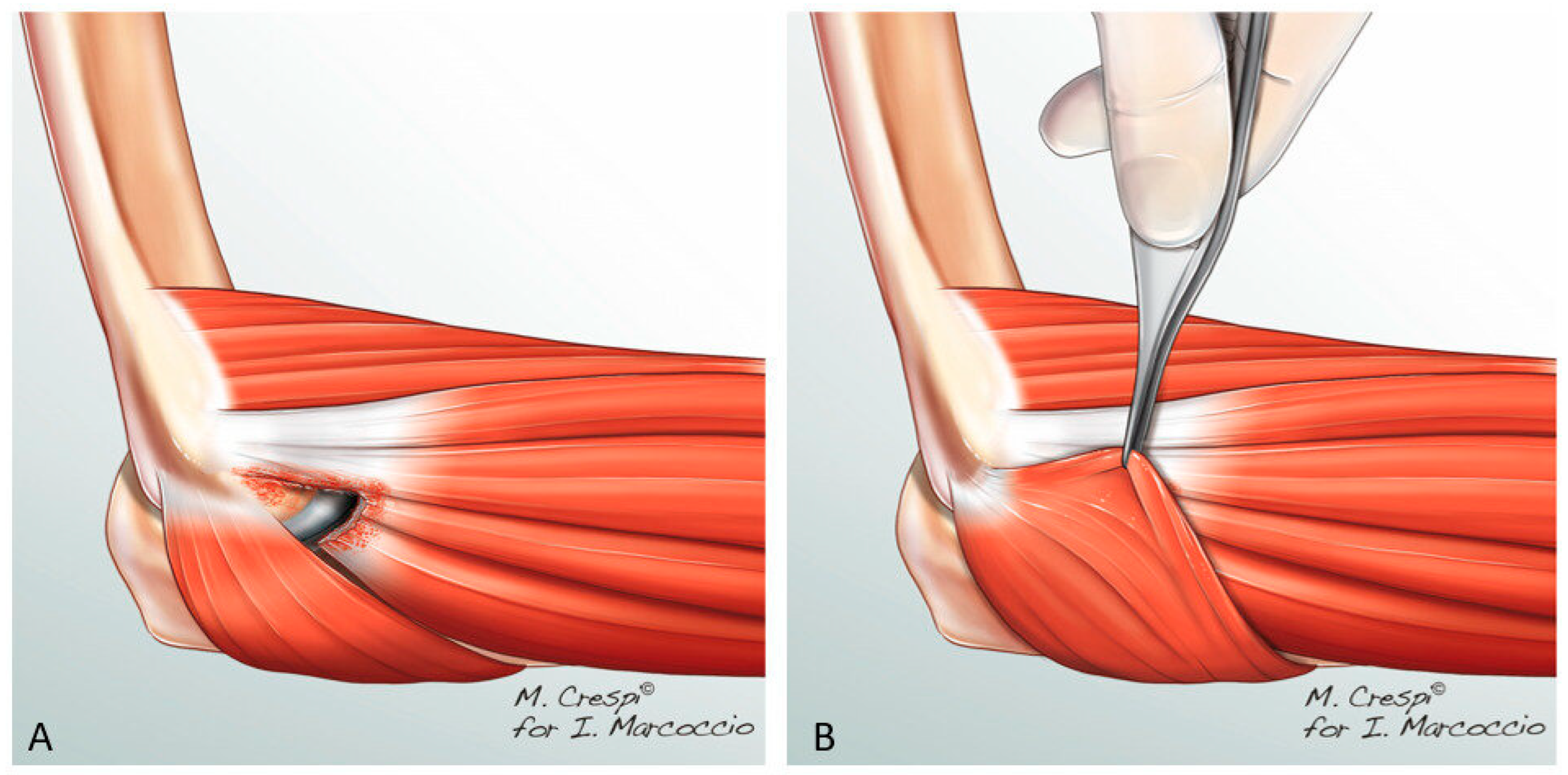
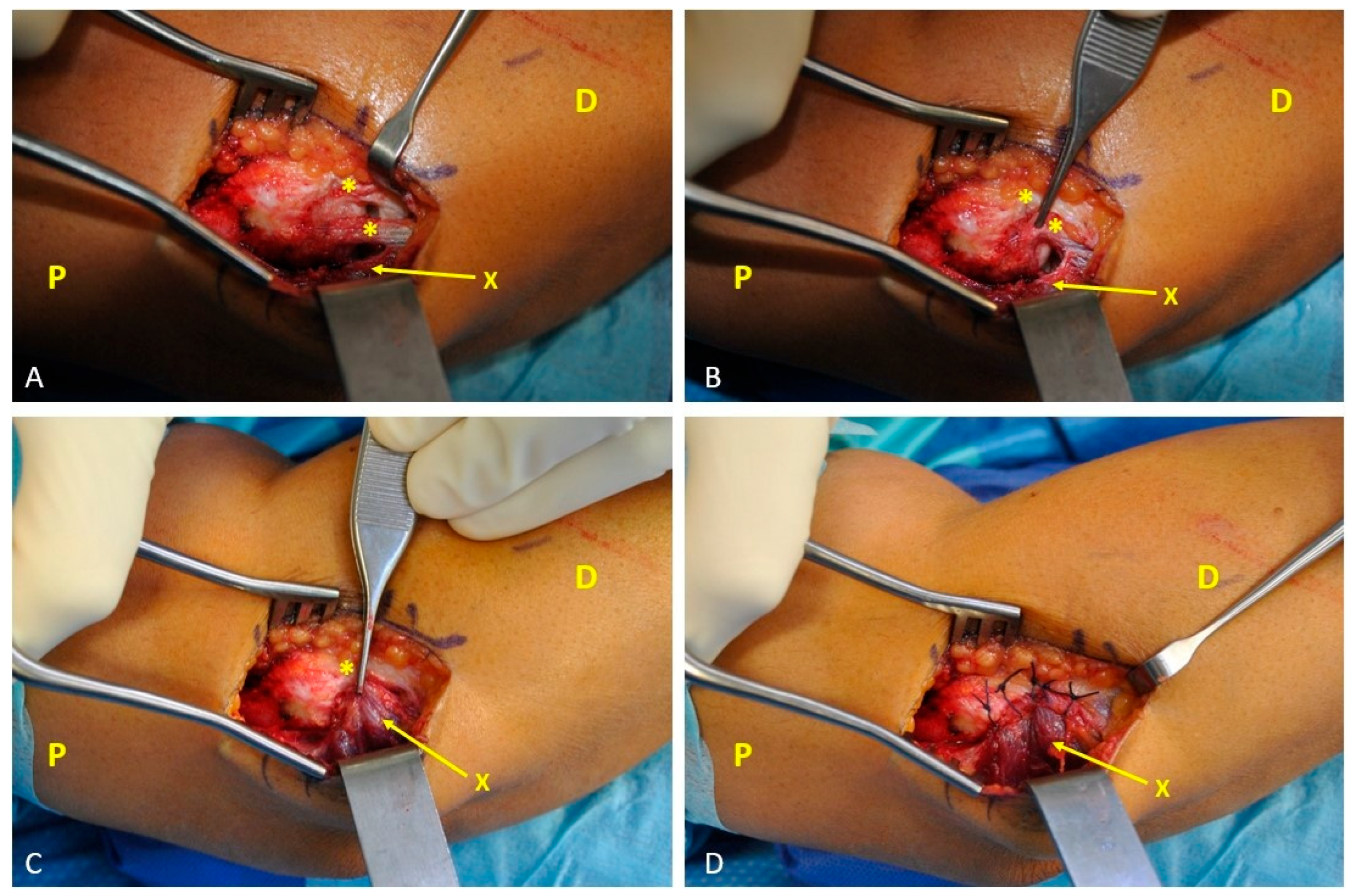
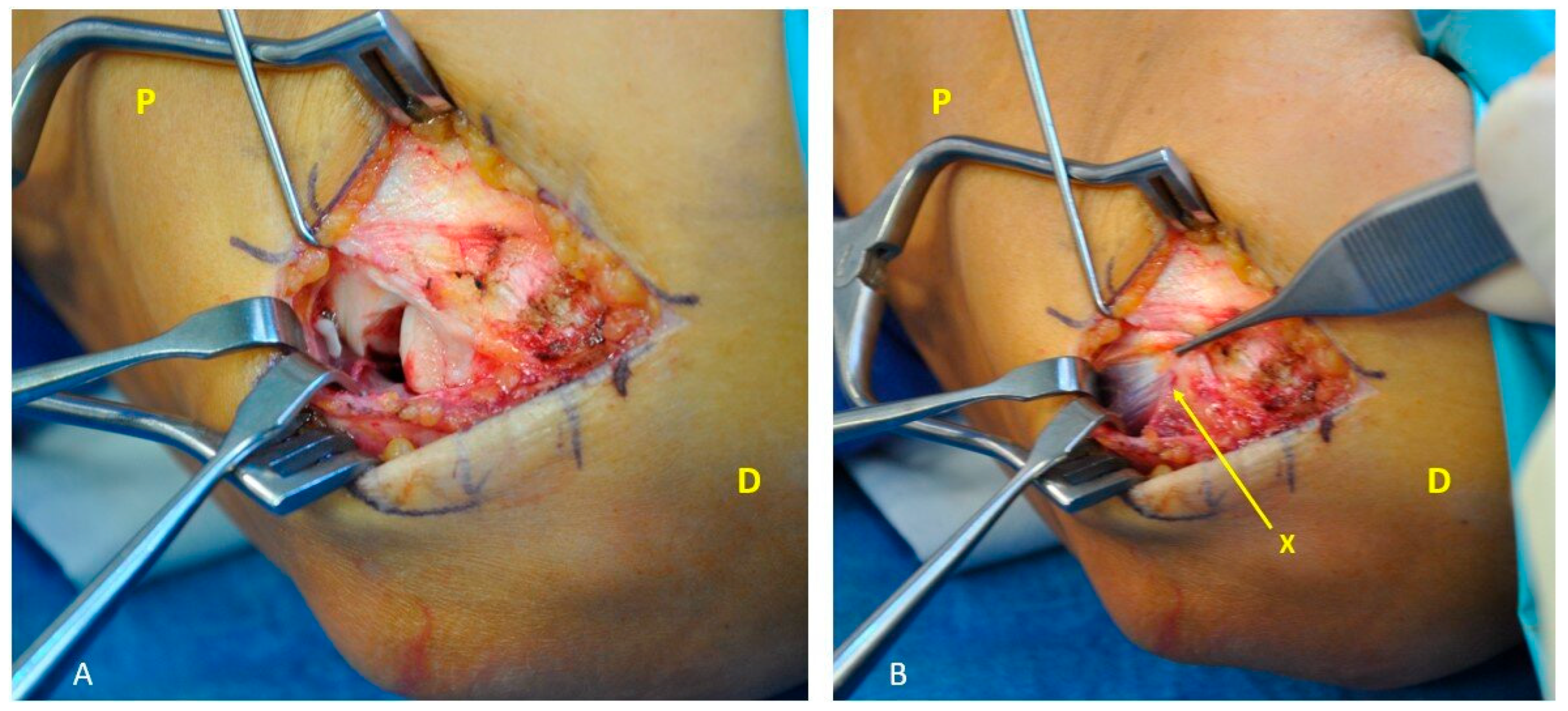
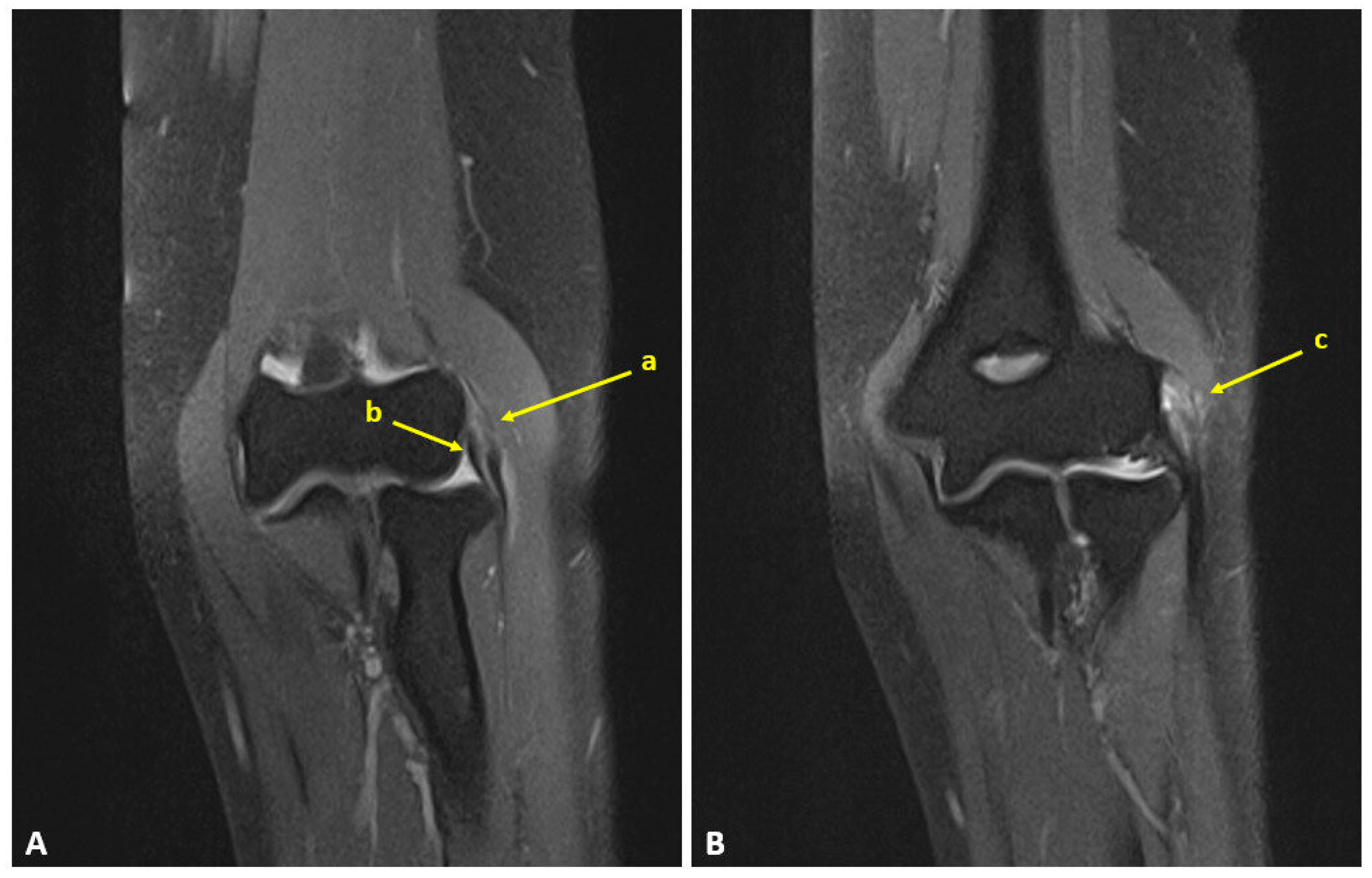
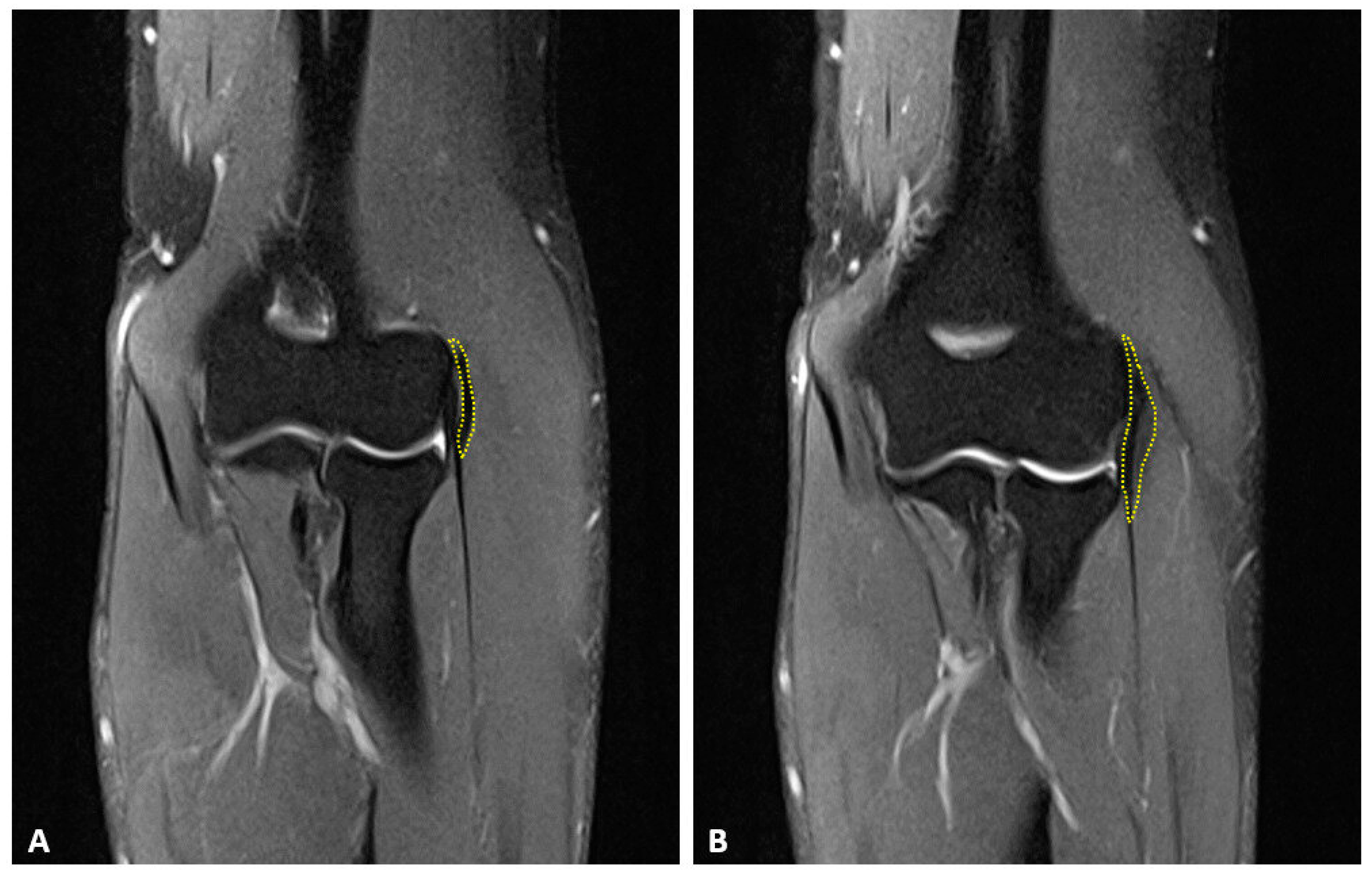
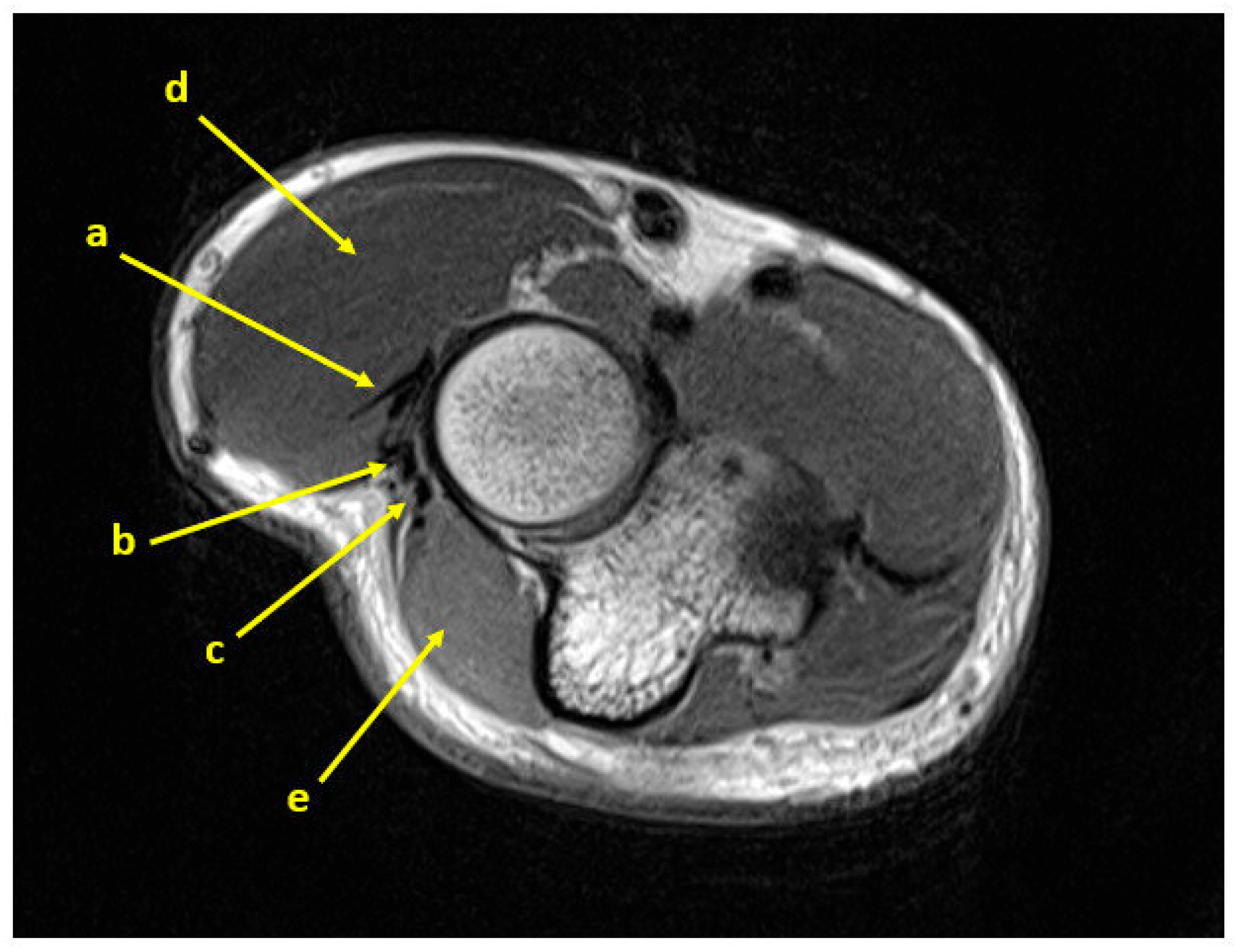
2.2. Surgical Technique
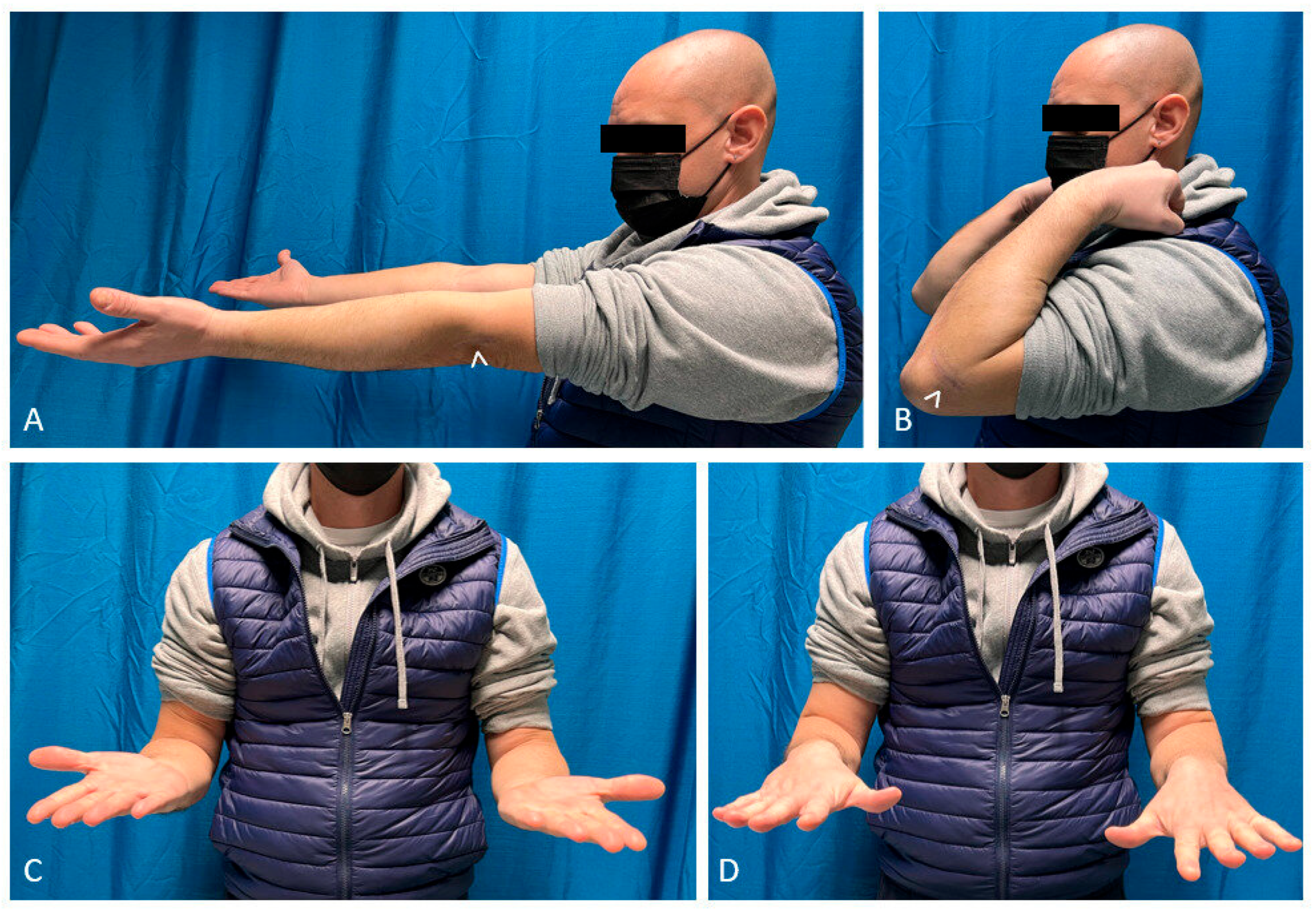
2.3. Outcome Measurements
2.4. Statistical Analysis
- MEPS: approximately 10–15 points depending on cohort and method [28]
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDC | Extensor digitorum communis |
| AMA | Anconeus muscle advancement |
| VAS | Visual analog pain scale |
| PROMs | Patient-reported outcome measures |
| DASH | Disabilities of the Arm, Shoulder and Hand |
| MEPS | Mayo Elbow Performance Score |
| PRTEE | Patient-Rated Tennis Elbow Evaluation |
| MCID | Minimal clinically important difference |
| LE | Lateral epicondylitis |
| ECRB | Extensor carpi radialis brevis |
| ECRL | Extensor carpi radialis longus |
| ECU | Extensor carpi ulnaris |
| PLRI | Posterolateral rotatory elbow instability |
| RPIA | Recurrent posterior interosseous artery |
| MCA | Medial collateral artery |
| PBRCA | Posterior branch of the radial collateral artery |
| LCL | Lateral ligament complex |
References
- Lenoir, H.; Mares, O.; Carlier, Y. Management of lateral epicondylitis. Orthop. Traumatol. Surg. Res. 2019, 105, S241–S246. [Google Scholar] [CrossRef] [PubMed]
- Bunata, R.E.; Brown, D.S.; Capelo, R. Anatomic factors related to the cause of tennis elbow. J. Bone Jt. Surg. Am. 2007, 89, 1955–1963. [Google Scholar] [CrossRef]
- Keijsers, R.; de Vos, R.J.; Kuijer, P.P.F.; van den Bekerom, M.P.; van der Woude, H.J.; Eygendaal, D. Tennis elbow. Shoulder Elb. 2019, 11, 384–392. [Google Scholar] [CrossRef]
- Kraushaar, B.S.; Nirschl, R.P. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J. Bone Jt. Surg. Am. 1999, 81, 259–278. [Google Scholar] [CrossRef]
- Nirschl, R.P.; Pettrone, F.A. Tennis elbow. The surgical treatment of lateral epicondylitis. J. Bone Jt. Surg. Am. 1979, 61, 832–839. [Google Scholar] [CrossRef]
- Regan, W.; Wold, L.E.; Coonrad, R.; Morrey, B.F. Microscopic histopathology of chronic refractory lateral epicondylitis. Am. J. Sports Med. 1992, 20, 746–749. [Google Scholar] [CrossRef]
- Fairbank, S.M.; Corlett, R.J. The role of the extensor digitorum communis muscle in lateral epicondylitis. J. Hand Surg. Br. 2002, 27, 405–409. [Google Scholar] [CrossRef]
- Calfee, R.P.; Patel, A.; DaSilva, M.F.; Akelman, E. Management of lateral epicondylitis: Current concepts. J. Am. Acad. Orthop. Surg. 2008, 16, 19–29. [Google Scholar] [CrossRef]
- Kacena-Merrell, C.; Ngo, S.; Chow, I. Mapping Origins of Tendons on the Lateral Epicondyle. J. Hand Surg. Am. 2025; in press. [Google Scholar] [CrossRef]
- Nimura, A.; Fujishiro, H.; Wakabayashi, Y.; Imatani, J.; Sugaya, H.; Akita, K. Joint capsule attachment to the extensor carpi radialis brevis origin: An anatomical study with possible implications regarding the etiology of lateral epicondylitis. J. Hand Surg. Am. 2014, 39, 219–225. [Google Scholar] [CrossRef]
- Ma, K.L.; Wang, H.Q. Management of Lateral Epicondylitis: A Narrative Literature Review. Pain Res. Manag. 2020, 2020, 6965381. [Google Scholar] [CrossRef]
- Ruch, D.S.; Orr, S.B.; Richard, M.J.; Leversedge, F.J.; Mithani, S.K.; Laino, D.K. A comparison of débridement with and without anconeus muscle flap for treatment of refractory lateral epicondylitis. J. Shoulder Elb. Surg. 2015, 24, 236–241. [Google Scholar] [CrossRef]
- Walz, D.M.; Newman, J.S.; Konin, G.P.; Ross, G. Epicondylitis: Pathogenesis, imaging, and treatment. Radiographics 2010, 30, 167–184. [Google Scholar] [CrossRef]
- Barnett, J.; Bernacki, M.N.; Kainer, J.L.; Smith, H.N.; Zaharoff, A.M.; Subramanian, S.K. The effects of regenerative injection therapy compared to corticosteroids for the treatment of lateral Epicondylitis: A systematic review and meta-analysis. Arch. Physiother. 2019, 9, 12. [Google Scholar] [CrossRef]
- Behrens, S.B.; Deren, M.E.; Matson, A.P.; Bruce, B.; Green, A. A review of modern management of lateral epicondylitis. Physician Sportsmed. 2012, 40, 34–40. [Google Scholar] [CrossRef]
- Kim, G.M.; Yoo, S.J.; Choi, S.; Park, Y.G. Current Trends for Treating Lateral Epicondylitis. Clin. Shoulder Elb. 2019, 22, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, I.T.; Lee, S.; Kim, T.S.; Jung, K.; Han, S.H. Long-term outcomes of the modified Nirschl technique for lateral Epicondylitis: A retrospective study. BMC Musculoskelet. Disord. 2021, 22, 205. [Google Scholar] [CrossRef]
- Baker, C.L.; Murphy, K.P.; Gottlob, C.A.; Curd, D.T. Arthroscopic classification and treatment of lateral epicondylitis: Two-year clinical results. J. Shoulder Elb. Surg. 2000, 9, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.M.; Kode, V.; Xu, M.C.; Shi, G.G.; Grindel, S.I. Anconeus muscle flap transfer for failed surgical treatment of recalcitrant chronic lateral epicondylitis. Orthoplastic Surg. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- Almquist, E.E.; Necking, L.; Bach, A.W. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J. Hand Surg. Am. 1998, 23, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, R.; Atzei, A.; Brunelli, F.; Fairplay, T. Anconeus muscle transposition for chronic lateral epicondylitis, recurrences, and complications. Tech. Hand Up. Extrem. Surg. 2005, 9, 105–112. [Google Scholar] [CrossRef]
- Degreef, I.; Van Raebroeckx, A.; De Smet, L. Anconeus muscle transposition for failed surgical treatment of tennis elbow: Preliminary results. Acta Orthop. Belg. 2005, 71, 154–156. [Google Scholar]
- Luyckx, T.; Decramer, A.; Luyckx, L.; Noyez, J. Modified anconeus muscle transfer as treatment of failed surgical release of lateral epicondylitis of the elbow. Acta Orthop. Belg. 2017, 83, 310–314. [Google Scholar]
- Stults, W.P.; Hanson, Z.C.; Lourie, G.M. A Combined Revision Surgical Technique for Failed Operative Lateral Epicondylitis with Concomitant Radial Tunnel Syndrome. Tech. Hand Up. Extrem. Surg. 2022, 26, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Padua, R.; Padua, L.; Ceccarelli, E.; Romanini, E.; Zanoli, G.; Amadio, P.C.; Campi, A. Italian version of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire. Cross-cultural adaptation and validation. J. Hand Surg. Br. 2003, 28, 179–186. [Google Scholar] [CrossRef]
- Evans, J.P.; Smith, C.D.; Fine, N.F.; Porter, I.; Gangannagaripalli, J.; Goodwin, V.A.; Valderas, J.M. Clinical rating systems in elbow research-a systematic review exploring trends and distributions of use. J. Shoulder Elb. Surg. 2018, 27, e98–e106. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.C.; Bonnaig, N.S.; Azar, F.M.; Mauck, B.M.; Smith, R.A.; Throckmorton, T.W. Accuracy and Reliability of the Mayo Elbow Performance Score. J. Hand Surg. 2014, 39, 1146–1150. [Google Scholar] [CrossRef]
- Rompe, J.D.; Overend, T.J.; MacDermid, J.C. Validation of the Patient-rated Tennis Elbow Evaluation Questionnaire. J. Hand Ther. 2007, 20, 3–10, quiz 11. [Google Scholar] [CrossRef]
- Cacchio, A.; Necozione, S.; MacDermid, J.C.; Rompe, J.D.; Maffulli, N.; Di Orio, F.; Santilli, V.; Paoloni, M. Cross-cultural adaptation and measurement properties of the italian version of the Patient-Rated Tennis Elbow Evaluation (PRTEE) questionnaire. Phys. Ther. 2012, 92, 1036–1045. [Google Scholar] [CrossRef]
- Macdermid, J. Update: The Patient-rated Forearm Evaluation Questionnaire is now the Patient-rated Tennis Elbow Evaluation. J. Hand Ther. 2005, 18, 407–410. [Google Scholar] [CrossRef]
- Todd, K.H.; Funk, K.G.; Funk, J.P.; Bonacci, R. Clinical significance of reported changes in pain severity. Ann. Emerg. Med. 1996, 27, 485–489. [Google Scholar] [CrossRef]
- Kelly, A.M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 2001, 18, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Sorensen, A.A.; Howard, D.; Tan, W.H.; Ketchersid, J.; Calfee, R.P. Minimal clinically important differences of 3 patient-rated outcomes instruments. J. Hand Surg. Am. 2013, 38, 641–649. [Google Scholar] [CrossRef]
- Polson, K.; Reid, D.; McNair, P.J.; Larmer, P. Responsiveness, minimal importance difference and minimal detectable change scores of the shortened disability arm shoulder hand (QuickDASH) questionnaire. Man. Ther. 2010, 15, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Skrepnik, N.V.; Edwards, S.G.; Jones, G.L.; Sampson, S.; Vermillion, D.A.; Ramsey, M.L.; Karli, D.C.; Rettig, A.C. Efficacy of platelet-rich plasma for chronic tennis elbow: A double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am. J. Sports Med. 2014, 42, 463–471. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, M.G.; Shin, S.J. What is the minimum clinically important difference in grip strength? Clin. Orthop. Relat. Res. 2014, 472, 2536–2541. [Google Scholar] [CrossRef]
- Pankovich, A.M. Anconeus approach to the elbow joint and the proximal part of the radius and ulna. J. Bone Jt. Surg. Am. 1977, 59, 124–126. [Google Scholar] [CrossRef]
- Mathes, S.J.; Nahai, F. Classification of the vascular anatomy of muscles: Experimental and clinical correlation. Plast. Reconstr. Surg. 1981, 67, 177–187. [Google Scholar] [CrossRef]
- Jeon, B.J.; Jwa, S.J.; Lee, D.C.; Roh, S.Y.; Kim, J.S. The Anconeus Muscle Free Flap: Clinical Application to Lesions on the Hand. Arch. Plast. Surg. 2017, 44, 420–427. [Google Scholar] [CrossRef]
- Magden, O.; Tayfur, V.; Edizer, M. Arterial Anatomy Of Anconeus Muscle Flap. Clin Med. 2010, 27, 24–25. [Google Scholar] [CrossRef]
- Hwang, K.; Han, J.Y.; Chung, I.H. Topographical anatomy of the anconeus muscle for use as a free flap. J. Reconstr. Microsurg. 2004, 20, 631–636. [Google Scholar] [CrossRef]
- Schmidt, C.C.; Kohut, G.N.; Greenberg, J.A.; Kann, S.E.; Idler, R.S.; Kiefhaber, T.R. The anconeus muscle flap: Its anatomy and clinical application. J. Hand Surg. Am. 1999, 24, 359–369. [Google Scholar] [CrossRef]
- Greco, V.E.; Wroblewski, A.; Kharlamov, A.; Miller, M.C.; Winek, N.; Hammarstedt, J.E.; Regal, S. Safe dissection parameters of the anconeus rotational flap for soft tissue coverage at the elbow. Shoulder Elb. 2023, 15, 436–441. [Google Scholar] [CrossRef]
- Meals, R.A. The use of a flexor carpi ulnaris muscle flap in the treatment of an infected nonunion of the proximal ulna. A case report. Clin. Orthop. Relat. Res. 1989, 240, 168–172. [Google Scholar] [CrossRef]
- Schneeberger, A.G.; Masquelet, A.C. Arterial vascularization of the proximal extensor carpi radialis brevis tendon. Clin. Orthop. Relat. Res. 2002, 398, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Iwasaki, N.; Funakoshi, T.; Motomiya, M.; Minami, A. Prevention of instability of the proximal end of the radius after radial head resection using an anconeus muscle flap. Hand Surg. 2012, 17, 25–31. [Google Scholar] [CrossRef]
- Baker, C.L.; Baker, C.L. Long-term follow-up of arthroscopic treatment of lateral epicondylitis. Am. J. Sports Med. 2008, 36, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Siddiqui, N.; Malik, S.S.; Abdus-Samee, M.; Tytherleigh-Strong, G.; Rushton, N. Lateral epicondylitis: A review of pathology and management. Bone Jt. J. 2013, 95, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.H.; Kim, J.J.; Davis, L.; Nirschl, R.P. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am. J. Sports Med. 2008, 36, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.J.; Govindaswamy, R.; Elbouni, T.; Chambler, A.F.W. Are “knife and fork” good enough for day case surgery of resistant tennis elbow? Int. Orthop. 2010, 34, 57–61. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | ||
|---|---|---|
| Age (years) | 48 ± 2.82 | |
| Gender | ||
| Male | 3 (25%) | |
| Female | 9 (75%) | |
| Side | ||
| Right | 9 (75%) | |
| Left | 3 (25%) | |
| Dominance | ||
| Yes | 10 (83.3%) | |
| No | 2 (16.6%) | |
| Time from symptoms onset to surgery | 36 ± 18 | |
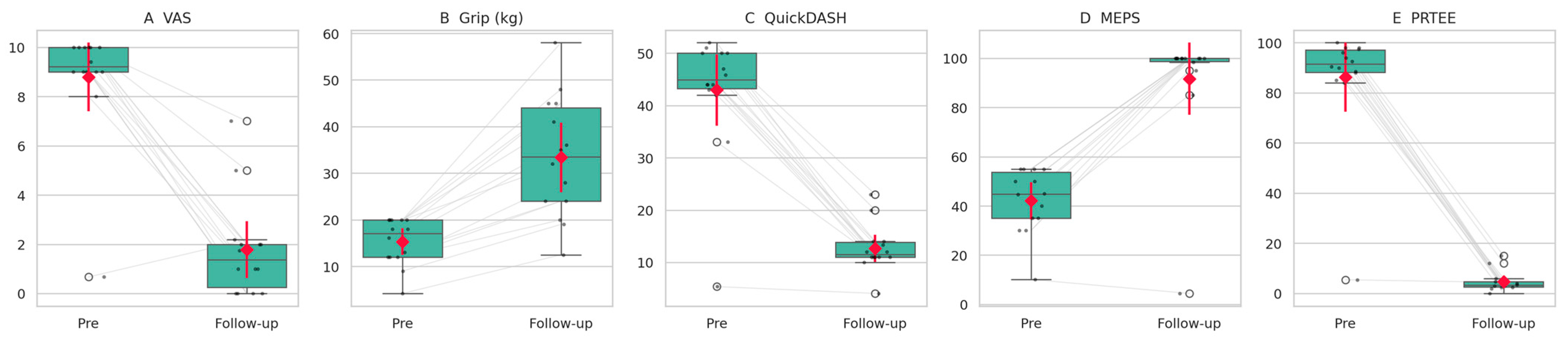
| Outcome | Preoperative (Mean ± SD) | Follow-Up (Mean ± SD) | p-Value | 95% CI of Δ (FU-Pre) | Effect Size |
|---|---|---|---|---|---|
| VAS | 9.42 ± 0.67 | 1.75 ± 2.18 | 0.0005 (Wilcoxon) | −8.67 to −6.50 | 1.0 |
| Grip Strength (kg) | 16.17 ± 4.20 | 35.0 ± 12.49 | <0.001 (paired t-test) | +13.02 to +24.64 | 2.06 |
| QuickDASH | 45.83 ± 5.36 | 13.33 ± 4.05 | <0.001 (paired t-test) | −35.76 to −29.24 | −6.34 |
| Mayo Elbow Score | 44.58 ± 10.10 | 98.33 ± 4.44 | 0.0005 (Wilcoxon) | +49.17 to +58.75 | 1.0 |
| PRTEE | 92.46 ± 5.49 | 4.71 ± 4.38 | <0.001 (paired t-test) | −90.73 to −84.77 | −18.72 |
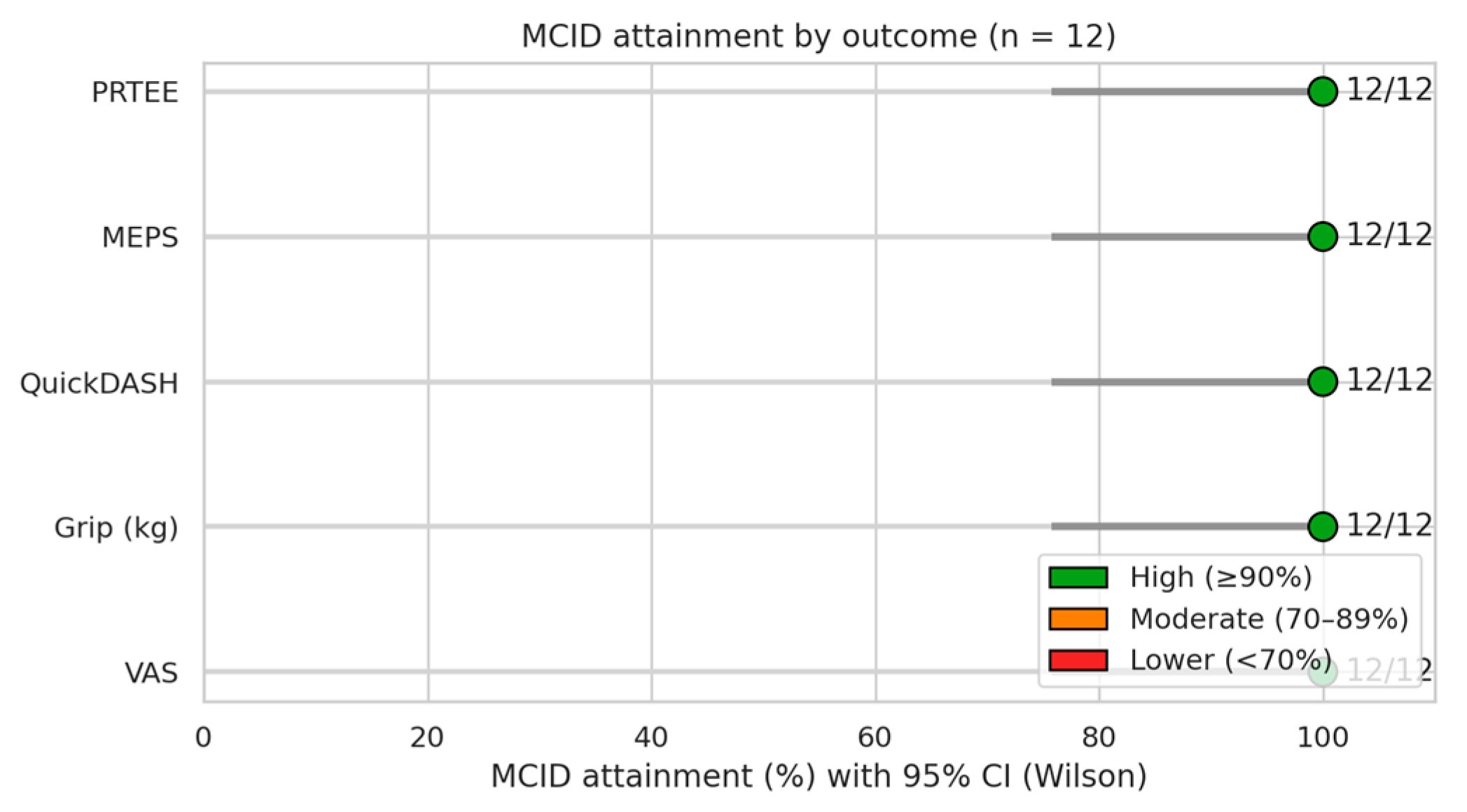
| Variable | Observed Mean Change (Δ) | Published MCID Range | Clinical Relevance | References |
|---|---|---|---|---|
| VAS (0–10) | −7.67 | 1.5–2.0 | Clinically meaningful | [32,33,34] |
| QuickDASH (0–100) | −32.50 | 8–15 | Clinically meaningful | [35,36] |
| PRTEE (0–100) | −87.75 | 11–15 | Clinically meaningful | [31,37] |
| Mayo Elbow Score | +53.75 | 10–15 | Clinically meaningful | [28] |
| Grip Strength (kg) | +18.83 | 5–6 | Clinically meaningful | [38,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcoccio, I.; Maffeis, J.; Gravina, P.; Civitenga, C.; Gervasio, A. A Novel Modification of Anconeus Muscle Flap for Extensor Digitorum Communis-Selective Lateral Epicondylitis: Preliminary Clinical Study. Surgeries 2025, 6, 105. https://doi.org/10.3390/surgeries6040105
Marcoccio I, Maffeis J, Gravina P, Civitenga C, Gervasio A. A Novel Modification of Anconeus Muscle Flap for Extensor Digitorum Communis-Selective Lateral Epicondylitis: Preliminary Clinical Study. Surgeries. 2025; 6(4):105. https://doi.org/10.3390/surgeries6040105
Chicago/Turabian StyleMarcoccio, Ignazio, Jacopo Maffeis, Pasquale Gravina, Carolina Civitenga, and Andrea Gervasio. 2025. "A Novel Modification of Anconeus Muscle Flap for Extensor Digitorum Communis-Selective Lateral Epicondylitis: Preliminary Clinical Study" Surgeries 6, no. 4: 105. https://doi.org/10.3390/surgeries6040105
APA StyleMarcoccio, I., Maffeis, J., Gravina, P., Civitenga, C., & Gervasio, A. (2025). A Novel Modification of Anconeus Muscle Flap for Extensor Digitorum Communis-Selective Lateral Epicondylitis: Preliminary Clinical Study. Surgeries, 6(4), 105. https://doi.org/10.3390/surgeries6040105






