1. Introduction
The hospital sector in Germany generates approximately 1.2 million tons of waste annually, much of which consists of single-use plastic products that are currently incinerated [
1]. In recent years, global research has increasingly focused on developing thermochemical recycling routes, such as pyrolysis, to recover valuable hydrocarbons from medical and packaging plastics. Several studies have demonstrated the technical feasibility of converting polypropylene (PP), polyethylene (PE), and mixed medical plastics into pyrolysis oils with yields typically between 60 and 80 wt%, depending on the feed composition and temperature profiles [
1,
2,
3]. Recent advances have focused on catalytic pyrolysis and reactor optimization to improve oil quality and reduce contaminants, such as chlorine and silicon, which pose challenges for downstream processing [
4,
5]. For example, one study reviewed pyrolysis pathways for healthcare plastics and reported that optimized batch and fluidized-bed reactors can achieve energy recovery efficiencies exceeding 75% with significant CO
2 reduction compared to incineration [
2]. However, contamination and regulatory constraints continue to hinder their direct reuse in medical-grade applications. While most previous work has focused on mixed municipal or packaging waste, systematic studies addressing standardized hospital waste streams—such as procedure-specific surgical trays—remain scarce.
Therefore, this study aimed to evaluate the pyrolysis of a well-defined medical plastic fraction derived from standardized surgical trays under controlled laboratory conditions, assessing both the yield and product quality in the context of a scalable circular economy model for healthcare.
In 2020, a cross-disciplinary expert group was established as a cross-industry cooperation dedicated to advancing the circular economy in medicine [
2]. The group operates under the legal framework of the non-profit association Ecology and Sustainability in Medicine e.V. and pursues the goals of waste reduction and high-quality plastic recycling from central operating rooms. We replaced the standard-packaged medical products with tray-based packaging in a comparative study of 64 operations [
3]. With tray-based packaging, we could demonstrate a 34% reduction in waste weight, a 32% reduction in waste volume, and a 43% reduction in operating room setup time. Workflow evaluation by the nursing staff was rated very positively, with a mean overall score of 9.75/10. The time savings and reduction in the workload stress were perceived very positively by nursing staff [
3]. To further enhance resource efficiency, the residual plastic waste from the trays was assessed for recyclability using thermochemical recycling. The aim of this study was to convert a standardized plastic fraction into synthetic oil. We conducted a feasibility study with one pyrolysis run.
4. Discussion
4.1. Recycling of Medical Waste via Pyrolysis
4.1.1. Fraction Analysis
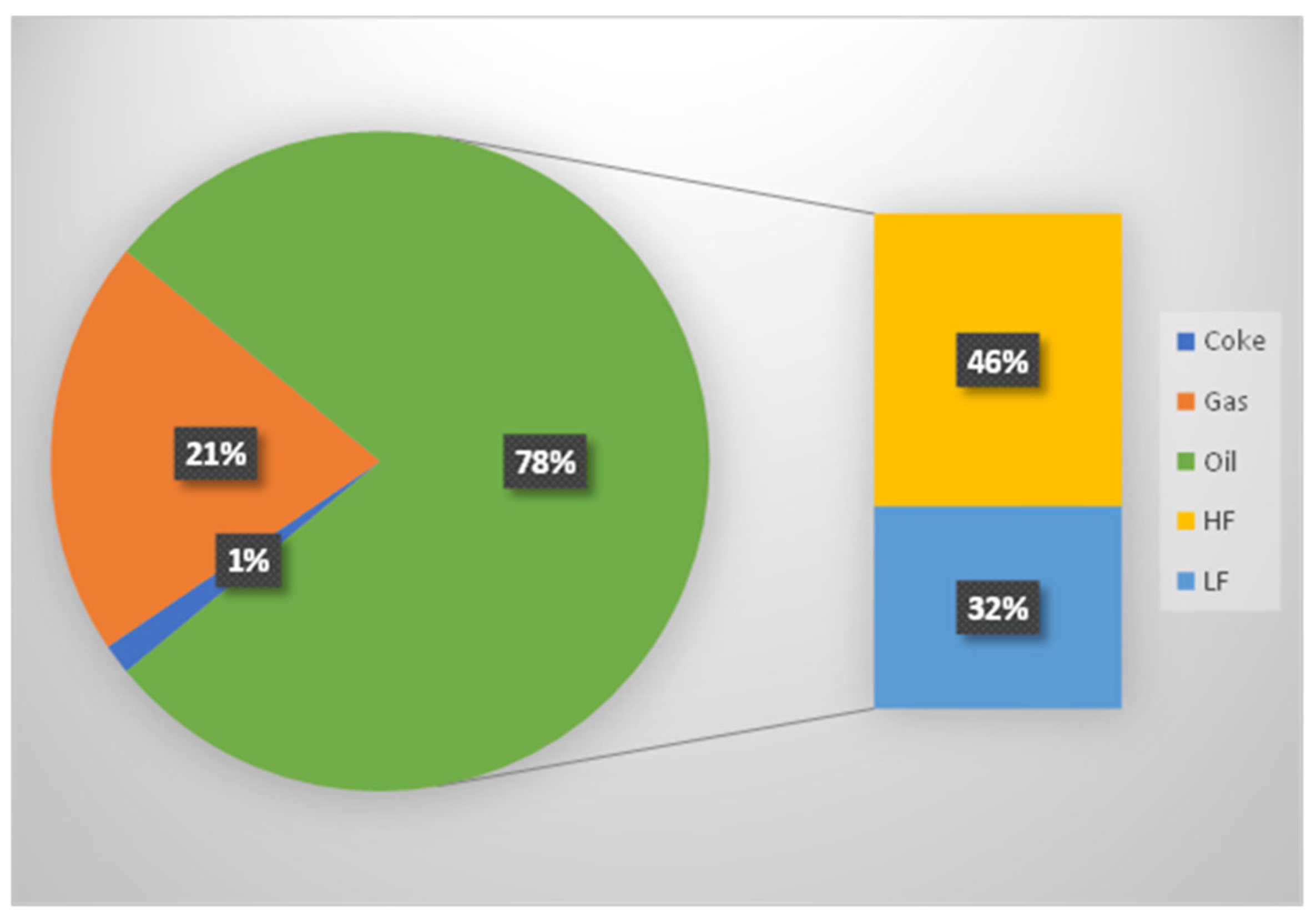
The experiment met the expectations for generating a high-quality and usable pyrolysis oil. From 100% hazardous waste, 78% oil was recovered, with a higher yield of heavy fraction compared to light fraction. The low coke content (1.5%) and the calculated gas fraction (20.5%) completed the overall mass balance. Importantly, the produced pyrolysis gas could potentially be applied in processes aimed at producing plastics for medical devices from a quality perspective. The light oil fraction acts as a renewable chemical feedstock, enabling the production of medical-grade high-purity polyethylene (PE) or polypropylene (PP), which are chemically and functionally identical to those derived from fossil naphtha. This establishes a technically and regulatory viable closed-loop recycling pathway for many non-implantable medical plastics—such as surgical trays, syringes, and packaging—within the circular economy framework. From a mechanistic perspective, the pyrolysis of polyolefin-rich medical plastics follows a free-radical chain scission pathway, in which C–C bonds in polypropylene (PP) and polyethylene (PE) undergo random cleavage at temperatures above 400 °C. This results in the formation of short-chain radicals that recombine to produce alkanes, alkenes, and small aromatic compounds in the C
5–C
20 range. The dominance of paraffinic and olefinic species in both heavy and light oil fractions corresponds with the observed high heating values (>46 MJ·kg
−1) and low oxygen content (<1 wt%). Compared with previous trials, the oil fractions fell within the expected range. For comparison, the density of gasoline lies between 720 and 775 kg/m
3 and that of diesel between 820 and 845 kg/m
3 [
5].
The energy balance of the process is favorable: approximately 78 wt% of the feedstock was converted into high-energy oil, with an average conversion efficiency exceeding 70%, comparable to the literature values for catalytic and non-catalytic polyolefin pyrolysis [
6,
7,
8,
9]. The integration of process gas combustion for heating—already implemented at the pilot facility—can further improve the overall thermal efficiency by 60–75%, reducing the need for external energy input. These mechanistic and energetic considerations confirm that the selected temperature profile (430–460 °C) is well-suited for maximizing the oil yield while ensuring complete polymer degradation.
The fractionation could be further improved by adding more condensation stages at dif-ferent temperatures, use higher surface areas to achieve thermodynamical balance faster or even use a fractional distillation.
The remaining coke fraction, representing 1.5% of the total mass, must be treated as hazardous waste and disposed of properly. Chemical analysis confirmed that the pyrolysis oils did not contain significant contamination originating from the medical plastics or their prior clinical use into the recovered pyrolysis. The absence of chemical residues or hazardous elements such as halogenated compounds, silicone residues, plasticizers and stabilizers, inorganic contaminants (e.g., metals), and residual pharmaceuticals was confirmed.
4.1.2. CHNS Analysis
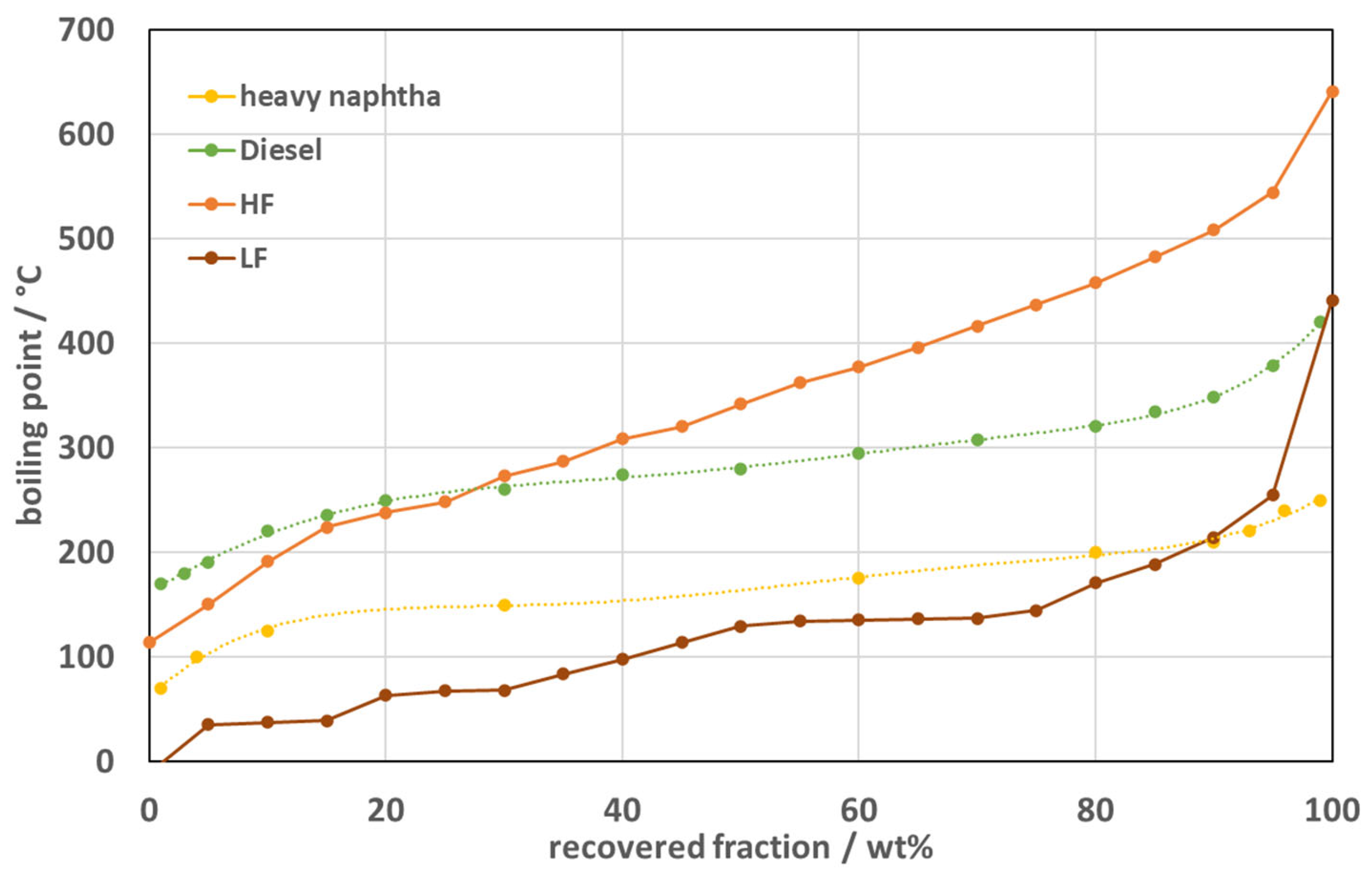
CHNS stands for carbon (C), hydrogen (H), nitrogen (N), and sulfur (S). These are the four key elements typically analyzed in organic compounds, particularly in the context of combustion analysis for characterizing the chemical composition of materials, such as pyrolysis oils, fuels, and biomass. The CHNS analysis involves determining the quantities of these elements present in a sample. Silicon primarily originates from silicone-based input materials. To reduce the Si content, separation of these feedstocks prior to pyrolysis could be considered. Nevertheless, both P and Si levels are acceptable and do not represent exclusion criteria for the further use of pyrolysis oil in medical device applications. A comparison of elemental concentrations between the heavy and light fractions revealed distinct distribution patterns: phosphorus (P) and silicon (Si) were both more concentrated in the light fraction (LF), while chlorine (Cl), although measured only in LF, is known to partition preferentially into the gas and light oil phases due to its volatility. These variations can be attributed to the differing thermal decomposition pathways and volatility of organosilicon and organophosphorus compounds present in the input plastics. Silicone-based tubing and coatings were identified as the principal sources of Si, whereas phosphorus-containing additives such as flame retardants or plasticizers likely account for the P content. The C/H ratio of the LF was 5.78 and therefore higher compared to the C/H ratio of 5.75 calculated for the HF. A higher C/H ratio can be attributed to several factors. Longer hydrocarbons have higher C/H ratios, as well as branched hydrocarbons. Another explanation might be the content of alkenes in the hydrocarbons. Alkenes have higher C/H ratios as their correspondent alkane. Therefore, both higher content of branched hydrocarbons and alkenes are a possible reason for the higher C/H ratio. The higher oxygen content in the LF hints towards accumulation of oxygenates in the lighter fraction of the oil. Nitrogen on the other hand is similarly high in both fractions and does not tend to accumulate in any of the fractions. The halogen content is a critical parameter for the steam cracking process. In the steam cracking process, the hydrocarbon chains are cracked down at high temperatures with the addition of steam to yield ethylene and propylene. Halogens like chlorine are highly corrosive to stainless-steel components, particularly at welding seams, in refinery and steam-cracking plants used for processing pyrolysis oils. Chlorine traces most probably originate from small quantities of chlorinated elastomers or labeling residues, even though PVC components were intentionally excluded. Controlling these heteroelements is crucial for the downstream use of pyrolysis oils in chemical or fuel applications. To mitigate contamination, presorting and feedstock homogenization—for example, separating silicone components and chlorinated polymers prior to pyrolysis—represent effective strategies. Future optimization will therefore focus on input stream refinement and process modifications to achieve further reductions in these elements, enhancing the oil’s suitability for closed-loop medical device production.
4.1.3. Input Materials
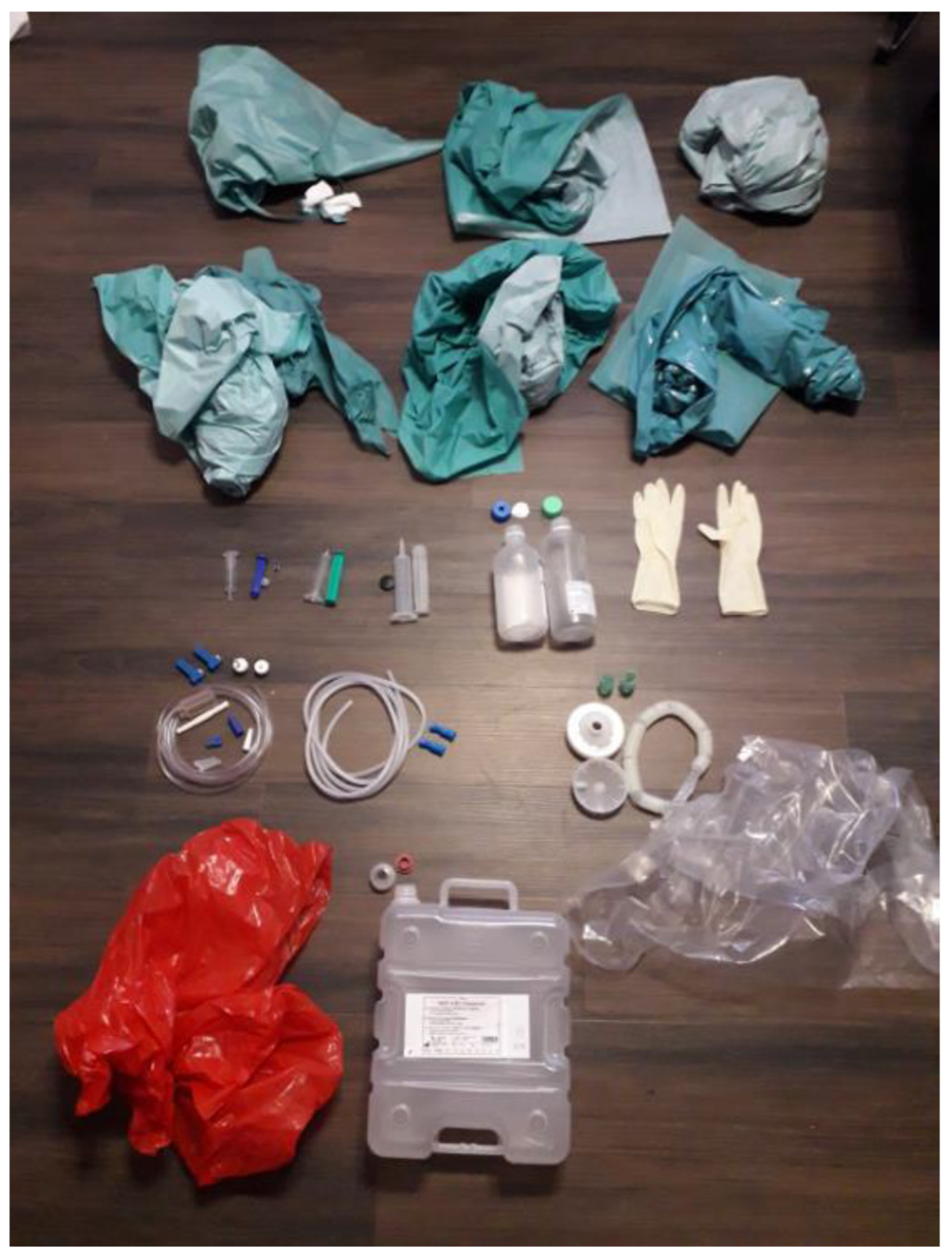
Materials not suitable for pyrolysis include PVC (polyvinyl chloride); silicone rubber and fluoropolymers; metal or composite parts, which do not thermally degrade; and paper or cellulose-based items, which char rather than produce usable oil. In the TUR tray used for this study, components such as PVC tubing, silicone lines, and rubber connectors were excluded from the pyrolysis input to ensure a clean feedstock. The goal was to establish a proof of concept using a homogeneous pyrolysis-compatible plastic fraction to produce high-quality oil. Future scalability will indeed depend on minimizing or eliminating presorting, ideally by standardizing OR trays to use only pyrolysis-compatible polymers.
Other recycling approaches cannot currently enable a true circular economy for medical products, since the risk of contamination remains a limiting factor in mechanical recycling. A commonly raised concern regarding pyrolysis relates to its economic and ecological implications, particularly the energy demand required to sustain the process.
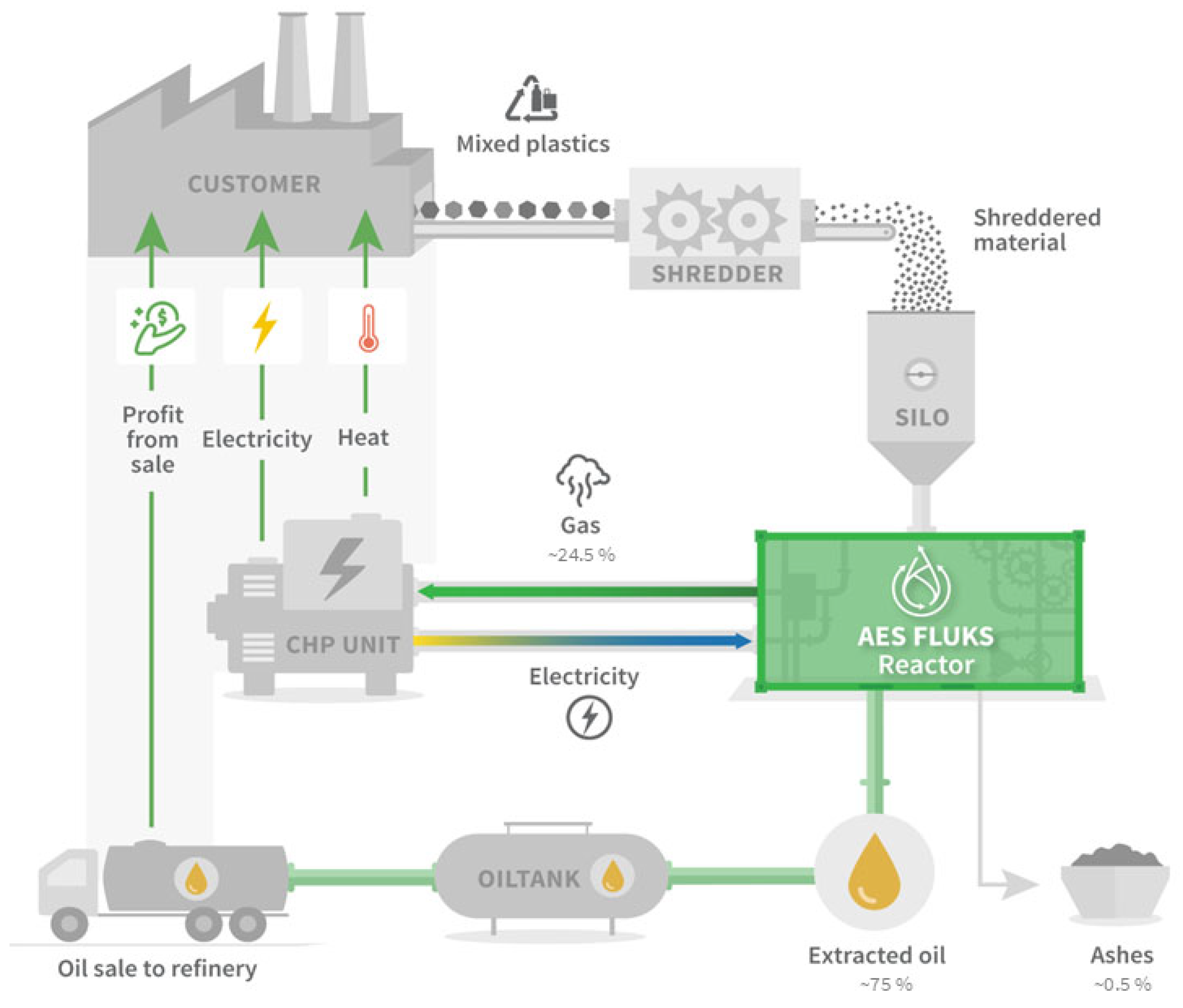
Medical plastic waste is currently almost exclusively incinerated, resulting in high emissions. From the results of this early feasibility study, a concept was derived, that reverses this logic: waste becomes a valuable resource. Instead of extracting fossil raw materials, a high-quality pyrolysis oil from standardized surgical trays is recovered—directly on site, in modular plants, with minimal effort (
Figure 6).
This approach represents a synergistic combination of R-strategies: reduce, recycle and reconsider. The use of trays reduces plastic packaging consumption in the operating room by 34% (R2 of the R-strategies). Subsequent utilization of the pyrolysis oil for the production of new medical devices represents a true circular economy (R8 of the R-strategies). The approach effectively addresses the commonly raised concerns regarding pyrolysis. The overall material recovery rate exceeded 70%. A large part of the success of pyrolysis at this time is dependent upon providing supplies made from the appropriate materials (i.e., feedstock) to the surgeons and eliminating those materials, which would significantly contaminate the feedstock. This reconsider strategy refers to rethinking and redesigning tray components to improve oil quality and reduce the need for presorting. However, this is an early feasibility study, and upscaling is needed to clarify the broad usage and the limitations.
4.2. Energy Content and Efficiency
The pyrolysis facility intends to utilize the processed gases to generate energy through an integrated combined heat and power (CHP) unit. These non-condensable processed gases produced during pyrolysis are represented in
Figure 4 by the orange section labeled ~20–21% (“gas”) of the total mass balance. This reduces the external energy demand of the plant by up to 75%. A CHP unit a system that simultaneously generates electricity and useful heat from a single energy source. While the CHP unit can significantly reduce the external energy demands of the pyrolysis process, it may not completely power the reactor depending on the scale of the reactor and the efficiency of the system. The reported figures for energy savings and CO
2 reduction were derived from process simulations and pilot-scale operational data of comparable medical plastic pyrolysis systems.
The used reactor setup shown in
Figure 2 has a maximum capacity of 3 kg of material per batch, with an input material being processed for about 4 h at temperatures ranging between 430 and 460 °C. However, in an industrial setting, scaling up to a 1000 kg/day capacity requires a larger reactor or multiple reactors in parallel. Larger reactors, either batch or continuous, do exist and are capable of processing much higher volumes of plastic waste, including 1000 kg per day. These reactors are designed with the infrastructure and energy systems necessary to handle industrial-scale operations, often requiring multiple units or advanced continuous processing systems.
4.3. Environmental Considerations
The environmental impacts also have to be taken into consideration. The gases, which were generated as a by-product with 21 wt%, can contain harmful compounds, such as carbon monoxide and methane, which are greenhouse gases. When released into the atmosphere without proper treatment, they contribute to air pollution and climate change. Some of the gases, especially carbon monoxide and other volatile compounds, are hazardous to human health if inhaled in significant quantities. For this reason, proper containment, filtering, or neutralizing measures should be in place before discharging the gases into the ventilation system. Otherwise, staff and personnel working in or around the area might be at risk of exposure. Depending on the local environmental regulations, discharging non-condensable gases without treatment or mitigation may violate air quality standards. In the herein presented concept, a CHP unit is used to oxidize the flammable gases, which leads to the formation of CO2 in case of the hydrocarbons.
An enclosed shredder design minimizes the noise emissions to LDEN < 50 decibels. For every ton of plastic waste processed, approximately 2.9 tons of CO2 emissions are avoided compared with conventional incineration. For the first pilot plant with a capacity of 350 tons, this corresponded to an annual reduction of more than 1000 tons of CO2. In absolute terms, the pyrolysis process emits approximately 0.4–0.5 t CO2 per ton of input plastic, whereas incineration emits around 3.3–3.5 t CO2 per ton.
These estimates include the direct process emissions and upstream substitution effects but exclude secondary logistics (such as the transport of collected trays to the pyrolysis site) since these are minor (<5% of total emissions).
4.4. Biological Contamination
One commonly raised concern when recycling medical-grade plastics, particularly single-use surgical materials, is the potential risk of biological contamination. Medical waste is often classified as hazardous, because it can contain residual organic matter such as blood, tissue, or microorganisms. This contamination risk is one of the main reasons why mechanical recycling is generally unsuitable for such waste streams. Mechanical recycling processes involve shredding, melting, and remolding the plastics at relatively low temperatures—typically below 250 °C—which are insufficient to destroy all biological agents. Consequently, the material remains microbiologically unsafe for reuse, and regulatory restrictions prohibit its reintegration into medical production cycles.
In contrast, pyrolysis offers a thermochemical process that inherently ensures complete decontamination. During pyrolysis, plastics are heated in a closed oxygen-free system to approximately 450 °C, a temperature at which all biological materials are irreversibly decomposed. Under these conditions, proteins, nucleic acids, lipids, and cell membranes undergo thermal cracking, while more resistant biological residues (such as bacterial spores or prions) are also destroyed [
10,
11]; all biological residues are thermally decomposed into simple gases and carbon residues. Pyrolysis is carried out in a sealed reactor system, eliminating the possibility of biological exposure or release into the environment. The resulting products—pyrolysis oil, non-condensable gases, and solid coke—are sterile and safe for further handling and industrial reuse. Decontamination is based on the process temperature and the chemistry of pyrolysis rather than direct microbial testing.
Consequently, biological contamination does not represent a concern in the context of pyrolysis-based recycling of medical plastics. The high temperature, inert atmosphere, and closed-system configuration ensure the full elimination of biological agents, meeting both hygienic and regulatory requirements for the safe recovery of materials from hospital waste.
4.5. Economic Impact
With a conservatively estimated waste generation of approximately 20 tons of plastic waste per hospital per year, the German healthcare sector produces more than 14,000 tons of medical plastic waste annually, most of which is currently incinerated [
12,
13]. This estimation aligns with recent assessments of healthcare waste volumes reported for OECD countries, highlighting the untapped recycling potential of standardized single-use medical products. The estimate of 20 metric tons of plastics per hospital per year seems low. Healthcare plastic emissions may be greater in the other countries, in the US it is estimated that each patient generates 13 kg of trash per day and that 25% of it may be plastic [
14,
15]. Based on this, a hospital generating only 20,000 kg of plastic annually would imply around 6000 patient days per year, which is unusually low for a typical hospital. This suggests that the estimate for German hospitals may need to be revised upwards.
By closely linking hospitals, logistics providers, waste management companies, regulatory authorities, and material manufacturers, a fully circular value chain emerges—planned, scalable, and sustainable. Our expert group “Circularity in Medicine” represents this value chain and has formally established itself as the association Ökologie & Nachhaltigkeit in der Medizin e.V.
The economic benefits of the proposed concept extend across all stakeholders. Hospitals benefit from cost reductions through waste minimization, while plastic waste is converted into valuable resources. The adoption of such a recycling system provides a marketing advantage as a “green hospital,” attractive to both staff and patients. Tray manufacturers profit from the distribution and use of eco-designed trays specifically developed for recycling. The application of surgical trays with pyrolysis-suitable materials also reduces the costs compared to single-packed standard trays, although the cost calculation was not part of the study. In addition, medical device manufacturers are able to meet the recycling quotas mandated by the EU-MDR, thereby strengthening and securing their market positions. Finally, pyrolysis plant operators gain direct economic value through the sale of recycled oil. The oil can achieve similar market prices as naphtha (approximately 520 USD per metric ton), or even higher prices in a regulated market if it is certified and accepted as recycled content by steam-cracking facilities.
This project, based on the pyrolysis of medical plastics, transforms sustainability from a regulatory obligation into a clinically verifiable reality. It creates the opportunity for every hospital to become an active part of the circular economy.
4.6. Limitations
The presented data represent results from a single controlled laboratory trial conducted under standardized conditions (430–460 °C, 150 °C·h−1, 4 h total duration). Due to the limited availability of medical-grade input material, replicates could not be performed within the initial study phase. However, the process parameters were continuously monitored and remained stable across the experiment, ensuring the reproducibility of the thermal and mass-balance conditions. Future work will include at least three independent replicates to quantify the variability and provide mean values with standard deviations for the oil yield, composition, and energy content, thereby strengthening the statistical confidence and comparability with other studies.
While the present study focused on physicochemical characterization, a detailed molecular analysis of the pyrolysis oils using gas chromatography–mass spectrometry (GC–MS) or Fourier-transform infrared spectroscopy (FTIR) was not included in this phase. In future work, GC–MS and FTIR spectra will be obtained to confirm the compositional distribution of the paraffinic, olefinic, and aromatic constituents, thereby enabling direct comparison with established petrochemical feedstocks and enhancing the robustness of the product’s qualification for medical-grade recycling. In addition, exact analyses of the process gases are needed to assess the environmental impact.
The chloride and silicon contents, as well as the coke fraction, could be further reduced by improved presorting of the input material, thereby enhancing the oil quality. This was addressed in our concept of using OR sets/trays containing only pyrolysis-suitable medical products. A subsequent sorting and collection system further optimized the input material.
The cost calculation and regulatory aspects were not part of the analyses.
Further validation trials are needed to prove the usability of large-scale pyrolysis for the recycling of medical waste.