Novel Approaches of Indocyanine Green and aPDT in the Treatment of Periodontitis: A Narrative Review
Abstract
1. Introduction
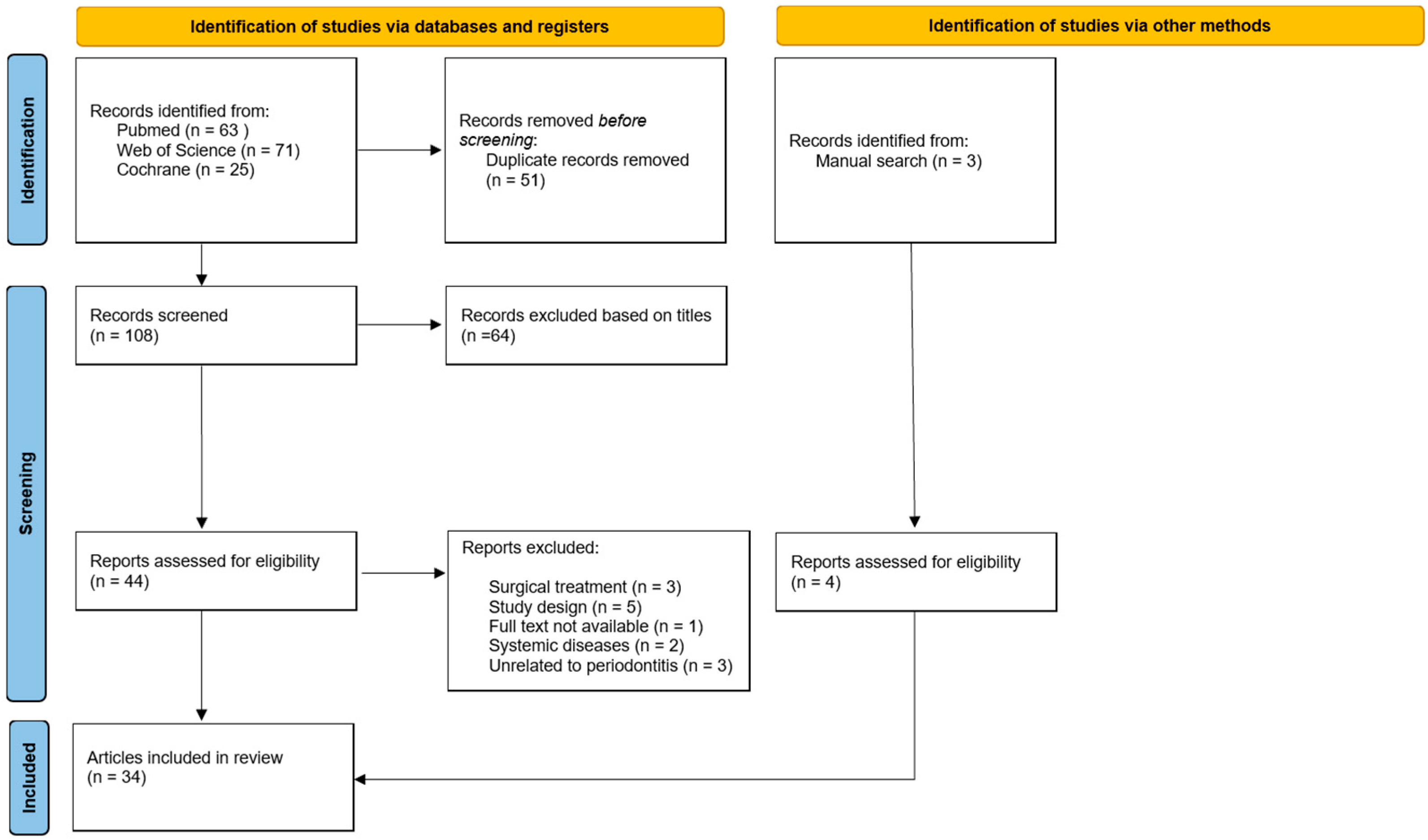
2. Materials and Methods
3. ICG Photosensitizer in aPDT Treatment of Periodontitis
3.1. Effect of Laser-Assisted aPDT with ICG on Human Gingival Fibroblast Cells
3.2. Antibacterial Effect of Laser-Assisted aPDT: In Vitro and In Vivo Data
3.3. Clinical Outcomes of Laser-Assisted ICG-aPDT in Periodontitis Treatment
3.4. Antimicrobial Effects of Novel Dual-Light aPDT
3.4.1. Evaluation of the Results of In Vitro Assessment
3.4.2. Clinical Evaluation of Dual-Light ICG aPDT in the Reduction in Dental Plaque
3.5. Investigations of the Clinical Effectiveness of Dual-Light aPDT in the Treatment of Periodontitis
4. Discussion
4.1. Laser-Assisted and Dual-Light ICG Antibacterial Photodynamic Therapy (aPDT)
4.2. Strengths and Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PI | Plaque Index |
| SD | Standard Deviation |
Appendix A
| Study, Year | Outcomes | Conclusions |
|---|---|---|
| Pourhajibagher M. et al., 2017 [24] | ICG-mediated aPDT at 62.5–125 µg/mL with 30–60 s diode laser irradiation (15.6–31.2 J/cm2) significantly reduced A. actinomycetemcomitans growth in a dose-dependent manner compared to untreated bacteria (p < 0.05). | ICG-aPDT effectively suppresses pathogen virulence genes, potentially limiting infectivity. |
| Pourhajibagher M., Bahador A., 2021 [25] | The results showed that 1000 and 125 μg/mL of ICG with 1 min diode laser irradiation significantly reduced A. actinomycetemcomitans (p < 0.05). | ICG-aPDT can reduce microbial load, metabolism, and gene expression effects, enhancing aPDT efficacy as an adjunct in nonsurgical periodontitis treatment. |
| Fekrazad R. et al., 2015 [26] | aPDT with indocyanine green effectively inactivated Candida albicans in vitro (p < 0.001). The same trend was observed for the light sources (810 nm vs. 630 nm), which did not significantly differ (p = 0.78). | ICG are effective against fungal biofilms. |
| Fekrazad R. et al., 2020 [27] | ICG-aPDT (810 nm) group showed significant reduction in the viability of P. gingivalis (p < 0.001) | aPDT can be used as an adjunctive method for controlling P. gingivalis infections. |
| Ahrari F. et al., 2018 [28] | Comparing the colony counts immediately and 24 h after treatment, the number of viable bacteria in the aPDT group (ICG combined with an 810 nm diode laser) was significantly reduced (p < 0.001) | L. acidophilus colonies were susceptible to photodynamic therapy after sensitization with ICG and exposure to an 810 nm diode laser. |
| Peeridogaheh H. et al., 2019 [29] | The maximal sub-lethal dose of ICG-PDT was 20.15 μM/mL ICG at 31.2 J/cm2. aPDT-treated A. actinomycetemcomitans biofilm-derived effectors altered cytokine production in human gingival fibroblasts (p < 0.05). | ICG-aPDT, with its antimicrobial effects, reduces inflammation and induces of tissue regeneration resulting from bacterial conditioned medium, can be considered an efficient adjunctive therapeutic method for the treatment of local infections. |
| Pourhajibagher M. et al., 2020 [30] | ICG with aPDT altered BCL-2 (B-cell lymphoma 2) and BAX (Bcl-2-associated X protein) gene expression in human gingival fibroblasts. aPDT with 500 µg/mL ICG significantly increased BAX gene expression by 8.5-fold, considerably higher than aPDT with 1500 and 2000 µg/mL ICG (~7- and 8.5-fold, respectively), indicating the induction of apoptosis in fibroblasts. | Different ICG concentrations may lead to varied BAX gene expression responses to aPDT in human gingival fibroblast cells. |
| Pourhajibagher M. et al., 2016 [31] | aPDT (810 nm) with ICG showed photocytotoxic effects on human gingival fibroblasts in vitro (p < 0.01). | Careful protocol selection is required due to potential cytotoxicity. |
Appendix B
| Study, Year | Group | Time Point | PPD ± SD (mm) | PPD ± SD Change (mm) | CAL ± SD (mm) | CAL ± SD Change (mm) | BOP ± SD (%) | PI ± SD (Score) |
|---|---|---|---|---|---|---|---|---|
| Sethi et al., 2019 [23] | Test | Baseline | 5.00 ± 0.73 * | - | 6.11 ± 0.64 * | - | - | 2.12 ± 0.33 * |
| 3 months | 3.14 ± 1.01 * | - | 4.70 ± 1.08 * | - | - | 0.99 ± 0.47 * | ||
| Control | Baseline | 5.30 ± 1.20 | - | 6.26 ± 0.89 | - | - | 2.08 ± 0.27 * | |
| 3 months | 4.60 ± 0.60 | - | 5.47 ± 0.18 | - | - | 1.28 ± 0.45 * | ||
| Joshi et al., 2020 [19] | Test | Baseline | 5.56 ± 0.55 | - | 5.68 ± 0.61 | - | - | 1.21 ± 0.25 |
| 3 months | 3.20 ± 0.54 | 2.36 ± 0.37 * | 3.34 ± 0.62 | 2.34 ± 0.37 * | - | |||
| Control | Baseline | 5.42 ± 0.47 | 2.10 ± 0.35 * | 5.70 ± 0.69 | - | - | ||
| 3 months | 3.32 ± 0.41 | - | 3.60 ± 0.63 | 2.10 ± 0.35 * | - | 1.27 ± 0.24 | ||
| Sukumar et al., 2020 [20] | Test | Baseline | 5.93 ± 0.82 | - | 5.73 ± 0.69 | - | 100 | 2.00 ± 0.00 |
| 3 months | 4.17 ± 0.83 * | - | 3.97 ± 0.80 * | - | 3.3 * | 1.00 ± 0.00 ** | ||
| 6 months | 3.40 ± 0.56 ** | - | 3.00 ± 0.91 * | - | 16.7 * | 0.13 ± 0.34 ** | ||
| Control | Baseline | 5.83 ± 0.64 | - | 5.60 ± 0.72 | - | 100 | 2.00 ± 0.00 | |
| 3 months | 4.60 ± 0.72 * | - | 4.47 ± 0.68 * | - | 30 * | 1.21 ± 0.17 ** | ||
| 6 months | 3.80 ± 0.40 ** | - | 3.70 ± 0.91 * | - | 46 * | 0.76 ± 0.40 ** | ||
| Wadhwa et al., 2021 [22] | Test | Baseline | 3.37 ± 0.47 | - | 3.44 ± 0.53 | - | - | 2.05 ± 0.28 |
| 3 months | 1.30 ± 0.36 ** | - | 1.37 ± 0.48 ** | - | - | 0.72 ± 0.27 ** | ||
| 6 months | 0.28 ± 0.29 ** | - | 0.35 ± 0.46 ** | - | - | 0.14 ± 0.08 ** | ||
| Control | Baseline | 3.37 ± 0.46 | - | 3.43 ± 0.52 | - | - | 2.06 ± 0.26 | |
| 3 months | 2.19 ± 0.48 ** | - | 2.20 ± 0.57 ** | - | - | 1.25 ± 0.23 ** | ||
| 6 months | 1.16 ± 0.48 ** | - | 1.26 ± 0.62 ** | - | - | 0.60 ± 0.15 ** | ||
| Karmkar et al., 2021 [18] | Test | Baseline | 6.5 ± 0.61 | - | 4.6 ± 0.67 | - | - | - |
| 3 months | 3.7 ± 0.86 ** | - | 1.8 ± 0.88 ** | - | - | - | ||
| Control | Baseline | 6.4 ± 0.75 | - | 4.5 ± 0.76 | - | - | - | |
| 3 months | 4.8 ± 0.77 ** | - | 2.9 ± 0.79 ** | - | - | - | ||
| Annunziata et al., 2023 [21] | Test | Baseline | 6.32 ± 0.66 | - | 6.77 ± 0.93 | - | 93.75 ± 11.31 | - |
| 3 months | - | 1.77 ± 0.77 * | - | 1.29 ± 0.89 * | - | - | ||
| 6 months | - | 1.81 ± 1.02 * | - | 1.06 ± 1.63 * | - | - | ||
| Control | Baseline | 6.63 ± 0.85 | - | 7.06 ± 0.89 | - | 95.83 ± 9.73 | - | |
| 3 months | - | 1.54 ± 1.10 * | - | 1.08 ± 0.9 * | - | - | ||
| 6 months | - | 1.25 ± 0.99 * | - | 0.77 ± 0.81 * | - | - |
Appendix C
| Study, Year | Outcomes | Conclusions |
|---|---|---|
| Sethi et al., 2019 [23] | Outcomes assessed: PPD, CAL, PI (at 3 months). Main finding: NSPT combined with ICG-PDT demonstrated significantly greater efficacy compared to NSPT alone. | These findings suggest that ICG in combination with an 810 nm diode laser may serve as a valuable adjunct to NSPT in the management of chronic periodontitis. |
| Joshi et al., 2020 [19] | aPDT resulted in significant improvement in PPD and CAL compared to control. A significant reduction in PI was observed in both groups. NSPT with ICG-PDT was significantly more efficacious than NSPT alone | The addition of indocyanine green–mediated antimicrobial photodynamic therapy to scaling and root planing resulted in significantly enhanced reductions in probing depth and gains in clinical attachment level. These findings highlight its role in augmenting the effectiveness of conventional nonsurgical periodontal therapy, particularly in decreasing periodontal pocket depth. |
| Sukumar et al., 2020 [20] | BOP decreased significantly in both groups at 3 and 6 months, with greater improvement in the aPDT group compared to control (p ≤ 0.05). The aPDT group also showed higher reductions in mean PI and GI scores at both time points (p ≤ 0.001). A statistically significant reduction in microbial load (copies/μL) of P. gingivalis, A. actinomycetemcomitans, T. forsythia, F. nucleatum, and T. denticola was observed at 3 and 6 months in the aPDT group (p ≤ 0.05). In the control group, only P. gingivalis showed a significant decrease at 6 months. | Multiple sessions of PDT in combination with SRP led to improved clinical parameters and more pronounced decreases in the primary periodontal pathogens relative to conventional treatment after six months. |
| Wadhwa et al., 2021 [22] | At baseline, PI, PPD, and CAL showed no significant difference between groups; however, at 3 and 6 months, these parameters were significantly lower in the aPDT group compared to the control. | Indocyanine green–based antimicrobial photodynamic therapy contributed to reductions in PI, PPD, CAL, demonstrating safety and efficacy as an adjunctive treatment for chronic periodontitis. |
| Karmkar et al., 2021 [18] | Sites receiving adjunctive ICG-mediated aPDT exhibited a statistically significant reduction in PPD and CAL compared to sites treated with SRP alone at 3 months. Both treatment protocols (SRP and SRP + ICG-PDT) decreased bacterial counts, with greater reductions observed in the SRP + ICG-PDT group; however, these differences were not statistically significant. | ICG along with PDT can be used for nonsurgical management of periodontal diseases. |
| Annunziata et al., 2023 [21] | Significant differences favoring the aPDT group were observed only at 6 months, with greater PPD reduction in initially deep pockets (PPD ≥ 6 mm) and a higher percentage of closed pockets (PPD ≤ 4 mm with no bleeding on probing). Microbiological changes were limited in both groups, with no inter-group differences, except for a more pronounced reduction in Aggregatibacter actinomycetemcomitans and Parvimonas micra levels in the test group at 3 months. | The combination of repeated ICG-aPDT and NSPT provided no benefits except for selective clinical and microbiological improvements compared to NSPT alone. |
References
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Romito, G.A.; Collins, J.R.; Hassan, M.A.; Benítez, C.; Contreras, A. Burden and impact of periodontal diseases on oral health-related quality of life and systemic diseases and conditions: Latin America and the Caribbean Consensus 2024. Braz. Oral Res. 2024, 38, e117. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients: A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Braz. Oral Res. 2021, 35, e095. [Google Scholar] [CrossRef]
- Pretzl, B.; Sälzer, S.; Ehmke, B.; Schlagenhauf, U.; Dannewitz, B.; Dommisch, H.; Eickholz, P.; Jockel-Schneider, Y. Administration of systemic antibiotics during non-surgical periodontal therapy-a consensus report. Clin. Oral. Investig. 2019, 23, 3073–3085. [Google Scholar] [CrossRef]
- Coelho, A.S.; Laranjo, M.; Gonçalves, A.C.; Paula, A.; Paulo, S.; Abrantes, A.M.; Caramelo, F.; Ferreira, M.M.; Silva, M.J.; Carrilho, E.; et al. Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblasts. Odontology 2020, 108, 260–270. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 176–198. [Google Scholar] [CrossRef]
- Bashir, N.Z.; Singh, H.; Virdee, S.S. Indocyanine green-mediated antimicrobial photodynamic therapy as an adjunct to periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 5699–5710. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann. Surg. 2022, 275, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Brinar, S.; Skvarča, A.; Gašpirc, B.; Schara, R. The effect of antimicrobial photodynamic therapy on periodontal disease and glycemic control in patients with type 2 diabetes mellitus. Clin. Oral Investig. 2023, 27, 6235–6244. [Google Scholar] [CrossRef]
- Wiench, R.; Fiegler-Rudol, J.; Latusek, K.; Brus-Sawczuk, K.; Fiegler, H.; Kasperski, J.; Skaba, D. Indocyanine Green as a Photosensitizer in Periodontitis Treatment: A Systematic Review of Randomized Controlled Trials. Life 2025, 15, 1015. [Google Scholar] [CrossRef]
- Giannelli, M.; Formigli, L.; Lorenzini, L.; Bani, D. Efficacy of Combined Photoablative-Photodynamic Diode Laser Therapy Adjunctive to Scaling and Root Planing in Periodontitis: Randomized Split-Mouth Trial with 4-Year Follow-Up. Photomed. Laser Surg. 2015, 33, 473–480. [Google Scholar] [CrossRef]
- Lähteenmäki, H.; Pätilä, T.; Räisänen, I.T.; Kankuri, E.; Tervahartiala, T.; Sorsa, T. Repeated Home-Applied Dual-Light Antibacterial Photodynamic Therapy Can Reduce Plaque Burden, Inflammation, and aMMP-8 in Peri-Implant Disease—A Pilot Study. Curr. Issues Mol. Biol. 2022, 44, 1273–1283. [Google Scholar] [CrossRef]
- Giannelli, M.; Materassi, F.; Fossi, T.; Lorenzini, L.; Bani, D. Treatment of severe periodontitis with a laser and light-emitting diode (LED) procedure adjunctive to scaling and root planing: A double-blind, randomized, single-center, split-mouth clinical trial investigating its efficacy and patient-reported outcomes at 1 year. Lasers Med. Sci. 2018, 33, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Huang, P.; Peng, P.; Shen, D.; Zhao, L.; Jiang, D.; Shen, Y.; Wei, L.; Bible, P.W.; Yang, J.; et al. Efficacy of photodynamic therapy as an adjunct to scaling and root planing on clinical parameters and microbial composition in subgingival plaque of periodontitis patients: A split-mouth randomized clinical trial. J. Periodontol. 2024, 95, 535–549. [Google Scholar] [CrossRef]
- Bourbour, S.; Darbandi, A.; Bostanghadiri, N.; Ghanavati, R.; Taheri, B.; Bahador, A. Effects of Antimicrobial Photosensitizers of Photodynamic Therapy (PDT) to Treat Periodontitis. Curr. Pharm. Biotechnol. 2024, 25, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Prakash, S.; Jagadeson, M.; Namachivayam, A.; Das, D.; Sarkar, S. Clinico-microbiological Efficacy of Indocyanine Green as a Novel Photosensitizer for Photodynamic Therapy among Patients with Chronic Periodontitis: A Split-mouth Randomized Controlled Clinical Trial. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), S143–S148. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Baiju, C.S.; Khashu, H.; Bansal, S. Clinical effectiveness of indocyanine green mediated antimicrobial photodynamic therapy as an adjunct to scaling root planing in treatment of chronic periodontitis- A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 29, 101591. [Google Scholar] [CrossRef]
- Sukumar, K.; Tadepalli, A.; Harinath, P.; Deepa, P. Evaluation of combined efficacy of photodynamic therapy using indocyanine green photosensitizer and non-surgical periodontal therapy on clinical and microbial parameters in the management of chronic periodontitis subjects: A randomized split-mouth design. Photodiagn. Photodyn. Ther. 2020, 31, 101949. [Google Scholar] [CrossRef]
- Annunziata, M.; Donnarumma, G.; Guida, A.; Nastri, L.; Persico, G.; Fusco, A.; Sanz-Sanchez, I.; Guida, L. Clinical and microbiological efficacy of indocyanine green-based antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Mallapragada, S.; Sharma, P. Novel indocyanine green mediated antimicrobial photodynamic therapy in the management of chronic periodontitis—A randomized controlled clinico-microbiological pilot study. J. Oral Biol. Craniofacial Res. 2021, 11, 57–62. [Google Scholar] [CrossRef]
- Sethi, K.S.; Raut, C.P. Antimicrobial photodynamic therapy using indocyanine green as a photosensitizer in treatment of chronic periodontitis: A clinico-microbial study. Indian J. Dent. Res. 2019, 30, 870–876. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Chiniforush, N.; Shahabi, S.; Sobhani, S.; Monzavi, M.M.; Monzavi, A.; Bahador, A. Monitoring gene expression of rcpA from Aggregatibacter actinomycetemcomitans versus antimicrobial photodynamic therapy by relative quantitative real-time PCR. Photodiagn. Photodyn. Ther. 2017, 19, 51–55. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Exploring Photoactivated Disinfection-Induced Bystander Effects on Microbial Biofilms of Aggregatibacter actinomycetemcomitans. Infect. Disord. Drug Targets 2021, 21, e170721187710. [Google Scholar] [CrossRef]
- Fekrazad, R.; Ghasemi Barghi, V.; Poorsattar Bejeh Mir, A.; Shams-Ghahfarokhi, M. In vitro photodynamic inactivation of Candida albicans by phenothiazine dye (new methylene blue) and Indocyanine green (EmunDo®). Photodiagn. Photodyn. Ther. 2015, 12, 52–57. [Google Scholar] [CrossRef]
- Fekrazad, R.; Khoei, F.; Bahador, A.; Hakimiha, N. Comparison of different modes of photo-activated disinfection against Porphyromonas gingivalis: An in vitro study. Photodiagn. Photodyn. Ther. 2020, 32, 101951. [Google Scholar] [CrossRef]
- Ahrari, F.; Shahabi, M.; Fekrazad, R.; Eslami, N.; Mazhari, F.; Ghazvini, K.; Emrani, N. Antimicrobial photodynamic therapy of Lactobacillus acidophilus by indocyanine green and 810-nm diode laser. Photodiagn. Photodyn. Ther. 2018, 24, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Peeridogaheh, H.; Pourhajibagher, M.; Barikani, H.R.; Bahador, A. The impact of Aggregatibacter actinomycetemcomitans biofilm-derived effectors following antimicrobial photodynamic therapy on cytokine production in human gingival fibroblasts. Photodiagn. Photodyn. Ther. 2019, 27, 1–6. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Gharesi, S.; Chiniforush, N.; Bahador, A. The Effect of Indocyanine Green Antimicrobial Photothermal/Photodynamic Therapy on the Expression of BCL-2 and BAX Messenger RNA Levels in Human Gingival Fibroblast Cells. Folia. Med. 2020, 62, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Chiniforush, N.; Parker, S.; Shahabi, S.; Ghorbanzadeh, R.; Kharazifard, M.J.; Bahador, A. Evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells: An in vitro photocytotoxicity investigation. Photodiagn. Photodyn. Ther. 2016, 15, 13–18. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hayashi, J.; Fujimura, T.; Iwamura, Y.; Yamamoto, G.; Nishida, E.; Ohno, T.; Okada, K.; Yamamoto, H.; Kikuchi, T.; et al. New Irradiation Method with Indocyanine Green-Loaded Nanospheres for Inactivating Periodontal Pathogens. Int. J. Mol. Sci. 2017, 18, 154. [Google Scholar] [CrossRef]
- Pakarinen, S.; Saarela, R.K.T.; Välimaa, H.; Heikkinen, A.M.; Kankuri, E.; Noponen, M.; Alapulli, H.; Tervahartiala, T.; Räisänen, I.T.; Sorsa, T.; et al. Home-Applied Dual-Light Photodynamic Therapy in the Treatment of Stable Chronic Periodontitis (HOPE-CP)-Three-Month Interim Results. Dent. J. 2022, 10, 206. [Google Scholar] [CrossRef]
- Londero, A.B.; Reiniger, A.P.P.; Tavares, R.C.R.; Ferreira, C.M.; Wikesjö, U.M.E.; Kantorski, K.Z.; Moreira, C.H.C. Efficacy of dental floss in the management of gingival health: A randomized controlled clinical trial. Clin. Oral Investig. 2022, 26, 5273–5280. [Google Scholar] [CrossRef]
- Mizutani, K.; Aoki, A.; Coluzzi, D.; Yukna, R.; Wang, C.; Pavlic, V.; Izumi, Y. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol. 2000 2016, 71, 185–212. [Google Scholar] [CrossRef]
- Hentilä, J.; Laakamaa, N.; Sorsa, T.; Meurman, J.; Välimaa, H.; Nikinmaa, S.; Kankuri, E.; Tauriainen, T.; Pätilä, T. Dual-Light Photodynamic Therapy Effectively Eliminates Streptococcus Oralis Biofilms. J. Pharm. Pharm. Sci. 2021, 24, 484–487. [Google Scholar] [CrossRef]
- Nikinmaa, S.; Podonyi, A.; Raivio, P.; Meurman, J.; Sorsa, T.; Rantala, J.; Kankuri, E.; Tauriainen, T.; Pätilä, T. Daily Administered Dual-Light Photodynamic Therapy Provides a Sustained Antibacterial Effect on Staphylococcus aureus. Antibiotics 2021, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, S.; Alapulli, H.; Auvinen, P.; Vaara, M.; Rantala, J.; Kankuri, E.; Sorsa, T.; Meurman, J.; Pätilä, T. Dual-light photodynamic therapy administered daily provides a sustained antibacterial effect on biofilm and prevents Streptococcus mutans adaptation. PLoS ONE 2020, 15, e0232775. [Google Scholar] [CrossRef]
- Nikinmaa, S.; Moilanen, N.; Sorsa, T.; Rantala, J.; Alapulli, H.; Kotiranta, A.; Auvinen, P.; Kankuri, E.; Meurman, J.H.; Pätilä, T. Indocyanine Green-Assisted and LED-Light-Activated Antibacterial Photodynamic Therapy Reduces Dental Plaque. Dent. J. 2021, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Baelum, V.; Sorsa, T.; Tervahartiala, T.; Skottrup, P.D.; López, R. Salivary levels of MPO, MMP-8 and TIMP-1 are associated with gingival inflammation response patterns during experimental gingivitis. Cytokine 2019, 115, 135–141. [Google Scholar] [CrossRef]
- Trujiilo, K.; Räisänen, I.T.; Sorsa, T.; Pätilä, T. Repeated Daily Use of Dual-Light Antibacterial Photodynamic Therapy in Periodontal Disease-A Case Report. Dent. J. 2022, 10, 163. [Google Scholar] [CrossRef]
- Felix Gomez, G.G.; Lippert, F.; Ando, M.; Zandona, A.F.; Eckert, G.J.; Gregory, R.L. Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light. Dent. J. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Yasui, M.; Shibata, Y.; Furuta, M.; Saeki, Y.; Eshima, N.; Yamashita, Y. Dental plaque development on a hydroxyapatite disk in young adults observed by using a barcoded pyrosequencing approach. Sci. Rep. 2015, 5, 8136. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, X.; Sun, X.; Li, C.; Tay, F.R.; Weir, M.D.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium. Dent. Mater. 2019, 35, 1665–1681. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 22 (Suppl. S22), 4–60, Erratum in: J. Clin. Periodontol. 2021, 48, 163. https://doi.org/10.1111/jcpe.13403. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Mangalekar, S.B.; Rai, J.; Vijapure, S.; Kumbhare, S.; Sawant, P. Comparative Evaluation of Antimicrobial Photodynamic Therapy Using Methylene Blue (660 nm Diode Laser) and Indocyanine Green (810 nm Diode Laser) in the Management of Chronic Periodontitis: A Clinical and Microbiological Study. J. Pharm. Bioallied Sci. 2025, 17 (Suppl. S2), S1436–S1438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study (Year) | Sample Size | Study Design | Test Group | Control Group | Follow-Up |
|---|---|---|---|---|---|
| Sethi et al., 2019 [23] | 30 | Split-mouth | ICG-aPDT + SRP | SRP alone | 3 months |
| Sukumar et al., 2020 [20] | 40 | Split-mouth | ICG-aPDT + SRP | SRP alone | 6 months |
| Joshi et al., 2020 [19] | 50 | Parallel | ICG-aPDT + SRP | SRP alone | 3 months |
| Karmakar et al., 2021 [18] | 20 | Split-mouth | ICG-aPDT + SRP | SRP alone | 3 months |
| Wadhwa et al., 2021 [22] | 40 | Parallel | ICG-aPDT + SRP | SRP alone | 6 months |
| Annunziata et al., 2023 [21] | 40 | Split-mouth | ICG-aPDT + SRP | SRP alone | 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šilė, R.; Mačiulskienė-Visockienė, V.; Šadzevičienė, R.; Pacauskienė, I.M. Novel Approaches of Indocyanine Green and aPDT in the Treatment of Periodontitis: A Narrative Review. Surgeries 2025, 6, 77. https://doi.org/10.3390/surgeries6030077
Šilė R, Mačiulskienė-Visockienė V, Šadzevičienė R, Pacauskienė IM. Novel Approaches of Indocyanine Green and aPDT in the Treatment of Periodontitis: A Narrative Review. Surgeries. 2025; 6(3):77. https://doi.org/10.3390/surgeries6030077
Chicago/Turabian StyleŠilė, Raimonda, Vita Mačiulskienė-Visockienė, Renata Šadzevičienė, and Ingrida Marija Pacauskienė. 2025. "Novel Approaches of Indocyanine Green and aPDT in the Treatment of Periodontitis: A Narrative Review" Surgeries 6, no. 3: 77. https://doi.org/10.3390/surgeries6030077
APA StyleŠilė, R., Mačiulskienė-Visockienė, V., Šadzevičienė, R., & Pacauskienė, I. M. (2025). Novel Approaches of Indocyanine Green and aPDT in the Treatment of Periodontitis: A Narrative Review. Surgeries, 6(3), 77. https://doi.org/10.3390/surgeries6030077







