Topical Tranexamic Acid Use Amongst Surgical Specialties: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
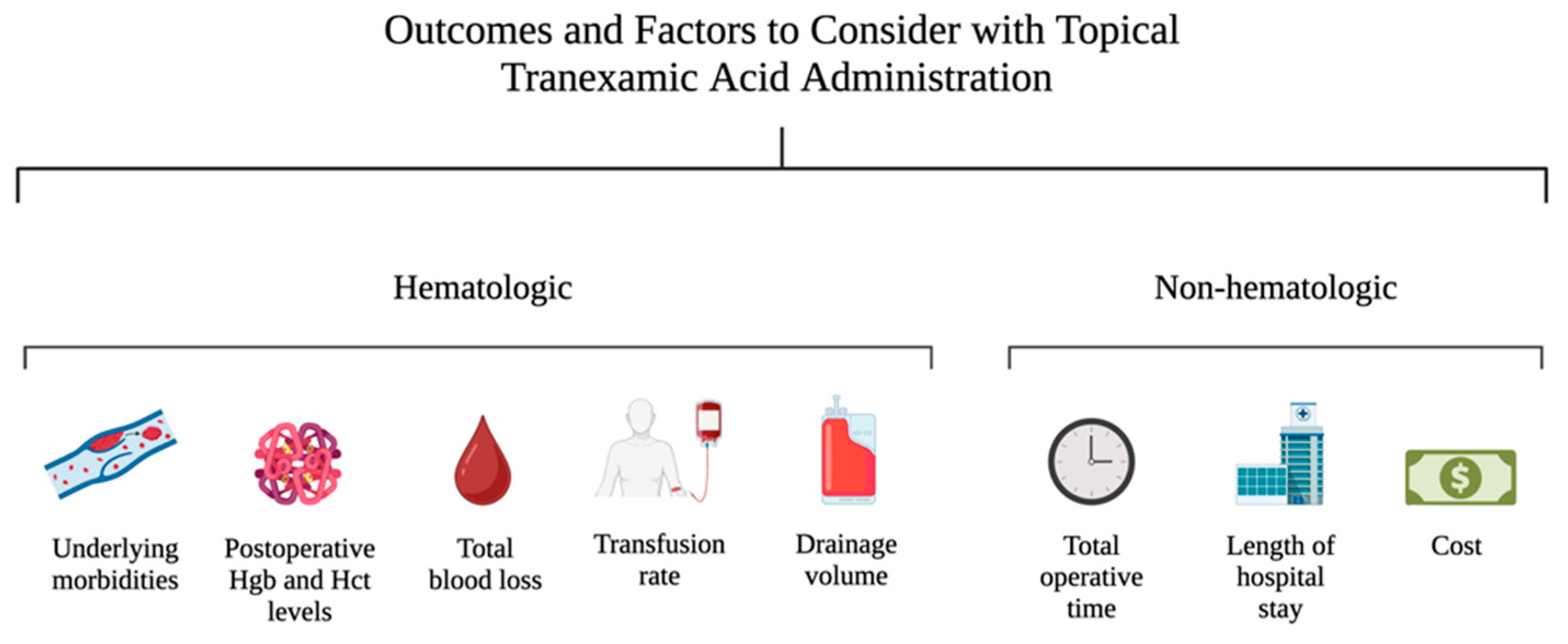
3. Results
3.1. Cardiac Surgery
3.2. Plastic Surgery
3.3. Ear, Nose, and Throat Surgery
3.4. Orthopedic Sugery
3.5. Obstetric and Gynecologic Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TXA | Tranexamic Acid |
| PPH | Postpartum Hemorrhage |
References
- Johnson, A.B.; Burns, B. Hemorrhage. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK542273/ (accessed on 8 December 2024).
- Sadiku, O.D.; Aina, S.A.; Odoemene, C.C.; Ogunmoyin, T.E.; Adedara, V.O.; Olasimbo, O.; Bernal, M.B.N. Approaches to the prevention and treatment of postpartum hemorrhage: A systematic review of past advances, recent developments, and best practices. Cureus 2024, 16, e65096. [Google Scholar] [CrossRef]
- Prutsky, G.; Domecq, J.P.; Salazar, C.A.; Accinelli, R. Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst. Rev. 2016, 11, CD008711. [Google Scholar] [CrossRef]
- Goobie, S.M.; Frank, S.M. Tranexamic acid: What is known and unknown, and where do we go from here. Anesthesiology 2017, 127, 405–407. [Google Scholar] [CrossRef]
- Shakur, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Loto, O. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef]
- Roberts, I.; Shakur, H.; Coats, T.; Hunt, B.; Balogun, E.; Barnetson, L.; Guerriero, C. The CRASH-2 trial: A randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol. Assess. 2013, 17, 1–79. [Google Scholar] [CrossRef]
- Nilsson, I.M. Clinical pharmacology of aminocaproic and tranexamic acids. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 1980, 14, 41. [Google Scholar] [CrossRef]
- Patel, S. Tranexamic acid-associated intrathecal toxicity during spinal anaesthesia: A narrative review of 22 recent reports. Eur. J. Anaesthesiol. 2023, 40, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ker, K.; Sentilhes, L.; Shakur-Still, H.; Madar, H.; Deneux-Tharaux, C.; Saade, G.; Roberts, I. Tranexamic acid for postpartum bleeding: A systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet 2024, 404, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Ker, K.; Beecher, D.; Roberts, I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst. Rev. 2013, CD010562. [Google Scholar] [CrossRef]
- Öztaş, S.; Öztürk, A.; Akalin, Y.; Şahin, N.; Özkan, Y.; Otuzbir, A.; Avcu, B. The effect of local and systemic application of tranexamic acid on the amount of blood loss and allogeneic blood transfusion after total knee replacement. Acta Orthop. Belg. 2015, 81, 698–707. [Google Scholar]
- Bolam, S.M.; O’Regan-Brown, A.; Paul Monk, A.; Musson, D.S.; Cornish, J.; Munro, J.T. Toxicity of tranexamic acid (TXA) to intra-articular tissue in orthopaedic surgery: A scoping review. Knee Surgery, Sports Traumatology. Arthroscopy 2021, 29, 1862–1871. [Google Scholar]
- Chaudhary, F.A.; Pervaz, Z.; Ilyas, S.; Niaz, M.N. Topical use of tranexamic acid in open heart surgery. J. Pak. Med. Assoc. 2018, 68, 538–542. [Google Scholar] [PubMed]
- Shah, M.A.; Asghar, M.I.; Siddiqi, R.; Chaudhri, M.S.; Janjua, A.M.; Iqbal, A. Topical application of tranexamic acid reduces postoperative bleeding in open-heart surgery: Myth or fact. J. Coll. Physicians Surg. Pak. 2015, 25, 161–165. [Google Scholar]
- Taksaudom, N.; Siwachat, S.; Tantraworasin, A. Additional effects of topical tranexamic acid in on-pump cardiac surgery. Asian Cardiovasc. Thorac. Ann. 2017, 25, 24–30. [Google Scholar] [CrossRef]
- Guo, J.; Gao, X.; Ma, Y.; Lv, H.; Hu, W.; Zhang, S.; Shi, J. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: A meta-analysis of randomized trials. BMC Anesthesiol. 2019, 19, 129. [Google Scholar] [CrossRef]
- Ockerman, A.; Vanassche, T.; Garip, M.; Vandenbriele, C.; Engelen, M.M.; Martens, J.; Verhamme, P. Tranexamic acid for the prevention and treatment of bleeding in surgery, trauma and bleeding disorders: A narrative review. Thromb. J. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Ausen, K.; Fossmark, R.; Spigset, O.; Pleym, H. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. J. Br. Surg. 2015, 102, 1348–1353. [Google Scholar] [CrossRef]
- Ausen, K.; Hagen, A.I.; Østbyhaug, H.S.; Olafsson, S.; Kvalsund, B.J.; Spigset, O.; Pleym, H. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: Randomized clinical trial. BJS Open 2020, 4, 216–224. [Google Scholar] [CrossRef]
- Yao, A.; Wang, F.; Benacquista, T.; Draper, L.B.; Garfein, E.S.; Monaco, C.; Ricci, J.A. Topical tranexamic acid does not reduce hematoma in reduction mammaplasty: A double-blind randomized controlled trial. Plast. Reconstr. Surg. 2024, 154, 30–37. [Google Scholar] [CrossRef]
- Achour, I.; Ben Rhaiem, Z.; Thabet, W.; Jdidi, J.; Mnejja, M.; Hammami, B.; Charfeddine, I. The effect of topical tranexamic acid in endoscopic sinus surgery: A triple blind randomized clinical trial. Ann. Otol. Rhinol. Laryngol. 2023, 132, 244–249. [Google Scholar] [CrossRef]
- Husain, S.; Ramos, J.A.; Karaf, J.H.A.; Zahedi, F.D.; Ahmad, N.; Abdullah, B. Efficacy of topical tranexamic acid to reduce bleeding in endoscopic sinus surgery for chronic rhinosinusitis with polyposis. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 737–741. [Google Scholar] [CrossRef]
- Whitworth, K.; Johnson, J.; Wisniewski, S.; Schrader, M. Comparative effectiveness of topically administered tranexamic acid versus topical oxymetazoline spray for achieving hemostasis in epistaxis. J. Emerg. Med. 2020, 58, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.A.; Hamed, E.M. Topical use of tranexamic acid versus epinephrine to optimise surgical field during exploratory tympanotomy. Anaesth. Crit. Care Pain. Med. 2020, 39, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Eftekharian, H.; Vahedi, R.; Karagah, T.; Tabrizi, R. Effect of tranexamic acid irrigation on perioperative blood loss during orthognathic surgery: A double-blind, randomized controlled clinical trial. J. Oral. Maxillofac. Surg. 2015, 73, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.K.; Singh, N.; Sudesh, P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: A prospective study. J. Arthroplast. 2016, 31, 1442–1448. [Google Scholar] [CrossRef]
- Gao, F.; Sun, W.; Guo, W.; Li, Z.; Wang, W.; Cheng, L. Topical administration of tranexamic acid plus diluted-epinephrine in primary total knee arthroplasty: A randomized double-blinded controlled trial. J. Arthroplast. 2015, 30, 1354–1358. [Google Scholar] [CrossRef]
- Prakash, J.; Seon, J.K.; Park, Y.J.; Jin, C.; Song, E.K. A randomized control trial to evaluate the effectiveness of intravenous, intra-articular and topical wash regimes of tranexamic acid in primary total knee arthroplasty. J. Orthop. Surg. 2017, 25, 2309499017693529. [Google Scholar] [CrossRef]
- Jordan, M.; Aguilera, X.; González, J.C.; Castillón, P.; Salomó, M.; Hernández, J.A.; Ruiz, L.; Mora, J.M.; Camacho-Carrasco, P.; Prat-Fabregat, S.; et al. Prevention of postoperative bleeding in hip fractures treated with prosthetic replacement: Efficacy and safety of fibrin sealant and tranexamic acid. A randomised controlled clinical trial (TRANEXFER study). Arch. Orthop. Trauma. Surg. 2019, 139, 597–604. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, J.; Liu, W.; Li, X.; Lu, H. Application of thromboelastography to evaluate the effect of different routes administration of tranexamic acid on coagulation function in total hip arthroplasty. J. Orthop. Surg. Res. 2019, 14, 430. [Google Scholar] [CrossRef]
- Masaryk, J.; Melus, V.; Vidan, J.; Steno, B. Comparison of intravenous and topical tranexamic acid in total joint arthroplasty. Acta Chir. Orthop. Traumatol. Cech. 2022, 89, 86–292. [Google Scholar] [CrossRef]
- Drakos, A.; Raoulis, V.; Karatzios, K.; Doxariotis, N.; Kontogeorgakos, V.; Malizos, K.; Varitimidis, S.E. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J. Orthop. Trauma. 2016, 30, 409–414. [Google Scholar] [CrossRef]
- Yee, D.K.H.; Wong, J.S.H.; Fang, E.; Wong, T.M.; Fang, C.; Leung, F. Topical administration of tranexamic acid in elderly patients undergoing short femoral nailing for intertrochanteric fracture: A randomised controlled trial. Injury 2022, 53, 603–609. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, W.; Xu, J.; Ding, J. Effect of topical tranexamic acid on post-traumatic elbow stiffness in patients treated with open arthrolysis: A prospective comparative study. J. Shoulder Elb. Surg. 2020, 29, 1375–1379. [Google Scholar] [CrossRef]
- Shen, J.; Yang, Z.; Fu, M.; Hao, J.; Jiang, W. The influence of topical use of tranexamic acid in reducing blood loss on early operation for thoracolumbar burst fracture: A randomized double-blinded controlled study. Eur. Spine J. 2021, 30, 3074–3080. [Google Scholar] [CrossRef]
- Pecold, J.; Al-Jeabory, M.; Krupowies, M.; Manka, E.; Smereka, A.; Ladny, J.R.; Szarpak, L. Tranexamic Acid for Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 11, 48. [Google Scholar] [CrossRef]
- Kachikis, A.; Gernsheimer, T.; Delaney, S. Tranexamic Acid for Prevention and Treatment of Postpartum Hemorrhage. University of Washington Obstetric Consensus Conference, June 2018. Anesth Analg. 2018. Available online: https://www.uwmedicine.org/sites/stevie/files/2020-11/Tranexamic-Acid-for-Prevention-and-Treatment-of-Postpartum-Hemorrhage-OB-Consensus-Conference.pdf (accessed on 8 January 2025).
- Rathore, M.; Gupta, A.; Kumari, N. Comparative study of topical and intravenous tranexamic acid for prevention of postpartum haemorrhage in placenta praevia cesarean section. Int. J. Clin. Obstet. Gynaecol. 2021, 5, 213–219. [Google Scholar] [CrossRef]
- Taha, A.A.; Shady, N.W.; Sallam, H.F.; Ahmed, M.M. Prophylactic use of temporary uterine packing combined with topical tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: A double-blind, randomized controlled trial. SVU-Int. J. Med. Sci. 2023, 6, 459–470. [Google Scholar] [CrossRef]
- Sallam, H.F.; Shady, N.W. Reducing blood loss during abdominal hysterectomy with intravenous versus topical tranexamic acid: A double-blind randomized controlled trial. J. Obstet. Gynecol. India 2019, 69, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Jain, K.; Singh, J.; Jindal, S.; Mehra, R.; Singh, S. Topical vs. intravenous administration of tranexamic acid to minimize blood loss in abdominal hysterectomy perioperatively: A randomized controlled study. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, M.; Tamai, H.; Miyake, M.; Shimizu, T. Uterine balloon tamponade in combination with topical administration of tranexamic acid for management of postpartum hemorrhage. Case Rep. Obstet. Gynecol. 2015, 2015, 195036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarris, I.; Arafa, A.; Konaris, L.; Kadir, R.A. Topical use of tranexamic acid to control perioperative local bleeding in gynaecology patients with clotting disorders: Two cases. Haemophilia 2007, 13, 115–116. [Google Scholar] [CrossRef]
- Ker, K.; Prieto-Merino, D.; Roberts, I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. J. Br. Surg. 2013, 100, 1271–1279. [Google Scholar] [CrossRef]
- Janbain, M.; Enjolras, N.; Bordet, J.C.; Bolbos, R.; Brevet, M.; Leissinger, C.; Dargaud, Y. Hemostatic effect of tranexamic acid combined with factor VIII concentrate in prophylactic setting in severe hemophilia A: A preclinical study. J. Thromb. Haemost. 2020, 18, 584–592. [Google Scholar] [CrossRef]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; AlSamkari, H.; DeLoughery, T.G.; Shatzel, J.J. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur. J. Haematol. 2020, 104, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Topical Hemostatic Agents at Time of Obstetric and Gynecologic Surgery. Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/10/topical-hemostatic-agents-at-time-of-obstetric-and-gynecologic-surgery (accessed on 8 January 2025).
- Zhong, Y.; Hu, H.; Min, N.; Wei, Y.; Li, X.; Li, X. Application and outlook of topical hemostatic materials: A narrative review. Ann. Transl. Med. 2021, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Steunenberg, T.A.; Bakker, N.C.; Wiersema, A.M.; Tournoij, E.; Yeung, K.K.; Jongkind, V. Efficacy and safety of tranexamic acid in non-cardiac arterial procedures: A systematic review and meta-analysis. Ann. Vasc. Surg. 2025, 116, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.M.; Clement, I.; Cheng, S.F.; Richards, T. Tranexamic acid does not cause clots: Experience in carotid endarterectomy. Br. J. Surg. 2023, 110, 1008–1010. [Google Scholar] [CrossRef]
- Nardi, K.; Pelone, G.; Bartolo, M.; Di Ruzza, M.R.; Storto, M.; Notte, A.; Vecchione, C. Ischaemic stroke following tranexamic acid in young patients carrying heterozygosity of MTHFR C677T. Ann. Clin. Biochem. 2011, 48, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, P.J.; Marcucci, M.; Painter, T.W.; Conen, D.; Lomivorotov, V.; Sessler, D.I.; Leslie, K. Tranexamic acid in patients undergoing noncardiac surgery. N. Engl. J. Med. 2022, 386, 1986–1997. [Google Scholar] [CrossRef]
- Şahin, Ö.; Taşcıoğlu, T.; Fırat, A.; Sürücü, H.S.; Çaydere, M. Topical tranexamic acid prevents scar tissue formation following craniectomy in a rat model. Eur. J. Med. Res. 2025, 30, 366. [Google Scholar] [CrossRef]
- Sipos, K.; Joensuu, K.; Kauhanen, S.; Ojala, K. Topical Tranexamic Acid and Chest Masculinization Surgeries—Impact on Postoperative Hematoma Incidence. JPRAS Open 2025, 43, 458–469. [Google Scholar] [CrossRef] [PubMed]
| Item | Specifications |
|---|---|
| Database | PubMed |
| Included search terms |
|
| Date range of published studies | Primarily 2015–2024, but earlier studies were included if relevant |
| Inclusion criteria | Peer review studies examining topical tranexamic acid use |
| Selection method | Two authors approved the inclusion of each study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarasinghe, R.; Sunoqrot, M.; Islam, S.; Gaddam, M.; Keivan, M.; Phillips, J.; Ahmadzia, H.K. Topical Tranexamic Acid Use Amongst Surgical Specialties: A Narrative Review. Surgeries 2025, 6, 69. https://doi.org/10.3390/surgeries6030069
Amarasinghe R, Sunoqrot M, Islam S, Gaddam M, Keivan M, Phillips J, Ahmadzia HK. Topical Tranexamic Acid Use Amongst Surgical Specialties: A Narrative Review. Surgeries. 2025; 6(3):69. https://doi.org/10.3390/surgeries6030069
Chicago/Turabian StyleAmarasinghe, Randilu, Mohammad Sunoqrot, Samita Islam, Medha Gaddam, Mona Keivan, Jaclyn Phillips, and Homa K. Ahmadzia. 2025. "Topical Tranexamic Acid Use Amongst Surgical Specialties: A Narrative Review" Surgeries 6, no. 3: 69. https://doi.org/10.3390/surgeries6030069
APA StyleAmarasinghe, R., Sunoqrot, M., Islam, S., Gaddam, M., Keivan, M., Phillips, J., & Ahmadzia, H. K. (2025). Topical Tranexamic Acid Use Amongst Surgical Specialties: A Narrative Review. Surgeries, 6(3), 69. https://doi.org/10.3390/surgeries6030069






