Achalasia and Gut Microbiota: Is Dysbiosis an Overlooked Factor in Postoperative Surgical Outcomes?
Abstract
1. Introduction
2. Materials and Methods
3. Esophageal Microbiota, Immune Response, and Surgical Implications
4. Post-Operative Complications and Dysbiosis: Evidence in General Surgery
4.1. Surgical Site Infections (SSI)
4.2. Postoperative Ileus and Neuroimmune Dysbiosis
4.3. Anastomotic Leakage
4.4. Systemic Dysbiosis and Aberrant Inflammatory Response
4.5. Relevance to Esophageal Achalasia
5. Esophageal Achalasia, Surgery, and Microbiota: A Theoretical but Plausible Interaction
5.1. Esophageal Microbial Environment in Achalasia: Stasis and Abnormal Bacterial Colonization
5.2. Effects of Surgery on the Gastroesophageal Microbiota
5.3. Analogous Findings from Related Surgeries: Fundoplication, Gastrectomy, Bariatric Surgery
5.4. Hypotheses for Future Investigations
6. Future Perspectives and Clinical Implications: Microbiota as an Emerging Target in Achalasia Surgery
6.1. Research Perspectives: Directions for Future Studies
6.1.1. Pre- and Postoperative Profiling of the Esophageal and Gastric Microbiota
6.1.2. Correlation with Local and Systemic Inflammatory Markers
6.1.3. Predictive Role of the Microbiota in Postoperative Outcomes
6.1.4. Microbiota Modulation Strategies
6.2. Translational Implications and Therapeutic Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| LES | Lower esophageal sphincter |
| POEM | Peroral endoscopic myotomy |
| HM | Heller myotomy |
| EF | Enterococcus faecalis |
| EC | Escherichia coli |
| TLRs | Activating Toll-like receptors |
| AM | Akkermansia muciniphila |
| SSI | Surgical site infections |
| GM | Gut microbiota |
| LPS | Lipopolysaccharides |
References
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of Functional Food Components on Gut Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The Gut Microbiota: A Treasure for Human Health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. Biomed. Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sood, U.; Gupta, V.; Singh, M.; Scaria, J.; Lal, R. Recent Advancements in the Development of Modern Probiotics for Restoring Human Gut Microbiome Dysbiosis. Indian. J. Microbiol. 2020, 60, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.; Jiang, Z. Dietary Additive Probiotics Modulation of the Intestinal Microbiota. Protein Pept. Lett. 2017, 24, 382–387. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Geng, Z.H.; Zhu, Y.; Chen, W.F.; Fu, P.Y.; Xu, J.Q.; Wang, T.Y.; Yao, L.; Liu, Z.Q.; Li, X.Q.; Zhang, Z.C.; et al. The Role of Type II Esophageal Microbiota in Achalasia: Activation of Macrophages and Degeneration of Myenteric Neurons. Microbiol. Res. 2023, 276, 127470. [Google Scholar] [CrossRef]
- Tustumi, F.; Arienzo, V.P.; Sunye, I.R.; Lucas, P.F.S.; Colonno, B.B.; Quintas, J.G.; Lisboa, E.N.; Szor, D.J. Esophageal Dysbiosis in Achalasia and Cancer Development: A Critical Review. Genes 2023, 14, 1521. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, D.; Zhuang, Q.; Hou, X.; Jia, X.; Chen, J.; Lin, H.; Zhang, M.; Tan, N.; Xiao, Y. Esophageal Microbial Dysbiosis Impairs Mucosal Barrier Integrity via Toll-like Receptor 2 Pathway in Patients with Gastroesophageal Reflux Symptoms. J. Transl. Med. 2024, 22, 1145. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sato, H.; Mizusawa, T.; Tominaga, K.; Ikarashi, S.; Hayashi, K.; Mizuno, K.I.; Hashimoto, S.; Yokoyama, J.; Terai, S. Comparison of Oral and Esophageal Microbiota in Patients with Achalasia before and after Peroral Endoscopic Myotomy. Turk. J. Gastroenterol. 2021, 32, 42–52. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut Microbiota Bridges Dietary Nutrients and Host Immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Abdalkareem Jasim, S.; Jade Catalan Opulencia, M.; Alexis Ramírez-Coronel, A.; Kamal Abdelbasset, W.; Hasan Abed, M.; Markov, A.; Raheem Lateef Al-Awsi, G.; Azamatovich Shamsiev, J.; Thaeer Hammid, A.; Nader Shalaby, M.; et al. The Emerging Role of Microbiota-Derived Short-Chain Fatty Acids in Immunometabolism. Int. Immunopharmacol. 2022, 110, 108983. [Google Scholar] [CrossRef]
- Guan, L.; Liu, R. The Role of Diet and Gut Microbiota Interactions in Metabolic Homeostasis. Adv. Biol. 2023, 7, e2300100. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Barranco, C. Microbiome-Targeted Diets That Alter Immune Status. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 594. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. Conference on Diet and Digestive Disease Symposium 2: Sensing and Signalling of the Gut Environment: Scfa: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-Related Changes in Intestinal Immunity and the Microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.S.; Bueno, S.M.; Kalergis, A.M. Contribution of Gut Microbiota to Immune Tolerance in Infants. J. Immunol. Res. 2021, 2021, 7823316. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B. The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection. Microbiol. Spectr. 2015, 3, MBP-0008-2014. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-Microbiota Interactions as Moderators of Human Metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Geagea, A.G.; Jurjus, A.; et al. Nutrition, Oxidative Stress and Intestinal Dysbiosis: Influence of Diet on Gut Microbiota in Inflammatory Bowel Diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Sugihara, K.; Kamada, N. Diet–Microbiota Interactions in Inflammatory Bowel Disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef]
- Ralls, M.W.; Miyasaka, E.; Teitelbaum, D.H. Intestinal Microbial Diversity and Perioperative Complications. J. Parenter. Enter. Nutr. 2014, 38, 392–399. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Babrowski, T.; Carlisle, E.M.; Olivas, A.; Romanowski, K.S.; Seal, J.B.; Liu, D.C.; Alverdy, J.C. The Human Microbiome and Surgical Disease. Ann. Surg. 2011, 253, 1094–1101. [Google Scholar] [CrossRef]
- Domingues, C.; Cabral, C.; Jarak, I.; Veiga, F.; Dourado, M.; Figueiras, A. The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review. Vaccines 2023, 11, 492. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.; Park, H.-M.; Kim, C.H.; Kim, H.R. Effect of Preoperative Immunonutrition on Fecal Microbiota in Colon Cancer Patients: A Secondary Analysis of a Randomized Controlled Trial. Nutr. Res. Pract. 2023, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, I.; Risaliti, M.; Ringressi, M.N.; Melli, F.; Nannini, G.; Amedei, A.; Muiesan, P.; Taddei, A. Role of Gut Microbiota-Immunity Axis in Patients Undergoing Surgery for Colorectal Cancer: Focus on Short and Long-Term Outcomes. World J. Gastroenterol. 2020, 26, 2498–2513. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, N.; Yan, X.; Kang, L.; Ou, G.; Zhou, Z.; Xu, C.; Feng, J.; Shi, T. Impact of Gut Microbiota on Colorectal Anastomotic Healing (Review). Mol. Clin. Oncol. 2025, 22, 52. [Google Scholar] [CrossRef]
- Willis, M.A.; Toews, I.; Soltau, S.L.V.; Kalff, J.C.; Meerpohl, J.J.; Vilz, T.O. Preoperative Combined Mechanical and Oral Antibiotic Bowel Preparation for Preventing Complications in Elective Colorectal Surgery. Cochrane Database Syst. Rev. 2023, 2023, CD014909. [Google Scholar] [CrossRef]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal Microbiota in Colorectal Cancer Surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Román-Sagüillo, S.; Porras, D.; García-Mediavilla, M.V.; Linares, P.; Ballesteros-Pomar, M.D.; Urioste-Fondo, A.; Álvarez-Cuenllas, B.; González-Gallego, J.; Sánchez-Campos, S.; et al. Long-Term Effects of Bariatric Surgery on Gut Microbiota Composition and Faecal Metabolome Related to Obesity Remission. Nutrients 2021, 13, 2519. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major Microbiota Dysbiosis in Severe Obesity: Fate after Bariatric Surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, K.N.; Pories, W.J. Bacteria with Potential: Improving Outcomes through Probiotic Use Following Roux-En-Y Gastric Bypass. Clin. Obes. 2023, 13, e12552. [Google Scholar] [CrossRef]
- Gentile, J.K.A.; Oliveira, K.D.; Pereira, J.G.; Tanaka, D.Y.; Guidini, G.N.; Cadona, M.Z.; Siriani-Ribeiro, D.W.; Perondini, M.T. The Intestinal Microbiome in Patients Undergoing Bariatric Surgery: A Systematic Review. Arq. Bras. De Cir. Dig. 2022, 35, e1707. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.S.M.; Rutegård, M.; Häggström, C.; Gylfe, Å.; Harlid, S.; Van Guelpen, B. Prior Antibiotics Exposure Is Associated with an Elevated Risk of Surgical Site Infections, Including Anastomotic Leakage, after Colon Cancer but Not Rectal Cancer Surgery: A Register-Based Study of 38,839 Patients. Int. J. Cancer 2025, 156, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Gonzalez, E.; Fragoso, G.; Oliero, M.; Alaoui, A.A.; Calvé, A.; Vennin Rendos, H.; Djediai, S.; Cuisiniere, T.; Laplante, P.; et al. Gut Microbiota Influence Anastomotic Healing in Colorectal Cancer Surgery through Modulation of Mucosal Proinflammatory Cytokines. Gut 2023, 72, 1143–1154. [Google Scholar] [CrossRef]
- Williamson, A.J.; Alverdy, J.C. Anastomotic Leaks in Colorectal Surgery: Influence of the Microbiome on Anastomotic Leak. Clin. Colon. Rectal Surg. 2021, 34, 439. [Google Scholar]
- Steyer, G.E.; Puchinger, M.; Pfeifer, J. Successful Clinical Avoidance of Colorectal Anastomotic Leakage through Local Decontamination. Antibiot. 2024, 13, 79. [Google Scholar] [CrossRef]
- Makanyengo, S.O.; Carroll, G.M.; Goggins, B.J.; Smith, S.R.; Pockney, P.G.; Keely, S. Systematic Review on the Influence of Tissue Oxygenation on Gut Microbiota and Anastomotic Healing. J. Surg. Res. 2020, 249, 186–196. [Google Scholar] [CrossRef]
- Chang, J.; Guyton, K. A Pathologic Microbiome Impacts Post-Operative Anastomotic Healing. Surg. Infect. 2023, 24, 238–244. [Google Scholar] [CrossRef]
- Arthur, J.C.; Jobin, C. The Struggle within: Microbial Influences on Colorectal Cancer. Inflamm. Bowel Dis. 2011, 17, 396–409. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- de Vos, W.M. Microbe Profile: Akkermansia Muciniphila: A Conserved Intestinal Symbiont That Acts as the Gatekeeper of Our Mucosa. Microbiology 2017, 163, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Evers, S.S.; Shin, J.H.; Ramakrishnan, S.K.; Bozadjieva-Kramer, N.; Yao, Q.; Shah, Y.M.; Sandoval, D.A.; Seeley, R.J. Vertical Sleeve Gastrectomy Increases Duodenal Lactobacillus Spp. Richness Associated with the Activation of Intestinal HIF2α Signaling and Metabolic Benefits. Mol. Metab. 2022, 57, 101432. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative Probiotics or Synbiotics in Adults Undergoing Elective Abdominal Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Q.; Liu, Y.; Fan, D. Effect of Perioperative Probiotics and Synbiotics on Postoperative Infections After Gastrointestinal Surgery: A Systematic Review With Meta-Analysis. J. Parenter. Enter. Nutr. 2017, 41, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Chen, C.C.; Chen, C.C.; Han, M.L.; Wu, J.F.; Wang, H.P.; Wu, M.S.; Tseng, P.H. Characteristics of the Esophageal Microbiome in Patients with Achalasia and Its Changes before and after Peroral Endoscopic Myotomy: A Pilot Study. J. Gastroenterol. Hepatol. 2023, 38, 1307–1315. [Google Scholar] [CrossRef]
- Lin, Y.J.; Liu, S.Z.; Li, L.S.; Han, K.; Shao, B.Z.; Linghu, E.Q.; Chai, N.L. Retrospective Study Repeat Peroral Endoscopic Myotomy with Simultaneous Submucosal and Muscle Dissection as a Salvage Option for Recurrent Achalasia. World J. Gastroenterol. 2023, 29, 2349–2358. [Google Scholar] [CrossRef]
- Sanaka, M.R.; Thota, P.N.; Parikh, M.P.; Hayat, U.; Gupta, N.M.; Gabbard, S.; Lopez, R.; Murthy, S.; Raja, S. Peroral Endoscopic Myotomy Leads to Higher Rates of Abnormal Esophageal Acid Exposure than Laparoscopic Heller Myotomy in Achalasia. Surg. Endosc. 2019, 33, 2284–2292. [Google Scholar] [CrossRef]
- Sasahira, M.; Matsumoto, H.; Go, T.T.; Yo, S.; Monden, S.; Ninomiya, T.; Oosawa, M.; Handa, O.; Umegaki, E.; Inoue, R.; et al. The Relationship Between Bacterial Flora in Saliva and Esophageal Mucus and Endoscopic Severity in Patients with Eosinophilic Esophagitis. Int. J. Mol. Sci. 2025, 26, 3026. [Google Scholar] [CrossRef]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the Microbiome: Advantages of Whole Genome Shotgun versus 16S Amplicon Sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef]
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial Biota in the Human Distal Esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255. [Google Scholar] [CrossRef]
- Norder Grusell, E.; Dahlén, G.; Ruth, M.; Bergquist, H.; Bove, M. The Cultivable Bacterial Flora of the Esophagus in Subjects with Esophagitis. Scand. J. Gastroenterol. 2018, 53, 650–656. [Google Scholar] [CrossRef]
- Blackett, K.L.; Siddhi, S.S.; Cleary, S.; Steed, H.; Miller, M.H.; MacFarlane, S.; MacFarlane, G.T.; Dillon, J.F. Oesophageal Bacterial Biofilm Changes in Gastro-Oesophageal Reflux Disease, Barrett’s and Oesophageal Carcinoma: Association or Causality? Aliment. Pharmacol. Ther. 2013, 37, 1084–1092. [Google Scholar] [CrossRef]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, T.; Nomoto, K.; Onodera, H. Significant Changes in the Intestinal Environment After Surgery in Patients with Colorectal Cancer. J. Gastrointest. Surg. 2013, 17, 1657–1664. [Google Scholar] [CrossRef]
- Bashir, H.; Singh, S.; Singh, R.P.; Agrewala, J.N.; Kumar, R. Age-mediated Gut Microbiota Dysbiosis Promotes the Loss of Dendritic Cells Tolerance. Aging Cell 2023, 22, e13838. [Google Scholar] [CrossRef]
- Elashiry, M.M.; Elashiry, M.; Zeitoun, R.; Elsayed, R.; Tian, F.; Saber, S.E.; Elashry, S.H.; Tay, F.R.; Cutler, C.W. Enterococcus Faecalis Induces Differentiation of Immune-Aberrant Dendritic Cells from Murine Bone Marrow-Derived Stem Cells. Infect. Immun. 2020, 88, e00338-20. [Google Scholar] [CrossRef] [PubMed]
- Schlechte, J.; Zucoloto, A.Z.; Yu, I.-L.; Doig, C.J.; Dunbar, M.J.; McCoy, K.D.; McDonald, B. Dysbiosis of a Microbiota–Immune Metasystem in Critical Illness Is Associated with Nosocomial Infections. Nat. Med. 2023, 29, 1017–1027. [Google Scholar] [CrossRef]
- Grasa, L.; Abecia, L.; Forcén, R.; Castro, M.; de Jalón, J.A.G.; Latorre, E.; Alcalde, A.I.; Murillo, M.D. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microb. Ecol. 2015, 70, 835–848. [Google Scholar] [CrossRef]
- Ma, T.R.; Xue, X.L.; Tian, H.; Zhou, X.X.; Wang, J.K.; Zhao, Z.W.; Wang, M.F.; Song, J.Y.; Feng, R.X.; Li, L.; et al. Effect of the Gut Microbiota and Their Metabolites on Postoperative Intestinal Motility and Its Underlying Mechanisms. J. Transl. Med. 2023, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Hussain, Z.; Lee, Y.J.; Park, H. An Altered Composition of Fecal Microbiota, Organic Acids, and the Effect of Probiotics in the Guinea Pig Model of Postoperative Ileus. Neurogastroenterol. Motil. 2021, 33, e13966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Zhou, Y.; Wu, X. Effects of Fiber and Probiotics on Diarrhea Associated with Enteral Nutrition in Gastric Cancer Patients. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef]
- Hussain, Z.; Park, H. Inflammation and Impaired Gut Physiology in Post-Operative Ileus: Mechanisms and the Treatment Options. J. Neurogastroenterol. Motil. 2022, 28, 517–530. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Wei, Y. New Understanding of Gut Microbiota and Colorectal Anastomosis Leak: A Collaborative Review of the Current Concepts. Front. Cell Infect. Microbiol. 2022, 12, 1022603. [Google Scholar] [CrossRef]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; An, G.; et al. Collagen Degradation and MMP9 Activation by Enterococcus Faecalis Contribute to Intestinal Anastomotic Leak. Sci. Transl. Med. 2015, 7, 286ra68. [Google Scholar] [CrossRef]
- Muske, J.; Knoop, K. Contributions of the Microbiota to the Systemic Inflammatory Response. Microbiota Host 2023, 1, e230018. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Kamolratanakul, S.; Schultz, M.J.; Leelahavanichkul, A. The Leaky Gut and the Gut Microbiome in Sepsis—Targets in Research and Treatment. Clin Sci 2023, 137, 645–662. [Google Scholar] [CrossRef]
- Jung, D.H.; Youn, Y.H.; Kim, D.H.; Lim, C.H.; Lim, H.S.; Moon, H.S.; Lee, J.Y.; Park, H.; Hong, S.J. Esophageal Microbiota and Nutritional Intakes in Patients With Achalasia Before and After Peroral Endoscopic Myotomy. J. Neurogastroenterol. Motil. 2022, 28, 237–246. [Google Scholar] [CrossRef]
- Palomba, G.; Capuano, M.; Basile, R.; Sorrentino, G.; Fernicola, A.; Anoldo, P.; Milone, M.; De Palma, G.D.; Aprea, G. Robotic Surgery in Achalasia: State of the Art. Chirurgia 2023, 118, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Freschi, G.; Ringressi, M.N.; Pallecchi, L.; Rossolini, G.M.; Bechi, P. The Esophageal Microbiota in Health and Disease. Ann. N. Y. Acad. Sci. 2017, 1381, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Feng, L.; Cai, X.; Qian, Y.; Xu, L. Esophageal Microflora in Esophageal Diseases. Front. Cell Infect. Microbiol. 2023, 13, 1145791. [Google Scholar] [CrossRef] [PubMed]
- Barchi, A.; Massimino, L.; Mandarino, F.V.; Vespa, E.; Sinagra, E.; Almolla, O.; Passaretti, S.; Fasulo, E.; Parigi, T.L.; Cagliani, S.; et al. Microbiota Profiling in Esophageal Diseases: Novel Insights into Molecular Staining and Clinical Outcomes. Comput. Struct. Biotechnol. J. 2023, 23, 626–637. [Google Scholar] [CrossRef]
- Amir, I.; Konikoff, F.M.; Oppenheim, M.; Gophna, U.; Half, E.E. Gastric Microbiota Is Altered in Oesophagitis and Barrett’s Oesophagus and Further Modified by Proton Pump Inhibitors. Environ. Microbiol. 2014, 16, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Fuccio, L.; Maselli, R.; Mazza, F.; Correale, L.; Mandolesi, D.; Bellisario, C.; Sethi, A.; Khashab, M.A.; Rösch, T.; et al. Gastroesophageal Reflux Disease after Per-Oral Endoscopic Myotomy as Compared with Heller’s Myotomy with Fundoplication: A Systematic Review with Meta-Analysis. Gastrointest. Endosc. 2018, 87, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.; Machado, M.; Barbosa, J.P.; Barbosa, J. Achalasia: Laparoscopic Heller Myotomy with Fundoplication versus Peroral Endoscopic Myotomy—A Systematic Review and Meta-Analysis. Esophagus 2024, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, S.A.; Burt, M.; Rwigema, J.-C.; Anderson, D.; Chao, J.; Kuchta, K.; Liu, N.; Hedberg, H.M.; Ujiki, M.B. Decision Regret for Patients Undergoing Laparoscopic Heller Myotomy and Per Oral Endoscopic Myotomy. J. Gastrointest. Surg. 2025. [Google Scholar] [CrossRef]
- Itskoviz, D.; Malnick, S.D.H. Gastroesophageal Reflux Following Peroral Endoscopic Myotomy for Achalasia: Bumps in the Road to Success. World J. Gastroenterol. 2024, 30, 3461–3464. [Google Scholar] [CrossRef]
- Harris, J.K.; Fang, R.; Wagner, B.D.; Choe, H.N.; Kelly, C.J.; Schroeder, S.; Moore, W.; Stevens, M.J.; Yeckes, A.; Amsden, K.; et al. Esophageal Microbiome in Eosinophilic Esophagitis. PLoS ONE 2015, 10, e0128346. [Google Scholar] [CrossRef]
- Liu, N.; Ando, T.; Ishiguro, K.; Maeda, O.; Watanabe, O.; Funasaka, K.; Nakamura, M.; Miyahara, R.; Ohmiya, N.; Goto, H. Characterization of Bacterial Biota in the Distal Esophagus of Japanese Patients with Reflux Esophagitis and Barrett’s Esophagus. BMC Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Vittori, A.; Capovilla, G.; Salvador, R.; Santangelo, M.; Provenzano, L.; Nicoletti, L.; Costantini, A.; Forattini, F.; Pittacolo, M.; Moletta, L.; et al. Laparoscopic Fundoplication Improves Esophageal Motility in Patients with Gastroesophageal Reflux Disease: A High-Volume Single-Center Controlled Study in the Era of High-Resolution Manometry and 24-Hour PH Impedance. J. Gastrointest. Surg. 2024. [Google Scholar] [CrossRef]
- Maksimaityte, V.; Bausys, A.; Kryzauskas, M.; Luksta, M.; Stundiene, I.; Bickaite, K.; Bausys, B.; Poskus, T.; Bausys, R.; Strupas, K. Gastrectomy Impact on the Gut Microbiome in Patients with Gastric Cancer: A Comprehensive Review. World J. Gastrointest. Surg. 2021. [Google Scholar] [CrossRef]
- Basso, N.; Soricelli, E.; Castagneto-Gissey, L.; Casella, G.; Albanese, D.; Fava, F.; Donati, C.; Tuohy, K.; Angelini, G.; La Neve, F.; et al. Insulin Resistance, Microbiota, and Fat Distribution Changes by a New Model of Vertical Sleeve Gastrectomy in Obese Rats. Diabetes 2016, 65, 2990–3001. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Nossa, C.W.; Francois, F.; Peek, R.M.; Pei, Z. Inflammation and Intestinal Metaplasia of the Distal Esophagus Are Associated With Alterations in the Microbiome. Gastroenterology 2009, 137, 588–597. [Google Scholar] [CrossRef]
- Deshpande, N.P.; Riordan, S.M.; Gorman, C.J.; Nielsen, S.; Russell, T.L.; Correa-Ospina, C.; Fernando, B.S.M.; Waters, S.A.; Castaño-Rodríguez, N.; Man, S.M.; et al. Multi-Omics of the Esophageal Microenvironment Identifies Signatures Associated with Progression of Barrett’s Esophagus. Genome Med. 2021, 13, 133. [Google Scholar] [CrossRef]
- Kinross, J.M.A.; Markar, S.; Karthikesalingam, A.; Chow, A.; Penney, N.; Silk, D.; Darzi, A. A Meta-Analysis of Probiotic and Synbiotic Use in Elective Surgery: Does Nutrition Modulation of the Gut Microbiome Improve Clinical Outcome? J. Parenter. Enter. Nutr. 2013, 37, 243–253. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.; Majaw, J.K.; Liang, L.; Chen, Y. Efficacy of Lactobacillus Reuteri Supplementation Therapy for Helicobacter Pylori Eradication: A Meta-Analysis of Randomised Controlled Trials. Med. Microecol. 2021, 8, 100036. [Google Scholar] [CrossRef]
- Yang, C.; Liang, L.; Lv, P.; Liu, L.; Wang, S.; Wang, Z.; Chen, Y. Effects of Non-Viable Lactobacillus Reuteri Combining with 14-Day Standard Triple Therapy on Helicobacter Pylori Eradication: A Randomized Double-Blind Placebo-Controlled Trial. Helicobacter 2021. [Google Scholar] [CrossRef]
- Emara, M.H.; Mohamed, S.Y.; Abdel Aziz, H.R. Lactobacillus Reuteri in Management of Helicobacter Pylori Infection in Dyspeptic Patients: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Ther. Adv. Gastroenterol. 2014. [Google Scholar] [CrossRef]
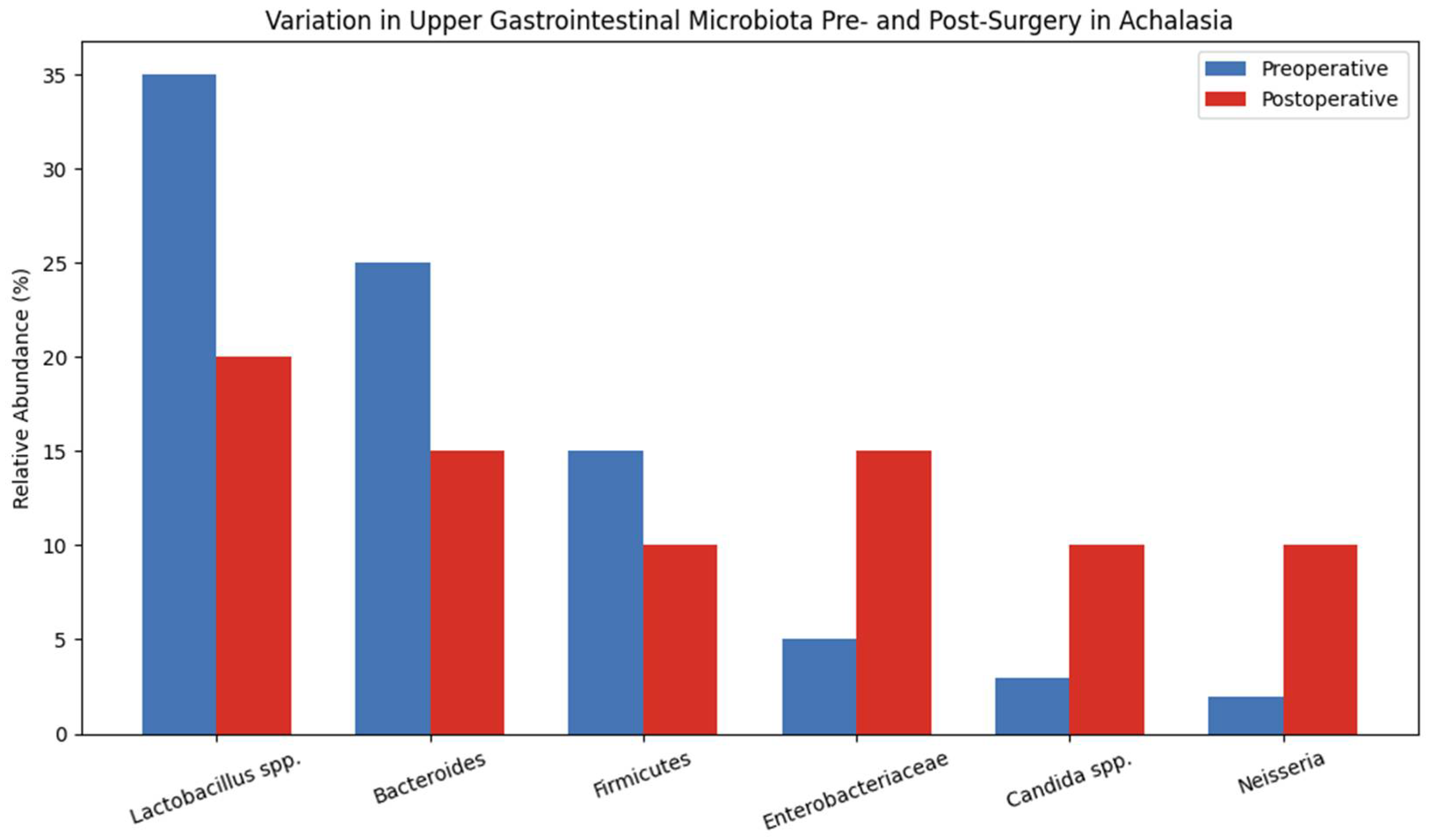
| Microorganism | Role in Microbiota/Dysbiosis | Clinical Implications | Notes in Achalasia/Postoperative Context |
|---|---|---|---|
| PREVOTELLA SPP. | Increased in esophageal stasis and inflammation | Potential pro-inflammatory role | High abundance in dilated esophagus |
| FUSOBACTERIUM SPP. | Associated with mucosal inflammatory processes | Potential promotion of chronic inflammation | Elevated in reflux and stasis |
| ENTEROBACTERIACEAE | Proliferation in bile reflux-altered environment | Associated with mucosal damage and potential infection | Increased post-POEM or biliary reflux |
| FIRMICUTES (REDUCTION) | Reduction in beneficial species | Loss of barrier and immune control | Reduced microbial diversity in achalasia |
| LACTOBACILLUS SPP. | Protective fermentative bacteria | Potential role in maintaining equilibrium | Decreased after reflux-inducing procedures |
| CANDIDA SPP. | Opportunistic fungi proliferating in dysbiosis | Risk of opportunistic infections in compromised esophagus | Reported in patients with stasis and esophageal dysfunction |
| Condition | Type of Dysbiosis Reported | Pathogenetic Mechanism | Relevance for Achalasia |
|---|---|---|---|
| CHRONIC ESOPHAGEAL STASIS | Increase in oral bacteria (Prevotella, Fusobacterium) | Slowed transit → retrograde bacterial growth | Typical scenario in untreated achalasia |
| GASTROESOPHAGEAL REFLUX | Reduced diversity, increase in pro-inflammatory microbes | Acid/bile reflux → hostile microenvironment | Possible after myotomy without fundoplication |
| EXPOSURE TO BILIARY SECRETIONS | Increase in Enterobacteriaceae, reduction in Firmicutes | Duodenal reflux → mucosal damage and microbial selection | Risk after POEM procedure |
| ADVANCED ACHALASIA | Scarce data (hypothetical increase in anaerobic microorganisms) | Stasis and esophageal dilation → fermentative environment | Central hypothesis to be tested in future studies |
| Surgical Technique | Anatomical–Functional Modifications | Potential Impact on Microbiota | Clinical Notes |
|---|---|---|---|
| HELLER MYOTOMY + FUNDOPLICATION | LES release + partial antireflux barrier | Minimal reflux, lower risk of dysbiosis | Protective standard against dysbiosis |
| POEM | LES release without antireflux protection | Increased acid/bile reflux → possible dysbiosis | May alter microbial environment long-term |
| LAPAROSCOPIC MYOTOMY | Like Heller but sometimes without fundoplication | Depends on presence of antireflux barrier | Postoperative microbiological studies lacking |
| Field | Possible Clinical Applications | Open Research Questions |
|---|---|---|
| DIAGNOSTICS | Microbiota profiling pre- and post-intervention | Are there dysbiotic phenotypes predictive of poor surgical outcomes? |
| PREVENTION | Prebiotic/probiotic interventions in selected patients | Can microbiota modulation reduce postoperative complications? |
| PERSONALIZED TREATMENT | Surgical technique choice based on individual microbiota | Can microbiota guide choice between POEM and Heller + fundoplication? |
| POSTOPERATIVE SURVEILLANCE | Use of microbial markers for monitoring | Can dysbiosis predict recurrent symptoms or persistent reflux? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernicola, A.; Palomba, G.; Calogero, A.; Sciarra, A.; Cavaliere, A.; Crocetto, F.; Sagnelli, C.; Alvigi, A.; Basile, R.; Pignatelli, D.; et al. Achalasia and Gut Microbiota: Is Dysbiosis an Overlooked Factor in Postoperative Surgical Outcomes? Surgeries 2025, 6, 63. https://doi.org/10.3390/surgeries6030063
Fernicola A, Palomba G, Calogero A, Sciarra A, Cavaliere A, Crocetto F, Sagnelli C, Alvigi A, Basile R, Pignatelli D, et al. Achalasia and Gut Microbiota: Is Dysbiosis an Overlooked Factor in Postoperative Surgical Outcomes? Surgeries. 2025; 6(3):63. https://doi.org/10.3390/surgeries6030063
Chicago/Turabian StyleFernicola, Agostino, Giuseppe Palomba, Armando Calogero, Antonella Sciarra, Annachiara Cavaliere, Felice Crocetto, Caterina Sagnelli, Antonio Alvigi, Raffaele Basile, Domenica Pignatelli, and et al. 2025. "Achalasia and Gut Microbiota: Is Dysbiosis an Overlooked Factor in Postoperative Surgical Outcomes?" Surgeries 6, no. 3: 63. https://doi.org/10.3390/surgeries6030063
APA StyleFernicola, A., Palomba, G., Calogero, A., Sciarra, A., Cavaliere, A., Crocetto, F., Sagnelli, C., Alvigi, A., Basile, R., Pignatelli, D., Paolillo, A., D’Alessio, F. M., Benassai, G., Quarto, G., & Santangelo, M. (2025). Achalasia and Gut Microbiota: Is Dysbiosis an Overlooked Factor in Postoperative Surgical Outcomes? Surgeries, 6(3), 63. https://doi.org/10.3390/surgeries6030063








