A Systematic Review of the Optilume® Drug-Coated Balloon in the Management of LUTS Secondary to BPH and Urethral Stricture
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
- Population: The study population consisted of male patients undergoing Optilume balloon dilation for anterior urethral strictures or BPO.
- Intervention: The placement of the Optilume drug-coated balloon.
- Comparison: Studies comparing Optilume with conventional treatments such as urethrotomy or mechanical dilation were included.
- Outcomes: The primary endpoint was the assessment of the efficacy of Optilume in treating BPO and US.
- Study Type: Randomised controlled trials (RCTs), cohort studies, and case reports evaluating the Optilume device were included. Reviews, commentaries, corrections, and updates were excluded. Only English-language studies were considered.
2.3. Data Extraction and Quality Assessment
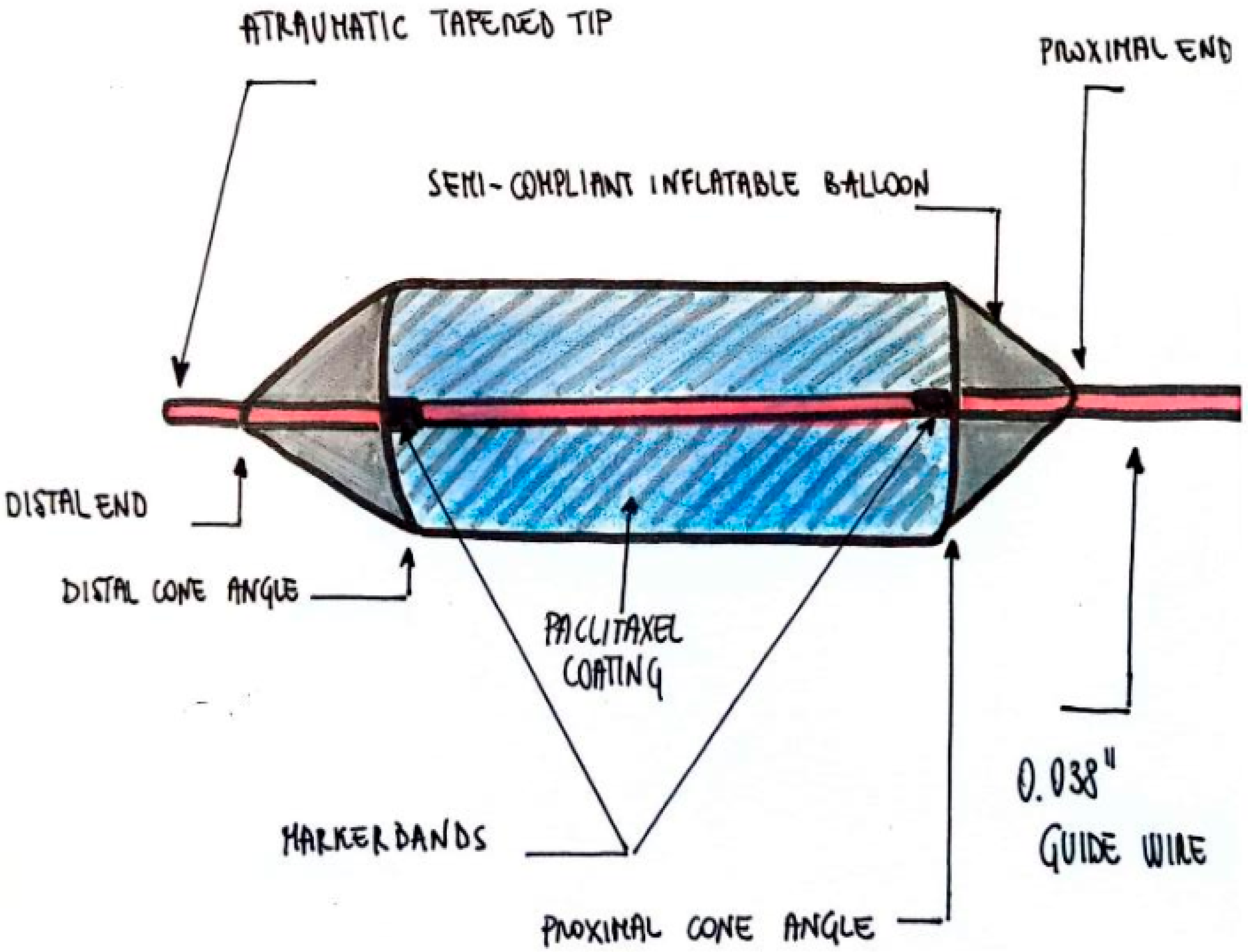
2.4. Device Description
- Inflatable balloon with a paclitaxel coating and a double-wall thickness
- Two marker bands at the ends of the balloon
- A distal end (bladder neck direction) and a proximal end (urethral meatus direction)
- Distal and proximal cone angles
- Atraumatic tapered tip [16]
2.5. Technical Aspects of the Procedure
- 18 and 24 Fr balloons: 12 ATM
- 30 Fr balloon: 10 ATM
- 36 Fr balloon: 8 ATM
2.6. Ethical Considerations
3. Results
- Immediate Post-operative Period: Monitoring for acute complications such as bleeding, pain, or urinary retention.
- 1 Week Post-Procedure: Assessment of early recovery and identification of any emerging side effects.
- 1 Month Post-Procedure: Evaluation of short-term outcomes and resolution of initial side effects.
- 3, 6, and 12 Months Post-Procedure: Long-term follow-up to assess the durability of the treatment effect and identify any late-onset complications.

| Author | Title | Type of Study | Primary Outcome | FU/yrs/mo |
|---|---|---|---|---|
| Delong, January 2022 | Robust II [25] | RCT-single blind | Functional improvement | 1 yr |
| Stuehmeier, March 2022 | Optilume drug-coated balloon dilation in complex female urethral stricture [26] | Case report | Functional improvement in female | 6 mo |
| Elliott, April 2022 | Robust III study [21] | RCT-single-blind | Functional improvement | 1 yr |
| Virasoro, May 2022 | Robust I Study [25] | RCT-single blind | Functional improvement | 3 yr |
| Kelly, April 2023 | Economic evaluation of Optilume, a drug-coated balloon for recurrent anterior male urethral stricture [23] | Study of cohort | Cost-saving |
3.1. Primary Results
3.1.1. Benign Prostatic Hyperplasia (BPH)
3.1.2. Urethral Stricture (US)
- Robust I established anatomical success as the ability to pass a 16 Fr flexible cystoscope or a 14 Fr Foley catheter. While cystoscopy was not performed beyond one year, functional success—defined as a ≥50% IPSS improvement without retreatment—was achieved in 70% of patients (32/46) at two years.
- Robust II confirmed the safety profile of Optilume, demonstrating sustained anatomical and symptomatic improvements at six months and one year.
- Robust III, a randomised controlled trial, reported one-year outcomes. The average time for drug-coated balloon (DCB) insertion and removal was 8 min and 42 s. At six months, anatomical success was significantly higher in the OCS group than in the control group (74.6% vs. 26.8%). The one-year recurrence-free rate also favoured OCS. Qmax initially improved in both groups, but while control patients exhibited deterioration after three months, the OCS cohort maintained a nearly twofold increase at one year. PVR levels remained higher in the control group at six months and one year compared to baseline.
3.2. Secondary Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elterman, D.; Gao, B.; Lu, S.; Bhojani, N.; Zorn, K.C.; Chughtai, B. New Technologies for Treatment of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2022, 49, 11–22. [Google Scholar] [CrossRef]
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines: Urethral Stricture. 2023. Available online: https://uroweb.org/guidelines/urethral-strictures/chapter/disease-management-in-males (accessed on 1 July 2025).
- de Kock, M.L.; Allen, F.J. Guidelines for the treatment of urethral strictures. S. Afr. J. Surg. Suid-Afr. Tydskr. Vir Chir. 1989, 27, 182–184. [Google Scholar] [PubMed]
- Chilton, C.P.; Shah, P.J.; Fowler, C.G.; Tiptaft, R.C.; Blandy, J.P. Optical urethrotomy, a modified technique: 6 years of experience. J. Urol. 1988, 140, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, J.W.; Heyns, C.F.; de Kock, M.L. The impact of optical urethrotomy on the management of urethral strictures. Br. J. Urol. 1983, 55, 705–710. [Google Scholar] [CrossRef]
- Steenkamp, J.W.; Heyns, C.F.; de Kock, M.L. Internal urethrotomy versus dilation as treatment for male urethral strictures: A prospective, randomized comparison. J. Urol. 1997, 157, 98–101. [Google Scholar] [CrossRef]
- Vyas, J.B.; Ganpule, A.P.; Muthu, V.; Sabnis, R.B.; Desai, M.R. Balloon dilatation for male urethral strictures “revisited”. Urol Ann. 2013, 5, 245–248. [Google Scholar] [CrossRef]
- Virasoro, R.; DeLong, J.M.; Mann, R.A.; Estrella, R.E.; Pichardo, M.; Lay, R.R.; Espino, G.; Roth, J.D.; Elliott, S.P. A drug-coated balloon treatment for urethral stricture disease: Interim results from the ROBUST I study. Can. Urol. Assoc. J. 2020, 14, 187–191. [Google Scholar] [CrossRef]
- Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhanel, M.; Spruss, T.; Bernhardt, G.; Graeff, C.; Farber, L.; Gschaidmeier, H.; et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Investig. 2002, 110, 1309–1318. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Donehower, R.C. Drug Therapy: Paclitaxel (Taxol®). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef]
- Axel, D.I.; Kunert, W.; Göggelmann, C.; Oberhoff, M.; Herdeg, C.; Küttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.; Köveker, G.; et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997, 96, 636–645. [Google Scholar] [CrossRef]
- Scheller, B.; Speck, U.; Abramjuk, C.; Bernhardt, U.; Böhm, M.; Nickenig, G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 2004, 110, 810–814. [Google Scholar] [CrossRef]
- Herten, M.; Torsello, G.B.; Schonefeld, E.; Stahlhoff, S. Critical appraisal of paclitaxel balloon angioplasty for femoral-popliteal arterial disease. Vasc. Health Risk Manag. 2016, 12, 341–356. [Google Scholar] [CrossRef]
- Will, T.A.; Polcari, A.J.; Garcia, J.G.; Ouwenga, M.K.; Voelzke, B.B.; Greisler, H.P.; Turk, T.M. Paclitaxel Inhibits Ureteral Smooth Muscle Cell Proliferation and Collagen Production in the Absence of Cell Toxicity. Turk. J. Urol. 2011, 185, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Dean SElterman, M.D.; Karl Coutinho, M.D.; Judith CHagedorn, M.D. How I Do It: The Optilume drug-coated balloon for urethral strictures. Can. J. Urol. 2020, 27, 10322–10328. [Google Scholar]
- Kaplan, S.A.; Pichardo, M.; Rijo, E.; Espino, G.; Lay, R.R.; Estrella, R. Estrella One-year outcomes after treatment with a drug-coated balloon catheter system for lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2021, 24, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- FDA Optilume® Urethral Drug Coated Balloon—P210020. 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P210020 (accessed on 1 July 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence. 2009. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 1 July 2025).
- Elliott, S.P.; Coutinho, K.; Robertson, K.J.; D’Anna, R.; Chevli, K.; Carrier, S.; Aube-Peterkin, M.; Cantrill, C.H.; Ehlert, M.J.; Te, A.E.; et al. One-Year Results for the ROBUST III Randomized Controlled Trial Evaluating the Optilume® Drug-Coated Balloon for Anterior Urethral Strictures. J. Urol. 2022, 207, 866–875. [Google Scholar] [CrossRef]
- McVary, K.T.; Gange, S.N.; Gittelman, M.C.; Goldberg, K.A.; Patel, K.; Shore, N.D.; Levin, R.M.; Rousseau, M.; Beahrs, J.R.; Kaminetsky, J.; et al. Minimally invasive prostate convective water vapor energy ablation: A multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2016, 195, 1529–1538. [Google Scholar] [CrossRef]
- MacDiarmid, S.A.; Harrigan, C.T.; Cottone, J.L.; McIntyre, W.J.; Johnson, D.E. Assessment of a new transurethral balloon dilation catheter in the treatment of urethral stricture disease. Urology 2000, 55, 408–413. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Moss, J.; Freedman, S.; Coutinho, K.; Wu, N.; Efros, M.; Elterman, D.; D’Anna, R.; Padron, O.; Robertson, K.J.; et al. The PINNACLE Study: A Double-blind, Randomized, Sham-controlled Study Evaluating the Optilume BPH Catheter System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J. Urol. 2023, 210, 500–509. [Google Scholar] [CrossRef]
- Mann, R.; Delong, J.; Virasoro, R.; Elliott, S. pd35-08 4 years of the optilume® drug coated balloon for recurrent anterior urethral strictures: A summary of robust I, II, III. J. Urol. 2023, 4, e977. [Google Scholar] [CrossRef]
- Stuehmeier, J.; Jelisejevas, L.A.; Kink, P.; Gulacsi, A.; Horninger, W.; Rehder, P. Optilume® drug-coated balloon dilation in complex female urethral stricture. Urol. Case Rep. 2022, 41, 101987. [Google Scholar] [CrossRef]
- Ebbing, J.; Bachmann, A. Anesthesia-free procedures for benign prostate obstruction: Worth it. Curr. Opin. Urol. 2015, 25, 32–39. [Google Scholar] [CrossRef]
- Westwood, J.; Geraghty, R.; Jones, P.; Rai, B.P.; Somani, B.K. Rezūm: A new transurethral water vapour therapy for benign prostatic hyperplasia. Ther. Adv. Urol. 2018, 10, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Cocci, A.; Bocchino, A.C.; Cito, G.; Lisa, A.; Russo, G.I.; Giudice, A.L.; Sessa, F.; Viola, L.; Cindolo, L.; Somani, B.K.; et al. Role of Rezum in the treatment of benign prostate hyperplasia: A review of the literature. Turk. J. Urol. 2021, 47, 452–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eardley, I. FDA approves the Optilume urethral drug coated balloon for the treatment of urethral strictures. In BJU International; John Wiley & Sons: Hoboken, NJ, USA. [CrossRef]
- Miller, L.E.; Chughtai, B.; Dornbier, R.A.; McVary, K.T. Surgical Reintervention Rate after Prostatic Urethral Lift: Systematic Review and MetaAnalysis Involving over 2000 Patients. J. Urol. 2020, 204, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; Gange, S.N.; Shore, N.D.; Giddens, J.L.; Bolton, D.M.; Cowan, B.E.; Brown, B.T.; McVary, K.T.; Te, A.E.; Gholami, S.S.; et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: The LIFT study. J. Urol. 2013, 190, 2161–2167. [Google Scholar] [CrossRef]
- Kelly, L.; Shore, J.; Wright, J.; Patrick, C.; Holmes, H. Economic evaluation of Optilume, a drug-coated balloon for recurrent anterior male urethral stricture. BJUI Compass 2023, 4, 430–436. [Google Scholar] [CrossRef]
- Cornu, J.N.; Zantek, P.; Burtt, G.; Martin, C.; Martin, A.; Springate, C.; Chughtai, B. Minimally Invasive Treatments for Benign Prostatic Obstruction: A Systematic Review and Network Meta-analysis. Eur. Urol. 2023, 83, 534–547. [Google Scholar] [CrossRef]
- Porto, J.G.; Titus, R.; Camargo, F.; Bhatia, A.; Ahie, N.; Blachman-Braun, R.; Malpani, A.; Lopategui, D.M.; Herrmann, T.R.; Marcovich, R.; et al. Minimally invasive techniques in quest of Holy Grail of surgical management of enlarged prostates: A narrative review. World J. Urol. 2024, 42, 35. [Google Scholar] [CrossRef] [PubMed]

| FDA Approval (OCS) | No FDA Approval (OCS) |
|---|---|
| Obstructive urinary symptoms associated with BPH or with anterior urethral stricture | Meatal stenosis |
| Adult male (age 50 and older) | Neck stenosis |
| Previous surgery for urethral stricture | Posterior urethral stricture |
| Anterior urethral stricture of ≤3 cm in length. | Urethro-vesical anastomosis post RP |
| Optilume™ DCB | mechanical dilation+ paclitaxel | - Minimally invasive procedure. - Potentially reduces recurrence rates - Short catheterisation | - Long-term efficacy - 70% anatomical success rate at 12 months for short bulbar strictures. | Minimally | Short bulbar strictures (<2 cm) and BPH | ~70% at 12 mo | Data emerging |
| Urethral Dilation | Gradual stretching of the urethra | - Simple and quick procedure. - Minimal equipment required. | High recurrence rates; - Risk of urethral trauma and false passages. | Minimally | Initial management of short strictures | Variable | High |
| DVIU | Endoscopic incision of the stricture to restore urethral patency | Minimally invasive with quick recovery. | - High recurrence rates - Not recommended for long or complex strictures | Minimally | Single, short bulbar strictures | ~20% long-term | High |
| Urethroplasty | Surgical reconstruction using grafts/flaps. | - High long-term success rates (>85%). - Definitive treatment with low recurrence. | - Invasive procedure. - Longer recovery period. | Invasive | Long, recurrent, or complex strictures | >85% | Low |
| Author | Title | Type of Study | Primary Outcome | FU/yr |
|---|---|---|---|---|
| Pichardo, August 2023 | EVEREST-I Study [22] | RCT-open-label, single-arm | Functional improvement | 2 yr |
| Kaplan, September 2023 | PINNACLE Study [24] | RCT-prospective, randomised, double-blind | Functional improvement | 1 yrs |
| Study Optilume BPH/US | Urinary Tract Infection | Haematuria | Urinary Retention | Urinary Incontinence | Dysuria | Ejaculation Disorder | Bladder Spasms | Urinary Urgency |
|---|---|---|---|---|---|---|---|---|
| EVEREST-1 (Tot. patients n. 80) [22] | 7 | 11 | 8 | 5 | 6 | 7 | - | - |
| PINNACLE (Tot. patients n. 98) [24] | 14 | 39 | 7 | 9 | 4 | 6 | 6 | |
| Robust III (tot. Patients n. 98) [20] | 1 | 11 | - | - | 2 | 0 | - | - |
| Robust II (tot. Patients n. 16) [25] | 4 | 1 | 1 | - | - | 0 | 1 | 2 |
| Robust I (tot. Patients n. 35) [25] | 1 | 1 | 1 | 1 | 1 | 0 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colalillo, G.; Ippoliti, S.; Asimakopoulos, A.D. A Systematic Review of the Optilume® Drug-Coated Balloon in the Management of LUTS Secondary to BPH and Urethral Stricture. Surgeries 2025, 6, 59. https://doi.org/10.3390/surgeries6030059
Colalillo G, Ippoliti S, Asimakopoulos AD. A Systematic Review of the Optilume® Drug-Coated Balloon in the Management of LUTS Secondary to BPH and Urethral Stricture. Surgeries. 2025; 6(3):59. https://doi.org/10.3390/surgeries6030059
Chicago/Turabian StyleColalillo, Gaia, Simona Ippoliti, and Anastasios D. Asimakopoulos. 2025. "A Systematic Review of the Optilume® Drug-Coated Balloon in the Management of LUTS Secondary to BPH and Urethral Stricture" Surgeries 6, no. 3: 59. https://doi.org/10.3390/surgeries6030059
APA StyleColalillo, G., Ippoliti, S., & Asimakopoulos, A. D. (2025). A Systematic Review of the Optilume® Drug-Coated Balloon in the Management of LUTS Secondary to BPH and Urethral Stricture. Surgeries, 6(3), 59. https://doi.org/10.3390/surgeries6030059







